-
PDF
- Split View
-
Views
-
Cite
Cite
Michael ToVinh, Gregor Hörr, Kristiyana Dobrikova, Christina Gotter, Clemens Rommel, Christoph Hoffmeister, Jan Raabe, Kim M Kaiser, Claudia Finnemann, Jenny Bischoff, Gereon J Rieke, Christoph Wilhelm, Vanessa Schmitt, Christoph Möhl, Mansoureh Aghabeig, Carolynne Schwarze-Zander, Christoph Boesecke, Kathrin van Bremen, Jan Christian Wasmuth, Christian P Strassburg, Jürgen K Rockstroh, Ulrich Spengler, Benjamin Krämer, Jacob Nattermann, Mitochondrial Dysfunction Contributes to Impaired Cytokine Production of CD56bright Natural Killer Cells From Human Immunodeficiency Virus–Infected Individuals Under Effective Antiretroviral Therapy, The Journal of Infectious Diseases, Volume 226, Issue 5, 1 September 2022, Pages 901–906, https://doi.org/10.1093/infdis/jiac103
Close - Share Icon Share
Abstract
Human immunodeficiency virus (HIV) infection is associated with impaired natural killer (NK) cell activity, which is only incompletely restored under antiretroviral therapy. Analyzing the bioenergetics profiles of oxygen consumption, we observed that several parameters were significantly reduced in HIV+ NK cells, indicating a mitochondrial defect. Accordingly, we found HIV+ CD56bright NK cells to display a decreased mitochondrial membrane potential and mitochondrial mass. Both parameters were positively correlated with interferon gamma (IFN-γ) production of NK cells. Finally, we demonstrated that stimulation of HIV+ NK cells with MitoTEMPO, a mitochondria-targeting antioxidant, significantly improved IFN-γ production. We identified mitochondrial dysfunction as a mechanism that contributes to impaired NK cell function.
AIDS-related mortality has decreased significantly since the introduction of antiretroviral therapy (ART). However, human immunodeficiency virus (HIV)–infected (HIV+) patients still have a shorter life expectancy than the HIV-uninfected (HIV–) population due to a significantly increased risk of non-AIDS-related morbidities. For example, HIV+ persons are less likely to spontaneously clear acute hepatitis C compared to HIV– patients and display a significantly faster progression of liver disease once chronic hepatitis C has been established [1].
Moreover, ART reduces the risk of some of the cancers considered “AIDS-associated,” such as Kaposi sarcoma of the skin, whereas “non-AIDS-associated” tumors, such as cervical cancer, lung cancer, or bladder cancer, seem to occur more frequently than in the general population [2].
Aside from the undesirable effects of ART, immunodeficiency, and immunodysregulation, which persist despite drug inhibition, seem to be important in this context. Natural killer (NK) cells play a special role here.
NK cells are a heterogeneous subpopulation of lymphocytes, characterized by their ability to lyse malignant transformed or virus-infected cells without prior “priming.” Accordingly, NK cells are considered to importantly contribute to antiviral immune responses and antitumor immune surveillance.
HIV has developed a number of mechanisms to evade recognition by NK cells such as altering expression of NK cell receptor ligands, which interferes with recognition by NK cells [3]. Apart from that, NK cells from HIV patients also appear to display an intrinsic functional defect independent of altered expression of NK cell receptors/ligands. In previous studies, we observed an impaired cytokine secretion in NK cells of HIV patients, which did not completely normalize under effective ART and was particularly found in interleukin 2 (IL-2)–stimulated cells. As a result, HIV+ NK cells were found to exhibit impaired antifibrotic and antiviral activity [4].
The underlying mechanisms are currently insufficiently understood. Here, we show that mitochondrial dysfunction may contribute to impaired NK cell activity.
MATERIALS AND METHODS
Patients
A total of 88 aviremic HIV patients under ART and 10 viremic treatment-naive HIV patients were recruited at the HIV outpatient clinic of the University Hospital Bonn, Germany, after written informed consent was obtained. Thirty-one HIV– donors served as controls. Further patient characteristics are given in Supplementary Table 1. This study was approved by the Institutional Review Board of the University Hospital Bonn (DZIF HIV cohort 279/14 and study cohort 035/14).
Cell Isolation
Peripheral blood mononuclear cells were isolated using Ficoll-Paque gradient centrifugation (Pan Biotech, Germany). Purification of NK cells was performed by isolation kit and negative magnetic separation (Miltenyi Biotech, Germany).
Analysis of Interferon-γ Production
A total of 25 000 IL-2 (25 U/mL)–preactivated NK cells were co-cultured with Jurkat target cells (E:T ratio 1:2) or stimulated with interleukin 12 (IL-12) (20 ng/mL) and interleukin 15 (IL-15) (50 ng/mL) (Immunotools, Germany) at 37°C. After 1 hour, Golgi-Stop (BD Bioscience) and brefeldin A (Enzo Biochem) were added and cells were cultured for an additional 5 hours. After washing with Dulbecco’s phosphate-buffered saline, NK cells were stained with Zombie-Aqua, anti-CD56-APC, anti-CD3-APC fire, and anti-CD16-PerCP (Biolegend). For detection of intracellular interferon gamma (IFN-γ), cells were permeabilized using Cytofix/Cytoperm (BD Bioscience) and stained with anti-IFN-γ-PE (R&D). When indicated, NK cells were treated with 10 µM MitoTEMPO (Sigma-Aldrich) for 1 hour at 37°C before overnight IL-2 stimulation. Samples were analyzed using FACSCanto II (BD Bioscience) and FlowJo software V10.7.1 (BD Bioscience).
In Vitro Analysis of Antiretroviral Drug Effects on NK Cells
For testing potential effects of ART on NK cell activity, NK cells were cultivated overnight with, respectively, therapeutic concentrations of ritonavir (1.0 mg/mL), emtricitabine/tenofovir alafenamide (1.0 mg/mL), tenofovir disoproxil fumarate/emtricitabine (0.2 mg/mL), or dolutegravir (4.0 mg/mL) before co-culturing with Jurkat cells.
Detection of Signaling Pathway Molecules
IL-2–stimulated NK cells were stained with anti-CD56-BV421 and anti-CD3-APC fire 700 (Biolegend), washed, mixed with ice-cold methanol, and incubated for 30 minutes at 4°C. Then, cells were resuspended in BD Permwash Buffer and stained with anti-pERK-fluorescein, anti-pAKT-AF488, anti-pSTAT5-PE, and anti-pSTAT4-AF488 antibodies (R&D/BD Bioscience).
Measurement of Mitochondrial Respiration
Real-time analyses of NK cell oxygen consumption rate (controls, n = 9; HIV+, n = 9) was performed using a Seahorse XFe-96 Analyzer (Agilent Technologies) according to the manufacturer’s protocol. In brief, purified IL-2–activated NK cells were resuspended in XF base and assay medium, adhered on a CellTaq-coated XF 96-well microplate (200 000 cells per well) and starved in a non–carbon dioxide chamber at 37°C for 1 hour. Oxygen consumption rate measurement was started under basal conditions, followed by the injection of oligomycin (1 µM), carbonylcyanide-4(trifluoromethoxy) phenylhydrazone (FCCP) (1 µM), and rotenone/antimycin A (both 500 nM).
Analyses of Mitochondrial Mass and Mitochondrial Membrane Potential
Purified NK cells (50 000 cells) were incubated with Zombie Aqua, followed by staining with anti-CD56-APC, anti-CD3-APC fire, and anti-CD16-PerCP/-FITC. Then NK cells were washed, stimulated with either 100 nM MitoTrackerGreen for mitochondrial mass (MTG; Thermo Fisher Scientific) or 25 nM tetramethylrhodamine-ethylester for mitochondrial membrane potential (TMRE; Cayman Chemicals) for 30 minutes at 37°C and finally analyzed using FACSCanto II and FlowJo software V10.7.1.
Mitochondrial Morphology
IL-2–preactivated NK cells (1 × 106 cells) from HIV– controls (n = 3) and HIV+ patients (n = 6) stained with Zombie Aqua, anti-CD56-BV421, anti-CD3-PE (Biolegend), and 10 nM MTG were analyzed by imaging flow cytometry using an Amnis ImageStreamX Mark II Imaging Flow Cytometer (Luminex) according to the manufacturer’s protocol. Data were acquired at ×60 magnification. Analysis of viable CD3–CD56+ MTG+ NK cells was performed using IDEAS Software (Luminex) and Fiji/ImageJ.
Statistical Analyses
After testing for normal distribution, differences between 2 groups were assessed using the (un)paired Wilcoxon-Mann-Whitney statistical test or Student t test. Differences among 3 or more groups were assessed using Kruskal–Wallis test corrected for multiple comparison by controlling false discovery rate (Benjamini–Krieger–Yekutieli method). Correlations were tested by Pearson or Spearman test. Statistical analysis was performed using Prism version 9.3.0 (GraphPad Software).
RESULTS
Both after co-culturing with target cells and after stimulation with IL-12/IL-15, decreased IFN-γ production was found in HIV+ NK cells (Figure 1A), indicating a general, stimulus-independent defect. Accordingly, analyzing the expression of pAKT, pERK, pSTAT4, and pSTAT5 did not indicate any defect in the IL-2 or IL-15 signaling pathway (Figure 1B). A similar NK cell dysfunction was also observed in treatment-naive HIV+ patients (Supplementary Figure 1A), whereas culturing of HIV– NK cells with typical therapeutic concentration of different antiretroviral drugs did not affect IFN-γ production (Supplementary Figure 1B). Thus, ART-induced impairment of NK cell activity could be excluded.
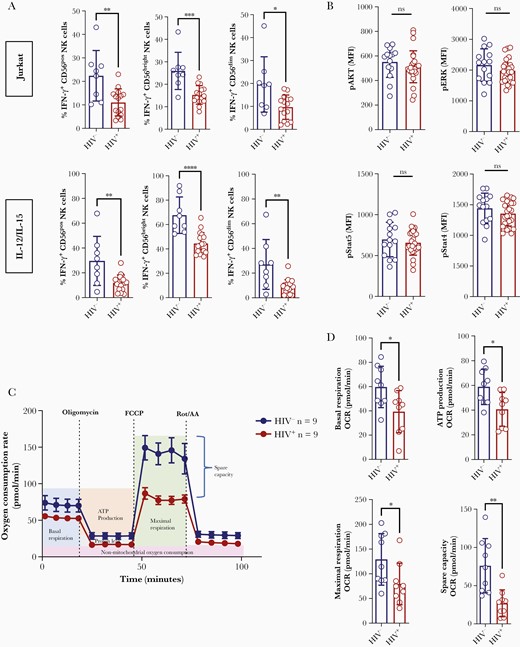
Mitochondrial dysfunction in HIV+ natural killer (NK) cells. A, Purified interleukin 2–preactivated NK cells from HIV– donors and HIV+ individuals under effective antiretroviral therapy were co-cultured with Jurkat cells (upper panels; HIV–, n = 8 and HIV+, n = 14) or stimulated with interleukin 12 (IL-12)/interleukin 15 (lower panels; HIV–, n = 8 and HIV+, n = 17) for 6 hours. Then, interferon-γ production was analyzed by flow cytometry. B, Expression of pAKT, pERK, pSTAT4, and pSTAT5 in HIV– (n = 14) and HIV+ (n = 26) NK cells analyzed by flow cytometry. C, Analysis of the bioenergetic profile by measurement of oxygen consumption rate in HIV– (n = 9, curve above) and HIV+ (n = 9, curve below) purified IL-2–preactivated NK cells. D, Comparison of basal respiration, ATP production, maximal respiration, and spare capacity in HIV– and HIV+ NK cells (each group, n = 9) based on the data display in C. Differences between groups in A, B, and D were analyzed by Student t test or Wilcoxon–Mann–Whitney statistical test. P > .05, not significant; *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001. Abbreviations: FCCP, carbonyl cyanide-4(trifluoromethoxy) phenylhydrazone; HIV–, human immunodeficiency virus negative; HIV+, human immunodeficiency virus positive; IFN-γ, interferon gamma; IL, interleukin; MFI, mean fluorescence intensity; NK, natural killer; ns, not significant; OCR, oxygen consumption rate.
Metabolic processes critically modulate for NK cell function, and metabolic reprogramming has been shown to be necessary for optimal NK cell responses to a cytokine stimulus [5]. Therefore, we speculated that dysregulation of intracellular metabolic processes might be involved in HIV-associated impairment of NK cell activity. To determine whether cellular metabolism was altered in HIV infection, we measured the bioenergetic profiles of oxygen consumption in HIV+ NK cells compared with HIV– controls, in the baseline condition and after addition of oligomycin (to block ATP synthesis), FCCP (to uncouple ATP synthesis from the electron transport chain), and rotenone/antimycin A (to block complexes I and III) (Figure 1C). These analyses indicated a significant mitochondrial defect in HIV+ NK cells as we observed significant reductions in oxygen consumption rate (an indicator of oxidative phosphorylation), mitochondrial spare respiratory capacity (an indicator of cell fitness), and maximal respiratory rate as well as ATP production in HIV+ patients (Figure 1D). To further corroborate these findings, we next analyzed mitochondrial membrane potential, a key readout of mitochondrial function, by TMRE staining. As shown in Figure 2A, we found HIV+ CD56bright NK cells to display a significantly decreased mitochondrial membrane potential compared to HIV– controls. Moreover, HIV infection was associated with a reduced mitochondrial mass in CD56bright NK cells (Figure 2A). No such effect were observed in CD56dim NK cells. Of note, both membrane potential and mitochondrial mass were positively correlated with IFN-γ production of CD56bright NK cells (Figure 2B). This association could also be confirmed when HIV– and HIV+ donors were analyzed separately (Supplementary Figure 2). To further assess HIV-associated morphological changes, we next analyzed NK cell mitochondria by imaging flow cytometry, which demonstrated a contracted and more spherical appearance in HIV+ NK cells compared to tubular-shaped mitochondria in HIV– controls. Quantitative analyses of these data confirmed HIV+ CD56bright NK cells to display a reduced mean mitochondrial area but increased mitochondrial fragmentation compared to controls (Figure 2C). Finally, we found that stimulation of HIV+ NK cells with MitoTEMPO, a mitochondria-targeting antioxidant, significantly improved IFN-γ production of CD56bright NK cells (Figure 2D), which further supported a role for HIV-associated mitochondria dysfunction in NK cell impairment.
![Correlation between natural killer (NK) cell interferon gamma (IFN-γ) production and mitochondrial function. A, Mitochondrial membrane potential (tetramethylrhodamine-ethylester [TMRE]) and mitochondrial mass (MitoTrackerGreen [MTG]) were assessed in interleukin 2 (IL-2)–preactivated purified NK cells from HIV+ individuals under effective antiretroviral therapy (ART) (TMRE, n = 20; MTG, n = 24) and HIV– controls (TMRE, n = 10; MTG, n = 14) by flow cytometry. B, Correlation (Pearson test) between CD56bright NK cell IFN-γ production and mitochondrial membrane potential (TMRE) (HIV–, n = 10; HIV+, n = 9) or mitochondrial mass (MTG) (HIV–, n = 11; HIV+, n = 12). C, Representative imaging of MTG-stained mitochondria in CD56bright NK cells from HIV– individuals and HIV+ donors under effective ART acquired by imaging flow cytometry (left) and average mitochondrial area (upper right graph) and fragmentation level (lower right graph) in CD56bright NK cells. For quantitative analysis, 25 randomly selected images from each individual (HIV–, n = 3; HIV+, n = 6) were analyzed using Fiji/ImageJ. D, Percentage of IFN-γ–producing CD56bright NK cells from HIV– controls (n = 8) and HIV+ individuals under effective ART (n = 15) after stimulation with the mitochondria-targeting antioxidant MitoTEMPO. Boxes indicate the interquartile range and whiskers the lower and upper quartiles. Differences between groups in A, C, and D were analyzed by Student t test or Wilcoxon–Mann–Whitney statistical test. P > .05, not significant; *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001. Abbreviations: HIV–, human immunodeficiency virus negative; HIV+, human immunodeficiency virus positive; IFN-γ, interferon gamma; MTG, MitoTrackerGreen; NK, natural killer; ns, not significant; RFI, relative fluorescence intensity; TMRE, tetramethylrhodamine-ethylester.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/226/5/10.1093_infdis_jiac103/1/m_jiac103_fig2.jpeg?Expires=1749856035&Signature=T7H9sAhG2PdqJp6uqJgvOxDP035oSyQcL34tQy5HgGK-IjWS-IzB5ftj-0wuyAj-GrSBoqhS4X06pQJpbkIw-H8MgwmLsMxkJ6WNwlEH-Wm-m1QVdJiVHv0kgvg1uHq4KmNW9524-4yJM-Mr1vqw-1B4X2n~X4lCNc5zav6ZAKaAzPWFdbGI-Yi1P~QJiAiHtlCHIMStSUyqjIXVlQ79gWl4e9stwEgQ5OoE3Pa43mVuB93KPyrjVNmBngKXxnjw0cLaqtf05zmG63yvCVFBHrC8s3xM9y7LfuPffMixbydn-WYV5Gbm9MnxHiI5nR5FfG7yl6jqQLFsUas8XbSF1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation between natural killer (NK) cell interferon gamma (IFN-γ) production and mitochondrial function. A, Mitochondrial membrane potential (tetramethylrhodamine-ethylester [TMRE]) and mitochondrial mass (MitoTrackerGreen [MTG]) were assessed in interleukin 2 (IL-2)–preactivated purified NK cells from HIV+ individuals under effective antiretroviral therapy (ART) (TMRE, n = 20; MTG, n = 24) and HIV– controls (TMRE, n = 10; MTG, n = 14) by flow cytometry. B, Correlation (Pearson test) between CD56bright NK cell IFN-γ production and mitochondrial membrane potential (TMRE) (HIV–, n = 10; HIV+, n = 9) or mitochondrial mass (MTG) (HIV–, n = 11; HIV+, n = 12). C, Representative imaging of MTG-stained mitochondria in CD56bright NK cells from HIV– individuals and HIV+ donors under effective ART acquired by imaging flow cytometry (left) and average mitochondrial area (upper right graph) and fragmentation level (lower right graph) in CD56bright NK cells. For quantitative analysis, 25 randomly selected images from each individual (HIV–, n = 3; HIV+, n = 6) were analyzed using Fiji/ImageJ. D, Percentage of IFN-γ–producing CD56bright NK cells from HIV– controls (n = 8) and HIV+ individuals under effective ART (n = 15) after stimulation with the mitochondria-targeting antioxidant MitoTEMPO. Boxes indicate the interquartile range and whiskers the lower and upper quartiles. Differences between groups in A, C, and D were analyzed by Student t test or Wilcoxon–Mann–Whitney statistical test. P > .05, not significant; *P ≤ .05; **P ≤ .01; ***P ≤ .001; ****P ≤ .0001. Abbreviations: HIV–, human immunodeficiency virus negative; HIV+, human immunodeficiency virus positive; IFN-γ, interferon gamma; MTG, MitoTrackerGreen; NK, natural killer; ns, not significant; RFI, relative fluorescence intensity; TMRE, tetramethylrhodamine-ethylester.
DISCUSSION
NK cells critically contribute to antiviral immune responses but are functionally impaired in HIV infection, even in patients under effective therapy.
Analyzing the bioenergetic profiles of NK cells, we observed oxygen consumption rate, mitochondrial spare respiratory capacity, maximal respiratory rate, and ATP production to be significantly reduced in HIV+ patients. Moreover, we found HIV infection to be associated with a significant decrease of NK cell mitochondrial membrane potential and mitochondrial mass, which all together indicated mitochondrial dysfunction in HIV+ NK cells.
As for all lymphocytes, metabolic processes and changes in metabolic activity after activation are essential for NK cells to exert effector functions [5, 6]. Mitochondria play a central role in this context. Recently, Surace and colleagues demonstrated mitochondrial dynamics and oxidative phosphorylation to orchestrate NK cell function [7]. Zheng et al showed polarization of mitochondria to be critical for the fitness of mature NK cells and demonstrated mitochondrial fragmentation to reduce the cytotoxic NK cell activity [8]. In line with these observations, we found membrane potential and mitochondrial mass to positively correlate with IFN-γ production of CD56bright NK cells, indicating mitochondrial dysfunction to contribute to impaired HIV+ NK cell activity. Accordingly, we found MitoTEMPO, a mitochondria-targeting antioxidant, to partly restore IFN-γ production of HIV+ NK cells.
The exact mechanisms underlying NK cell mitochondrial dysfunction in HIV patients remain to be clarified. An effect of antiretroviral drugs seems unlikely, as we observed similar NK cell dysfunction in treatment-naive patients and no effect of ART drugs on the function of healthy NK cells was detectable in vitro. Alternatively, a direct HIV-mediated effect would be conceivable, as several HIV-encoded proteins have been shown to affect mitochondrial integrity and functions [9]. However, we observed NK cell mitochondrial dysfunction also in patients under ART with viral loads below the level of detection, which might argue against such a mechanism. However, we cannot exclude that reactivation of latent virus reservoirs may play a role here, as data obtained in the simian immunodeficiency virus–infected nonhuman primate model suggest that NK cells in lymph nodes are continuously activated, likely by ongoing low-level virus replication [10]. Alternatively, HIV-associated chronic immune activation may play a role in this context. Cytokines such as tumor necrosis factor–α can impede mitochondrial oxidative phosphorylation and stimulate the production of reactive oxygen species, leading to mitochondrial membrane permeabilization and changes in mitochondrial dynamics [11, 12]. Unfortunately, neither lymph node nor colon tissue was available to address these hypotheses. Moreover, we did not delineate a biochemical cascade leading to metabolic dysregulation and dysfunction. Further studies are needed to clarify these questions.
Our observation of NK cell mitochondrial dysfunction in HIV infection resembled earlier studies analyzing CD8+ T-cell functions in viral hepatitis, which might indicate that both innate and adaptive immune responses are affected by metabolic alterations in viral infection. For example, Fisicaro et al demonstrated exhausted hepatitis B virus–specific CD8+ T cells to display mitochondrial dysfunction with reduced mitochondrial mass and membrane potential [13], whereas Barili et al found also metabolic exhausted T cells in hepatitis C infection [14].
The identification of mitochondrial dysfunction as a mechanism contributing to impaired NK cell function points to a potential new approach to restore HIV+ NK cell activity. Accordingly, we observed treatment of HIV+ NK cells with MitoTEMPO, a mitochondria-targeting antioxidant [15], to significantly improve IFN-γ production of CD56bright NK cells.
Therefore, further studies exploring the mechanisms underlying HIV-associated defects in NK cell mitochondria and testing approaches to overcome these dysfunctions appear as interesting concepts to improve NK cell function in HIV+ patients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Presented in part: Third International Conference on Innate Lymphoid Cells, Tokyo, Japan, 2018 (abstract P-96); 35th Annual Meeting of the German Association of the Study of the Liver, Heidelberg, Germany, February 2019 (abstract 5.45); and 18th Meeting of the Society for Natural Immunity, Luxembourg City, Luxembourg (abstract 209).
Acknowledgments. We thank the Flow Cytometry Core Facility of the Medical Faculty at the University of Bonn for providing support, especially Maximilian Germer, and instrumentation funded by the German Research Foundation (project number 389568007). We are also grateful to the patients and donors who volunteered to participate in this study, making this research possible.
Financial support. This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) under Germany’s Excellence Strategy (EXC2151–390873048 to C. W. and J. N.; DFG SPP1937 and SFB TR57 to J. N.); the German Liver Foundation (S163/10103/2014-BN to C. B.); the Hector Foundation (PrEP-M90 to C. B. and M88 to J. N.); the BONFOR program of the University of Bonn (2020-1A-08 to J. B. and 2019-1A-09 to G. J. R.); NEAT ID (ProbeC 2014 and NEAT ID Integration Grant 2016 to C. B.); and the German Centre for Infection Research (05.811_00 to C. B. and TTU 04.816, 04.817, and 04.819 to J. N.).
References
Author notes
B. K. and J. N. contributed equally to this work as joint senior authors.
Potential conflicts of interest. G. J. R. has received travel expenses and honoraria from Gilead. C. B. has received honoraria for consulting or speaking at educational events from AbbVie, Gilead, Janssen, MSD, and ViiV. J. K. R. has received honoraria for consulting or speaking at educational events from Abivax, Gilead, Janssen, Merck, Theratechnologies, and ViiV. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




