-
PDF
- Split View
-
Views
-
Cite
Cite
Richard Long, Angela Lau, Mary Lou Egedahl, Catherine Paulsen, Courtney Heffernan, Brett Edwards, Ryan Cooper, Local Transmission Plays No Important Role in the Occurrence of Multidrug-Resistant Tuberculosis in Immigrants to Canada: An In-depth Epidemiologic Analysis, The Journal of Infectious Diseases, Volume 224, Issue 6, 15 September 2021, Pages 1029–1038, https://doi.org/10.1093/infdis/jiab045
Close - Share Icon Share
Abstract
Multidrug-resistant (MDR) tuberculosis has increased among migrants in Canada. The cause(s) of this increase is unknown.
We performed a retrospective cohort study in a Canadian province with substantially increased immigration between 1982–2001 and 2002–2019. The proportion of MDR tuberculosis among migrants arriving from high MDR (HMDR) tuberculosis burden countries during these 2 periods was used to estimate the proportion of cases due to immigration versus change in proportion in the country of birth. Epidemiologic, spatiotemporal, and drug resistance pattern data were used to confirm local transmission.
Fifty-two of 3514 (1.48%) foreign-born culture-positive tuberculosis patients had MDR tuberculosis: 8 (0.6%) in 1982–2001 and 44 (2.0%) in 2002–2019. Between time periods, the proportion of MDR tuberculosis among migrants with tuberculosis from HMDR tuberculosis countries increased from 1.11% to 3.62%, P = .003; 31.6% attributable to recent immigration and 68.4% to a higher proportion of MDR tuberculosis in cases arrived from HMDR tuberculosis countries. No cases of MDR tuberculosis were attributable to local transmission.
In stark contrast to HMDR tuberculosis countries, local transmission plays no important role in the occurrence of MDR tuberculosis in Canada. Improved tuberculosis programming in HMDR tuberculosis countries is urgently needed.
Over the past 60 years, the country of birth of migrants to Canada has shifted from low–tuberculosis-incidence countries of Western Europe to high–tuberculosis-incidence countries of Asia and Africa [1, 2]. Moreover, in the last 25 years there has been a sharp rise, from approximately 150 000 to 250 000, in the absolute number of new immigrants accepted into Canada each year [3–5]. These trends have had a major influence on the epidemiology of tuberculosis in Canada and on drug-resistant tuberculosis in particular.
From 1970 to 2017, the percentage of total tuberculosis cases reported among foreign-born persons in Canada increased from 18% to 72% [1, 6]. Early on, it was apparent that foreign-born cases were more likely than Canadian-born cases to be drug resistant [7], attributable, it was understood, to a combination of factors in immigrants’ country of birth. These included drugs being improperly prescribed, properly prescribed but unavailable, inadequately supervised, or possibly malabsorbed [8]. High rates of drug resistance were observed in countries with high rates of tuberculosis and widely available, but poorly organized, access to health care and medications [9]. The use of standardized regimens in lieu of individualized regimens based upon drug susceptibility test results quite likely exacerbated the problem [10]. Resistance to the 2 most important first-line drugs, isoniazid and rifampin, with or without resistance to other drugs (multidrug-resistant, MDR), emerged as a grave threat to tuberculosis prevention and care. Treatment requires longer, more costly, and often more toxic drug regimens. Once established in an infectious patient, resistant bacteria may spread to others through airborne droplets as primary or transmitted drug resistance. In 2010, it was estimated that 54% of MDR tuberculosis cases in low- and middle-income countries were due to transmitted drug resistance [11].
High-income, low–tuberculosis-incidence countries like Canada are not isolated from global increases in MDR tuberculosis as emigrants from high MDR (HMDR) tuberculosis burden countries may be infected with MDR tuberculosis strains prior to immigration and develop active disease after arrival in Canada. In Canada, most MDR tuberculosis cases are reported in Ontario, Quebec, British Columbia, and Alberta, the 4 major immigrant-receiving provinces. The proportion of the tuberculosis case load that is foreign born and the standard of care in these provinces is similar [2]. This study used conventional and molecular epidemiologic data over an extended period of time to explain the proximate cause of MDR tuberculosis in foreign-born persons in Canada—is it attributable to an increase in the local transmission of MDR tuberculosis strains, increase in the number of newly arrived immigrants, or increase in the proportion of MDR tuberculosis in those newly arrived, or some combination of these factors? As a case study it looks specifically at the Province of Alberta (population 4 252 900 in 2016 [3], which, over the past 18 years, has seen a sharp rise in immigration (Figure 1A) and, compared to the previous 20 years, a 5-fold increase in the number of foreign-born MDR tuberculosis cases.
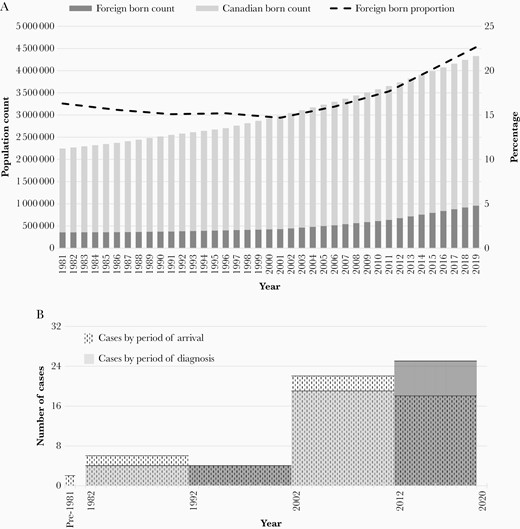
A, Population of Alberta and proportion foreign born, 1981–2019 (Statistics Canada). B, Number of foreign-born persons with multidrug-resistant tuberculosis by period of arrival and diagnosis.
METHODS
We used a retrospective cohort study design. We identified cases in the Provincial tuberculosis registry and collected appropriate demographic and clinical features from their records. We abstracted laboratory data from the Provincial Laboratory for Public Health (ProvLab), where all mycobacteriology in the province is performed. Demographic data included age at diagnosis, sex, country of birth, and year of arrival. Countries of birth were divided into those that were HMDR tuberculosis burden, according to World Health Organization (WHO) estimates in 2008, and those that were not (Table 1) [12]. The year 2008 was the first year that the WHO formally identified HMDR tuberculosis countries; it was also the median year of arrival of our MDR cases. Clinical data included disease type (new active vs relapse/retreatment as defined in the Canadian Tuberculosis Standards) [2], disease site (respiratory vs nonrespiratory), and human immunodeficiency virus (HIV) serostatus.
Estimated Proportion of MDR Tuberculosis Cases Among Incident Tuberculosis Cases in the 27 High MDR Tuberculosis Burden Countries, 2008a
| WHO Region . | Country . | Source of Estimates . | % MDR Among New Tuberculosis Cases (95% CI) . | % MDR Among Previously Treated Tuberculosis Cases (95% CI) . |
|---|---|---|---|---|
| Africa | DR Congo | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) |
| Ethiopia | DRS, 2005 | 1.6 (.9–2.7) | 11.8 (6.4–21.0) | |
| Nigeria | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) | |
| South Africa | DRS, 2002 | 1.8 (1.5–2.3) | 6.7 (5.5–8.1) | |
| Europe | Armenia | DRS, 2007 | 9.4 (7.3–12.1) | 43.2 (38.1–48.5) |
| Azerbaijan | DRS, 2007b | 22.3 (19.0–26.0) | 55.8 (51.6–59.9) | |
| Belarus | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Bulgaria | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Estonia | DRS, 2008 | 15.4 (11.6–20.1) | 42.7(32.1–53.9) | |
| Georgia | DRS, 2006 | 6.8 (5.2–8.7) | 27.4 (23.7–31.4) | |
| Kazakhstan | DRS, 2001 | 14.2 (11.0–18.2) | 56.4 (50.9–61.8) | |
| Kyrgyzstan | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Latvia | DRS, 2008 | 12.1 (9.9–14.8) | 31.9 (24.9–39.9) | |
| Lithuania | DRS, 2008 | 9.0 (7.5–10.7) | 47.5 (42.9–52.2) | |
| Republic of Moldova | DRS, 2006 | 19.4 (16.8–22.2) | 50.8 (48.7–53.0) | |
| Russian Federation | DRS, 2008b | 15.8 (11.9–19.7) | 42.4 (38.1–46.7) | |
| Tajikistan | DRS, 2008b | 16.5 (11.3–23.6) | 61.6 (52.9–69.7) | |
| Ukraine | DRS, 2002b | 16.0 (13.8–18.3) | 44.3 (40.0–48.7) | |
| Uzbekistan | DRS, 2005b | 14.2 (10.4–18.1) | 49.8 (35.8–63.8) | |
| Eastern Mediterranean | Pakistan | Model | 2.9 (.0–8.0) | 35.4 (.0–75.1) |
| South-East Asia | Bangladesh | Model | 2.2 (.0–5.6) | 14.7 (.0–39.6) |
| India | DRS, 2005b | 2.3 (1.8–2.8) | 17.2 (14.9–19.5) | |
| Indonesia | DRS, 2004b | 2.0 (.5–6.9) | 14.7 (.0–39.6) | |
| Myanmar | DRS, 2007 | 4.2 (3.2–5.6) | 10.0 (7.1–14.0) | |
| Western Pacific | China | DRS, 2007 | 5.7 (5.0–6.6) | 25.6 (22.6–28.3) |
| Philippines | DRS, 2004 | 4.0 (3.0–5.5) | 20.9 (14.8–28.7) | |
| Viet Nam | DRS, 2006 | 2.7 (2.0–3.6) | 19.3 (14.5–25.2) |
| WHO Region . | Country . | Source of Estimates . | % MDR Among New Tuberculosis Cases (95% CI) . | % MDR Among Previously Treated Tuberculosis Cases (95% CI) . |
|---|---|---|---|---|
| Africa | DR Congo | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) |
| Ethiopia | DRS, 2005 | 1.6 (.9–2.7) | 11.8 (6.4–21.0) | |
| Nigeria | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) | |
| South Africa | DRS, 2002 | 1.8 (1.5–2.3) | 6.7 (5.5–8.1) | |
| Europe | Armenia | DRS, 2007 | 9.4 (7.3–12.1) | 43.2 (38.1–48.5) |
| Azerbaijan | DRS, 2007b | 22.3 (19.0–26.0) | 55.8 (51.6–59.9) | |
| Belarus | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Bulgaria | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Estonia | DRS, 2008 | 15.4 (11.6–20.1) | 42.7(32.1–53.9) | |
| Georgia | DRS, 2006 | 6.8 (5.2–8.7) | 27.4 (23.7–31.4) | |
| Kazakhstan | DRS, 2001 | 14.2 (11.0–18.2) | 56.4 (50.9–61.8) | |
| Kyrgyzstan | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Latvia | DRS, 2008 | 12.1 (9.9–14.8) | 31.9 (24.9–39.9) | |
| Lithuania | DRS, 2008 | 9.0 (7.5–10.7) | 47.5 (42.9–52.2) | |
| Republic of Moldova | DRS, 2006 | 19.4 (16.8–22.2) | 50.8 (48.7–53.0) | |
| Russian Federation | DRS, 2008b | 15.8 (11.9–19.7) | 42.4 (38.1–46.7) | |
| Tajikistan | DRS, 2008b | 16.5 (11.3–23.6) | 61.6 (52.9–69.7) | |
| Ukraine | DRS, 2002b | 16.0 (13.8–18.3) | 44.3 (40.0–48.7) | |
| Uzbekistan | DRS, 2005b | 14.2 (10.4–18.1) | 49.8 (35.8–63.8) | |
| Eastern Mediterranean | Pakistan | Model | 2.9 (.0–8.0) | 35.4 (.0–75.1) |
| South-East Asia | Bangladesh | Model | 2.2 (.0–5.6) | 14.7 (.0–39.6) |
| India | DRS, 2005b | 2.3 (1.8–2.8) | 17.2 (14.9–19.5) | |
| Indonesia | DRS, 2004b | 2.0 (.5–6.9) | 14.7 (.0–39.6) | |
| Myanmar | DRS, 2007 | 4.2 (3.2–5.6) | 10.0 (7.1–14.0) | |
| Western Pacific | China | DRS, 2007 | 5.7 (5.0–6.6) | 25.6 (22.6–28.3) |
| Philippines | DRS, 2004 | 4.0 (3.0–5.5) | 20.9 (14.8–28.7) | |
| Viet Nam | DRS, 2006 | 2.7 (2.0–3.6) | 19.3 (14.5–25.2) |
Modified from Table 6 in WHO Multidrug and extensively drug-resistant TB: 2010 global report on surveillance and response and used with permission [12].
Abbreviations: CI confidence interval; DR, Democratic Republic; DRS, drug resistance surveillance or survey data; MDR, multidrug resistant; WHO, World Health Organization.
aThe 27 high MDR tuberculosis burden countries refer to WHO member states that were estimated by the WHO in 2008 to have had an annual incidence of at least 4000 MDR tuberculosis cases and/or at least 10% of newly registered tuberculosis cases with MDR tuberculosis.
bEstimates were based on subnational drug resistance data.
Estimated Proportion of MDR Tuberculosis Cases Among Incident Tuberculosis Cases in the 27 High MDR Tuberculosis Burden Countries, 2008a
| WHO Region . | Country . | Source of Estimates . | % MDR Among New Tuberculosis Cases (95% CI) . | % MDR Among Previously Treated Tuberculosis Cases (95% CI) . |
|---|---|---|---|---|
| Africa | DR Congo | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) |
| Ethiopia | DRS, 2005 | 1.6 (.9–2.7) | 11.8 (6.4–21.0) | |
| Nigeria | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) | |
| South Africa | DRS, 2002 | 1.8 (1.5–2.3) | 6.7 (5.5–8.1) | |
| Europe | Armenia | DRS, 2007 | 9.4 (7.3–12.1) | 43.2 (38.1–48.5) |
| Azerbaijan | DRS, 2007b | 22.3 (19.0–26.0) | 55.8 (51.6–59.9) | |
| Belarus | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Bulgaria | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Estonia | DRS, 2008 | 15.4 (11.6–20.1) | 42.7(32.1–53.9) | |
| Georgia | DRS, 2006 | 6.8 (5.2–8.7) | 27.4 (23.7–31.4) | |
| Kazakhstan | DRS, 2001 | 14.2 (11.0–18.2) | 56.4 (50.9–61.8) | |
| Kyrgyzstan | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Latvia | DRS, 2008 | 12.1 (9.9–14.8) | 31.9 (24.9–39.9) | |
| Lithuania | DRS, 2008 | 9.0 (7.5–10.7) | 47.5 (42.9–52.2) | |
| Republic of Moldova | DRS, 2006 | 19.4 (16.8–22.2) | 50.8 (48.7–53.0) | |
| Russian Federation | DRS, 2008b | 15.8 (11.9–19.7) | 42.4 (38.1–46.7) | |
| Tajikistan | DRS, 2008b | 16.5 (11.3–23.6) | 61.6 (52.9–69.7) | |
| Ukraine | DRS, 2002b | 16.0 (13.8–18.3) | 44.3 (40.0–48.7) | |
| Uzbekistan | DRS, 2005b | 14.2 (10.4–18.1) | 49.8 (35.8–63.8) | |
| Eastern Mediterranean | Pakistan | Model | 2.9 (.0–8.0) | 35.4 (.0–75.1) |
| South-East Asia | Bangladesh | Model | 2.2 (.0–5.6) | 14.7 (.0–39.6) |
| India | DRS, 2005b | 2.3 (1.8–2.8) | 17.2 (14.9–19.5) | |
| Indonesia | DRS, 2004b | 2.0 (.5–6.9) | 14.7 (.0–39.6) | |
| Myanmar | DRS, 2007 | 4.2 (3.2–5.6) | 10.0 (7.1–14.0) | |
| Western Pacific | China | DRS, 2007 | 5.7 (5.0–6.6) | 25.6 (22.6–28.3) |
| Philippines | DRS, 2004 | 4.0 (3.0–5.5) | 20.9 (14.8–28.7) | |
| Viet Nam | DRS, 2006 | 2.7 (2.0–3.6) | 19.3 (14.5–25.2) |
| WHO Region . | Country . | Source of Estimates . | % MDR Among New Tuberculosis Cases (95% CI) . | % MDR Among Previously Treated Tuberculosis Cases (95% CI) . |
|---|---|---|---|---|
| Africa | DR Congo | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) |
| Ethiopia | DRS, 2005 | 1.6 (.9–2.7) | 11.8 (6.4–21.0) | |
| Nigeria | Model | 1.8 (.0–4.3) | 7.7 (.0–18.1) | |
| South Africa | DRS, 2002 | 1.8 (1.5–2.3) | 6.7 (5.5–8.1) | |
| Europe | Armenia | DRS, 2007 | 9.4 (7.3–12.1) | 43.2 (38.1–48.5) |
| Azerbaijan | DRS, 2007b | 22.3 (19.0–26.0) | 55.8 (51.6–59.9) | |
| Belarus | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Bulgaria | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Estonia | DRS, 2008 | 15.4 (11.6–20.1) | 42.7(32.1–53.9) | |
| Georgia | DRS, 2006 | 6.8 (5.2–8.7) | 27.4 (23.7–31.4) | |
| Kazakhstan | DRS, 2001 | 14.2 (11.0–18.2) | 56.4 (50.9–61.8) | |
| Kyrgyzstan | Model | 12.5 (.0–25.3) | 42.1 (11.9–72.2) | |
| Latvia | DRS, 2008 | 12.1 (9.9–14.8) | 31.9 (24.9–39.9) | |
| Lithuania | DRS, 2008 | 9.0 (7.5–10.7) | 47.5 (42.9–52.2) | |
| Republic of Moldova | DRS, 2006 | 19.4 (16.8–22.2) | 50.8 (48.7–53.0) | |
| Russian Federation | DRS, 2008b | 15.8 (11.9–19.7) | 42.4 (38.1–46.7) | |
| Tajikistan | DRS, 2008b | 16.5 (11.3–23.6) | 61.6 (52.9–69.7) | |
| Ukraine | DRS, 2002b | 16.0 (13.8–18.3) | 44.3 (40.0–48.7) | |
| Uzbekistan | DRS, 2005b | 14.2 (10.4–18.1) | 49.8 (35.8–63.8) | |
| Eastern Mediterranean | Pakistan | Model | 2.9 (.0–8.0) | 35.4 (.0–75.1) |
| South-East Asia | Bangladesh | Model | 2.2 (.0–5.6) | 14.7 (.0–39.6) |
| India | DRS, 2005b | 2.3 (1.8–2.8) | 17.2 (14.9–19.5) | |
| Indonesia | DRS, 2004b | 2.0 (.5–6.9) | 14.7 (.0–39.6) | |
| Myanmar | DRS, 2007 | 4.2 (3.2–5.6) | 10.0 (7.1–14.0) | |
| Western Pacific | China | DRS, 2007 | 5.7 (5.0–6.6) | 25.6 (22.6–28.3) |
| Philippines | DRS, 2004 | 4.0 (3.0–5.5) | 20.9 (14.8–28.7) | |
| Viet Nam | DRS, 2006 | 2.7 (2.0–3.6) | 19.3 (14.5–25.2) |
Modified from Table 6 in WHO Multidrug and extensively drug-resistant TB: 2010 global report on surveillance and response and used with permission [12].
Abbreviations: CI confidence interval; DR, Democratic Republic; DRS, drug resistance surveillance or survey data; MDR, multidrug resistant; WHO, World Health Organization.
aThe 27 high MDR tuberculosis burden countries refer to WHO member states that were estimated by the WHO in 2008 to have had an annual incidence of at least 4000 MDR tuberculosis cases and/or at least 10% of newly registered tuberculosis cases with MDR tuberculosis.
bEstimates were based on subnational drug resistance data.
Laboratory data included susceptibilities to isoniazid, rifampin, ethambutol, and streptomycin on all initial isolates dating from 1982 and to pyrazinamide on all initial isolates dating from 1991. From 1982 to 1990, the resistance ratio method was used to determine susceptibilities [13]. Strains with a resistance ratio of ≤ 2 were considered sensitive, while strains with a ratio of ≥ 8 were considered resistant. From 1991 to 2010, the BACTEC radiometric system (BACTEC 460TB, Becton-Dickinson Diagnostic Instrument Systems), and from 2010 to 2019, the BACTEC nonradiometric system (BACTEC MGIT 960, Becton Dickinson), were used to determine susceptibilities. All strains found to be resistant by BACTEC 460TB or BACTEC MGIT 960 were retested using the resistance ratio method to confirm drug resistance. All isolates determined to be multidrug resistant were tested for second-line resistance at both ProvLab and the National Microbiology Laboratory, Public Health Agency of Canada. Second-line drugs included amikacin, kanamycin, capreomycin, ofloxacin, ethionamide, para-aminosalicylic acid, rifabutin, and, in more recent years, linezolid and moxifloxacin.
Between January 1990 and June 2016, all initial isolates of Mycobacterium tuberculosis in Alberta were DNA fingerprinted using IS6110 restriction fragment-length polymorphism (RFLP), supplemented by spoligotyping in isolates with fewer than 6 copies of IS6110. Between January 2014 and June 2020, they were fingerprinted with 24-loci mycobacterial interspersed repetitive units (MIRU)-variable number tandem repeats (VNTR) [14–16]. Over these 30.5 years, the DNA fingerprints of MDR tuberculosis isolates were compared to each other and to those of non-MDR tuberculosis isolates grown from both foreign-born and Canadian-born cases. Any cases with matching isolates (ie, isolates that shared an identical DNA fingerprint; for low IS6110 copy number isolates this required that both the RFLP and spoligotype match) were carefully examined to determine (1) whether they had been identified as a contact of one another; (2) if not a contact, whether they could be linked to one another in time (diagnosed either 6 months before or 24 months after an MDR tuberculosis case) and place (resided in the same forward sortation area—a geographic unit associated with a postal facility from which mail delivery originates—as determined by the first 3 digits of their postal code) [17, 18]; or (3) whether the matching isolates shared the same first- (and second-) line antituberculosis drug resistance pattern.
During the last 18 years (2002–2019), all contacts of adult (age > 14 years) culture-positive pulmonary MDR tuberculosis cases were identified. Contacts were grouped according to those that were close (household or nonhousehold) or casual, and these in turn were grouped according to the outcome of their investigations as follows: (1) tuberculin skin test (TST) or interferon-γ release assay (IGRA; QuantiFERON) positive; (2) TST/IGRA negative; or (3) TST/IGRA unknown (investigation either not performed or incomplete) as per the Canadian Tuberculosis Standards [2]. If latent tuberculosis infection was identified, its management was described; if treated, the drug or drug combination used was reported. All contacts were then crossmatched with the tuberculosis registry database up to the end of June 2020 using their name, date of birth, personal health care number, and tuberculosis registry number, to determine whether they had ever been reported as a case, culture positive or negative, prevalent (diagnosed 180 days before or after the index case), or incident (diagnosed later).
Analysis
The DNA fingerprint database (1990–2020) and the contact tracing database (2002–2019), as described above, were used to document the presence or absence of local transmission. In the absence of any local transmission the proportion of MDR tuberculosis in immigrants who arrived from HMDR tuberculosis countries in 1982–2001, and were diagnosed with culture-positive tuberculosis in those years, was used to estimate the number of MDR tuberculosis cases to be expected in 2002–2019, based on the number of immigrants newly arrived from HMDR tuberculosis countries and diagnosed with culture-positive tuberculosis in those years. In the absence of any increase in the proportion of cases that had arrived from HMDR tuberculosis countries in the 2 time periods, any further increase in the number of cases in 2002–2019, over and above that expected on the basis of increased immigration, was concluded to be due to a higher proportion of MDR tuberculosis in the tuberculosis cases arrived from HMDR tuberculosis countries of birth. Furthermore, it was concluded that this higher proportion likely reflected a higher prevalence of MDR tuberculosis in the country of birth. Observations are summarized below, the 2-sided P values corresponding to the χ 2 test or Fisher exact test using a 5% level of significance. Study approval was obtained from the Health Research Ethics Board of the University of Alberta.
RESULTS
Fifty-two (1.48%) of the 3514 culture-positive, foreign-born tuberculosis cases in Alberta from 1982 to 2019 had MDR tuberculosis (Table 2). Overall, MDR tuberculosis was associated with younger age, HMDR tuberculosis burden country of birth, and relapse/retreatment disease type, but not sex or disease site. Migrants from the Philippines represented the highest number of MDR tuberculosis cases, accounting for 20 (38.5%) cases.
Characteristics of Foreign-Born MDR and Non-MDR Tuberculosis Cases in Alberta, Canada, 1982–2019
| Case Characteristics . | Cases . | MDR, No. (%) . | RR (95% CI) . | P Value . |
|---|---|---|---|---|
| Overall | 3514 | 52 (1.48) | ||
| Age, y | ||||
| <35 | 1229 | 28 (2.28) | 4.51 (1.75–11.66) | .0006d |
| 35–64 | 1295 | 19 (1.47) | 2.90 (1.09–7.75) | .0254d |
| >64 | 990 | 5 (0.51) | 1.0 | |
| Sex | ||||
| Female | 1714 | 24 (1.40) | 0.90 (.52–1.55) | .7031 |
| Male | 1800 | 28 (1.56) | 1.0 | |
| Country of birtha | ||||
| High MDR tuberculosis burdenb | 2551 | 47 (1.85) | 3.53 (1.41–8.86) | .0039 |
| Other | 959 | 5 (0.52) | 1.0 | |
| Disease typec | ||||
| Relapse/retreatment | 231 | 24 (10.39) | 12.0 (7.07–20.36) | <.0001 |
| New active | 3234 | 28 (0.87) | 1.0 | |
| Disease site | ||||
| Respiratory | 2482 | 39 (1.57) | 1.25 (.67–2.33) | .4859 |
| Nonrespiratory | 1032 | 13 (1.26) | 1.0 |
| Case Characteristics . | Cases . | MDR, No. (%) . | RR (95% CI) . | P Value . |
|---|---|---|---|---|
| Overall | 3514 | 52 (1.48) | ||
| Age, y | ||||
| <35 | 1229 | 28 (2.28) | 4.51 (1.75–11.66) | .0006d |
| 35–64 | 1295 | 19 (1.47) | 2.90 (1.09–7.75) | .0254d |
| >64 | 990 | 5 (0.51) | 1.0 | |
| Sex | ||||
| Female | 1714 | 24 (1.40) | 0.90 (.52–1.55) | .7031 |
| Male | 1800 | 28 (1.56) | 1.0 | |
| Country of birtha | ||||
| High MDR tuberculosis burdenb | 2551 | 47 (1.85) | 3.53 (1.41–8.86) | .0039 |
| Other | 959 | 5 (0.52) | 1.0 | |
| Disease typec | ||||
| Relapse/retreatment | 231 | 24 (10.39) | 12.0 (7.07–20.36) | <.0001 |
| New active | 3234 | 28 (0.87) | 1.0 | |
| Disease site | ||||
| Respiratory | 2482 | 39 (1.57) | 1.25 (.67–2.33) | .4859 |
| Nonrespiratory | 1032 | 13 (1.26) | 1.0 |
Values in bold are statistically significant at p < .05.
Abbreviations: CI, confidence interval; MDR, multidrug resistant; RR, relative risk.
aThe country of birth was unknown for 4 cases that were not MDR.
bHigh MDR tuberculosis burden countries that were associated with MDR tuberculosis cases in this study were: Philippines (20 cases); India (9 cases); China, including Hong Kong, Macau, and Taiwan (5 cases); Vietnam (4 cases); Ethiopia (4 cases); Nigeria (2 cases); Congo (1 case); Pakistan (1 case); and Russian Federation (1 case).
cDisease type was missing for 49 non-MDR cases.
dP value for the age groups overall (ie, χ 2 value for the 3 × 2 table) is .003.
Characteristics of Foreign-Born MDR and Non-MDR Tuberculosis Cases in Alberta, Canada, 1982–2019
| Case Characteristics . | Cases . | MDR, No. (%) . | RR (95% CI) . | P Value . |
|---|---|---|---|---|
| Overall | 3514 | 52 (1.48) | ||
| Age, y | ||||
| <35 | 1229 | 28 (2.28) | 4.51 (1.75–11.66) | .0006d |
| 35–64 | 1295 | 19 (1.47) | 2.90 (1.09–7.75) | .0254d |
| >64 | 990 | 5 (0.51) | 1.0 | |
| Sex | ||||
| Female | 1714 | 24 (1.40) | 0.90 (.52–1.55) | .7031 |
| Male | 1800 | 28 (1.56) | 1.0 | |
| Country of birtha | ||||
| High MDR tuberculosis burdenb | 2551 | 47 (1.85) | 3.53 (1.41–8.86) | .0039 |
| Other | 959 | 5 (0.52) | 1.0 | |
| Disease typec | ||||
| Relapse/retreatment | 231 | 24 (10.39) | 12.0 (7.07–20.36) | <.0001 |
| New active | 3234 | 28 (0.87) | 1.0 | |
| Disease site | ||||
| Respiratory | 2482 | 39 (1.57) | 1.25 (.67–2.33) | .4859 |
| Nonrespiratory | 1032 | 13 (1.26) | 1.0 |
| Case Characteristics . | Cases . | MDR, No. (%) . | RR (95% CI) . | P Value . |
|---|---|---|---|---|
| Overall | 3514 | 52 (1.48) | ||
| Age, y | ||||
| <35 | 1229 | 28 (2.28) | 4.51 (1.75–11.66) | .0006d |
| 35–64 | 1295 | 19 (1.47) | 2.90 (1.09–7.75) | .0254d |
| >64 | 990 | 5 (0.51) | 1.0 | |
| Sex | ||||
| Female | 1714 | 24 (1.40) | 0.90 (.52–1.55) | .7031 |
| Male | 1800 | 28 (1.56) | 1.0 | |
| Country of birtha | ||||
| High MDR tuberculosis burdenb | 2551 | 47 (1.85) | 3.53 (1.41–8.86) | .0039 |
| Other | 959 | 5 (0.52) | 1.0 | |
| Disease typec | ||||
| Relapse/retreatment | 231 | 24 (10.39) | 12.0 (7.07–20.36) | <.0001 |
| New active | 3234 | 28 (0.87) | 1.0 | |
| Disease site | ||||
| Respiratory | 2482 | 39 (1.57) | 1.25 (.67–2.33) | .4859 |
| Nonrespiratory | 1032 | 13 (1.26) | 1.0 |
Values in bold are statistically significant at p < .05.
Abbreviations: CI, confidence interval; MDR, multidrug resistant; RR, relative risk.
aThe country of birth was unknown for 4 cases that were not MDR.
bHigh MDR tuberculosis burden countries that were associated with MDR tuberculosis cases in this study were: Philippines (20 cases); India (9 cases); China, including Hong Kong, Macau, and Taiwan (5 cases); Vietnam (4 cases); Ethiopia (4 cases); Nigeria (2 cases); Congo (1 case); Pakistan (1 case); and Russian Federation (1 case).
cDisease type was missing for 49 non-MDR cases.
dP value for the age groups overall (ie, χ 2 value for the 3 × 2 table) is .003.
Forty-four (84.6%) foreign-born MDR tuberculosis cases were reported in the last 18 years (2002–2019), a 5.5-fold increase over the number reported in the previous 20 years (1982–2001). The time period of arrival and diagnosis of MDR tuberculosis cases are described in Figure 1B [19]. Compared to cases reported in 1982–2001, cases reported in 2002–2019 were more likely to be <35 years of age, although given the small number of cases this result was not statistically significant (Table 3). They did not differ by sex, disease type, disease site, or pattern of resistance to other first-line drugs. Fluoroquinolone resistance was uncommon (4/44, 9.1%). Only 1 of 44 HIV-tested MDR tuberculosis patients was determined to be HIV positive (data not shown).
Characteristics of Foreign-Born Multidrug-Resistant Tuberculosis Cases in Alberta, 1982–2001 and 2002–2019
| . | Time Period . | . | |
|---|---|---|---|
| Case Characteristics . | 1982–2001, n = 8, No. (%) . | 2002–2019, n = 44, No. (%) . | P Valuea . |
| Age, y | |||
| <35 | 2 (25.0) | 26 (59.1) | .123 |
| 35–64 | 5 (62.5) | 14 (31.8) | |
| >64 | 1 (12.5) | 4 (9.1) | |
| Sex | |||
| Female | 4 (50.0) | 20 (45.5) | 1.0 |
| Male | 4 (50.0) | 24 (54.5) | |
| Disease type | |||
| Relapse/retreatment | 5 (62.5) | 19 (43.2) | .447 |
| New active | 3 (37.5) | 25 (56.8) | |
| Disease site | |||
| Respiratory | 8 (100) | 31 (70.5) | .177 |
| Nonrespiratory | 0 (0.0) | 13 (29.5) | |
| Other drug resistance | |||
| Pyrazinamide | 2 (50.0)b | 19 (43.2) | 1.0 |
| Ethambutol | 3 (37.5) | 18 (40.9) | |
| Streptomycin | 4 (50.0) | 25 (56.8) | |
| Ofloxacin | NA | 4 (9.1) | |
| . | Time Period . | . | |
|---|---|---|---|
| Case Characteristics . | 1982–2001, n = 8, No. (%) . | 2002–2019, n = 44, No. (%) . | P Valuea . |
| Age, y | |||
| <35 | 2 (25.0) | 26 (59.1) | .123 |
| 35–64 | 5 (62.5) | 14 (31.8) | |
| >64 | 1 (12.5) | 4 (9.1) | |
| Sex | |||
| Female | 4 (50.0) | 20 (45.5) | 1.0 |
| Male | 4 (50.0) | 24 (54.5) | |
| Disease type | |||
| Relapse/retreatment | 5 (62.5) | 19 (43.2) | .447 |
| New active | 3 (37.5) | 25 (56.8) | |
| Disease site | |||
| Respiratory | 8 (100) | 31 (70.5) | .177 |
| Nonrespiratory | 0 (0.0) | 13 (29.5) | |
| Other drug resistance | |||
| Pyrazinamide | 2 (50.0)b | 19 (43.2) | 1.0 |
| Ethambutol | 3 (37.5) | 18 (40.9) | |
| Streptomycin | 4 (50.0) | 25 (56.8) | |
| Ofloxacin | NA | 4 (9.1) | |
Abbreviation: NA not available.
aFisher exact test.
bOnly 4 of the 8 multidrug-resistant tuberculosis isolates were tested for susceptibility to pyrazinamide, with 2 (50%) of these cases having resistance.
Characteristics of Foreign-Born Multidrug-Resistant Tuberculosis Cases in Alberta, 1982–2001 and 2002–2019
| . | Time Period . | . | |
|---|---|---|---|
| Case Characteristics . | 1982–2001, n = 8, No. (%) . | 2002–2019, n = 44, No. (%) . | P Valuea . |
| Age, y | |||
| <35 | 2 (25.0) | 26 (59.1) | .123 |
| 35–64 | 5 (62.5) | 14 (31.8) | |
| >64 | 1 (12.5) | 4 (9.1) | |
| Sex | |||
| Female | 4 (50.0) | 20 (45.5) | 1.0 |
| Male | 4 (50.0) | 24 (54.5) | |
| Disease type | |||
| Relapse/retreatment | 5 (62.5) | 19 (43.2) | .447 |
| New active | 3 (37.5) | 25 (56.8) | |
| Disease site | |||
| Respiratory | 8 (100) | 31 (70.5) | .177 |
| Nonrespiratory | 0 (0.0) | 13 (29.5) | |
| Other drug resistance | |||
| Pyrazinamide | 2 (50.0)b | 19 (43.2) | 1.0 |
| Ethambutol | 3 (37.5) | 18 (40.9) | |
| Streptomycin | 4 (50.0) | 25 (56.8) | |
| Ofloxacin | NA | 4 (9.1) | |
| . | Time Period . | . | |
|---|---|---|---|
| Case Characteristics . | 1982–2001, n = 8, No. (%) . | 2002–2019, n = 44, No. (%) . | P Valuea . |
| Age, y | |||
| <35 | 2 (25.0) | 26 (59.1) | .123 |
| 35–64 | 5 (62.5) | 14 (31.8) | |
| >64 | 1 (12.5) | 4 (9.1) | |
| Sex | |||
| Female | 4 (50.0) | 20 (45.5) | 1.0 |
| Male | 4 (50.0) | 24 (54.5) | |
| Disease type | |||
| Relapse/retreatment | 5 (62.5) | 19 (43.2) | .447 |
| New active | 3 (37.5) | 25 (56.8) | |
| Disease site | |||
| Respiratory | 8 (100) | 31 (70.5) | .177 |
| Nonrespiratory | 0 (0.0) | 13 (29.5) | |
| Other drug resistance | |||
| Pyrazinamide | 2 (50.0)b | 19 (43.2) | 1.0 |
| Ethambutol | 3 (37.5) | 18 (40.9) | |
| Streptomycin | 4 (50.0) | 25 (56.8) | |
| Ofloxacin | NA | 4 (9.1) | |
Abbreviation: NA not available.
aFisher exact test.
bOnly 4 of the 8 multidrug-resistant tuberculosis isolates were tested for susceptibility to pyrazinamide, with 2 (50%) of these cases having resistance.
Local transmission did not explain any MDR tuberculosis case. From 1990 forward, most M. tuberculosis isolates were DNA fingerprinted (MDR, 47/48, 97.9% and non-MDR, 3924/4009, 97.9%). There was a total of 6 genotypic clusters. There was 1 cluster of 5 and 1 cluster of 30, each of which contained 2 MDR tuberculosis cases, and there were 2 clusters of 2 and 2 clusters of 5, each of which contained only 1 MDR tuberculosis case (Table 4 and Table 5). The genotypically matched MDR tuberculosis isolates in clusters 1 and 2 could not be convincingly linked to one another by conventional epidemiology, spatiotemporal links, or drug-resistance pattern. The largest cluster of 30 cases, 2 of which were MDR, consisted of 28 Philippine-born persons, 1 Canadian-born person who had lived in the Philippines, and 1 Vietnam-born person. The first 12 MIRU/VNTR loci of this cluster genotype were homologous with the ubiquitous Manila family strain [20].
| Cluster . | Genotyping Method . | No. of Cases in Cluster . | No, of Non-MDR Cases With History of Contact With MDR Tuberculosis Case . | No. of Non-MDR Cases With Spatial/Temporal Links to MDR Tuberculosis Case . | Resistance Patterns of Non-MDR Cases With Matching Genotype . |
|---|---|---|---|---|---|
| 1 | MIRU/VNTR | 5 (2 MDR) | 0 | 1 N/Y, 2 N/N | 3 Drug susceptible |
| 2 | MIRU/VNTR | 30 (2 MDR) | 0 | 1 Y/N, 6 N/Y | 2 H Resistant |
| 21 N/N | 1 R and RIFAB resistant | ||||
| 25 Drug susceptible | |||||
| 3 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 4 | RFLP (low copy number) | 5 (1 MDR) | 0 | 3 N/Y, 1 N/N | 4 Drug susceptible |
| 5 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 6 | MIRU/VNTR | 5 (1 MDR) | 0 | 1 N/Y, 3 N/N | 4 Drug susceptible |
| Cluster . | Genotyping Method . | No. of Cases in Cluster . | No, of Non-MDR Cases With History of Contact With MDR Tuberculosis Case . | No. of Non-MDR Cases With Spatial/Temporal Links to MDR Tuberculosis Case . | Resistance Patterns of Non-MDR Cases With Matching Genotype . |
|---|---|---|---|---|---|
| 1 | MIRU/VNTR | 5 (2 MDR) | 0 | 1 N/Y, 2 N/N | 3 Drug susceptible |
| 2 | MIRU/VNTR | 30 (2 MDR) | 0 | 1 Y/N, 6 N/Y | 2 H Resistant |
| 21 N/N | 1 R and RIFAB resistant | ||||
| 25 Drug susceptible | |||||
| 3 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 4 | RFLP (low copy number) | 5 (1 MDR) | 0 | 3 N/Y, 1 N/N | 4 Drug susceptible |
| 5 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 6 | MIRU/VNTR | 5 (1 MDR) | 0 | 1 N/Y, 3 N/N | 4 Drug susceptible |
Abbreviations: H, isoniazid; MDR, multidrug resistant; MIRU, mycobacterial interspersed repetitive units; N, no; R, rifampin; RFLP, restriction fragment-length polymorphism; RIFAB, rifabutin; VNTR, variable number of tandem repeats; Y, yes.
| Cluster . | Genotyping Method . | No. of Cases in Cluster . | No, of Non-MDR Cases With History of Contact With MDR Tuberculosis Case . | No. of Non-MDR Cases With Spatial/Temporal Links to MDR Tuberculosis Case . | Resistance Patterns of Non-MDR Cases With Matching Genotype . |
|---|---|---|---|---|---|
| 1 | MIRU/VNTR | 5 (2 MDR) | 0 | 1 N/Y, 2 N/N | 3 Drug susceptible |
| 2 | MIRU/VNTR | 30 (2 MDR) | 0 | 1 Y/N, 6 N/Y | 2 H Resistant |
| 21 N/N | 1 R and RIFAB resistant | ||||
| 25 Drug susceptible | |||||
| 3 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 4 | RFLP (low copy number) | 5 (1 MDR) | 0 | 3 N/Y, 1 N/N | 4 Drug susceptible |
| 5 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 6 | MIRU/VNTR | 5 (1 MDR) | 0 | 1 N/Y, 3 N/N | 4 Drug susceptible |
| Cluster . | Genotyping Method . | No. of Cases in Cluster . | No, of Non-MDR Cases With History of Contact With MDR Tuberculosis Case . | No. of Non-MDR Cases With Spatial/Temporal Links to MDR Tuberculosis Case . | Resistance Patterns of Non-MDR Cases With Matching Genotype . |
|---|---|---|---|---|---|
| 1 | MIRU/VNTR | 5 (2 MDR) | 0 | 1 N/Y, 2 N/N | 3 Drug susceptible |
| 2 | MIRU/VNTR | 30 (2 MDR) | 0 | 1 Y/N, 6 N/Y | 2 H Resistant |
| 21 N/N | 1 R and RIFAB resistant | ||||
| 25 Drug susceptible | |||||
| 3 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 4 | RFLP (low copy number) | 5 (1 MDR) | 0 | 3 N/Y, 1 N/N | 4 Drug susceptible |
| 5 | RFLP | 2 (1 MDR) | 0 | 1 N/N | 1 H Resistant |
| 6 | MIRU/VNTR | 5 (1 MDR) | 0 | 1 N/Y, 3 N/N | 4 Drug susceptible |
Abbreviations: H, isoniazid; MDR, multidrug resistant; MIRU, mycobacterial interspersed repetitive units; N, no; R, rifampin; RFLP, restriction fragment-length polymorphism; RIFAB, rifabutin; VNTR, variable number of tandem repeats; Y, yes.
| Cluster . | Strain (MIRU/VNTR) . | No. of Cases . | Country of Birth . | Disease Site . | History of Contact . | Diagnosis Date, mm/yyyy . | Spatial/Temporal Link . | Drug Resistance Pattern . |
|---|---|---|---|---|---|---|---|---|
| 1 | 22 33 25 15 35 33 44 56 44 42 33 82 | 2 | Russian Federation | Pulmonary (S−) | N | 08/2016 | … | H, R, E, S, ETH, PAS, RIFAB |
| Indiaa | Extrapulmonary | N | 11/2019a | N/N | H, R, S, O, ETH, RIFAB, M | |||
| 2 | 25 43 26 22 34 32 14 10 9 43 26 32 71 | 2 | Philippines | Pulmonary (S−) | N | 07/2019 | … | H, R ETH, RIFAB |
| Philippines | Pulmonary (S−) | N | 09/2019 | N/Y | H, R, RIFAB |
| Cluster . | Strain (MIRU/VNTR) . | No. of Cases . | Country of Birth . | Disease Site . | History of Contact . | Diagnosis Date, mm/yyyy . | Spatial/Temporal Link . | Drug Resistance Pattern . |
|---|---|---|---|---|---|---|---|---|
| 1 | 22 33 25 15 35 33 44 56 44 42 33 82 | 2 | Russian Federation | Pulmonary (S−) | N | 08/2016 | … | H, R, E, S, ETH, PAS, RIFAB |
| Indiaa | Extrapulmonary | N | 11/2019a | N/N | H, R, S, O, ETH, RIFAB, M | |||
| 2 | 25 43 26 22 34 32 14 10 9 43 26 32 71 | 2 | Philippines | Pulmonary (S−) | N | 07/2019 | … | H, R ETH, RIFAB |
| Philippines | Pulmonary (S−) | N | 09/2019 | N/Y | H, R, RIFAB |
Abbreviations: E, ethambutol; ETH, ethionamide; H, isoniazid; M, moxifloxacin; MIRU, mycobacterial interspersed repetitive units; N, no; O, ofloxacin; PAS, para-aminosalicylic acid; S−, smear negative; R, rifampin; RIFAB, rifabutin; S, streptomycin; VNTR, variable number of tandem repeats; Y, yes.
aThis patient arrived in Canada 3 years after the first multidrug-resistant tuberculosis case was diagnosed.
| Cluster . | Strain (MIRU/VNTR) . | No. of Cases . | Country of Birth . | Disease Site . | History of Contact . | Diagnosis Date, mm/yyyy . | Spatial/Temporal Link . | Drug Resistance Pattern . |
|---|---|---|---|---|---|---|---|---|
| 1 | 22 33 25 15 35 33 44 56 44 42 33 82 | 2 | Russian Federation | Pulmonary (S−) | N | 08/2016 | … | H, R, E, S, ETH, PAS, RIFAB |
| Indiaa | Extrapulmonary | N | 11/2019a | N/N | H, R, S, O, ETH, RIFAB, M | |||
| 2 | 25 43 26 22 34 32 14 10 9 43 26 32 71 | 2 | Philippines | Pulmonary (S−) | N | 07/2019 | … | H, R ETH, RIFAB |
| Philippines | Pulmonary (S−) | N | 09/2019 | N/Y | H, R, RIFAB |
| Cluster . | Strain (MIRU/VNTR) . | No. of Cases . | Country of Birth . | Disease Site . | History of Contact . | Diagnosis Date, mm/yyyy . | Spatial/Temporal Link . | Drug Resistance Pattern . |
|---|---|---|---|---|---|---|---|---|
| 1 | 22 33 25 15 35 33 44 56 44 42 33 82 | 2 | Russian Federation | Pulmonary (S−) | N | 08/2016 | … | H, R, E, S, ETH, PAS, RIFAB |
| Indiaa | Extrapulmonary | N | 11/2019a | N/N | H, R, S, O, ETH, RIFAB, M | |||
| 2 | 25 43 26 22 34 32 14 10 9 43 26 32 71 | 2 | Philippines | Pulmonary (S−) | N | 07/2019 | … | H, R ETH, RIFAB |
| Philippines | Pulmonary (S−) | N | 09/2019 | N/Y | H, R, RIFAB |
Abbreviations: E, ethambutol; ETH, ethionamide; H, isoniazid; M, moxifloxacin; MIRU, mycobacterial interspersed repetitive units; N, no; O, ofloxacin; PAS, para-aminosalicylic acid; S−, smear negative; R, rifampin; RIFAB, rifabutin; S, streptomycin; VNTR, variable number of tandem repeats; Y, yes.
aThis patient arrived in Canada 3 years after the first multidrug-resistant tuberculosis case was diagnosed.
In 2002–2019, there were 30 MDR pulmonary tuberculosis cases: 12 smear-positive, 18 smear-negative (median age, 35.5 years; interquartile range [IQR], 30.0–45.3). Together they had 482 contacts: 214 (44.4%) close and 268 (55.6%) casual (Figure 2). The median number of close contacts per case was 5 (IQR, 3.0–11.5). Most contacts were completely assessed (88.8% of close and 82.1% of casual). Completely assessed close contacts were more likely than completely assessed casual contacts to have a positive TST or IGRA (42.1% vs 18.6%, P < .0001). Most TST and/or IGRA-positive contacts were foreign born (85.1%); few were younger than 5 years (4.1%). Among TST- or IGRA-positive contacts (excluding 4 who had relocated), 54 (46.2%) were offered treatment of latent tuberculosis infection, 31 (26.5%) were recommended for annual surveillance, and 32 (27.4%) were put to no further follow-up or lost to follow-up. Of those offered treatment of latent tuberculosis infection, 28 (51.9%) completed treatment; of these, 25 (89.3%) were treated with a fluoroquinolone with or without ethambutol. No contact was determined to be a secondary case. The median and minimum duration of follow-up of contacts, assuming all remained in Alberta, was 54.1 (IQR, 21.8–115.3) months and 9 months, respectively.
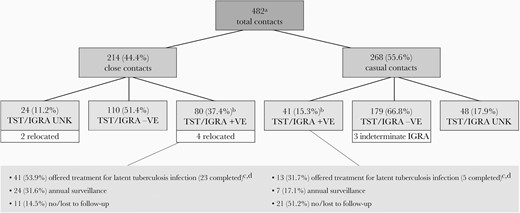
Investigation and management of contacts of adult pulmonary MDR tuberculosis cases (12 smear positive; 18 smear negative) diagnosed between 2002 and 2019. Abbreviations: + VE, positive; − VE, negative; IGRA, interferon-γ release assay; MDR, multidrug resistant; TST, tuberculin skin test; UNK, unknown. aOne smear-positive MDR tuberculosis case had a disproportionate number of contacts (n = 180; 9 close, 171 casual). Close or casual TST-positive contacts who had also undergone an IGRA (n = 46) and were IGRA-negative (n = 21, 45.7%) were grouped as TST/IGRA-negative. bAmong TST/IGRA-positive close contacts (excluding those who had relocated) there were 10 converters, 53 new positives, and 13 previous positives; among TST/IGRA-positive casual contacts there were 1 converter, 29 new positives, and 11 previous positives. cAmong the 54 close and casual contacts who were offered treatment of latent tuberculosis infection, 28 (51.9%) accepted and completed, 14 (25.9%) accepted but did not complete on account of side effects, and 12 (22.2%) declined. dAmong the 28 TST/IGRA-positive close and casual contacts who completed treatment of latent tuberculosis infection, 1 received rifabutin for 4 months (index case isolate rifabutin susceptible), 2 received isoniazid and rifampin for 4 months, 7 received levofloxacin or moxifloxacin with ethambutol for 9 months, and 18 received levofloxacin or moxifloxacin monotherapy for 4 to 12 months.
The extent to which the large increase in MDR tuberculosis in the second time period was attributable to immigration and/or a changed proportion of MDR tuberculosis in immigrants with tuberculosis who had arrived from HMDR tuberculosis countries in the 2 time periods, is shown in Table 6. In the 2 time periods, similar proportions of tuberculosis cases had arrived from HMDR tuberculosis countries: 80.1% (539/673) in 1982–2001 and 74.5% (1049/1408) in 2002–2019. The proportion of MDR tuberculosis among tuberculosis cases from HMDR tuberculosis countries that had arrived in the 2 time periods increased significantly from the first to the last time period (1.11 to 3.62, P = .003). Based on the proportion of MDR tuberculosis in immigrants with tuberculosis who arrived from HMDR tuberculosis countries in 1982–2001, we estimated that the number of MDR tuberculosis cases in immigrants with tuberculosis who arrived from HMDR tuberculosis countries in 2002–2019 would be 12 (0.011 × 1049), if the only factor that had changed was the number of immigrants. However, the actual number of cases was 38. Thus, 26 (ie, 38 − 12), 68.4% of the cases, that is a higher proportion in the second time period, were attributable to a changed proportion of MDR tuberculosis in the cases arrived from HMDR tuberculosis countries of birth. This was very likely a reflection of a higher prevalence of MDR tuberculosis in those countries. Among those emigrating from the Philippines, 100% of the MDR tuberculosis cases in the second time period were due to a change in proportion as no emigrants from the Philippines in the first time period had MDR tuberculosis. The chances of a Philippine-born case that arrived in 2002–2019 being MDR was 4.19%.
Proportion of MDR Tuberculosis in Foreign-Born Tuberculosis Cases in Alberta, 1982–2001 and 2002–2019a
| . | . | Time Period of Diagnosis . | . | |
|---|---|---|---|---|
| Foreign-born Tuberculosis Case Grouping . | Total . | 1982–2001 . | 2002–2019 . | P Value . |
| All cases | ||||
| Tuberculosis cases | 3514 | 1335 | 2179 | |
| MDR tuberculosis cases | 52 | 8 | 44 | <.001 |
| Group MDR tuberculosis, % | 1.48 | 0.60 | 2.02 | |
| Tuberculosis cases that arrived in the period | 2081 | 673 | 1408 | |
| MDR tuberculosis cases that arrived in the period | 46 | 6 | 40 | .004 |
| Group MDR tuberculosis, % | 2.21 | 0.89 | 2.84 | |
| Cases born in HMDRC | ||||
| Tuberculosis cases | 2546 | 930 | 1616 | |
| MDR tuberculosis cases | 47 | 7 | 40 | .001 |
| Group MDR tuberculosis, % | 1.85 | 0.75 | 2.48 | |
| Tuberculosis cases that arrived in the period | 1588 | 539 | 1049 | |
| MDR tuberculosis cases that arrived in the period | 44 | 6 | 38 | .003 |
| Group MDR tuberculosis, % | 2.77 | 1.11 | 3.62 | |
| Philippine-born cases | ||||
| Tuberculosis cases | 736 | 145 | 591 | |
| MDR tuberculosis cases | 20 | 0 | 20 | .020 |
| Group MDR tuberculosis, % | 2.72 | 0.0 | 3.38 | |
| Tuberculosis cases that arrived in the period | 574 | 97 | 477 | |
| MDR tuberculosis cases that arrived in the period | 20 | 0 | 20 | .034 |
| Group MDR tuberculosis, % | 3.48 | 0.0 | 4.19 | |
| . | . | Time Period of Diagnosis . | . | |
|---|---|---|---|---|
| Foreign-born Tuberculosis Case Grouping . | Total . | 1982–2001 . | 2002–2019 . | P Value . |
| All cases | ||||
| Tuberculosis cases | 3514 | 1335 | 2179 | |
| MDR tuberculosis cases | 52 | 8 | 44 | <.001 |
| Group MDR tuberculosis, % | 1.48 | 0.60 | 2.02 | |
| Tuberculosis cases that arrived in the period | 2081 | 673 | 1408 | |
| MDR tuberculosis cases that arrived in the period | 46 | 6 | 40 | .004 |
| Group MDR tuberculosis, % | 2.21 | 0.89 | 2.84 | |
| Cases born in HMDRC | ||||
| Tuberculosis cases | 2546 | 930 | 1616 | |
| MDR tuberculosis cases | 47 | 7 | 40 | .001 |
| Group MDR tuberculosis, % | 1.85 | 0.75 | 2.48 | |
| Tuberculosis cases that arrived in the period | 1588 | 539 | 1049 | |
| MDR tuberculosis cases that arrived in the period | 44 | 6 | 38 | .003 |
| Group MDR tuberculosis, % | 2.77 | 1.11 | 3.62 | |
| Philippine-born cases | ||||
| Tuberculosis cases | 736 | 145 | 591 | |
| MDR tuberculosis cases | 20 | 0 | 20 | .020 |
| Group MDR tuberculosis, % | 2.72 | 0.0 | 3.38 | |
| Tuberculosis cases that arrived in the period | 574 | 97 | 477 | |
| MDR tuberculosis cases that arrived in the period | 20 | 0 | 20 | .034 |
| Group MDR tuberculosis, % | 3.48 | 0.0 | 4.19 | |
Data are No. except where indicated. Values in bold are statistically significant at p < .05.
Abbreviations: MDR, multidrug resistant; HMDRC, high multidrug-resistant tuberculosis burden country.
aCulture-positive tuberculosis cases only.
Proportion of MDR Tuberculosis in Foreign-Born Tuberculosis Cases in Alberta, 1982–2001 and 2002–2019a
| . | . | Time Period of Diagnosis . | . | |
|---|---|---|---|---|
| Foreign-born Tuberculosis Case Grouping . | Total . | 1982–2001 . | 2002–2019 . | P Value . |
| All cases | ||||
| Tuberculosis cases | 3514 | 1335 | 2179 | |
| MDR tuberculosis cases | 52 | 8 | 44 | <.001 |
| Group MDR tuberculosis, % | 1.48 | 0.60 | 2.02 | |
| Tuberculosis cases that arrived in the period | 2081 | 673 | 1408 | |
| MDR tuberculosis cases that arrived in the period | 46 | 6 | 40 | .004 |
| Group MDR tuberculosis, % | 2.21 | 0.89 | 2.84 | |
| Cases born in HMDRC | ||||
| Tuberculosis cases | 2546 | 930 | 1616 | |
| MDR tuberculosis cases | 47 | 7 | 40 | .001 |
| Group MDR tuberculosis, % | 1.85 | 0.75 | 2.48 | |
| Tuberculosis cases that arrived in the period | 1588 | 539 | 1049 | |
| MDR tuberculosis cases that arrived in the period | 44 | 6 | 38 | .003 |
| Group MDR tuberculosis, % | 2.77 | 1.11 | 3.62 | |
| Philippine-born cases | ||||
| Tuberculosis cases | 736 | 145 | 591 | |
| MDR tuberculosis cases | 20 | 0 | 20 | .020 |
| Group MDR tuberculosis, % | 2.72 | 0.0 | 3.38 | |
| Tuberculosis cases that arrived in the period | 574 | 97 | 477 | |
| MDR tuberculosis cases that arrived in the period | 20 | 0 | 20 | .034 |
| Group MDR tuberculosis, % | 3.48 | 0.0 | 4.19 | |
| . | . | Time Period of Diagnosis . | . | |
|---|---|---|---|---|
| Foreign-born Tuberculosis Case Grouping . | Total . | 1982–2001 . | 2002–2019 . | P Value . |
| All cases | ||||
| Tuberculosis cases | 3514 | 1335 | 2179 | |
| MDR tuberculosis cases | 52 | 8 | 44 | <.001 |
| Group MDR tuberculosis, % | 1.48 | 0.60 | 2.02 | |
| Tuberculosis cases that arrived in the period | 2081 | 673 | 1408 | |
| MDR tuberculosis cases that arrived in the period | 46 | 6 | 40 | .004 |
| Group MDR tuberculosis, % | 2.21 | 0.89 | 2.84 | |
| Cases born in HMDRC | ||||
| Tuberculosis cases | 2546 | 930 | 1616 | |
| MDR tuberculosis cases | 47 | 7 | 40 | .001 |
| Group MDR tuberculosis, % | 1.85 | 0.75 | 2.48 | |
| Tuberculosis cases that arrived in the period | 1588 | 539 | 1049 | |
| MDR tuberculosis cases that arrived in the period | 44 | 6 | 38 | .003 |
| Group MDR tuberculosis, % | 2.77 | 1.11 | 3.62 | |
| Philippine-born cases | ||||
| Tuberculosis cases | 736 | 145 | 591 | |
| MDR tuberculosis cases | 20 | 0 | 20 | .020 |
| Group MDR tuberculosis, % | 2.72 | 0.0 | 3.38 | |
| Tuberculosis cases that arrived in the period | 574 | 97 | 477 | |
| MDR tuberculosis cases that arrived in the period | 20 | 0 | 20 | .034 |
| Group MDR tuberculosis, % | 3.48 | 0.0 | 4.19 | |
Data are No. except where indicated. Values in bold are statistically significant at p < .05.
Abbreviations: MDR, multidrug resistant; HMDRC, high multidrug-resistant tuberculosis burden country.
aCulture-positive tuberculosis cases only.
DISCUSSION
This study investigates culture-positive tuberculosis in foreign-born persons of a major immigrant-receiving province in Canada over 38 years. There were 52 MDR tuberculosis cases; a stable 4 cases per decade over the first 20 years (1982–2001) and 44 cases (84.6% of the total) over the last 18 years (2002–2019). Most MDR tuberculosis cases (90.4%) were from HMDR tuberculosis burden countries. Those diagnosed in 2002–2019 were more likely to be younger (<35 years) than those diagnosed in 1982–2001 (59.1% vs 25.0%). The substantial increase in the number of MDR tuberculosis cases in recent years coincided with a substantial increase in immigration into the province. However, immigration alone accounted for only 31.6% of the increase while the balance (68.4%) was due to an increase in the proportion of MDR tuberculosis in tuberculosis patients arrived from HMDR tuberculosis burden countries in the 2 time periods—almost certainly a reflection of an increased prevalence of MDR tuberculosis in their countries of birth. An in-depth analysis of both conventional and molecular epidemiologic data over an extended period found no MDR tuberculosis cases attributable to local transmission. These findings are interpreted within a global and historical context.
Tuberculosis prevention and care programs in high-income/low-incidence countries are generally robust, which provide an advantage that guards against local transmission. This extends to the transmission of MDR tuberculosis strains. Indeed, the fact that there were no MDR tuberculosis cases whose disease could be attributed to local transmission, rather that these cases occurred due to circumstances beyond the reach of the local program—notably, federally established immigration policies and events overseas allowing acquisition and spread of MDR tuberculosis—is no doubt due to factors that reflect this privilege. These include active case finding—44.2% of our MDR tuberculosis cases had a past history of tuberculosis and by virtue of having such a history, were referred by immigration authorities for medical surveillance postarrival—and contact tracing with administration of preventive therapy to those infected (see below). By happenstance, most contacts were probably at low risk of progressing from infection to disease, as 95.9% were >4 years of age and 85.1% were foreign-born, increasing the probability that their infection was remote or that they had been bacille Calmette-Guérin (BCG) vaccinated. While BCG vaccination has been shown to offer some protection against MDR tuberculosis [21], alternatively it may have caused their TST to be falsely positive (of the TST-positive contacts who had an IGRA, 46.7% were IGRA negative). Contacts were not systematically screened for HIV but the seroprevalence of HIV in the province is very low and only 1 of 44 HIV-tested MDR cases was determined to be HIV positive [22]. GeneXpert MTB/RIF has been in use since 2014, cases are managed by a consilium of physicians, and treatment is directly observed, all of which may have interrupted transmission sooner [23, 24]. A conventional epidemiologic study in the neighboring province of British Columbia also found no transmission-related MDR secondary cases over a 19-year period [25]. More recently, combined conventional and molecular epidemiologic studies in the United Kingdom and France found evidence of limited in-country, transmission-related, MDR tuberculosis in foreign-born persons [26, 27].
These considerations aside, it is nevertheless remarkable that no contact of an MDR-pulmonary tuberculosis case was reported to have prevalent active or incident tuberculosis during a median 54.1 (IQR, 21.8–115.3) months of follow-up. Of those contacts that were TST/IGRA positive, only 28 (23.9%) completed preventive therapy. This raises the question of whether there is a fitness cost to MDR tuberculosis—as is suggested in one but not another prospective study of MDR tuberculosis transmission [28, 29]. The relationship between resistance-conferring mutations and the fitness of M. tuberculosis—the ability to survive, reproduce, be transmitted, and cause disease—is complex, however, and unlikely to be the same or similar across the many MDR tuberculosis strains grown from our patients [30]. If there is such a cost, it is relative as the role of transmission and the importance of primary MDR tuberculosis drug resistance to the global burden of MDR tuberculosis is undisputed; the evidence is especially compelling in children [31, 32]. Current estimates of MDR tuberculosis prevalence among tuberculosis notifications are consistent with a hypothesis that more than 80% of incident MDR tuberculosis cases in most present-day epidemic settings result from transmission of MDR tuberculosis rather than selection of de novo resistance during previous treatment of the index case [33]. In comparisons across countries made at the beginning of the new millennium, the MDR tuberculosis rate among previously untreated cases was found to be inversely correlated with treatment success using short-course chemotherapy [34].
Predeparture, proximate causes within HMDR tuberculosis burden countries are thus strikingly different from those in their high-income, low-incidence country of destination. What has transpired and what is the way forward? Seventy years ago, when triple therapy (isoniazid, streptomycin, and para-aminosalicylic acid) and a pharmacologic cure for tuberculosis were first discovered, René and Jean Dubos, in their remarkably prescient book, The White Plague: Tuberculosis, Man and Society warned of the mutability and adaptability of pathogens in the face of shifting disease ecologies and changing social patterns [35]. Twenty years later, first-generation multidrug-resistance (ie, resistance to all 3 triple-therapy drugs) began to emerge and programs looked to the then new drugs rifampin and ethambutol to provide a cure [36]. Now we look to the fluoroquinolones and yet newer drugs like the diarylquinoline, bedaquiline to provide a cure for second-generation MDR tuberculosis, but behold, third generation multidrug-resistance, that is extensively drug-resistant tuberculosis (MDR plus resistance to the fluoroquinolones and a second-line injectable agent) is now on the rise and bedaquiline resistance is being reported [37, 38]. Breaking this cycle in HMDR tuberculosis burden countries will require the implementation of a proper patient-centered approach to the clinical, laboratory, and programmatic management of MDR tuberculosis, as well as a recognition of the role played by HIV [39, 40]. Even more critical long term, is the need to resist the temptation to medicalize—to treat social issues (and natural events) as biological problems requiring medical treatment.
Strengths of this study include the breadth and completeness of the data, in particular those data that relate to the exclusion of local transmission. Limitations include our use of a fixed assignation date (2008) to label countries of birth as HMDR tuberculosis burden when this may change over time, the use of year of arrival of new immigrants as a proxy for year of departure from their country of birth, and not accounting for return travel [41]. Another limitation is the assumption that contacts remained in Alberta and did not relocate and reactivate in another province/territory (a limitation that might have been overcome by having a national DNA fingerprint database [42]) or country.
In conclusion, despite substantially increased immigration into Canada from many low- and middle-income countries, the main reason for the increase in MDR tuberculosis in recent years is not immigration but rather an increase in the proportion of MDR tuberculosis in the cases arrived from HMDR tuberculosis burden countries. Local transmission of MDR tuberculosis was not a contributor. Overseas investment in a patient-centered approach to clinical and programmatic management in HMDR tuberculosis burden countries is necessary in the short term and commitment to a more equitable, sustainable, and resilient future in the long term.
Notes
Acknowledgments. The authors are very grateful to the staff of the Provincial, Edmonton and Calgary TB Clinics, and the Provincial Laboratory for Public Health (Alberta Health Services), and the staff of the TB Program Evaluation and Research Unit (University of Alberta) for their generous support of this project.
Financial support. This work was supported by the University of Alberta Hospital Foundation, Edmonton, Alberta, Canada.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
R. L. and A. L. contributed equally.




