-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Pichler, Matthias Baumgartner, Janine Kimpel, Annika Rössler, Lydia Riepler, Katie Bates, Verena Fleischer, Dorothee von Laer, Wegene Borena, Reinhard Würzner, Marked Increase in Avidity of SARS-CoV-2 Antibodies 7–8 Months After Infection Is Not Diminished in Old Age, The Journal of Infectious Diseases, Volume 224, Issue 5, 1 September 2021, Pages 764–770, https://doi.org/10.1093/infdis/jiab300
Close - Share Icon Share
Abstract
The kinetics of immunoglobulin G (IgG) avidity maturation during severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection obtained from 217 participants of the Ischgl cohort, Austria, was studied 0.5–1.5 months (baseline) and 7–8 months (follow-up) after infection. The IgG avidity assay, using a modified IgG enzyme-linked immunosorbent assay (ELISA) and 5.5 M urea, revealed that old age does not diminish the increase in avidity, detected in all participants positive at both time points, from 18% to 42%. High avidity was associated with a marked residual neutralization capacity in 97.2.% of participants (211/217), which was even higher in the older age group, revealing an important role of avidity assays as easy and cheap surrogate tests for assessing the maturation of the immune system conveying potential protection against further SARS-CoV-2 infections without necessitating expensive and laborious neutralization assays.
The RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a member of the severe acute respiratory syndrome-related coronavirus family, together with SARS-CoV-1, which was responsible for the SARS outbreak in 2002–2003 [1]. Described for the first time in Wuhan, Hubei, China, as having originated from a seafood wholesale market at the end of December 2019, SARS-CoV-2 spread globally within a couple of months, attaining the status of pandemic by mid-March 2020 [2]. Since then, more than 122 million confirmed cases and more than 2.7 million deaths have been reported worldwide (as of 26 March 2021) [3]. The disease caused by the virus (coronavirus disease 2019, COVID-19) is characterized by mild to moderate symptoms including dry cough, fever, fatigue, and loss of smell and taste in the majority of cases [4, 5]. In various risk groups and especially older age groups, however, severe illnesses occur requiring hospitalization and intensive care unit management with a death toll of 1%–5% [4].
Austria is among the countries hit by the pandemic with more than half a million cases recorded (as of 22 March 2021) [3]. The first cases reported in Austria were on 25 February 2020. The first significant outbreak of SARS-CoV-2 was described in Ischgl, Tyrol, at the beginning of March. On the 13 March 2020, the government placed the town of Ischgl under quarantine and on the 16 March 2020 the whole nation was locked down under the COVID-19 Measures Act 2020. The outbreak in the ski resort Ischgl played a major role in the spread of SARS-CoV-2 throughout Austria and Northern Europe [6]. An epidemiological cluster analysis carried out by the Austrian Agency for Health and Food Safety showed that up to 40% of the Austrian SARS-CoV-2 infections by 20 April 2020 had their origins in Ischgl [7]. In total, 45% of the adult population had antibodies 0.5–1.5 months after the infection [8].
As stringent preventive measures continue to curb the uncontrolled spread of the virus, it is of paramount importance to understand the hallmarks of immune response among the infected. A plethora of previous studies have shown that SARS-CoV-2 infection induces virus-specific antibodies and a marked T-cellular immune response. Whereas the T-cell response helps prevent severe illnesses following an infection [9, 10], antibodies (in particular neutralizing ones) bind specifically to viral targets blocking an entry into host cells, thus preventing a cell from becoming infected with the virus. For the latter, avidity diagnostics of antibodies are of particular value [11]. Avidity is the binding strength of an antibody-antigen complex and thus elucidates the quality of the immune response [12]. Generally speaking, the avidity is low at the beginning of an infection. After a maturation process that takes a couple of months, avidity increases and indicates a functionally active antibody pool, as shown for SARS-CoV-1 [13].
Now that recent data on longevity of the immune response to SARS CoV-2 prove persistence of antibodies over a period of 7–8 months [14], characterizing the avidity of SARS-CoV-2–specific antibodies is of key interest. To date only a few studies have investigated avidity, and only for a much shorter period.
The data from Ischgl 7–8 months after the initial infection [14] could represent a particular basis to understand aspects of the dynamics of the immune response to SARS-CoV-2 infections due to the above-mentioned factors, especially the high infection rate. Furthermore, the willingness to participate in this study was high. Nearly the whole town has taken part (83%) in the first survey in April 2020 [8] (baseline) and 217 informative adults were included in the second survey in November 2020 (follow-up).
The main aim of the study was to assess in a large longitudinal study, with one of the longest follow-up times currently available, whether the marked increase in avidity of antibodies we observed 7–8 months after an infection [14] was also substantial in older people. Other aims were to assess dependence on sex and whether a high avidity is correlated with a high neutralization capacity, which would then convey an important role for avidity tests in assessing potential protection against further SARS-CoV-2 infections without necessitating expensive and laborious neutralization assays.
METHODS
Study Population and Plasma Samples
Our study population is a subpopulation of a large longitudinal seroprevalence study in Ischgl, Austria, targeting the entire adult population of Ischgl (n = 1527, at the time of data collection). Both the baseline and the follow-up study were assessed by the local Ethics Committee of Medical University of Innsbruck (1100/2020, 21 April 2020 and 1330/2020, 27 October 2020). Informed consent was given by all participants. Blood was drawn at 2 time points, April 2020 (baseline) and November 2020 (follow-up). We included all the 217 adults positive for anti-S1 immunoglobulin G (IgG) at both time points; only 6 of them reported a hospital stay due to SARS-CoV2 infection (Table 1). Only 11 subjects (5%) of the baseline cohort presented with negligible antibody titers at follow-up and could not be used for the calculation of avidity. All plasma samples were stored at + 4°C for short-term use or at −20°C when stored for longer than 4 weeks.
| Variables . | Values . |
|---|---|
| No. of participants (%) | |
| Total | 217 (100) |
| Female | 127 (58.5) |
| Male | 90 (41.5) |
| Age, y, mean (SD), median | |
| Female | 47.7 (16.3), 50 |
| Male | 46.7 (16.3), 48 |
| Age, y, n (%) | |
| 18–24 | 26 (12) |
| 25–34 | 28 (12.9) |
| 35–44 | 37 (17) |
| 45–54 | 44 (20.3) |
| 55–64 | 52 (24) |
| 65–74 | 20 (9.2) |
| ≥75 | 10 (4.6) |
| Reported hospitalization due to SARS-CoV-2, n (%) | |
| Female | 2 (0.9) |
| Male | 4 (1.8) |
| Variables . | Values . |
|---|---|
| No. of participants (%) | |
| Total | 217 (100) |
| Female | 127 (58.5) |
| Male | 90 (41.5) |
| Age, y, mean (SD), median | |
| Female | 47.7 (16.3), 50 |
| Male | 46.7 (16.3), 48 |
| Age, y, n (%) | |
| 18–24 | 26 (12) |
| 25–34 | 28 (12.9) |
| 35–44 | 37 (17) |
| 45–54 | 44 (20.3) |
| 55–64 | 52 (24) |
| 65–74 | 20 (9.2) |
| ≥75 | 10 (4.6) |
| Reported hospitalization due to SARS-CoV-2, n (%) | |
| Female | 2 (0.9) |
| Male | 4 (1.8) |
Race and ethnicity were not part of the questionnaire, but >>95% of subjects were white. Assessment was done 1–7 weeks (median 30 days) after SARS-CoV2 infection, which was confirmed by positive PCR of a nasopharyngeal swab.
Abbreviations: PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
| Variables . | Values . |
|---|---|
| No. of participants (%) | |
| Total | 217 (100) |
| Female | 127 (58.5) |
| Male | 90 (41.5) |
| Age, y, mean (SD), median | |
| Female | 47.7 (16.3), 50 |
| Male | 46.7 (16.3), 48 |
| Age, y, n (%) | |
| 18–24 | 26 (12) |
| 25–34 | 28 (12.9) |
| 35–44 | 37 (17) |
| 45–54 | 44 (20.3) |
| 55–64 | 52 (24) |
| 65–74 | 20 (9.2) |
| ≥75 | 10 (4.6) |
| Reported hospitalization due to SARS-CoV-2, n (%) | |
| Female | 2 (0.9) |
| Male | 4 (1.8) |
| Variables . | Values . |
|---|---|
| No. of participants (%) | |
| Total | 217 (100) |
| Female | 127 (58.5) |
| Male | 90 (41.5) |
| Age, y, mean (SD), median | |
| Female | 47.7 (16.3), 50 |
| Male | 46.7 (16.3), 48 |
| Age, y, n (%) | |
| 18–24 | 26 (12) |
| 25–34 | 28 (12.9) |
| 35–44 | 37 (17) |
| 45–54 | 44 (20.3) |
| 55–64 | 52 (24) |
| 65–74 | 20 (9.2) |
| ≥75 | 10 (4.6) |
| Reported hospitalization due to SARS-CoV-2, n (%) | |
| Female | 2 (0.9) |
| Male | 4 (1.8) |
Race and ethnicity were not part of the questionnaire, but >>95% of subjects were white. Assessment was done 1–7 weeks (median 30 days) after SARS-CoV2 infection, which was confirmed by positive PCR of a nasopharyngeal swab.
Abbreviations: PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Antibody Avidity Assay
Plasma samples were tested for antibody avidity by an Anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA; Euroimmun, Ref EI 2606–9601). Microplate wells coated with an S1-domain of the spike protein of SARS-CoV-2 were used in this study. The antigen was expressed recombinantly in the human cell line HEK 293 [15].
To determine the relative avidity index (RAI), 2 microplate wells next to each other were used for each subject. In 1 well the anti-SARS-CoV-2 ELISA was carried out according to the manufacturer’s instructions and in the other an additional urea treatment (5.5 M for 10 minutes) was performed. The urea treatment leads to the detachment of low-avidity antibodies from the antigen [13].
Plasma samples were initially diluted 1:101 in sample buffer. If the photometric measurement showed an extinction higher than 2.0 in the well without urea treatment, a higher dilution of 1:401, 1:801, or 1:1601 was used to bring the extinction into the linear range. Otherwise, the ELISA was done according to manufacturer’s instructions [15]. The RAI was calculated from the ratio of the absorbance with and without urea incubation and is expressed as percentage.
Neutralizing Antibody Assay
The assay was based on a replication-defective variant of the vesicular stomatitis virus expressing green fluorescent protein (VSVΔG-GFP), as vector, as described previously [16]. The virus was produced on 293T cells stably expressing the SARS-CoV-2 spike protein (Wuhan isolate), leading to single-cycle infectious virus particles pseudotyped with SARS-CoV-2 spike. Patient samples were incubated with the vector for 1 hour at 37°C, in a 4-fold serum dilution starting with a 1:16 dilution. Subsequently, 293T-ACE2 cells, seeded the day before, were infected with the vector-serum mixtures and incubated for 16 hours. The plates were then analyzed using an Immunospot reader (CTL Europe) to count the infected cells; 50% neutralization titers were calculated using a nonlinear regression as described previously [17]. In our study, samples with a neutralization titer ≥1:16 were considered as definitely neutralizing, samples that did not neutralize at 1:16 (ie, <1:16) were considered negative or not neutralizing [16].
Statistical Analysis
Statistical analysis was conducted in SPSS version 26.0 (IBM ) and figures were created with GraphPad Prism version 9.1.0.221.
Quantitative variables were compared across groups using parametric (Student t test and analysis of variance [ANOVA]) or nonparametric methods (Mann-Whitney U test and Kruskal-Wallis test), where appropriate. Linear regression models were used to assess the relationship between difference in RAI at baseline and follow-up by age and sex controlling for baseline RAI. Associations between categorical variables were tested using χ 2, with Fishers exact test where appropriate. A 2-tailed value of P < .05 was considered statistically significant for all comparisons.
Simple linear regression models for age and neutralization titer, and RAI (%) with neutralization titer, at both baseline and follow-up, were fitted separately. For all regressions, neutralization titer variables were transformed using log2 base to meet the assumptions of the linear regression model and outliers were removed.
RESULTS
Comparable Increase in Avidity Across All Age Groups During the Study Period
We observed increasing avidity during the observation period of 6.5 months from 18% to 42% in all samples (217/217), which was independent of age and certainly not impaired in older adults (r = −0.09 at baseline and r = −0.1 at follow-up; Figure 1). The slight tendency towards lower avidities with increasing age was influenced by just 1 outlier (Figure 1; arrow) and influenced by the smaller amount of data in very old age group (see limitations in “Discussion” section). We divided the cohort into 3 age groups, demonstrating a significant increase in avidity at 6.5 months postinfection and comparable avidity in participants older than 61 years (P < .0001 for all 3 age groups). No significantly lower avidity was detectable in older age groups (P > .2; Figure 2). There was also no significant difference in avidity between men and women at baseline or at follow-up (P > .1 at baseline and P > .6 at follow-up; Supplementary Figure 1).
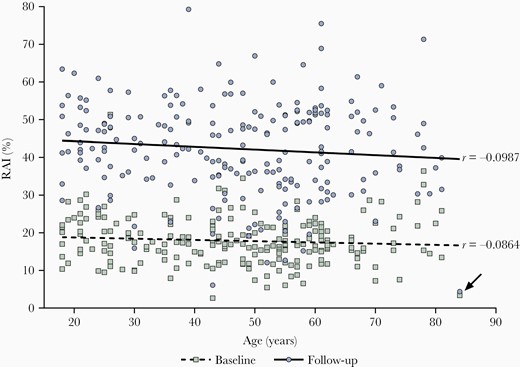
Correlation between age and relative avidity indices (RAI, %) at baseline and at follow-up with Spearman correlation coefficient (r). Squares (Green) represent RAIs of participants obtained at baseline (0.5–1.5 months after infection). Circles (Blue) show RAIs of the same participants at follow-up (7–8 months after infection). The lower dashed line shows mean RAIs across age at baseline, the upper continuous line the mean RAIs across age at follow-up, n = 217. The arrow indicates 1 outlier.
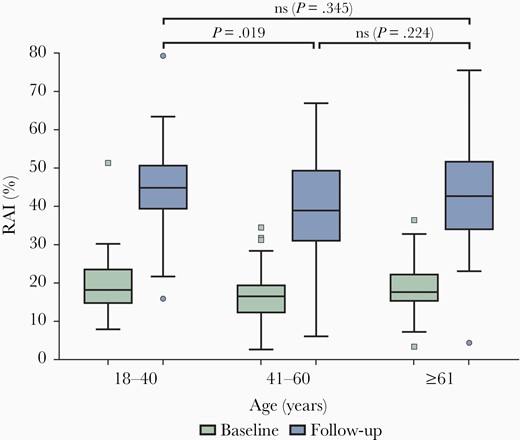
Box plots showing the RAI of subjects divided into 3 age groups of similar size. The lower (green) box plots represent RAI at baseline and upper (blue) ones at follow-up. The numbers of subjects in age groups 18–40, 41–60, and ≥61 are 75, 89, and 53, respectively. Abbreviations: ns, nonsignificant; RAI, relative avidity index.
Association of Avidity With Residual Neutralization Capacity and Increase in Neutralisation Capacity in Older People at Follow-up
Although there was a marked decrease in neutralization capacity at 6.5 months, 97.2% of all participant plasma samples had neutralizing capacity (≥1:16; Figure 3). In univariate regression analyses no relationship was found between neutralization capacity and avidity at baseline. However, at follow-up a significant association was found; a 2-fold increase in neutralization capacity was significantly associated with a 1.99% increase in RAI (95% confidence interval [CI], .76–3.22; Figure 3). There was only a slight significant difference in neutralization capacity between men (81/90) and women (121/127) at baseline but not at follow-up (Supplementary Figure 2). Of note, the 6 samples with a negative neutralization titer (<1:16) still exhibited a marked avidity, not different from those positive for neutralizing antibodies (P > .2; Figure 3).
![Correlation between relative avidity indices (RAIs) and neutralization capacities at baseline (squares [green] boxes, not significant) and at follow-up (circles [blue], significant). The dotted line indicates the threshold for positivity.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/224/5/10.1093_infdis_jiab300/1/m_jiab300f0003.jpeg?Expires=1749810238&Signature=CCSuGahcrwFvhubo5tdtNgo0-hYt5fXRYYN18f62cULx-7fnf8LU3ROYixsSRuZpttBcl46L2cQ1Qkxg9Yb~Ef3OFzWL8u9lmqr4WbZ~JON7UAtQYI9jyLzI25lrOBIL~hRLBEGXkuhAcru-6uJG00v37pBfyYbiPJjDlNN5ZUL6Sz7xih6f8zi1H~HjcYoiWqgGN3PWnzpZSvVOE4qkji21Wy0a7n4ruK12EIyEy1JgQFWXb65m4xfgB~ziZa0cnsvfr8-pnu63poWPxtJ89TuJyUih8NAbk~c5PE5e-JTkYgyVFmSM5guW4BY~7KbU68eA7~5VSpr-dSFeEVOefg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation between relative avidity indices (RAIs) and neutralization capacities at baseline (squares [green] boxes, not significant) and at follow-up (circles [blue], significant). The dotted line indicates the threshold for positivity.
The effect of age on neutralization capacity was both significant and similar at baseline and follow-up (Figure 4). A 1-year increase in age was found to be associated with a 1.01-fold (1%) increase in neutralization capacity at baseline (95% CI, 1.00–1.02); at follow-up, a 1-year increase in age was found to be associated with a 1.01-fold increase in neutralization titer (95% CI, 1.01–1.02). Thus, neutralization capacity was not decreased, but even increased in the older age group.
![Correlation between neutralization capacities and age at baseline (squares [green], significant) and at follow-up (circles [blue], significant). The dotted line indicates the threshold for positivity.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/224/5/10.1093_infdis_jiab300/1/m_jiab300f0004.jpeg?Expires=1749810238&Signature=Dw3m0uvlUS~9sHNnnGtH5k9Pk-WX5~mUi3FVvRb3~gZvscPlS6mmv7fFyCUXiOYGpGFbdPRScR4yLZm8WnShJL-J-ZCnfBDPVT0WUrUG22Hi2L4tMkQSObV2WLudQ5Y1z8-vVPTbcyPpEm4sD2QE7k1WZ6WvT2txJ1blJedyUnz0BiuV1HHtIZqbj-n0bIoCwW4PS3rNNXVJ3r7v4WIojpquumHhTZLM7~q5ES5UTxmIwfuZfDGlOSYsmcTwXoHeNUDbuYUgRj4b0G6lO1JUx1hb5WbT074fyOsx4D3kVcuUVwnWEOd9m5e6pl9mkCA0MR9eWJjaYMM1TviSMXQSNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation between neutralization capacities and age at baseline (squares [green], significant) and at follow-up (circles [blue], significant). The dotted line indicates the threshold for positivity.
Discussion
Although there have been studies and indications in the past that avidity may rise weeks after active COVID-19 disease (detailed below), our present study on a subgroup of 217 participants from a large cohort [14], comprising all antibody-positive subjects at both time points, clearly confirmed the rise of avidity and revealed that it is not only significant, but marked. The substantial increase in median avidity from 18% only 0.5–1.5 months after the onset of COVID-19 to 42% half a year later is clear evidence that robust immunity persists at least 7–8 months after an infection with SARS CoV-2. This immunity was sustained and not impaired in older participants.
The domain of avidity testing is clearly an assessment of whether an infectious disease commenced very recently [18]. Antibodies developed shortly after a primary infection exhibit a low avidity and bind weakly to the specific antigenic target. Over time, affinity towards the antigen increases as antibodies mature through clonal expansion, hypermutation, and affinity selection in the germinal center. This selection process guarantees an efficient immune defense in case of a repeat encounter with the pathogen. Aside from this, the time required to achieve a certain level of affinity maturation is utilized in clinical diagnostics to estimate the approximate time of a first contact with the pathogen. Whereas antibodies with low binding affinity indicate a recent infection, a high binding affinity speaks of a primary infection that took place at least 3–4 months earlier. This is of particular importance in pregnancy, where a primary infection plays a major role in intrauterine infection, which is especially relevant for rubella infections [18]: a high avidity of IgG antibodies in the first weeks of pregnancy, without medical history of a transfusion, would then clearly point towards an onset of the infection months before the pregnancy, even in the presence of positive IgM, implying that the unborn cannot be affected by this infection, thus avoiding an unnecessary termination of pregnancy.
To assess the immune response, avidity provides important and additional information on the immune response, revealing functionality of the persisting antibodies. Concerning the time course, the group of Liu found that IgG against SARS-CoV-2 S1 and receptor binding domain showed a low avidity 6–45 days postinfection [19], comparable to the 18% obtained during our baseline. Benner and coworkers found that anti-spike IgG avidity increased over days after symptom onset and appeared to peak around day 21 before beginning to plateau [20]. Our data could not elucidate when the peak of avidity had been reached, but there was an overall increase from 18% to 42% and there is thus the possibility that the maximum was even higher, as Benner and colleagues have also shown a decrease in the patients in their study, who were all hospitalized. In contrast, Luo and coworkers found that IgG avidity significantly increases from 1 to 90 days after symptom onset, without indications of a decrease [21]. Our study demonstrates continued avidity at 7–8 months postinfection. Given the fact that a high proportion of anti-S antibodies are neutralizing in nature, a stronger binding affinity of these antibodies corroborates the assumption that an efficient immune defense normally persists at least 7 months after an initial infection with SARS-CoV-2.
Concerning avidity in old age in other infectious diseases, antibody response to influenza vaccination in older people has been shown to be considerably lower than in younger adults [22], but age affects quantity, but not quality, of antibody responses after vaccination against tick-borne encephalitis [23]. Despite their overall impairment of immune functions, older people are still able to produce high-affinity antibodies that have similar functional activities to those produced at a young age [23]. Others claim that the reduction in number and size of germinal centers, where antibody affinity maturation processes occur, leads to a decrease of antibody affinity [24]. Along the same line, Banerjee and coworkers argue that older people are less able to make high-affinity antibodies to foreign antigens [25]. For pertussis, avidity against filamentous hemagglutinin was lower in older people, whereas there was no significant difference in avidity against the toxin [26].
For COVID-19 patients, higher levels of anti-spike avidity were associated with older age, male sex, and hospitalization [20]. This may be because men have increased disease severity compared to women. Additionally, increased risk for severity of disease is associated with advancing age, resulting in a more severe form of COVID-19 and therefore in a higher anti-spike IgG titer and avidity [20]. This was corroborated by another study detailing that avidity was significantly higher in severe compared to mild disease cases [21]. In our cohort, there was no stronger avidity in older people or men, but most of our study population was less severely affected by COVID-19 [8]. On the other hand, it is of public health interest to characterize the quality of immune response among the majority of a population, known to acquire normally mild or asymptomatic, rather than severe, SARS-CoV-2 infection. For this reason, a limitation of our study, namely that older people (60–80 years) but not very old people (>80 years) were included, may not be too serious as the total number of cases, but not the relative death toll, is higher among people aged 60–80 years compared to those aged >80 years [6].
In addition to antibody binding avidity, neutralization capacity is also an important marker of immune response. Higher levels of anti-spike IgG titers and higher levels of anti-spike IgG avidity were significantly associated with a higher prevalence of a neutralizing antibody titer [20]. However, those who generated a high neutralizing antibody titer still had high neutralizing antibody titers after 3 months, with only a negligible decline [27]. Only 5% of the original cohort (11 subjects, not included here) did not have sufficient antibody concentrations for calculation of avidity and another 2.8% of the baseline cohort (6 subjects) presented with a nondetectable neutralization capacity at follow-up. This implies that more than 92.5% of the baseline antibody-positive cohort neutralized at follow-up after 6.5 months.
As a rise in avidity confirms the maturation of the entire immune system, detailed above, we propose that the high avidity will likely compensate for a slight decrease in neutralization activity. Furthermore, given that neutralization capacity is just an in vitro surrogate marker, although an important one, the proof of a strong avidity of antibodies, confirming that the immune system has matured, may be a better universal immunologic parameter to predict protection from a reinfection, especially after almost three-quarters of a year.
It is of interest to note that elderly and middle-aged patients have been found to have significantly higher plasma neutralizing antibodies than young patients [28], which was also found in our study. In the previous study, samples were collected from 175 COVID-19 recovered patients with mild symptoms 10–24 days after symptom onset [28]. Even higher numbers may be found in patients with severe symptoms, as those with lower disease severity were reported to show a decline to undetectable neutralizing antibodies [27]. Likewise, individuals who recover from COVID-19 without becoming hospitalized typically do not have high neutralizing antibody activity [20] and individuals with severe disease exhibited elevated virus-neutralizing titers [29]. Ripperger and colleagues have also confirmed that disease severity correlates with neutralization capacity, but they did not find a correlation with age or sex, despite the difference in outcome [29]. Again, we cannot draw precise conclusions on associations with disease severity, but at baseline we had many participants with high neutralization titers (Figure 3), although only a few participants in the cohort had been hospitalized. Interestingly, although older male patients are more seriously affected by COVID-19, we did not see a sex difference in our large cohort for avidity and only a weak one for neutralization, which was only at baseline.
Studies investigating the sustainability of neutralization have shown a prolonged immunity over 6 months; the authors conclude that these individuals could mount a rapid and effective response to the virus upon reexposure [30]. This is corroborated by Ripperger and colleagues who showed that neutralizing antibodies are stably produced for at least 5–7 months after SARS-CoV-2 infection [29], as also supported by our study showing a neutralization capacity for more than 7 months postinfection, although titers decreased.
In summary, it is very interesting that older people can mount a similar avidity to young people and that the assessment of avidity may actually represent an excellent surrogate marker for a sustained immunity, replacing the laborious and expensive neutralization assays. A follow-up study over an even longer time may confirm our findings, in particular that high avidity is a better and more universal marker indicating protection from infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Ms Elisabeth Vetter, Euroimmun, for her support and help and Euroimmun, Lübeck, Germany. We thank Bianca Neurauter for excellent assistance in organizing the study, and Lisa-Maria Raschbichler and Albert Falch for excellent technical support.
Financial support. This work was supported by the Land of Tyrol Doctoral Programme of Excellence HOROS, Fonds zur Förderung der wissenschaftlichen Forschung (FWF), Vienna, Austria (grant number ZFW12530); and the Marie Skłodowska Curie Action project CORVOS, European Union (grant number EU-H2020-MSCA-ITN-2019, 860064). K. B. has been supported by a FWF Austrian Science Fund Lise Meitner Award (grant number M-3069-B).
Potential conflict of interest. None. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
D. P., M. B., and J. K. contributed equally to this work.




