-
PDF
- Split View
-
Views
-
Cite
Cite
Danuta M Skowronski, Solmaz Setayeshgar, Macy Zou, Natalie Prystajecky, John R Tyson, Hind Sbihi, Chris D Fjell, Eleni Galanis, Monika Naus, David M Patrick, Shiraz El Adam, May A Ahmed, Shinhye Kim, Bonnie Henry, Linda M N Hoang, Manish Sadarangani, Agatha N Jassem, Mel Krajden, Comparative Single-Dose mRNA and ChAdOx1 Vaccine Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2, Including Variants of Concern: Test-Negative Design, British Columbia, Canada, The Journal of Infectious Diseases, Volume 226, Issue 3, 1 August 2022, Pages 485–496, https://doi.org/10.1093/infdis/jiac023
Close - Share Icon Share
Abstract
In British Columbia, Canada, most adults 50–69 years old became eligible for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in April 2021, with chimpanzee adenoviral vectored vaccine (ChAdOx1) restricted to ≥55-year-olds and second doses deferred ≥6 weeks to optimize single-dose coverage.
Among adults 50–69 years old, single-dose messenger RNA (mRNA) and ChAdOx1 vaccine effectiveness (VE) against SARS-CoV-2 infection and hospitalization, including variant-specific, was assessed by test-negative design between 4 April and 2 October 2021.
Single-dose VE included 11 861 cases and 99 544 controls. Median of postvaccination follow-up was 32 days (interquartile range, 15–52 days). Alpha, Gamma, and Delta variants comprised 23%, 18%, and 56%, respectively, of genetically characterized viruses. At 21–55 days postvaccination, single-dose mRNA and ChAdOx1 VE (95% confidence interval [CI]) was 74% (71%–76%) and 59% (53%–65%) against any infection and 86% (80%–90%) and 94% (85%–97%) against hospitalization, respectively. VE (95% CI) was similar against Alpha and Gamma infections for mRNA (80% [76%–84%] and 80% [75%–84%], respectively) and ChAdOx1 (69% [60%–76%] and 66% [56%–73%], respectively). mRNA VE was lower at 63% (95% CI, 56%–69%) against Delta but 85% (95% CI, 71%–92%) against Delta-associated hospitalization (nonestimable for ChAdOx1).
A single mRNA or ChAdOx1 vaccine dose gave important protection against SARS-CoV-2, including early variants of concern. ChAdOx1 VE was lower against infection, but 1 dose of either vaccine reduced the hospitalization risk by >85% to at least 8 weeks postvaccination. Findings inform program options, including longer dosing intervals.
In Canada, 2 messenger RNA (mRNA) vaccines against SARS-CoV-2 were approved in December 2020 according to a 2-dose schedule with an interval between doses of 3 weeks for BNT162b2 (Pfizer-BioNTech) and 4 weeks for mRNA-1273 (Moderna) [1]. On 26 February 2021, a chimpanzee adenoviral vectored (ChAdOx1) vaccine (Covishield, AstraZeneca) was authorized as 2 doses spaced 4–12 weeks apart [1]. In randomized controlled trial (RCT) analyses, single-dose efficacy against symptomatic SARS-CoV-2 illness was 92%–93% for mRNA vaccines [2, 3] and 76% for ChAdOx1 [4].
In early January 2021, confronted with elevated SARS-CoV-2 pandemic activity and constrained vaccine supplies, the province of British Columbia (BC), Canada, extended the interval between first and second doses to 6 weeks to enable more people to benefit from substantial single-dose protection. On 2 March 2021 (epi-week 9), Canada’s National Advisory Committee on Immunization (NACI) extended the interval up to 16 weeks between doses [5]. BC followed this NACI recommendation but as vaccine supply improved, shorter intervals and a preference for 6–8 weeks between doses resumed in August 2021 [6]. On 29 March 2021 (epi-week 13), NACI further recommended that ChAdOx1 be limited to adults ≥55 years old due to emerging global reports of vaccine-associated thrombosis with thrombocytopenia, lowering that age limit to ≥30 years on 23 April 2021 (epi-week 16) [7, 8].
Community vaccination in BC, Canada, followed a sequential age-based strategy, prioritizing mRNA vaccines for the eldest citizens beginning around epi-week 10 (7 March 2021). The spring 2021 pandemic wave in BC peaked in mid-April and was comprised of unique Alpha and Gamma variant of concern (VOC) co-circulation [9–11]. Thereafter, the Delta VOC increased through the summer months, comprising virtually all characterized viruses from August and through the autumn 2021 wave [9–11].
Using a test-negative design (TND), we previously reported single-dose mRNA vaccine effectiveness (VE) among adults ≥70 years old, showing the infection risk was reduced by about two-thirds overall and comparably against Alpha and Gamma variants [12]. Too few older adults had received ChAdOx1 to enable its VE estimation, but this became feasible when most adults 50–69 years old next became vaccine-eligible around epi-week 14 (4 April 2021). Herein we apply the same TND approach to compare single-dose mRNA and ChAdOx1 VE against SARS-CoV-2 infection and hospitalization, including VOC-associated, among adults 50–69 years old in BC, Canada.
METHODS
Study Design and Analysis Period
We estimated single-dose VE by TND, using multivariable logistic regression to derive the adjusted odds ratio (AOR) for vaccination among SARS-CoV-2 test-positive cases vs test-negative controls. VE and 95% confidence intervals (CIs) were computed as (1 – AOR) × 100%. Adjusted models included age group (50–59, 60–69 years), sex (male/female), individual epi-week of specimen collection (categorical), and region (5 health authority categories). Analyses spanned epi-weeks 14–39 (4 April–2 October) of 2021, taking into account single-dose coverage, second-dose rollout and censoring, and VOC contribution to variant-specific estimation.
Data Sources
There are approximately 1.4 million adults aged 50–69 years in BC, of whom about half are 50–59 years and half are women [13]. A publicly funded, foremost symptom-based approach for SARS-CoV-2 diagnostic testing was broadly accessible in BC during the study period. Specimens were sampled from the provincial database capturing all nucleic acid amplification tests (NAATs) for SARS-CoV-2 in BC along with client, collection, and testing details. Because symptoms and onset dates are not consistently captured, we assessed VE against any infection timed upon specimen collection date. Hospitalized cases were identified through linkage with the notifiable disease list. Vaccination details were obtained from the provincial immunization registry. Individual-level database linkages were achieved through unique personal identifiers.
Vaccine Status Definition
Clients with record of a single dose of mRNA or ChAdOx1 vaccine on or before specimen collection were considered vaccinated. Individuals were censored from specimen contribution upon receipt of a second dose. Those with no record of SARS-CoV-2 vaccination on or before specimen collection were considered unvaccinated.
In RCT analyses, a lag of ≥14 days between first dose and symptom onset is typically required to consider a case vaccine-preventable [2, 3]. Among adults 50–69 years old with both dates available in our study, the median interval and interquartile range (IQR) between symptom onset and specimen collection was 2 (1–5) days. Taking additional lag between onset and specimen collection into account, we defined vaccine status by receipt ≥21 days before specimen collection while exploring a range of intervals. We primarily used the period 21–55 days postvaccination to standardize follow-up time in comparing single-dose VE by vaccine type and VOC, notably given the later circulation of Delta compared to Alpha or Gamma variants.
Case Definition and VOC Analyses
Cases included any NAAT-confirmed infection. Hospitalized cases were any cases identified to have been hospitalized on or within 30 days following specimen collection. Individuals could contribute a single test-positive specimen and were censored thereafter.
The methods and sampling frame for genetic characterization of viruses evolved in response to changing epidemic patterns, variant emergence, case load, and laboratory capacity (Supplementary Material 1) [10, 11, 14, 15]. Between 30 May and 31 August 2021 (epi-weeks 22–35), the BC Centre for Disease Control Public Health Laboratory (BCCDC PHL) attempted whole-genome sequencing (WGS) of all SARS-CoV-2 detections provincially. In support of variant-specific VE estimates, the BCCDC PHL additionally characterized contributing case viruses from epi-week 17, by which time about half of 50–69 year olds had received at least 1 vaccine dose. From 1 September to 2 October (epi-weeks 35–39), WGS was applied to a subset only of SARS-CoV-2 detections provincially, of which virtually all were Delta (Supplementary Tables 1 and 2; Supplementary Figure 1) [10]. Variant-specific analyses thus spanned epi-weeks 17–39, with all viruses between epi-weeks 35–39 of unknown characterization considered Delta. All SARS-CoV-2 virus sequences of adequate quality are routinely posted by the BCCDC PHL to GISAID as hCoV-19/Canada/BC-BCCDC-######/2021.
Control Selection
Four approaches were explored for test-negative control selection: (1) all test-negative specimens or (2) the single earliest, (3) single latest, or (4) single randomly selected test-negative specimen per individual (Supplementary Figure 2). The same chosen set of test-negative controls was used for VE estimation against infection and hospitalization.
Exclusions
Specimens excluded were those collected from individuals with invalid or missing linkage, exposure, outcome or covariate information; identified as cases before the analysis period; residents of assisted-living, independent-living, or long-term care facilities; or privately tested or screened outside of public funding (thereby also excluding individuals screened for travel abroad).
Ethics Statement
Data linkages and analyses were authorized by the Provincial Health Officer under the Public Health Act and exempt from research ethics board review.
RESULTS
Vaccination Profiles
Overall, 52 517 of 111 405 (47%) contributing specimens were from individuals who received a single vaccine dose, of whom approximately 80% received mRNA vaccine (63% BNT162b2 and 18% mRNA-1273). ChAdOx1 vs mRNA recipients were slightly younger (median, 58 vs 60 years), and less often women (48% vs 54%) (Supplementary Table 3).
Without 2-dose censoring, the weekly percentage vaccinated among all control specimens closely tracked the estimated provincial vaccine coverage in the general population 50–69 years old (Figure 1). With increase in 2-dose coverage and associated censoring, the percentage of study controls who were single-dose recipients steadily decreased. Of 78 748 control specimens collected from twice-vaccinated 50- to 69-year-olds, nearly half (34 489/78 748 [44%]) received their second dose 8–9 weeks after the first, with 62 319 (79%) revaccinated ≥8 weeks after the first dose (Supplementary Table 4). Of contributing specimens from individuals who received a single vaccine dose on/before specimen collection, the median (IQR) follow-up since vaccination was 32 (15–52) days, with 79% having <8 weeks of single-dose follow-up (Table 1). Median (IQR) follow-up of single-dose mRNA and ChAdOx1 was 33 (15–53) days and 28 (13–47) days, respectively, with 77% and 85%, respectively, followed <8 weeks postvaccination (Supplementary Table 3).
Participant Characteristics by Case and Control Status, Including Profile of Single-Dose–Vaccinated Participants, Adults 50–69 Years Old, British Columbia, Canada, Weeks 14–39 (4 April–2 October 2021)
| Characteristic . | Overall No. (Column %) . | Single-Dose Vaccinated, No. (Row %)a . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | All Cases . | Hospitalized Casesb . | Controlsc . | Total . | All Cases . | Hospitalized Casesb . | Controlsc . | |
| Total | 111 405 (100) | 11 861 (11) | 1355 (11)d | 99 544 (89) | 52 517 (47) | 3040 (26) | 272 (20) | 49 477 (50) |
| Age group, y | ||||||||
| 50–59 | 62 890 (56) | 7208 (61) | 651 (48) | 55 682 (56) | 26 527 (42) | 1668 (23) | 111 (17) | 24 859 (45) |
| 60–69 | 48 515 (44) | 4653 (39) | 704 (52) | 43 862 (44) | 25 990 (54) | 1372 (29) | 161 (23) | 24 618 (56) |
| Median (IQR) | 58 (54–63) | 58 (53–62) | 60 (55–64) | 58 (54–63) | 59 (55–64) | 59 (54–63) | 61 (56–66) | 59 (55–64) |
| Sex | ||||||||
| Male | 53 604 (48) | 6160 (52) | 835 (62) | 47 444 (48) | 24 640 (46) | 1591 (26) | 174 (21) | 23 049 (49) |
| Female | 57 801 (52) | 5701 (48) | 520 (38) | 52 100 (52) | 27 877 (48) | 1449 (25) | 98 (19) | 26 428 (51) |
| Epidemiological week of specimen collection, 2021 | ||||||||
| 14–16 (4–24 Apr) | 35 299 (32) | 4179 (35) | 398 (29) | 31 120 (31) | 8599 (24) | 659 (16) | 64 (16) | 7940 (26) |
| 17–20 (25 Apr–22 May) | 33 460 (30) | 2965 (25) | 320 (24) | 30 495 (31) | 19 738 (59) | 1253 (42) | 107 (33) | 18 485 (61) |
| 21–26 (23 May–3 Jul) | 22 850 (21) | 864 (7) | 98 (7) | 21 986 (22) | 17 594 (77) | 465 (54) | 35 (36) | 17 129 (78) |
| 27–30 (4–31 Jul) | 4866 (4) | 202 (2) | 23 (2) | 4664 (5) | 2421 (50) | 55 (27) | 2 (9) | 2366 (51) |
| 31–34 (1–28 Aug) | 6151 (6) | 1284 (11) | 169 (12) | 4867 (5) | 1819 (30) | 225 (18) | 19 (11) | 1594 (33) |
| 35–39 (29 Aug–2 Oct) | 8779 (8) | 2367 (20) | 347 (26) | 6412 (6) | 2346 (27) | 383 (16) | 45 (13) | 1963 (31) |
| Health authority | ||||||||
| Fraser | 54 713 (49) | 5731 (48) | 597 (44) | 48 982 (49) | 27 869 (51) | 1645 (29) | 119 (20) | 26 224 (54) |
| Interior | 19 120 (17) | 2354 (20) | 282 (21) | 16 766 (17) | 7677 (40) | 449 (19) | 35 (12) | 7228 (43) |
| Northern | 4038 (4) | 847 (7) | 118 (9) | 3191 (3) | 1647 (41) | 175 (21) | 21 (18) | 1472 (46) |
| Vancouver Coastal | 21 897 (20) | 2332 (20) | 299 (22) | 19 565 (20) | 10 517 (48) | 644 (28) | 85 (28) | 9873 (50) |
| Vancouver Island | 11 637 (10) | 597 (5) | 59 (4) | 11 040 (11) | 4807 (41) | 127 (21) | 12 (20) | 4680 (42) |
| Single-dose vaccinatede,f | ||||||||
| BNT162b2 (Pfizer-BioNTech) | NA | NA | NA | NA | 33 004 (63) | 1819 (60) | 176 (65) | 31 185 (63) |
| mRNA-1273 (Moderna) | NA | NA | NA | NA | 9375 (18) | 537 (18) | 50 (18) | 8838 (18) |
| ChAdOx1 (AstraZeneca) | NA | NA | NA | NA | 10 136 (19) | 684 (22) | 46 (17) | 9452 (19) |
| Time since first dose of vaccinee | ||||||||
| 0–13 d (0–1 wk) | NA | NA | NA | NA | 11 774 (22) | 1311 (43) | 143 (53) | 10 463 (21) |
| 14–20 d (2 wk) | NA | NA | NA | NA | 6045 (12) | 413 (14) | 30 (11) | 5632 (11) |
| 21–27 d (3 wk) | NA | NA | NA | NA | 5490 (10) | 266 (9) | 21 (8) | 5224 (11) |
| 28–34 d (4 wk) | NA | NA | NA | NA | 5095 (10) | 191 (6) | 9 (3) | 4904 (10) |
| 35–41 d (5 wk) | NA | NA | NA | NA | 4668 (9) | 129 (4) | 9 (3) | 4539 (9) |
| 42–55 d (6–7 wk) | NA | NA | NA | NA | 8398 (16) | 240 (8) | 11 (4) | 8158 (16) |
| 56–69 d (8–9 wk) | NA | NA | NA | NA | 5191 (10) | 161 (5) | 8 (3) | 5030 (10) |
| 70–83 d (10–11 wk) | NA | NA | NA | NA | 2464 (5) | 78 (3) | 7 (3) | 2386 (5) |
| 84–97 d (12–13 wk) | NA | NA | NA | NA | 1419 (3) | 63 (2) | 34 (3) | 1356 (3) |
| 98–111 d (14–15 wk) | NA | NA | NA | NA | 748 (1) | 64 (2) | 8 (3) | 684 (1) |
| ≥112 d (≥16 wk) | NA | NA | NA | NA | 1225 (2) | 124 (4) | 18 (7) | 1101 (2) |
| Time since first dose of vaccinee | ||||||||
| Median (IQR), d | NA | NA | NA | NA | 32 (15–52) | 16 (8–40) | 13 (8–35) | 32 (16–52) |
| Range, d | NA | NA | NA | NA | 0–259 | 0–252 | 0–235 | 0–259 |
| Characteristic . | Overall No. (Column %) . | Single-Dose Vaccinated, No. (Row %)a . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | All Cases . | Hospitalized Casesb . | Controlsc . | Total . | All Cases . | Hospitalized Casesb . | Controlsc . | |
| Total | 111 405 (100) | 11 861 (11) | 1355 (11)d | 99 544 (89) | 52 517 (47) | 3040 (26) | 272 (20) | 49 477 (50) |
| Age group, y | ||||||||
| 50–59 | 62 890 (56) | 7208 (61) | 651 (48) | 55 682 (56) | 26 527 (42) | 1668 (23) | 111 (17) | 24 859 (45) |
| 60–69 | 48 515 (44) | 4653 (39) | 704 (52) | 43 862 (44) | 25 990 (54) | 1372 (29) | 161 (23) | 24 618 (56) |
| Median (IQR) | 58 (54–63) | 58 (53–62) | 60 (55–64) | 58 (54–63) | 59 (55–64) | 59 (54–63) | 61 (56–66) | 59 (55–64) |
| Sex | ||||||||
| Male | 53 604 (48) | 6160 (52) | 835 (62) | 47 444 (48) | 24 640 (46) | 1591 (26) | 174 (21) | 23 049 (49) |
| Female | 57 801 (52) | 5701 (48) | 520 (38) | 52 100 (52) | 27 877 (48) | 1449 (25) | 98 (19) | 26 428 (51) |
| Epidemiological week of specimen collection, 2021 | ||||||||
| 14–16 (4–24 Apr) | 35 299 (32) | 4179 (35) | 398 (29) | 31 120 (31) | 8599 (24) | 659 (16) | 64 (16) | 7940 (26) |
| 17–20 (25 Apr–22 May) | 33 460 (30) | 2965 (25) | 320 (24) | 30 495 (31) | 19 738 (59) | 1253 (42) | 107 (33) | 18 485 (61) |
| 21–26 (23 May–3 Jul) | 22 850 (21) | 864 (7) | 98 (7) | 21 986 (22) | 17 594 (77) | 465 (54) | 35 (36) | 17 129 (78) |
| 27–30 (4–31 Jul) | 4866 (4) | 202 (2) | 23 (2) | 4664 (5) | 2421 (50) | 55 (27) | 2 (9) | 2366 (51) |
| 31–34 (1–28 Aug) | 6151 (6) | 1284 (11) | 169 (12) | 4867 (5) | 1819 (30) | 225 (18) | 19 (11) | 1594 (33) |
| 35–39 (29 Aug–2 Oct) | 8779 (8) | 2367 (20) | 347 (26) | 6412 (6) | 2346 (27) | 383 (16) | 45 (13) | 1963 (31) |
| Health authority | ||||||||
| Fraser | 54 713 (49) | 5731 (48) | 597 (44) | 48 982 (49) | 27 869 (51) | 1645 (29) | 119 (20) | 26 224 (54) |
| Interior | 19 120 (17) | 2354 (20) | 282 (21) | 16 766 (17) | 7677 (40) | 449 (19) | 35 (12) | 7228 (43) |
| Northern | 4038 (4) | 847 (7) | 118 (9) | 3191 (3) | 1647 (41) | 175 (21) | 21 (18) | 1472 (46) |
| Vancouver Coastal | 21 897 (20) | 2332 (20) | 299 (22) | 19 565 (20) | 10 517 (48) | 644 (28) | 85 (28) | 9873 (50) |
| Vancouver Island | 11 637 (10) | 597 (5) | 59 (4) | 11 040 (11) | 4807 (41) | 127 (21) | 12 (20) | 4680 (42) |
| Single-dose vaccinatede,f | ||||||||
| BNT162b2 (Pfizer-BioNTech) | NA | NA | NA | NA | 33 004 (63) | 1819 (60) | 176 (65) | 31 185 (63) |
| mRNA-1273 (Moderna) | NA | NA | NA | NA | 9375 (18) | 537 (18) | 50 (18) | 8838 (18) |
| ChAdOx1 (AstraZeneca) | NA | NA | NA | NA | 10 136 (19) | 684 (22) | 46 (17) | 9452 (19) |
| Time since first dose of vaccinee | ||||||||
| 0–13 d (0–1 wk) | NA | NA | NA | NA | 11 774 (22) | 1311 (43) | 143 (53) | 10 463 (21) |
| 14–20 d (2 wk) | NA | NA | NA | NA | 6045 (12) | 413 (14) | 30 (11) | 5632 (11) |
| 21–27 d (3 wk) | NA | NA | NA | NA | 5490 (10) | 266 (9) | 21 (8) | 5224 (11) |
| 28–34 d (4 wk) | NA | NA | NA | NA | 5095 (10) | 191 (6) | 9 (3) | 4904 (10) |
| 35–41 d (5 wk) | NA | NA | NA | NA | 4668 (9) | 129 (4) | 9 (3) | 4539 (9) |
| 42–55 d (6–7 wk) | NA | NA | NA | NA | 8398 (16) | 240 (8) | 11 (4) | 8158 (16) |
| 56–69 d (8–9 wk) | NA | NA | NA | NA | 5191 (10) | 161 (5) | 8 (3) | 5030 (10) |
| 70–83 d (10–11 wk) | NA | NA | NA | NA | 2464 (5) | 78 (3) | 7 (3) | 2386 (5) |
| 84–97 d (12–13 wk) | NA | NA | NA | NA | 1419 (3) | 63 (2) | 34 (3) | 1356 (3) |
| 98–111 d (14–15 wk) | NA | NA | NA | NA | 748 (1) | 64 (2) | 8 (3) | 684 (1) |
| ≥112 d (≥16 wk) | NA | NA | NA | NA | 1225 (2) | 124 (4) | 18 (7) | 1101 (2) |
| Time since first dose of vaccinee | ||||||||
| Median (IQR), d | NA | NA | NA | NA | 32 (15–52) | 16 (8–40) | 13 (8–35) | 32 (16–52) |
| Range, d | NA | NA | NA | NA | 0–259 | 0–252 | 0–235 | 0–259 |
Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine (AstraZeneca, Covishield); d, days; IQR, interquartile range; mRNA, messenger RNA; NA, Not applicable; wk, weeks.
Unless otherwise specified, displayed is the percentage by row category who received 1 vaccine dose on or before specimen collection regardless of time since first dose.
Hospitalized on or within 30 days following specimen collection.
As per approach 1 for control selection: includes all test-negative specimens.
Displayed is the percentage of cases who were hospitalized.
All percentages displayed below this row are column %. Vaccinated are those who received 1 vaccine dose on or before specimen collection date regardless of time since first dose.
Two specimens from vaccinated individuals with product name unspecified.
Participant Characteristics by Case and Control Status, Including Profile of Single-Dose–Vaccinated Participants, Adults 50–69 Years Old, British Columbia, Canada, Weeks 14–39 (4 April–2 October 2021)
| Characteristic . | Overall No. (Column %) . | Single-Dose Vaccinated, No. (Row %)a . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | All Cases . | Hospitalized Casesb . | Controlsc . | Total . | All Cases . | Hospitalized Casesb . | Controlsc . | |
| Total | 111 405 (100) | 11 861 (11) | 1355 (11)d | 99 544 (89) | 52 517 (47) | 3040 (26) | 272 (20) | 49 477 (50) |
| Age group, y | ||||||||
| 50–59 | 62 890 (56) | 7208 (61) | 651 (48) | 55 682 (56) | 26 527 (42) | 1668 (23) | 111 (17) | 24 859 (45) |
| 60–69 | 48 515 (44) | 4653 (39) | 704 (52) | 43 862 (44) | 25 990 (54) | 1372 (29) | 161 (23) | 24 618 (56) |
| Median (IQR) | 58 (54–63) | 58 (53–62) | 60 (55–64) | 58 (54–63) | 59 (55–64) | 59 (54–63) | 61 (56–66) | 59 (55–64) |
| Sex | ||||||||
| Male | 53 604 (48) | 6160 (52) | 835 (62) | 47 444 (48) | 24 640 (46) | 1591 (26) | 174 (21) | 23 049 (49) |
| Female | 57 801 (52) | 5701 (48) | 520 (38) | 52 100 (52) | 27 877 (48) | 1449 (25) | 98 (19) | 26 428 (51) |
| Epidemiological week of specimen collection, 2021 | ||||||||
| 14–16 (4–24 Apr) | 35 299 (32) | 4179 (35) | 398 (29) | 31 120 (31) | 8599 (24) | 659 (16) | 64 (16) | 7940 (26) |
| 17–20 (25 Apr–22 May) | 33 460 (30) | 2965 (25) | 320 (24) | 30 495 (31) | 19 738 (59) | 1253 (42) | 107 (33) | 18 485 (61) |
| 21–26 (23 May–3 Jul) | 22 850 (21) | 864 (7) | 98 (7) | 21 986 (22) | 17 594 (77) | 465 (54) | 35 (36) | 17 129 (78) |
| 27–30 (4–31 Jul) | 4866 (4) | 202 (2) | 23 (2) | 4664 (5) | 2421 (50) | 55 (27) | 2 (9) | 2366 (51) |
| 31–34 (1–28 Aug) | 6151 (6) | 1284 (11) | 169 (12) | 4867 (5) | 1819 (30) | 225 (18) | 19 (11) | 1594 (33) |
| 35–39 (29 Aug–2 Oct) | 8779 (8) | 2367 (20) | 347 (26) | 6412 (6) | 2346 (27) | 383 (16) | 45 (13) | 1963 (31) |
| Health authority | ||||||||
| Fraser | 54 713 (49) | 5731 (48) | 597 (44) | 48 982 (49) | 27 869 (51) | 1645 (29) | 119 (20) | 26 224 (54) |
| Interior | 19 120 (17) | 2354 (20) | 282 (21) | 16 766 (17) | 7677 (40) | 449 (19) | 35 (12) | 7228 (43) |
| Northern | 4038 (4) | 847 (7) | 118 (9) | 3191 (3) | 1647 (41) | 175 (21) | 21 (18) | 1472 (46) |
| Vancouver Coastal | 21 897 (20) | 2332 (20) | 299 (22) | 19 565 (20) | 10 517 (48) | 644 (28) | 85 (28) | 9873 (50) |
| Vancouver Island | 11 637 (10) | 597 (5) | 59 (4) | 11 040 (11) | 4807 (41) | 127 (21) | 12 (20) | 4680 (42) |
| Single-dose vaccinatede,f | ||||||||
| BNT162b2 (Pfizer-BioNTech) | NA | NA | NA | NA | 33 004 (63) | 1819 (60) | 176 (65) | 31 185 (63) |
| mRNA-1273 (Moderna) | NA | NA | NA | NA | 9375 (18) | 537 (18) | 50 (18) | 8838 (18) |
| ChAdOx1 (AstraZeneca) | NA | NA | NA | NA | 10 136 (19) | 684 (22) | 46 (17) | 9452 (19) |
| Time since first dose of vaccinee | ||||||||
| 0–13 d (0–1 wk) | NA | NA | NA | NA | 11 774 (22) | 1311 (43) | 143 (53) | 10 463 (21) |
| 14–20 d (2 wk) | NA | NA | NA | NA | 6045 (12) | 413 (14) | 30 (11) | 5632 (11) |
| 21–27 d (3 wk) | NA | NA | NA | NA | 5490 (10) | 266 (9) | 21 (8) | 5224 (11) |
| 28–34 d (4 wk) | NA | NA | NA | NA | 5095 (10) | 191 (6) | 9 (3) | 4904 (10) |
| 35–41 d (5 wk) | NA | NA | NA | NA | 4668 (9) | 129 (4) | 9 (3) | 4539 (9) |
| 42–55 d (6–7 wk) | NA | NA | NA | NA | 8398 (16) | 240 (8) | 11 (4) | 8158 (16) |
| 56–69 d (8–9 wk) | NA | NA | NA | NA | 5191 (10) | 161 (5) | 8 (3) | 5030 (10) |
| 70–83 d (10–11 wk) | NA | NA | NA | NA | 2464 (5) | 78 (3) | 7 (3) | 2386 (5) |
| 84–97 d (12–13 wk) | NA | NA | NA | NA | 1419 (3) | 63 (2) | 34 (3) | 1356 (3) |
| 98–111 d (14–15 wk) | NA | NA | NA | NA | 748 (1) | 64 (2) | 8 (3) | 684 (1) |
| ≥112 d (≥16 wk) | NA | NA | NA | NA | 1225 (2) | 124 (4) | 18 (7) | 1101 (2) |
| Time since first dose of vaccinee | ||||||||
| Median (IQR), d | NA | NA | NA | NA | 32 (15–52) | 16 (8–40) | 13 (8–35) | 32 (16–52) |
| Range, d | NA | NA | NA | NA | 0–259 | 0–252 | 0–235 | 0–259 |
| Characteristic . | Overall No. (Column %) . | Single-Dose Vaccinated, No. (Row %)a . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | All Cases . | Hospitalized Casesb . | Controlsc . | Total . | All Cases . | Hospitalized Casesb . | Controlsc . | |
| Total | 111 405 (100) | 11 861 (11) | 1355 (11)d | 99 544 (89) | 52 517 (47) | 3040 (26) | 272 (20) | 49 477 (50) |
| Age group, y | ||||||||
| 50–59 | 62 890 (56) | 7208 (61) | 651 (48) | 55 682 (56) | 26 527 (42) | 1668 (23) | 111 (17) | 24 859 (45) |
| 60–69 | 48 515 (44) | 4653 (39) | 704 (52) | 43 862 (44) | 25 990 (54) | 1372 (29) | 161 (23) | 24 618 (56) |
| Median (IQR) | 58 (54–63) | 58 (53–62) | 60 (55–64) | 58 (54–63) | 59 (55–64) | 59 (54–63) | 61 (56–66) | 59 (55–64) |
| Sex | ||||||||
| Male | 53 604 (48) | 6160 (52) | 835 (62) | 47 444 (48) | 24 640 (46) | 1591 (26) | 174 (21) | 23 049 (49) |
| Female | 57 801 (52) | 5701 (48) | 520 (38) | 52 100 (52) | 27 877 (48) | 1449 (25) | 98 (19) | 26 428 (51) |
| Epidemiological week of specimen collection, 2021 | ||||||||
| 14–16 (4–24 Apr) | 35 299 (32) | 4179 (35) | 398 (29) | 31 120 (31) | 8599 (24) | 659 (16) | 64 (16) | 7940 (26) |
| 17–20 (25 Apr–22 May) | 33 460 (30) | 2965 (25) | 320 (24) | 30 495 (31) | 19 738 (59) | 1253 (42) | 107 (33) | 18 485 (61) |
| 21–26 (23 May–3 Jul) | 22 850 (21) | 864 (7) | 98 (7) | 21 986 (22) | 17 594 (77) | 465 (54) | 35 (36) | 17 129 (78) |
| 27–30 (4–31 Jul) | 4866 (4) | 202 (2) | 23 (2) | 4664 (5) | 2421 (50) | 55 (27) | 2 (9) | 2366 (51) |
| 31–34 (1–28 Aug) | 6151 (6) | 1284 (11) | 169 (12) | 4867 (5) | 1819 (30) | 225 (18) | 19 (11) | 1594 (33) |
| 35–39 (29 Aug–2 Oct) | 8779 (8) | 2367 (20) | 347 (26) | 6412 (6) | 2346 (27) | 383 (16) | 45 (13) | 1963 (31) |
| Health authority | ||||||||
| Fraser | 54 713 (49) | 5731 (48) | 597 (44) | 48 982 (49) | 27 869 (51) | 1645 (29) | 119 (20) | 26 224 (54) |
| Interior | 19 120 (17) | 2354 (20) | 282 (21) | 16 766 (17) | 7677 (40) | 449 (19) | 35 (12) | 7228 (43) |
| Northern | 4038 (4) | 847 (7) | 118 (9) | 3191 (3) | 1647 (41) | 175 (21) | 21 (18) | 1472 (46) |
| Vancouver Coastal | 21 897 (20) | 2332 (20) | 299 (22) | 19 565 (20) | 10 517 (48) | 644 (28) | 85 (28) | 9873 (50) |
| Vancouver Island | 11 637 (10) | 597 (5) | 59 (4) | 11 040 (11) | 4807 (41) | 127 (21) | 12 (20) | 4680 (42) |
| Single-dose vaccinatede,f | ||||||||
| BNT162b2 (Pfizer-BioNTech) | NA | NA | NA | NA | 33 004 (63) | 1819 (60) | 176 (65) | 31 185 (63) |
| mRNA-1273 (Moderna) | NA | NA | NA | NA | 9375 (18) | 537 (18) | 50 (18) | 8838 (18) |
| ChAdOx1 (AstraZeneca) | NA | NA | NA | NA | 10 136 (19) | 684 (22) | 46 (17) | 9452 (19) |
| Time since first dose of vaccinee | ||||||||
| 0–13 d (0–1 wk) | NA | NA | NA | NA | 11 774 (22) | 1311 (43) | 143 (53) | 10 463 (21) |
| 14–20 d (2 wk) | NA | NA | NA | NA | 6045 (12) | 413 (14) | 30 (11) | 5632 (11) |
| 21–27 d (3 wk) | NA | NA | NA | NA | 5490 (10) | 266 (9) | 21 (8) | 5224 (11) |
| 28–34 d (4 wk) | NA | NA | NA | NA | 5095 (10) | 191 (6) | 9 (3) | 4904 (10) |
| 35–41 d (5 wk) | NA | NA | NA | NA | 4668 (9) | 129 (4) | 9 (3) | 4539 (9) |
| 42–55 d (6–7 wk) | NA | NA | NA | NA | 8398 (16) | 240 (8) | 11 (4) | 8158 (16) |
| 56–69 d (8–9 wk) | NA | NA | NA | NA | 5191 (10) | 161 (5) | 8 (3) | 5030 (10) |
| 70–83 d (10–11 wk) | NA | NA | NA | NA | 2464 (5) | 78 (3) | 7 (3) | 2386 (5) |
| 84–97 d (12–13 wk) | NA | NA | NA | NA | 1419 (3) | 63 (2) | 34 (3) | 1356 (3) |
| 98–111 d (14–15 wk) | NA | NA | NA | NA | 748 (1) | 64 (2) | 8 (3) | 684 (1) |
| ≥112 d (≥16 wk) | NA | NA | NA | NA | 1225 (2) | 124 (4) | 18 (7) | 1101 (2) |
| Time since first dose of vaccinee | ||||||||
| Median (IQR), d | NA | NA | NA | NA | 32 (15–52) | 16 (8–40) | 13 (8–35) | 32 (16–52) |
| Range, d | NA | NA | NA | NA | 0–259 | 0–252 | 0–235 | 0–259 |
Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine (AstraZeneca, Covishield); d, days; IQR, interquartile range; mRNA, messenger RNA; NA, Not applicable; wk, weeks.
Unless otherwise specified, displayed is the percentage by row category who received 1 vaccine dose on or before specimen collection regardless of time since first dose.
Hospitalized on or within 30 days following specimen collection.
As per approach 1 for control selection: includes all test-negative specimens.
Displayed is the percentage of cases who were hospitalized.
All percentages displayed below this row are column %. Vaccinated are those who received 1 vaccine dose on or before specimen collection date regardless of time since first dose.
Two specimens from vaccinated individuals with product name unspecified.
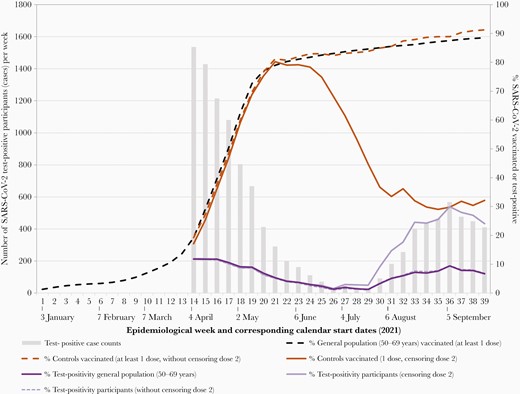
Weekly case counts, percentage of specimens testing positive, and percentage vaccinated among controls and the general population of adults 50–69 years old, British Columbia, Canada, epi-weeks 14–39 (4 April–2 October) of 2021. Shown are the weekly number of test-positive study cases (gray bars). Shown also are the percentage of contributing specimens that were test-positive among study participants including with (light purple solid line) and without (light purple dashed line) second-dose censoring, compared to the weekly test positivity among the general population of adults 50–69 years old (dark purple line). Shown also are the weekly percentage of test-negative controls who had received at least 1 vaccine dose (without second-dose censoring, dashed orange line) or just 1 dose (solid orange line), compared to the general population of adults 50–69 years old who had received at least 1 vaccine dose (dashed black line). The horizontal axis is based on individual epidemiological week of 2021, with the calendar date corresponding to the start of the first full epidemiological week of each month displayed. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Case and Control Profiles
In total, 111 405 SARS-CoV-2 specimens contributed to VE analyses, including 11 861 (11%) test-positive cases and 99 544 test-negative controls in approach 1 (Table 1; Supplementary Figure 2). Without second-dose censoring, the weekly percentage of study specimens that were test-positive closely reflected positivity among specimens collected from the general population 50–69 years old (Figure 1). With second-dose censoring, the weekly percentage of study specimens that were test-positive was higher than the general population, as expected in the context of effective 2-dose vaccination.
Case and control distributions by age subgroup and sex reflected general population distributions, with variation by region reflecting epidemic intensity provincially (Table 1) [9, 13]. The percentage of study cases hospitalized within 30 days of specimen collection (1355/11 861 [11%]) was the same as among the general population 50–69 years old during epi-weeks 14–39 (11%) (not shown) [9]. Among individuals contributing control specimens, 87% provided just 1 and 97% provided ≤ 2 test-negative specimens across the analysis period, with similar distribution of negative specimens contributed per individual for vaccinated and unvaccinated controls (not shown).
VOC Profiles
Between epi-weeks 17 and 39 (25 April–2 October 2021), 7682 cases contributed (Table 1). Of these, 5541 (72%) overall were genetically characterized (Supplementary Table 1). Between epi-weeks 31–34, >90% of case viruses were characterized (1191/1284 [93%]), of which 99% (1174/1191) were Delta. Although less than half of case viruses thereafter to epi-week 39 were characterized (1084/2367 [46%]), virtually all (1081/1084 [99%]) were Delta. Assuming all 1283 noncharacterized case viruses between epi-weeks 35 and 39 (29 August–2 October 2021) were Delta, we were able to genetically subgroup nearly 90% of case viruses (6824/7682) between epi-weeks 17 and 39 for inclusion in variant-specific VE analyses. Of these, 6589 of 6824 (97%) were considered VOCs, including 1547 (23%) Alpha, 5 (<1%) Beta, 1227 (18%) Gamma, and 3810 (56%) Delta (2527 confirmed). Of 235 of 6824 (3%) case viruses that were non-VOCs, 20 bore the pivotal E484K substitution considered potentially influential on vaccine protection. Of the 215 other non-VOCs, 208 (97%) accrued between weeks 17 and 20 (25 April–22 May 2021), during which about 80% were B.1.438.1 (47%) or Epsilon (B.1.429) (34%) (Supplementary Table 2).
The percentage distribution by VOC status did not vary meaningfully between participant age subgroup or sex (Supplementary Figure 1). Whereas from epi-week 17, virtually all Alpha (1509/1547 [98%]) and Gamma (1188/1227 [97%]) VOCs were detected before epi-week 27 (4 July 2021), virtually all confirmed Deltas accrued thereafter (2371/2527 [94%]) (Supplementary Tables 1 and 2). VOC case distribution by time and region mirrored that reported provincially [10].
VE Estimates
VE estimates did not vary substantially by approach for control selection (Supplementary Tables 5–10). In particular, using all (approach 1) or a single randomly selected test-negative specimen per individual (approach 4), absolute differences in adjusted VE estimates were minimal in primary analyses (21–55 or ≥21 days postvaccination) for mRNA or ChAdOx1 vaccines (±2%). Greater differences were observed with finer stratification by time since vaccination, notably at longer postvaccination follow-up when CIs were also wider. VE estimates in Figures 2–4 are based on all test-negative specimens as controls.
VE by Time Since Vaccination
At 0–3 days following dose 1, when no vaccine protection is expected, VE estimates were positively biased for both mRNA and ChAdOx1. This skew resolved by 4–7 days postvaccination for mRNA (5% [95% CI, –7% to 15%]) but not ChAdOx1 (24% [95% CI, 3%–40%]) (Figure 2; Supplementary Tables 7 and 10). By 8–13 days, VE was comparably low for mRNA (11% [95% CI, 2%–19%]) and ChAdOx1 (18% [95% CI, 2%–31%]). VE increased for both vaccines across 14–20, 21–27, and 28–34 days postvaccination, but sooner and more substantially for mRNA (58% [95% CI, 52%–63%], 68% [95% CI, 63%–73%], and 74% [95% CI, 69%–78%], respectively) than ChAdOx1 (25% [95% CI, 10%–37%], 49% [95% CI, 36%–60%], and 58% [95% CI, 45%–68%], respectively), consistently lower for ChAdOx1 regardless of approach for control selection (Supplementary Tables 7 and 10. Higher mRNA than ChAdOx1 VE then persisted weekly up to at least 7 weeks postvaccination, more comparable thereafter but with overlapping CIs. At ≥10 weeks postvaccination, mRNA VE was 67% (95% CI, 63%–71%) using all controls and 62% (95% CI, 57%–66%) with single randomly selected control; ChAdOx1 VE was 55% (95% CI, 34%–69%) with either method of control selection.
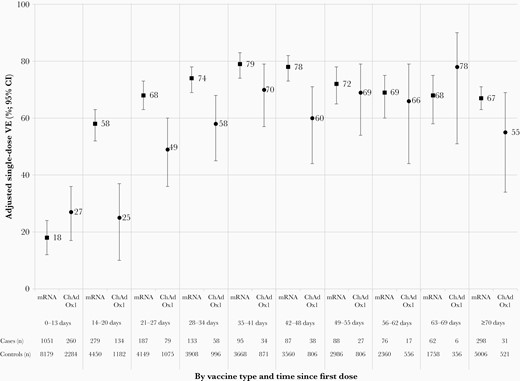
Adjusted single-dose vaccine effectiveness (VE) against severe acute respiratory syndrome coronavirus 2 infection by vaccine type and time since receipt of the first dose, adults 50–69 years old, British Columbia, Canada, epi-weeks 14–39 (4 April–2 October) of 2021. Displayed are single-dose VE estimates against infection by time since vaccination, with accompanying sample sizes (approach one using all controls). The number of unvaccinated cases (8821) and controls (50 067) is the same for all analyses. See Supplementary Tables 7 and 10 for details. Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine (AstraZeneca, Covishield); CI, confidence interval; mRNA, messenger RNA; VE, vaccine effectiveness.
Primary VE, Infection, and Hospitalization
To standardize for time since vaccination, VE comparisons by vaccine type and VOC are displayed in Figures 3 and 4 for the period from 21 to 55 days postvaccination. Estimates were similar at ≥21 days postvaccination overall, with details for both analysis periods provided in the corresponding Supplementary Material.
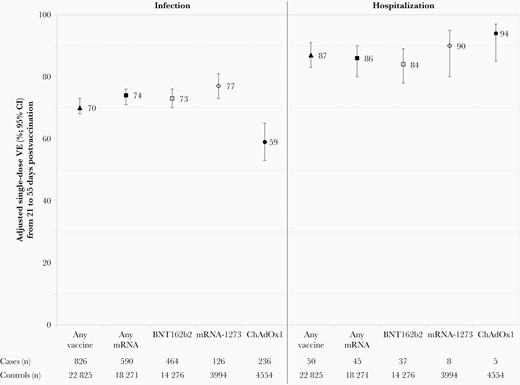
Adjusted single-dose vaccine effectiveness (VE) against severe acute respiratory syndrome coronavirus 2 infection and hospitalization, by vaccine type, adults 50–69 years old, British Columbia, Canada, epi-weeks 14–39 (4 April–2 October) of 2021. Displayed are single-dose VE estimates from 21 to 55 days postvaccination against infection and hospitalization, with accompanying sample sizes (approach one using all controls). The number of unvaccinated cases (8821 overall, 1083 hospitalized) and unvaccinated controls (50 067) is the same for all analyses. See Supplementary Tables 5 and 11 for details. Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine (AstraZeneca, Covishield); CI, confidence interval; mRNA, messenger RNA; VE, vaccine effectiveness.
During epi-weeks 14–39, VE against any infection (21–55 days postvaccination) was higher for mRNA recipients at 74% (95% CI, 71%–76%) than for ChAdOx1 recipients at 59% (95% CI, 53%–65%) (Figure 3; Supplementary Table 5). VE was significantly higher against hospitalization than infection for both mRNA (86% [95% CI, 80%–90%]) and ChAdOx1 (94% [95% CI, 85%–97%]), exceeding 85% to at least 8 weeks postvaccination for both mRNA and ChAdOx1 (Figure 3; Supplementary Tables 11 and 12).
VE did not vary meaningfully by age subgroup or sex (Supplementary Tables 13 and 14). mRNA vs ChAdOx1 VE differences were similar with restriction to those aged 55–69 years (Supplementary Table 13). Estimates were similar by epidemic period for epi-weeks 14–20 (4 April–22 May 2021, when non-VOCs contributed) vs 21–30 (23 May–31 July 2021, when Alpha and Gamma predominated). mRNA VE was lower against infection, but comparable against hospitalization, during epi-weeks 31–39 (1 August–2 October) of 2021 when Delta predominated (nonestimable for ChAdOx1) (Supplementary Table 11).
VE by VOC
During epi-weeks 17–20 (25 April–22 May 2021), single-dose VE (21–55 days postvaccination) against non-VOC infections tended to be higher than against Alpha or Gamma for both mRNA (88% [95% CI, 73%–94%] vs 80% [95% CI, 73%–84%] vs 84% [95% CI, 78%–88%], respectively) and ChAdOx1 (93% [95% CI, 70%–98%] vs 71% [95% CI, 61%–78%] vs 62% [95% CI, 50%–72%], respectively), but with overlapping CIs (nonestimable for Delta) (Figure 4; Supplementary Table 15).
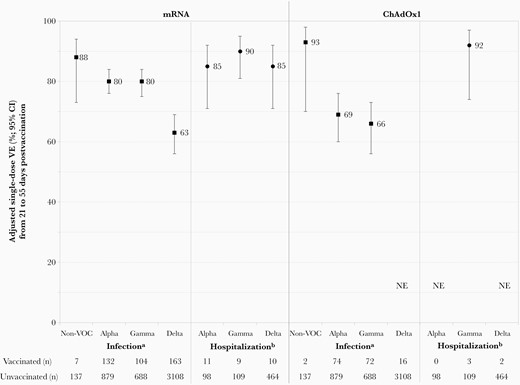
Adjusted vaccine effectiveness (VE) against infection due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) by vaccine type, adults 50–69 years old, British Columbia, Canada, epi-weeks 17–39 (25 April–2 October) of 2021. Displayed are single-dose VE estimates from 21 to 55 days postvaccination against infection and hospitalization associated with SARS-CoV-2 non-VOCs and VOCs, with accompanying sample sizes (approach one using all controls). See Supplementary Tables 15–17 for details. aAnalysis period for VE against non-VOC infections spans epi-weeks 17–20 owing to few detections thereafter, with 5533 mRNA vaccinated and 2677 ChAdOx1 vaccinated controls and 12 009 unvaccinated controls; VE against VOC infections spans epi-weeks 17–39 with 15 974 mRNA vaccinated and 4305 ChAdOx1 vaccinated controls, and 26 886 unvaccinated controls. bAnalysis period for VE against hospitalization associated with Alpha and Gamma VOCs spans epi-weeks 17–30 owing to few associated hospitalizations thereafter with 14 981 mRNA vaccinated and 4300 ChAdOx1 vaccinated controls, and 19 164 unvaccinated controls; VE against hospitalization associated with Delta spans epi-weeks 17–39 with 15 974 mRNA vaccinated and 4305 ChAdOx1 vaccinated controls and 26 886 unvaccinated controls. Abbreviations: ChAdOx1, chimpanzee adenoviral vectored vaccine (AstraZeneca, Covishield); CI, confidence interval; mRNA, messenger RNA; NE, not estimable or total span of confidence interval around vaccine effectiveness estimate is ≥100%; VE, vaccine effectiveness; VOC, variant of concern.
During epi-weeks 17–39 (25 April–2 October 2021), single-dose VE (21–55 days postvaccination) did not differ against Alpha or Gamma infections for mRNA (80% [95% CI, 76%–84%] and 80% [95% CI, 75%–84%]) or ChAdOx1 vaccines (69% [95% CI, 60%–76%] and 66% [95% CI, 56%–73%]) (Figure 4; Supplementary Table 16). mRNA VE was lower against Delta (63% [95% CI, 56%–69%]) overall (Figure 4; Supplementary Table 16) or with restriction to confirmed cases only (62% [95% CI, 54%–69%]) (not shown; nonestimable for ChAdOx1). mRNA VE against Alpha-, Gamma-, and Delta-associated hospitalizations were similar at 85% (95% CI, 71%–92%), 90% (95% CI, 81%–95%), and 85% (95% CI, 71%–92%), respectively (Figure 4; Supplementary Table 17) (nonestimable for ChAdOx1).
DISCUSSION
In this postmarketing observational study, we compared single-dose effectiveness of 2 major vaccine innovations (mRNA and vector-based), deployed for the first time on a mass population scale during the coronavirus disease 2019 (COVID-19) pandemic. Among adults aged 50–69 years who were eligible for both vaccines in BC, 1 dose reduced the SARS-CoV-2 infection risk by about three-quarters for mRNA vaccines and 60% for ChAdOx1, with VE exceeding 85% against hospitalization to at least 8 weeks postvaccination with either vaccine. Such protection is particularly meaningful because it was observed when a variety of newly emergent VOCs were contributing, including early Alpha and Gamma co-circulation followed by later Delta dominance.
Although no head-to-head RCT efficacy comparisons are yet available, the higher mRNA vs ChAdOx1 effectiveness we report is consistent with indirect comparison across product-specific RCTs, similarly showing higher efficacy with a single dose of mRNA (92%–93%) vs ChAdOx1 (76%) vaccine against clinical illness [2–4]. Our point estimate of ChAdOx1 VE against any infection (61%) is moreover similar to the single-dose estimate of ChAdOx1 efficacy against any infection (64%) in pooled analysis of premarket RCTs (22–90 days postvaccination) [4]. The more rapid increase in protection we observe among mRNA recipients also aligns with the faster antibody responses reported for mRNA vs ChAdOx1 in head-to-head immunogenicity comparison [16]. We qualify the lower estimates of ChAdOx1 efficacy by showing that 1 dose was nevertheless at least as protective as mRNA vaccines against severe outcomes. Improved ChAdOx1 protection against more severe outcomes also aligns with findings from immunogenicity comparisons, showing lower antibody but comparable or even higher T-cell responses with a single dose of ChAdOx1 vs mRNA vaccine [16, 17].
Our study also adds to the understanding of single-dose VE against early VOCs, unavailable from premarket RCTs. As recommended to reduce selection bias [18], we were able to genetically characterize most (nearly 90%) case viruses contributing to variant-specific VE, relying foremost upon WGS and imputing from September 2021 only, when Delta already comprised >99% of characterized viruses. Both mRNA and ChAdOx1 vaccines protected best against viruses lacking key spike mutations (such as E484K) that are the hallmarks of some VOCs. In that regard, our single-dose mRNA VE of about 90% against non-VOCs is most comparable to RCT estimates [2, 3]. We acknowledge, however, that non-VOCs were displaced early, reducing their available sample size and resulting in wider confidence intervals and more uncertainty in the estimates. As in our previous analysis of mRNA vaccines among adults ≥70 years old [12], VE was comparable against Alpha and Gamma variants, here shown for both mRNA (80% against both variants) and ChAdOx1 (69% and 66%, respectively). Because the Delta variant contributed later, we standardized all VE comparisons for the period 21–55 days postvaccination to distinguish the effects of differential vaccine relatedness from the potential effects of waning immunity. On that basis, we report significantly lower mRNA VE against infection due to Delta (63%), whereas single-dose VE against hospitalization was comparably preserved at ≥85% against VOC-associated hospitalization, including Delta.
Elsewhere within Canada, Chung et al used the TND to estimate single-dose VE in the province of Ontario between mid-December 2020 and mid-April 2021 [19]. In a subset analysis of adults 40–69 years, they reported lower single-dose BNT162b2 VE (≥14 days postvaccination) than we do against symptomatic illness (64%) but similarly exceeding 80% against hospitalization. Their analyses, however, did not include ChAdOx1. In a preprint from Ontario, Nasreen et al provide variant-specific estimates to May 2021, including for ChAdOx1 [20]. During a period of changing VOC contribution, their analysis was largely based on probabilistic model imputation rather than empirical genetic characterization of individual case viruses. They also combined Beta/Gamma analyses, although these VOCs may not be comparable in their potential for vaccine escape: Reductions in neutralizing antibody for Beta are more severe and, in clinical trials, ChAdOx1 showed no efficacy against mild-to-moderate Beta illness [21–23]. Among adults aged <60 years, Nasreen et al reported lower single-dose BNT162b2 VEs against non-VOCs (65%), Alpha (71%), and Beta/Gamma (65%) that were comparable against Delta (62%). Their estimates for ChAdOx1 were similar to BNT162b2 against Alpha (67%) and Delta (67%), and lower only against Beta/Gamma (43%). Estimates from Canada are nevertheless aligned in each being higher than reported in TND analysis from the United Kingdom [24, 25]. Among individuals ≥16 years old in England between October 2020 and May 2021 [24], Lopez Bernal et al reported comparable single-dose BNT162b2 and ChAdOx1 VE estimates against Alpha (48% and 49%, respectively) and Delta (36% and 30%, respectively) that were also lower for both vaccines against Alpha than they had reported previously (60%–70%) [26, 27]. Among individuals ≥15 years old in Scotland between April and June 2021, Sheikh et al report similarly low single-dose VE for BNT162b2 and ChAdOx1 against Alpha (38% and 37%) and Delta (30% and 18%) [25]. In both studies, the distinction between Alpha and Delta variants was primarily based upon presumptive S-gene screen [24, 25]. In comparing across studies, other differences in target populations and methods should also be considered.
The main limitation of our study, as elsewhere, is reliance on general laboratory submissions and surveillance data subject to missing information, misclassification, and selection bias. The TND has the advantage over other observational study designs (eg, cohort) of standardizing for the likelihood of being tested, but exposure risk and/or case ascertainment may still differ between the vaccinated and unvaccinated. We attempted to standardize for the likelihood of being found to be a case by excluding specimens tested for nonclinical screening indications (eg, privately funded) or flagged as within residential care settings. Such efforts, however, may have been incomplete and the reason for testing was not otherwise routinely captured. Testing for vaccine-associated adverse events mimicking but not due to COVID-19 (eg, flu-like symptoms) may explain our positively biased VE estimates during the first few days following vaccination, persisting longer for ChAdOx1 perhaps owing to heightened safety concerns with that product. Aligned with that hypothesis, we highlight lower test positivity among vaccinated individuals during days 0–3 vs 4–7 postvaccination (Supplementary Tables 6–10). Our analyses included limited covariate adjustment, and we cannot rule out residual bias and confounding. We note that in the VE analysis from Ontario, the single most important confounder was calendar time, whereas, comorbidity and other covariates, beyond those for which we also adjust, made little difference to VE estimates [19]. Restricting to a discrete age band of adults earliest eligible for both kinds of vaccine enabled us to compare single-dose VE by vaccine type and VOC over a prolonged period while standardizing for potential age-associated variations in risk behaviors; however, this may limit generalizability to younger adults. In BC, hospitalization information is available within the notifiable disease case list but not available for test-negative controls; accordingly, we used the same control set for VE estimation against infection and hospitalization. As hospitalized controls may be more likely to be vaccinated, our inclusion of community controls may have given lower VE against hospitalization than if we had used hospitalized controls only. Unlike mRNA vaccines, ChAdOx1 was available in BC through pharmacies without awaiting invitation; those at higher risk may have preferentially received this vaccine, contributing to lower VE. Conversely, if ChAdOx1 recipients were generally more risk-averse in their behaviors, our ChAdOx1 VE may be an overestimate. Reassuring against bias in either direction, our estimates align well with clinical trial findings. We cannot reliably comment on single-dose protection beyond our primary analysis period up to 8 weeks postvaccination, notably for ChAdOx1, nor against subsequently emergent VOCs potentially more distinct from the vaccine strain (eg Omicron). Finally, we highlight that our estimates of single-dose protection are lower than the 2-dose VE we report elsewhere for this age group, approximating 90% for 2 mRNA doses and 70%–80% for 2 ChAdOx1 doses against infection, and 95% for both vaccines against hospitalization [28].
In conclusion, a single dose of mRNA or ChAdOx1 vaccine gave important protection against SARS-CoV-2, including early VOCs. VE was lower against any infection for ChAdOx1 and against the Delta variant for mRNA vaccine, but 1 dose of either vaccine reduced the COVID-19 hospitalization risk by >85% to at least 8 weeks postvaccination. A second dose is ultimately required to improve the strength and durability of vaccine protection but these findings inform the option of using a longer interval between doses to extend single-dose coverage and reduce the overall COVID-19 burden, in particular during periods of constrained vaccine supplies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Yayuk Joffres and Braeden Klaver for their contributions to laboratory data assembly and Hanna Caird for her contributions to provincial surveillance comparisons, each of the British Columbia (BC) Centre for Disease Control. Manish Sadarangani acknowledges general salary support provided to him by awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program, and the Michael Smith Foundation for Health Research. Finally, we thank the many frontline, regional, and provincial practitioners, including clinical, laboratory and public health providers, epidemiologists, Medical Health Officers, laboratory staff, vaccinators, participants, and others who contributed to the epidemiological, virological, and genetic characterization data underpinning these analyses.
Potential conflicts of interest. D. M. S. is Principal or Co-Investigator on grants from the Michael Smith Foundation for Health Research, the Public Health Agency of Canada, and the Canadian Institutes of Health Research paid to her institution and unrelated to the current work. M. K. received grants/contracts paid to his institution from Roche, Hologic, and Siemens, unrelated to this work. M. S. has been an investigator on projects, unrelated to the current work, funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo, and VBI Vaccines; all funds have been paid to his institute, and he has not received any personal payments. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




