-
PDF
- Split View
-
Views
-
Cite
Cite
Grahame S Davis, Patrick J Horner, Malcolm J Price, Holly D Mitchell, Kate Soldan, What Do Diagnoses of Pelvic Inflammatory Disease in Specialist Sexual Health Services in England Tell Us About Chlamydia Control?, The Journal of Infectious Diseases, Volume 224, Issue Supplement_2, 15 August 2021, Pages S113–S120, https://doi.org/10.1093/infdis/jiab175
Close - Share Icon Share
Abstract
Pelvic inflammatory disease (PID) is an outcome measure for the evaluation of chlamydia screening programs. We explore PID diagnoses in specialist sexual health services (SSHSs) in England to inform the evaluation of the National Chlamydia Screening Programme, which was implemented nationally in 2008.
We conducted descriptive analyses using data on diagnoses of PID—with and without Chlamydia trachomatis (CT) and/or Neisseria gonorrhoeae (GC)—by age and year of birth, in SSHSs between 2009 and 2019 from the GUMCAD STI Surveillance System database. Rates were calculated per 100 000 females residing in England.
CT screening activity peaked in 2010. The rates of all PID diagnoses decreased between 2009 and 2019 by 39%. CT-associated PID (CT-PID) declined by 58%, and nonspecific PID declined by 37%. GC-PID increased by 34%. CT-PID decreased across all age groups with the highest observed decline, 71%, in 15- to 19-year-olds. A dose-response relationship was observed between CT-PID rates and screening, with rates lowest in those with the greatest exposure to screening.
There was a marked decline in diagnoses of CT-PID, and nonspecific PID, at SSHSs after the introduction of widespread chlamydia screening, whereas GC-PID diagnoses increased. This ecological trend was broadly consistent with what we would have expected to see if widespread screening reduced the incidence of chlamydia-associated PID (and of nonspecific PID), as has been observed in randomized controlled trials of screening.
The National Chlamydia Screening Programme (NCSP) was established in England in 2003 and nationally implemented by 2008 with the aim of controlling chlamydia in young, sexually active people, among whom incidence was known to be higher. Together with the introduction of nucleic acid amplification testing technologies from 2003, this resulted in large increases in chlamydia screening and diagnoses: 2.3 million tests were reported in 2010 among 15- to 24-year-olds, equivalent to 44% of females and 24% of males in this age group, if only 1 test per person.
Pelvic inflammatory disease (PID) encompasses a range of upper genital tract inflammatory disorders in females that result from the spread of microorganisms from the lower to the upper genital tract [1, 2]. Genital infection with Chlamydia trachomatis (CT) is 1 of the principal causes of PID and has been estimated to account for around 35% of cases in females aged 16–24 years, decreasing to around 11% in females aged 25–44 years in England [1–3]. Other sexually transmitted infections (STIs) that can cause PID include Neisseria gonorrhoeae (GC) and Mycoplasma genitalium [1, 2, 4, 5]. The importance of PID as an outcome measure for the evaluation of STI control lies not only in its direct impact on health, but also as a precursor of more serious conditions including ectopic pregnancy and infertility [1, 2, 6]. However as the proportion of PID caused by chlamydia decreases with age, changes in all-cause PID, which is what is usually reported nationally, tells us little about the effectiveness of chlamydia control programs [3, 7].
In England, females with PID can present to primary care, hospital or specialist genitourinary medicine, and integrated genitourinary medicine/sexual and reproductive health specialist sexual health services (SSHSs) [3, 6]. While it is unclear what proportion of females with PID are diagnosed at SSHSs, these services are available free of charge to all females at risk of STIs [3, 6]. Attendance at SSHSs increased year on year, particularly among asymptomatic males and females requesting screening. It is unknown if this could affect access by symptomatic females. We explore available data about PID diagnoses and associated infections in SSHSs in England to inform evaluation of chlamydia screening.
METHODS
Data Source
We used data from the GUMCAD STI Surveillance System, the national STI surveillance system in England. It is a pseudo-anonymized patient-level dataset that includes information on all attendances, tests, and diagnoses at SSHSs in England. Each attendance is reported with demographic information including age, gender, sexual orientation, ethnicity, and patient area of residence. Patient records can be linked within, but not across clinics, using a clinic patient number that is unique to each individual [8, 9].
We included data on all diagnoses of PID—with and without CT and/or GC—of bacterial vaginosis and of anogenital candidosis made at SSHSs between 2009 and 2019. Counts and rates of diagnoses were calculated for 15- to 34-year-olds in 5-year age groups, for females only.
Validation and Data Management
To explore the potential effect of changes in access to (or use of) SSHSs over this time, we used data on the total number of female attendances and changes in diagnoses of vaginosis/vaginitis (bacterial vaginosis and candidosis). This measure was chosen as a symptomatic clinical presentation with no active control program, with the assumption that the frequency of these conditions in the population is relatively stable and therefore likely—we proposed—to be reflective of variations in service use for symptomatic conditions. Attendances were used as a measure of access for symptomatic and asymptomatic care.
To investigate the potential impact of changes in service use, indicated by attendance for symptomatic conditions with stable epidemiology, the ratio of CT PID and nonspecific PID to any vaginosis/vaginitis diagnoses was calculated.
Individuals who had a diagnosis of nonspecific PID and CT on the same day were considered to have CT-associated PID (herein referred to as CT-PID), and those who had a diagnosis of nonspecific PID and GC were considered to have GC-PID. PID codes were de-duplicated with a patient only able to have 1 diagnosis of PID in a 42-day period, reflecting the standard duration of an episode of care in SSHSs [8, 10]; if there were multiple codes within a 42-day period then a CT/GC PID diagnosis was kept over any nonspecific PID diagnosis, and a GC-PID coding was kept over a CT-PID coding.
Chlamydia Screening Activity Data
Pre-2012, community (non-SHSS) chlamydia tests and diagnoses in England were reported using 2 systems: the NCSP core data return and an aggregate laboratory reporting system. In 2012, these 2 data sources were replaced by a single laboratory reporting system, the Chlamydia Testing Activity Dataset (CTAD). CTAD now provides detailed reports at national and local levels on chlamydia screening activity in 15- to 24-year olds. Data were extracted for 2009–2019 to show trends in this wider screening activity alongside testing data from SSHS.
Population Data
Rates were calculated per 100 000 females residing in England using Office for National Statistics midyear population estimates as denominators [11].
Birth Cohorts
To evaluate the dose-response relationship between PID and chlamydia screening, birth-cohort groupings were defined by exposure to widespread screening through the NCSP. The groups were defined as:
Full exposure: females aged ≤10 years in 2008 (born after 1997) who should have had the greatest access to screening since sexual debut.
High exposure: females aged ≤16 years in 2008 (born between 1992 and 1997) who should have had good access to screening since sexual debut.
Partial exposure: females aged between 17 and 20 years in 2008 (born between 1988 and 1991) who should have had some access to screening while under 20 years of age.
Low exposure: females aged between 21 and 24 years in 2008 (born between 1984 and 1987), who should have had access to screening over 20 years of age.
Very low exposure: females aged >24 years in 2008 (born between 1976 and 1983), who would only have had access to screening during the initial roll-out phase of the NCSP (from 2003).
No exposure: females aged >33 years in 2008 (born between 1965 and 1975) who would have reached 24 years before any screening was offered through the NCSP.
Ethical Considerations
No specific consent was required from the patients whose data were used in these analyses. In its role providing infectious disease surveillance, Public Health England has permission to handle data obtained by the GUMCAD STI Surveillance System and the CTAD Chlamydia Surveillance System under Regulation 3 of the Health Service (Control of Patient Information) Regulations 2002.
RESULTS
Changes in Chlamydia Testing Activity in England
Testing activity in 15- to 24-year-olds varied over the time period (2009–2019) with a peak in testing seen in 2010 of around 2.3 million tests. Subsequent years saw a decline with 1.3 million tests recorded in 2019, a 39% decline since 2010. Data by gender were available since 2012 and the percentage decline since 2012 was greater in males than in females (35% and 24%, respectively).
Testing was more stable in SSHSs; in 15- to 24-year-olds, there were around 580 000 tests carried out in SSHSs in 2019 and this was a 2.4% decrease since a peak (n = 595 222) in 2014. Testing in females was also stable in SSHSs with 372 305 tests in 2019, a negligible increase since 2014 (n = 371 821).
Changes in Population Utilizing SHSSs
The rate of all-age female attendances (including new and follow-up consultations) at SHSSs increased by 50% between 2009 and 2019 to 6275 per 100 000 population: Consultation rates increased in 15- to 24-year-old and 25- to 34-year-old females by 53% and 46%, respectively.
Rates of any vaginosis/vaginitis overall peaked in 2012 at 662 diagnoses per 100 000 female population, before decreasing to 490 per 100 000 in 2019, a drop of 21% between 2009 and 2019.
Trends in PID Diagnoses
The rates of PID diagnoses decreased during the study period by 38%, although the scale of the decline varied by PID type (Figure 1). CT-PID declined by 58%, and nonspecific PID declined by 37%. GC-PID fluctuated but showed an overall increase of 34% between 2009 and 2019 (Figure 1B). During the initial 2009–2012 period (during which the NCSP recorded highest levels of screening), CT-PID diagnoses decreased by 36%, and nonspecific PID decreased by 4% whereas GC-PID diagnoses increased by 5%.
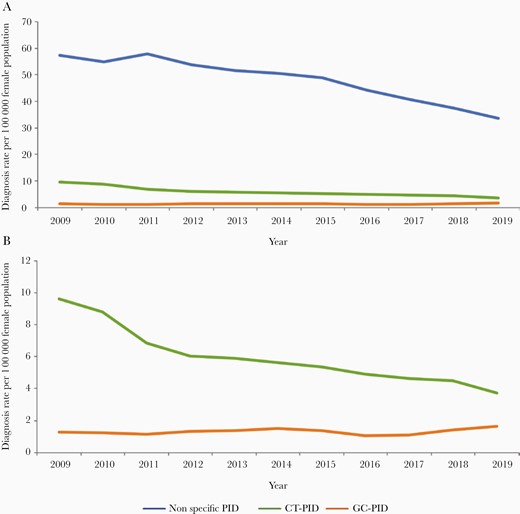
A, All-age pelvic inflammatory disease (PID) diagnosis rates per 100 000 female population between 2009 and 2019 for nonspecific PID, Chlamydia trachomatis (CT)–associated PID, and Neisseria gonorrhoeae (GC)–associated PID. B, CT-PID and GC-PID rates per 100 000 female population between 2009 and 2019 presented on a smaller scale.
CT-PID decreased across all age groups (Figure 2); the decline was highest in 15- to 19-year-olds, 71%, compared to declines of between 44% and 54% in the older age groups. A similar trend was observed in nonspecific PID, as diagnoses decreased in all age groups but more so in the younger age groups, ranging from 60% (in 15- to 19-year-olds) to 21% (in 30- to 34-year-olds). GC-PID did not follow the same pattern: It also had a decline, though less, of around 6% in 15- to 19-year-olds but increases in the age groups 20–24, 25–29, and 30–34 years by 22%, 77%, and 128%, respectively.
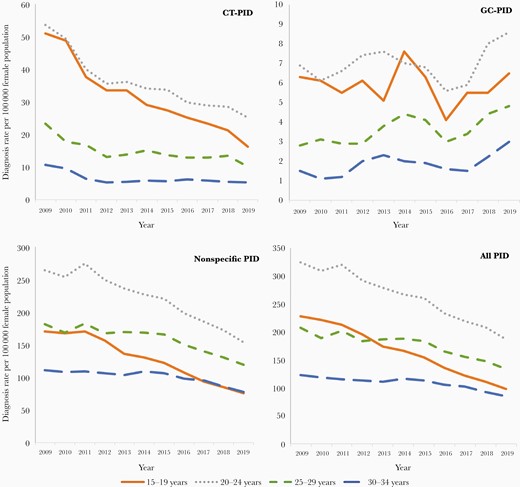
Pelvic inflammatory disease (PID) diagnosis rates per 100 000 female population between 2009 and 2019 for Chlamydia trachomatis (CT)–associated PID, Neisseria gonorrhoeae (GC)–associated PID, nonspecific PID, and all PID by age group (15–19 years, 20–24 years, 25–29 years, and 30–34 years).
Focusing on the age group eligible for screening, CT-PID declined between 2009 and 2019 with a greater decrease in 15- to 24-year-olds than in 25- to 34-year-olds (62% and 52%, respectively). Both age groups showed a reduction between 2009 and 2012; however the trends post 2012 differ. Diagnoses in the 15- to 24-year-old age group reduced consistently after 2012, a decrease of 43% by 2019, whereas there was a decrease of 14% in the 25- to 34-year-old age group between 2012 and 2019.
Adjusting for changes in attendance for symptomatic conditions using the ratio of nonspecific PID and of CT-PID to any vaginosis/vaginitis diagnoses, the declining trend in CT PID persisted but was lessened (51% for this ratio compared to 58% for the rate, 2009–2019). For both CT-PID and nonspecific PID, the decline post-2012 was lessened when this ratio was considered; however, the declining trend from 2009 to 2012 remained and the lower rate was maintained after 2012 (Figure 3).
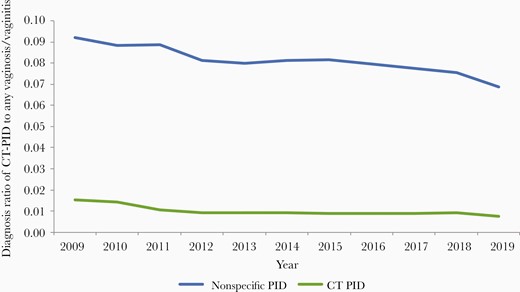
Diagnosis ratio of all-age nonspecific pelvic inflammatory disease (PID) and Chlamydia trachomatis (CT)–associated PID to any vaginosis/vaginitis in females between 2009 and 2019.
Trends in NCSP Birth Cohorts
CT-PID rates were lowest in the cohort with the greatest exposure to the NCSP program, with each cohort with less exposure showing higher observed rates. In 20-year-olds, the “full exposure” cohort had a lower CT-PID rate of 36 per 100 000 population compared to the “high exposure” and the “partial exposure” cohorts (43 and 61 per 100 000 population, respectively; Figure 4).
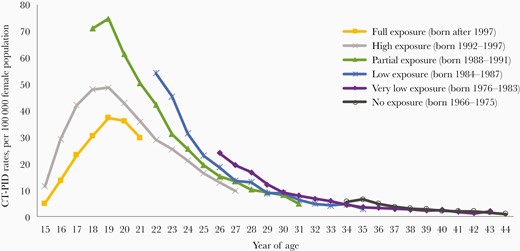
Chlamydia trachomatis (CT)–associated pelvic inflammatory disease (PID) rates per 100 000 female population aged 15–44 years by year of age and birth cohort with varying exposure to widespread chlamydia screening through the National Chlamydia Screening Programme, 2009–2019.
A similar dose-response pattern was observed with nonspecific PID rates, but not with GC-PID (Supplementary Figure 5).
Discussion
Between 2009 and 2019, PID diagnoses in females attending SSHSs decreased by 39% with CT-PID diagnoses decreasing by 58% and nonspecific PID by 37%. The proportion of all PID that was CT-PID fell from 14.1% to 9.6% (data not shown). This decline in CT-PID was greatest in females aged 15–24 years (62%), with the greatest decline observed between 2009 and 2011. The decrease in the number of CT-PID cases persisted when the number and types of attendances were controlled for by examining the population-based PID rates and adjusting for the annual number of females diagnosed with vaginal candidiasis and/or bacterial vaginosis. The declines in CT-PID and nonspecific PID show a dose-response relationship with access to NCSP-driven screening during years of sexually active young adulthood.
Strengths
This was a large study that used comprehensive national surveillance data which records all attendances at SSHSs in England. All attendances were coded using standardized definitions based on the clinical and/or microbiological diagnosis [9]. The guidelines used in these settings have, since 2005, advised a low index of clinical suspicion when diagnosing PID, particularly in females <25 years of age, as the symptoms and signs lack sensitivity and specificity [2, 12, 13]. Thus, these criteria have remained essentially unchanged over the study period. The fraction of PID associated with CT decreases with increasing age, being greatest in females <25 years old [3]. In this study we were able to look at changes in CT-PID rates in 15- to 24-year-olds and 25- to 34-year-olds, enabling us to adjust for potential changes in age distribution of female attendees over time.
Access to SSHSs has changed over the study time period with the number of attendances increasing [14, 15]. Changes in access—that is, reduction in clinical capacity associated with rising demand for asymptomatic screening—could potentially reduce the number of PID attendances. We were able to explore this by examining the changes in PID diagnoses compared to vaginal discharge caused by candidosis and bacterial vaginosis, rates of which should remain constant within the population. Falls in CT-PID were still observed after adjusting for changes in these other diagnoses, although they were not as great.
Limitations
This was an ecological study and although the changes in PID rates were consistent with what one would expect to see if higher levels of screening, as facilitated by the NCSP, were effective it was not possible to infer that the CT-PID rate fall was caused by the NCSP policy and its implementation since 2008. We were not able to analyze changes in PID in the same way prior to 2009 due to changes in data collection. An increase in all PID and CT-PID was observed between 2004 and 2009; however, this was alongside increased attendance following the government’s 2004 white paper [16], which identified improving sexual health services as a priority and was associated with an increase in funding. Similar increases in capacity following service improvements have been reported as the cause of increase in PID rates in SSHSs in Australia [17].
Other studies have looked at changes in PID over time; however, the associated infections were often not available and many studies used rates for females with PID treated in hospital [6, 7, 18–20]. The majority of females diagnosed with PID are managed in an outpatient or community setting with some evidence that CT-PID may have a milder clinical presentation than other causes and thus be less likely to require admission to hospital [6, 19]. In addition, the proportion of PID caused by CT decreases with age [3]. It is thus difficult to use such data to examine the effect of CT control measures when using all PID as an outcome measure [7].
Nevertheless, our results are not inconsistent with the findings from the following studies. The pooled risk ratio for all-cause PID after 1 year of follow-up in females invited to have a chlamydia screening test in 4 randomized controlled trials (RCTs) was 0.64 (95% confidence interval [CI], .45–.90; I2 = 20%) after 1 year [21]. In the recent Australian Chlamydia Control Effectiveness Pilot (ACCEPt) trial, the incidence of PID diagnosed in hospital decreased by 1.37 per 100 000 women (95% CI, .5–26.9) [20]. However, there was no change in the incidence of PID diagnosed in clinics.
The NCSP was nationally implemented in 2008 and it is unclear why a drop was only observed from 2009. The official estimated coverage in females (assuming 1 test per female) increased from 30.9% in 2008–2009 to 42.1% in 2010–2011 but is likely to be lower as some females will have tested more than once in any given year [22, 23]. The NCSP promotes annual testing, which in the first year would only prevent 55%–66% of CT-PID cases in those screened [24]. It is possible that any reductions in CT-PID in 2008 and 2009 may have been obscured by the increases in SSHS attendances between 2004 and 2009 as described above.
Finally, CT positivity in females with PID attending SSHSs is lower than that estimated through a recent multiparameter evidence (MPES) synthesis [3, 6]. It is unclear why that is, but the CT positivity is similar to that observed in Australian SSHSs [25]. This may reflect a low index of suspicion as recommended in the British Association for Sexual Health and HIV PID guidelines and would include females with “possible” PID whereas the MPES analysis considered only females with probable or definite PID and used data obtained before the introduction of screening [6, 26].
Our data and analyses show an ecological trend that is broadly consistent with widespread screening through the NCSP reducing the incidence of CT-PID as observed in previous RCTs [21]. It is of interest that nonspecific PID rates also decreased, but to a lesser extent and later than the CT-PID rates with the proportion of CT- PID, decreasing from 14.1% to 9.6% between 2009 and 2019, while GC-PID increased. One explanation as discussed by Horner et al elsewhere in this special supplement is that CT infection of the fallopian tubes can result in a persistent epithelial-to-mesenchymal transition (EMT) state as a result of epigenetic changes [27]. Such a state is proinflammatory and could increase the risk of nonspecific PID developing in females whose upper genital tract is colonized by bacterial vaginosis–associated bacteria (BVAB) [27, 28]. EMT reduces the integrity of the epithelium, potentially making it more susceptible to invasion and disease from BVAB [27]. BVAB are the most common cause of nonspecific PID [1, 2, 27]. It is likely if CT-PID rates decreased so did upper genital tract CT infection, which is not always symptomatic [6, 27]. This would then reduce the subsequent risk of developing PID from any cause [18, 19, 27]. Horner et al argue that this hypothesis merits further investigation as it would increase our understanding of the risks of sequelae associated with chlamydial genital tract infection and thus better inform public health interventions and cost-effectiveness models of interventions such as screening and vaccination [27, 29, 30].
Consideration should be given to investigating whether serology as discussed by Horner elsewhere in this supplement can be used to evaluate whether there is an observable birth cohort effect on CT tubal factor infertility similar to what we have demonstrated for CT-PID [31]. Most females with infertility present years after the inciting infection has resolved and would no longer be detected by nucleic acid amplification testing or other tests for active urogenital infection [6, 31].
Conclusions
There was a marked decline in diagnoses of CT PID, and nonspecific PID, at SSHSs after the introduction of widespread chlamydia screening, while GC-PID diagnoses increased. This occurred despite an increase in attendances at SSHSs and alongside a far smaller decline in diagnoses of vaginosis/vaginitis due to bacterial vaginosis and candidosis. Further work is needed, including to explore trends in general practice and hospital admissions, to determine whether the frequency of CT-PID (rates, or more likely as a proportion of all PID) in SHSSs might offer a useful national and local metric for the success of chlamydia control.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Paula Blomquist, Bersabeh Sile, and Hamish Mohammed for their support with accessing and analyzing the GUMCAD STI Surveillance System data.
Disclaimer. The views expressed are those of the author and not necessarily those of the National Institute for Health Research (NIHR), the Department of Health and Social Care, or Public Health England.
Financial support. P. H. was funded by the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol. M. J. P. is supported by the NIHR Birmingham Biomedical Research Centre.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: STI & HIV 2019 World Congress, Joint Meeting of the 23rd International Society for Sexually Transmitted Diseases Research and 20th International Union Against Sexually Transmitted Infections, Vancouver, Canada, 14–17 July 2019.




