-
PDF
- Split View
-
Views
-
Cite
Cite
Purbita Bandopadhyay, Ranit D’Rozario, Abhishake Lahiri, Jafar Sarif, Yogiraj Ray, Shekhar Ranjan Paul, Rammohan Roy, Rajshekhar Maiti, Kausik Chaudhuri, Saugata Bagchi, Ayan Maiti, Mohammed Masoom Perwez, Biswanath Sharma Sarkar, Devlina Roy, Rahul Chakraborty, Janani Srinivasa Vasudevan, Sachin Sharma, Durba Biswas, Chikam Maiti, Bibhuti Saha, Prasun Bhattacharya, Rajesh Pandey, Shilpak Chatterjee, Sandip Paul, Dipyaman Ganguly, Nature and Dimensions of Systemic Hyperinflammation and its Attenuation by Convalescent Plasma in Severe COVID-19, The Journal of Infectious Diseases, Volume 224, Issue 4, 15 August 2021, Pages 565–574, https://doi.org/10.1093/infdis/jiab010
Close - Share Icon Share
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), has led to significant morbidity and mortality. While most suffer from mild symptoms, some patients progress to severe disease with acute respiratory distress syndrome (ARDS) and associated systemic hyperinflammation.
First, to characterize key cytokines and their dynamics in this hyperinflammatory condition, we assessed abundance and correlative expression of a panel of 48 cytokines in patients progressing to ARDS as compared to patients with mild disease. Then, in an ongoing randomized controlled trial of convalescent plasma therapy (CPT), we analyzed rapid effects of CPT on the systemic cytokine dynamics as a correlate for the level of hypoxia experienced by the patients.
We identified an anti-inflammatory role of CPT independent of its neutralizing antibody content.
Neutralizing antibodies, as well as reductions in circulating interleukin-6 and interferon-γ–inducible protein 10, contributed to marked rapid reductions in hypoxia in response to CPT.
CTRI/2020/05/025209. http://www.ctri.nic.in/
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to significant morbidity and mortality worldwide. The disease caused by SARS-CoV-2, coronavirus disease 2019 (COVID-19), has a spectrum of symptoms spread over 2 distinct phases in symptomatic individuals. The variable symptomatology in the first milder phase is usually followed by recovery [1]. In a fraction of infected individuals this milder phase progresses to a more severe disease characterized by gradually worsening hypoxia and in some to acute respiratory distress syndrome (ARDS), leading to fatal outcomes in a proportion of patients [1, 2]. A hyperimmune activation response is associated with severe symptoms, characterized by a systemic deluge of inflammatory cytokines [3–5].
Different therapeutic approaches are being explored, either by repurposing specific antiviral agents, that is remdesivir [6], or by using corticosteroids to affect immunomodulation [7]. In addition, convalescent plasma therapy (CPT) has emerged as a widely tried strategy against COVID-19, and is being explored in a number of clinical trials worldwide [8, 9, 10]. CPT is an age-old strategy for passive immunization, with the primary intention to supplement nonrecovering patients with antibodies against specific pathogens [9, 11]. Several previous pandemics of respiratory infections saw successful use of CPT, starting from the 1918 Spanish influenza to the more recent SARS and Ebola epidemics [12–15].
In the present study, first, we aimed at an in-depth characterization of the nature and dimensions of the cytokines involved in the systemic hyperinflammation encountered in COVID-19 patients with ARDS as compared to mild disease, and then, in a randomized control clinical trial of COVID-19 CPT, we explored the effects of convalescent plasma (CP), if any, on mitigation of this systemic immune hyperactivation phenotype. We revealed the multidimensional nature of the systemic hyperinflammation in severe COVID-19. Moreover, we identified an anti-inflammatory effect of CPT in terms of attenuation of the systemic cytokine deluge, which contributed to rapid mitigation of hypoxia, in addition to the neutralizing antibody content of CP.
METHODS
Ethical Approval
The randomized control trial (RCT) on CPT and all associated studies, and the required human sampling, were carried out with informed consent from the patients as per ethical approval from the Institutional Review Boards of the CSIR-Indian Institute of Chemical Biology, Kolkata, India (IICB/IRB/2020/3P), Medical College Hospital, Kolkata (MC/KOL/IEC/NON-SPON/710/04/2020), India, and Infectious Disease and Beleghata General Hospital (ID and BG Hospital), Kolkata, India (IDBGH/Ethics/2429). The RCT was approved by Central Drugs Standard Control Organisation, Ministry of Health and Family Welfare, Government of India (approval No. CT/BP/09/2020) and registered with the Clinical Trial Registry of India (CTRI/2020/05/025209).
Plasma Cytokine Analysis
Plasma was isolated from peripheral blood of patients collected in EDTA vials and cytokine levels (pg/mL) were measured using the Bio-Plex Pro Human Cytokine Screening Panel 48-Plex Assay (Bio-Rad), which quantitates 48 cytokines: fibroblast growth factor (FGF) basic, eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-1ra, IL-1α, IL-2Rα, IL-3, IL-12 (p40), IL-16, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, growth-related oncogene-α (GRO-α), hepatocyte growth factor (HGF), IFN-α2, leukemia inhibitory factor (LIF), monocyte chemoattractant protein-3 (MCP-3), IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, IL-18, IFN-γ–inducible protein-10 (IP-10), MCP-1, monokine induced by interferon-γ (MIG), nerve growth factor-β (NGF-β), stem cell factor (SCF), stem cell growth factor-β (SCGF-β), stromal cell-derived factor-1α (SDF-1α), macrophage-inflammatory protein-1α (MIP-1α), MIP-1β, platelet-derived growth factor-BB (PDGF-BB), regulated upon activation normal T-cell expressed and secreted (RANTES), tumor necrosis factor-α (TNF-α), TNF-β, vascular endothelial growth factor (VEGF), cutaneous T cell-attracting chemokine (CTACK), migration inhibitory factor (MIF), TNF-related apoptosis-inducing ligand (TRAIL), and M-CSF. The patient plasma samples were diluted (1:3) in sample diluent and the assay was performed using the manufacturer’s protocol. The plate was run and analyzed using the Bio-Plex 200 System (Bio-Rad).
RNA Isolation and RT-PCR for SARS-CoV-2
Nasopharyngeal swabs were collected from all patients on the day of enrolment and directly put in TRIzol. RNA from these TRIzol samples were extracted using the chloroform-isopropanol method followed by quantitation using NanoDrop (Thermo Fisher Scientific). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) for SARS-CoV-2 detection was performed using the STANDARD M nCoV Real-Time Detection kit (SD Biosensor), approved by the Indian Council of Medical Research. A cycle threshold (Ct) cutoff mean value for both RdRp and E genes was considered for interpreting the results. A cyanine 5-labeled internal control was used. Average Ct values for 2 SARS-CoV-2 targets (RdRp and E) were used in the study as a surrogate for viral load.
SARS-CoV-2 Surrogate Virus Neutralization Assay
Neutralizing antibodies (nAb) against SARS-CoV-2 in CP samples from peripheral blood of convalescent donors were detected using GenScript SARS-CoV-2 Surrogate Virus Neutralization kit (catalog No. L00847). The kit consists of recombinant SARS-CoV-2 spike protein receptor-binding domain fragment conjugated with horseradish peroxidase (HRP-RBD) and human angiotensin-converting enzyme (ACE2) receptor protein. Assay was performed according to the manufacturer’s protocol. Plasma samples were diluted at a ratio of 1:10. Because presence of SARS-CoV-2 nAb in the CP samples will result in inhibition of interaction between HRP-RBD and plate-bound human ACE2 protein, and subsequent development of color, assay results are interpreted as the inhibition rate of the assay reaction. Inhibition rate is calculated as: (1 – [OD of sample/OD of negative control]) × 100. Inhibition values ≥20% signify positive detection of nAb. In recipient patients, the amount of nAb received was calculated considering the amount (mL) of CP transfused.
ELISA for Anti–SARS-CoV-2 IgG
Levels of immunoglobulin G (IgG) specific for SARS-CoV-2 in the CP were detected using EUROIMMUN anti–SARS-CoV-2 (IgG) enzyme-linked immunosorbent assay (ELISA) kit (catalog No. EI 2606–9601 G). Assay was performed according to the manufacturer’s protocol. The assay wells were precoated with recombinant S1 domain of the SARS-CoV-2 spike protein. CP samples were diluted with the provided sample dilution buffer at a ratio of 1:101 (vol:vol). Presence of anti–SARS-CoV-2 IgG antibodies in the CP was measured using the following formula: ratio = extinction of the control or CP samples/extinction of calibrator. Ratio ≥1.1 is interpreted as positive. In recipient patients, the amount of IgG received was calculated considering the amount (mL) of CP transfused.
Standard of Care
At the clinical trial site (ID and BG Hospital, Kolkata, India) standard of care (SOC) in all patients with evidence for ARDS was: oxygen therapy as per requirement in all patients; dexamethasone or equivalent corticosteroid in all patients; for patients with D-dimer <1000 fibrinogen equivalent units (FEU) prophylactic anticoagulation and for patients with D-dimer >1000 ng/mL FEU therapeutic anticoagulation using either low molecular weight heparin or unfractionated heparin; appropriate broad-spectrum antibiotic therapy based on clinical and microbiological assessment; blood sugar maintained below 200 mg/dL using appropriate antidiabetic therapy; and appropriate antihypertensive agents to maintain systolic blood pressure 100–140 mmHg, diastolic blood pressure 70–90 mmHg, and mean arterial pressure >65 mmHg. Awake proning for 6–8 hours/day was attempted in all patients with ARDS. Oxygen therapy was designed to maintain Spo2 >95% using different devices with different efficiencies in oxygen supplementation (as denoted by Fio2), that is nasal cannula, face mask, face mask with reservoir, and in patients unable to maintain Spo2 above 90% with face mask with reservoir, high-flow nasal cannula, or in some cases mechanical ventilation. For Spo2/Fio2 ratio kinetics, a value of 89.99 was used for data points where either high-flow nasal cannula or mechanical ventilation was in use. Of note, Spo2/Fio2 ratio kinetics was analyzed only in patients with data for at least 1 intervening time point in addition to day 1 and day 5.
Convalescent Plasma Therapy
CP was collected from convalescent donors (recovered from RT-PCR–positive SARS-CoV-2 infection at least 28 days prior to donation) by apheresis at the Department of Blood Transfusion and Immunohematology, Medical College Hospital, Kolkata, India. All donors were tested for their anti-spike IgG content in addition to routine screening tests to exclude major blood-borne pathogens before apheresis. We randomized the ARDS patients into either the SOC group as controls or the CPT group, who received 2 200-mL doses of ABO-matched CP on 2 consecutive days in addition to standard care, the first transfusion being on the day of recruitment (day 1).
Co-occurrence Analyses
Co-occurrence among each pair of cytokines in patient plasma was calculated using Pearson correlation (r). Absolute values of the cytokines were used for the calculation of correlation network and threshold was set to r > 0.5, P < .01 for the complete set of cytokines from mild disease (n = 13) and ARDS (n = 33) conditions, and to r > 0.3, P < .05 for the significantly different cytokines between mild disease and ARDS and between SOC (n = 16) and CPT (n = 17) sets at time point 2 (T2). All calculations were done using Hmisc R package and converted to a network file using igraph. Visualization of the network was performed using Cytoscape. Each cytokine was color coded and node size was set in proportion to the median fold change as compared to the same cytokine in the mild disease datasets.
Statistical Analyses
Differential abundance of cytokines was evaluated by Mann-Whitney U test and Wilcoxon matched pairs test for categorical and ordinal data, respectively. To test the monotonous relationship between Ct value and plasma cytokine, Spearman rank correlation was used, as was done between nAb content and IgG antibody content for CP. To explain the area under the curve of Spo2/Fio2 ratio measured for 5 days (SFR5DAUC) as a function of a combination of cytokines and nAb, linear regression was applied on the ranks of each of the parameters and was implemented using R. The statistical significance was defined as a P value <.05 and P value <.01 with 2-sided tests, unless otherwise noted. Statistical tests were also performed in Statistica 64 (StatSoft).
RESULTS
Subject Recruitment and Sampling
We recruited patients with COVID-19 at the ID and BG Hospital, Kolkata, India, who either had mild COVID-19 disease (WHO Clinical Progression Score 1–4; n = 13, 12 men and 1 woman, aged 41.1 ± 11.2 years, enrolled 2.5 ± 2 days after hospitalization) or more severe disease showing evidence for ARDS with Pao2/Fio2 ratio between 100 and 300 (WHO Clinical Progression Score 5–6; n = 33, 26 men and 7 women, aged 60.03 ± 11.5 years, enrolled 4 ± 2.8 days after hospitalization). We randomized the recruited ARDS patients into either the SOC group or CPT group who received 2 doses of CP, 200 mL, on 2 consecutive days in addition to standard care. The first transfusion of ABO-matched CP was done on the day of enrolment (day 1), followed by another transfusion on the next day (day 2). Patient plasma samples were taken on day 1 before CP transfusion (time point 1 [T1]) and again on day 3 or 4 posttransfusion (T2) to assess the rapid effects of CP transfusion on the systemic cytokine milieu.
Nature of Systemic Hyperinflammation and its Relationship With Viral Load in Severe COVID-19
First, we analyzed a panel of 48 cytokines in plasma at T1 for deeper characterization of the systemic hyperinflammation through comparison between patients with milder disease and patients with evidence of ARDS. We identified a panel of 14 molecules that were significantly higher in ARDS patients (Figure 1A and Supplementary Figure 1). In addition, we found a significant decrease in plasma abundance of TRAIL in ARDS.
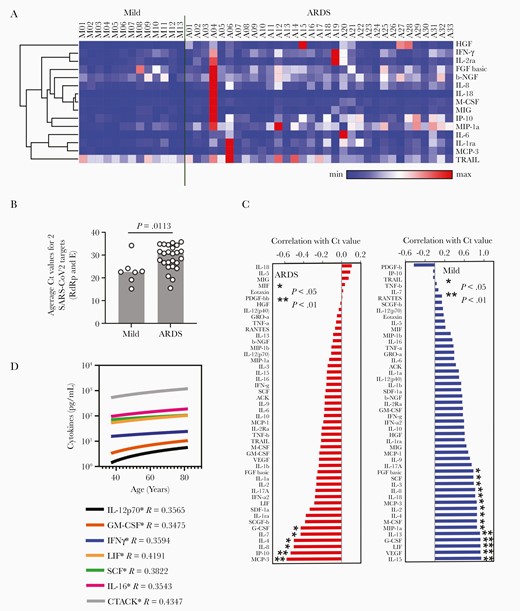
Nature of the systemic hyperinflammation in severe COVID-19 disease. A, Heatmap representing normalized plasma abundance of 15 major cytokines compared between patients with mild disease (n = 13) or ARDS (n = 33) at enrolment, made with Morpheus. Rows are clustered using 1 minus Pearson correlation distance and average linkage. B, Comparison between the Ct values of RT-PCR for SARS-CoV-2 target genes from nasopharyngeal swabs in patients with mild disease and patients progressing to ARDS. C, Extent of correlation between the plasma levels of cytokines and concomitant viral loads measured by average Ct for real-time PCR of 2 SARS-CoV-2 target genes, compared between patients with mild disease and ARDS. Spearman correlation values are arranged in ascending order and significances are * P < .05 or ** P < .01. D, Plasma level of cytokines having significant (P < .05) correlation with age of patients with ARDS. The Spearman R value is given for each of the cytokines with age. Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; Ct, cycle threshold value; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; see text for definitions of cytokines.
The viral load (estimated by average Ct values from real-time PCR for 2 viral genes from nasopharyngeal swabs collected at the same time as plasma sampling) was significantly higher (ie, lower Ct values) in patients with milder disease, perhaps due to earlier sampling in their disease course (Figure 1B). Plasma abundance of a number of cytokines in mild disease was negatively correlated with concurrent viral load, presumably representing the cytokine component of an efficient protective immunity in the milder phase (Figure 1C). Interestingly, in patients with ARDS there was a significant positive correlation of viral load with a few specific cytokines (Figure 1C). Increasing age of the patients was significantly correlated with higher plasma abundance of a number of cytokines, although apart from IFN-γ none of them were significantly associated with ARDS (Figure 1D).
Severe COVID-19 Is Characterized by Correlated Induction of Multiple Cytokines
Next, to gather some insight on the dimensions of the systemic hyperinflammation, we constructed the correlative networks (Pearson correlation, with a cutoff of R > 0.5, P < .01) among individual members of the whole panel of cytokines and compared between mild disease and ARDS patients (Supplementary Figure 2A and 2B). We also separately constructed similar correlative networks (Pearson correlation, with a cutoff of R > 0.5, P < .05) among the cytokines that were found to be significantly dysregulated in ARDS (Figure 2). Interestingly, in ARDS we found more robust assimilation of correlative networks among the cytokines with greater numbers of edges compared to mild disease (156 edges in mild vs 356 edges in ARDS among the whole panel of 48 cytokines and 23 edges in mild disease vs 52 edges in ARDS among the cytokines significantly dysregulated in ARDS). In this analysis, most notable was a 5-member cytokine module, comprising of IL-6, MCP-3, MIP-1α, IL-1ra, and IP-10, showing robust correlative upregulation. On the other hand, a notable absence from this correlative network, despite a significantly higher abundance in ARDS, was the mesenchymal cell-derived pleiotropic cytokine HGF.
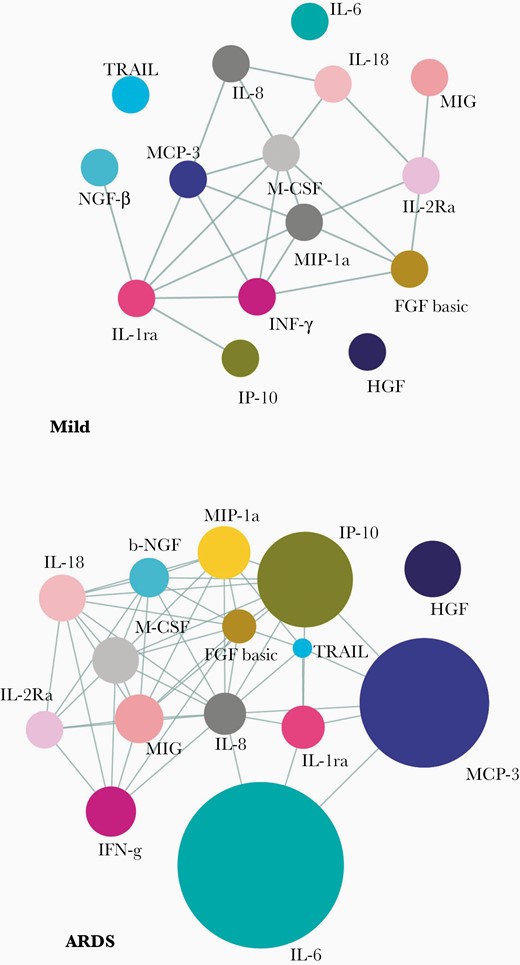
Correlative dynamics of the plasma cytokine abundance in severe COVID-19. Correlation networks of major cytokines at time point 1 for the mild disease group (n = 13) and ARDS group (n = 33) are shown. Pearson correlations with R > 0.3 and P < .05 are considered to draw the edges. Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; see text for definitions of cytokines.
Rapid Attenuation of Systemic Cytokine Levels in Response to Therapy
We then analyzed the aforementioned cytokine panel in all ARDS patients at T2 and explored if the T1 to T2 change was different between SOC and CPT groups. Intriguingly, on analyzing the panel of 15 cytokines depicted in Figure 1A, we found a notable effect of CPT, as compared to SOC, in reducing the levels of a number of them, that is IL-6, IP-10, and MCSF (Figure 3A and 3B and Supplementary Table 1). On the other hand, none of the major cytokines driving the hyperinflammatory state was found to be significantly reduced at T2 in patients receiving SOC (Supplementary Table 1). This anti-inflammatory effect was dependent on neither the anti–SARS-CoV-2 spike IgG content of the transfused CP nor its nAb content (Figure 3A). Of note, we found a strong correlation between anti-spike IgG content of CP and its nAb content, as measured in an in vitro assay [16] (R = 0.8564, P < .0001; Figure 3C).
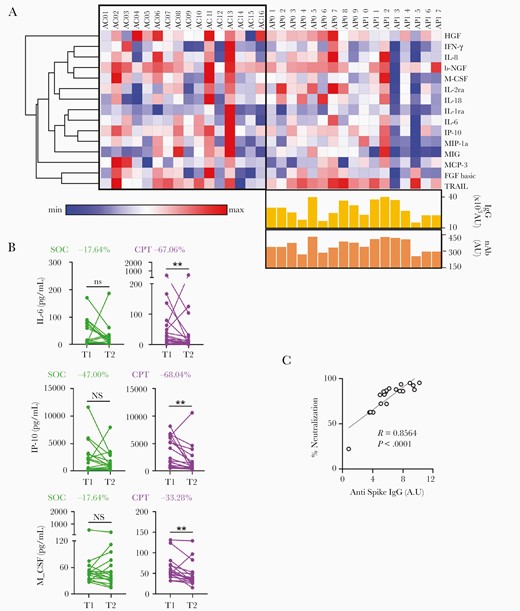
Attenuation of hyperinflammation and mitigation of hypoxia in response to convalescent plasma. A, Heatmap representing log2 fold change between T1 and T2 in the plasma abundance of major cytokines associated with ARDS compared between SOC (n = 16) and CPT (n = 17) groups. Heatmap was made with Morpheus and rows are clustered using 1 minus Pearson correlation distance and average linkage. Anti-spike IgG content and nAb content of the convalescent plasma transfused are represented for the respective patients in the CPT group. AU represents the total units of either IgG or the Neutralizing contents received through two units of CP transfused to a given recipient, based on the corresponding values for each unit of CP. B, Reduction in plasma levels of IL-6, IP-10, and M-CSF from T1 to T2 compared between SOC and CPT groups. The percentage difference in median value at T2 compare to T1 was also calculated for 3 of the cytokines. C, Correlation between the anti–SARS-CoV-2 spike IgG content of convalescent plasma transfused into patients and their neutralization efficacy in the in vitro assay. Abbreviations: ARDS, acute respiratory distress syndrome; CPT, convalescent plasma therapy; IgG, immunoglobulin G; nAb, neutralizing antibody; ns, nonsignificant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOC, standard of care; T, time point; see text for definitions of cytokines.
This anti-inflammatory effect of CP was also apparent when we compared the correlative networks at T2 of both the whole panel of cytokines (Supplementary Figure 2C and 2D), as well as when we did the same among the 15 cytokines found to be significantly dysregulated in ARDS (Figure 4) and compared them between the SOC and CPT groups. Residual correlative edges among the cytokines at T2 were significantly fewer in case of CPT in contrast to the SOC group (334 edges in SOC group vs 135 edges in CPT group among the whole panel of 48 cytokines and 47 edges in SOC group vs 33 edges in CPT group among cytokines significantly dysregulated in ARDS), representing an attenuation of systemic hyperinflammation in response to CPT and a trend toward a cytokine milieu quite similar to one found in the early milder phase.
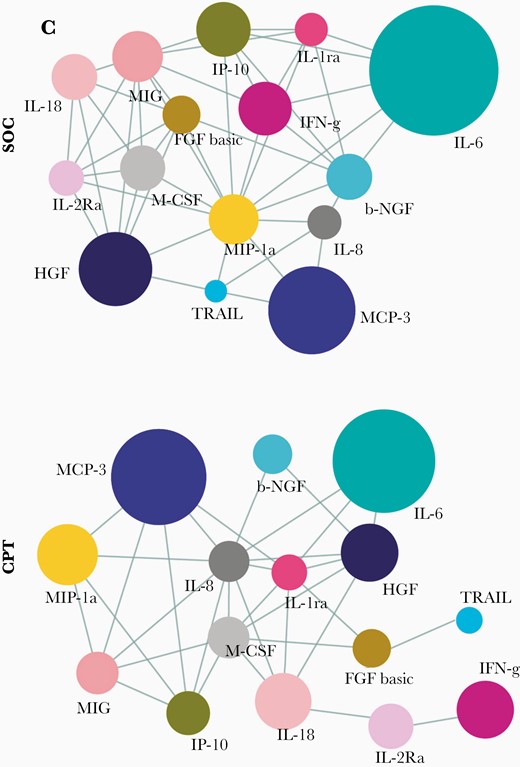
Rapid effect of therapy on the correlative dynamics of the systemic cytokine deluge in severe COVID-19. Correlation networks of major cytokines at time point 2 compared between the SOC (n = 16) and CPT (n = 17) groups are shown. Pearson correlations with R > 0.3 and P < .05 are considered. Abbreviations: COVID-19, coronavirus disease 2019; CPT, convalescent plasma therapy; SOC, standard of care; see text for definitions of cytokines.
Effect of Convalescent Plasma on Rapid Mitigation of Hypoxia
To explore if the effect of therapy on systemic hyperinflammation was correlated with the concomitant clinical status of the ARDS patients, we decided to assess their requirement for oxygen supplementation (as measured by fraction of inspired oxygen, Fio2) for maintaining oxygen saturation of circulating hemoglobin at a physiological level (oxygen saturation as measured by pulse oximetry, Spo2). This offered a quantifiable, comparable, and relevant clinical parameter across all ARDS patients. Thus, the short-term clinical outcome in the ARDS patients were assessed by the kinetics of the Spo2/Fio2 ratio for 5 days following enrolment (Figure 5A). This data was then processed to represent clinical improvement over 5 days with respect to day 1 by calculating the AUC for the Spo2/Fio2 ratio curve (SFR5DAUC) (Figure 5B). Mitigation of hypoxia was faster in the CPT group compared to patients receiving SOC only (Figure 5A and 5B). We noted gradual abrogation of this differential response in reduction of hypoxia from the third day onwards after the second dose of CPT, indicating that a sustained benefit may require additional transfusions of CPT in some patients.
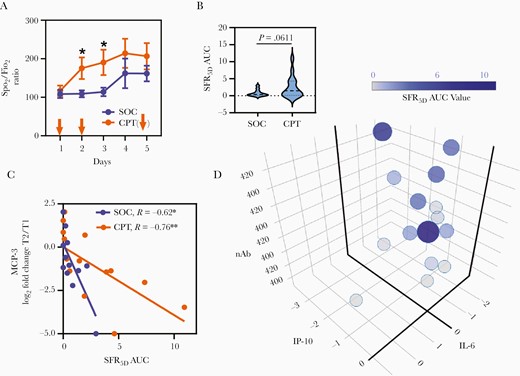
Role of convalescent plasma on rapid mitigation of hypoxia in severe COVID-19. A, Kinetics of Spo2/Fio2 ratio in patients during 5 days postenrolment compared between SOC and CPT groups. Comparison of daily means was performed by Welch t test and significant differences are indicated by * P < .05. The plasma transfusion days are indicated by arrows. B, Violin plot representing the comparison of SFR5DAUC for patients in the SOC and CPT groups. C, Correlation of log2 fold change between T1 and T2 for plasma level of MCP-3 and SFR5DAUC. The Spearman correlations values for SOC and CPT groups are given with their significance level. D, SFR5DAUC relationships with nAb content of transfused plasma, and log2 fold change of plasma levels of IL-6 and IP-10 in the CPT group. The plot was generated by plotly package in R. Abbreviations: COVID-19, coronavirus disease 2019; CPT, convalescent plasma therapy; IL-6, interleukin-6; IP-10, interferon-γ-inducible protein-10; MCP-3, monocyte chemoattractant protein-3; nAb, neutralizing antibody; SFR5DAUC, area under the curve of Spo2/Fio2 ratio measured for 5 days; SOC, standard of care; T, time point.
Reduction in the chemokine MCP-3 at T2 as compared to T1 was significantly associated with mitigation of hypoxia, irrespective of whether patients received CPT or not, thus identifying a major pathogenic molecule underlying COVID-19 ARDS (Figure 5C). In patients receiving CPT, as expected, improvements in Spo2/Fio2 ratio was significantly correlated with the nAb content of CP they were transfused with. Interestingly, in a linear regression analysis we identified that IL-6 and IP-10, the 2 major ARDS-associated cytokines that were affected by the anti-inflammatory effect of CPT as described in Figure 3A, also played a major role in the rapid mitigation of hypoxia in combination with the nAb content (Figure 5D and Supplementary Table 2).
DISCUSSION
In this study we have been able to chart the nature of the characteristic immune hyperactivation associated with diseases severity in COVID-19 patients, to a considerable resolution, by looking at a large number of cytokines at 2 time points. A panel of 14 cytokines dominated the systemic deluge in patients with severe disease, in terms of statistically significant increases in their plasma abundances compared to mild disease. Plasma abundance of a single cytokine, TRAIL, was found to be significantly reduced in severe COVID-19 patients. TRAIL is known to be expressed in cytotoxic T cells and NK cells and is involved in killing of virus-infected host cells [17]. Decrease in circulating TRAIL may signify obviation of infected host-cell–directed cytotoxicity in this later phase of the disease.
In patients with ARDS, there was, in contrast to patients with mild disease, a significant positive correlation of viral load with a few specific cytokines. Among these, IL-8 and G-CSF presumably represent the usual neutrophil recruitment response triggered by residual virus-infected cells. Notably, most of the major cytokine components of systemic hyperinflammation, that is IL-6, IFN-γ, IL-1ra, MIP-1α, seemed not to correlate in any way with the concomitant viral load in patients with ARDS. But notable exceptions here were MCP-3 and IP-10, which may represent pathogenic links between host-virus interactions in the earlier phase of the disease and the hyperimmune activation that ensues later in a fraction of patients and warrant further mechanistic studies. Although the number of patients recruited in the study was small, the statistical robustness makes the findings notable.
As discussed above, despite a significantly higher abundance in ARDS, the mesenchymal cell-derived pleiotropic cytokine HGF was interestingly not part of the correlative network of cytokines at any time point. Systemic abundance of HGF perhaps represents an anti-inflammatory as well as a tissue regeneration response in response to the systemic inflammatory assault, as shown earlier [18]. Although a proinflammatory role of HGF, targeting neutrophils through c-MET receptor signaling, has also been described recently and thus may be of interest to explore in the context of severe COVID-19 [19].
On analyzing the kinetics of the cytokine components of the systemic hyperinflammation in both the SOC and CPT groups after enrolment, we noted reduced abundance of a number of major cytokines preferentially in patients receiving CPT. Moreover, this cytokine attenuation effect was not found to be correlated with the anti–SARS-CoV-2 spike IgG content or the nAb content of the transfused CP. Of note, beyond providing specific pathogen-neutralizing antibodies, CP has the potential to drive other biological effects, for example immunomodulation as well as endothelial stabilization [20, 21]. This may result from other constituents of CP, that is nonspecific immunoglobulins acting through the Fc receptors, anti-inflammatory components of the complement system, and coagulation factors, in addition to other anti-inflammatory proteins and cytokines [20].
A number of clinical trials, with varying study designs and scopes, are ongoing in different parts of the world that are evaluating therapeutic efficacy of CPT in COVID-19. Therapeutic use of CPT was not found to have any considerable safety issues in a large study in the United States [8]. But there have been contradictory reports in terms of measurable clinical benefits offered by CPT. A number of RCTs could not register any clinical benefit of CPT in severe COVID-19 patients [22, 23]. On the other hand, in several case series early in the pandemic, as well as in a few RCTs, therapeutic benefits were recorded [24–26]. In view of the variable reports it is imperative to explore if there are patient subgroups who may or may not benefit from CPT.
We report here that the beneficial effect of CPT perhaps goes beyond just passive immunization of the recipients and thus should further be explored mechanistically to identify other anti-inflammatory factors in CP. We envisage that the anti-inflammatory effect of CPT may also mitigate longer-term systemic sequelae of the hyperinflammation in severe COVID-19 full appreciation of which awaits end-point analyses in our trial as well as further meta-analyses of data from other clinical trials on CPT completed or ongoing elsewhere.
Thus, this study characterizes the nature and dimensions of the systemic hyperinflammation in patients with ARDS, which could identify a number of hitherto unappreciated features of the disease pathogenesis in severe COVID-19. Moreover, we report here an anti-inflammatory effect of COVID-19 CP, independent of, but acting in synergy with, its neutralizing antibody content, which may prove to be a composite predictor of response to CPT in COVID-19 and should be explored when analyzing the clinical outcomes of trials ongoing throughout the world.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. G. conceptualized the study. D. G. and Y. R. designed the study protocol. P. Ba. and R. D. did the plasma cytokine analysis. J. S. performed serological studies. S. P. and A. L. contributed to the analysis and computation. Y. R. and S. R. P. recruited patients, maintained clinical data, and supervised clinical management. R. R., R. M., K. C., S. B., A. M., M. M. R., B. S. S., D. R., R. C., and B. S. contributed to patient management. J. S. V., S. S., and R. P. did the RT-PCR for SARS-CoV-2. S. C. contributed immunological studies. S. P. and P. Bh. recruited convalescent donors. D. B., C. M., and P. Bh. performed donor screening, apheresis, and biobanking of convalescent plasma. D. G. and S. P. interpreted data. D. G. wrote the manuscript. All authors approved the manuscript.
Acknowledgments. Authors express their gratitude to Anurag Agrawal and Kamakshi Sureka for critical reading of the manuscript and Shantanu Sengupta for help with the neutralizing antibody assay.
Financial support. This work was supported by the Council of Scientific Industrial Research (CSIR), India (grant numbers MLP-129 to D. G. and MLP-2005 to R. P.); and Foundation Botnar. The authors hold fellowships from the Department of Science and Technology, India (Swarnajayanti Fellowship to D. G.); Science and Engineering Research Board, India (Ramanujan Fellowship to S. P.); Department of Biotechnology, India (DBT)-Welcome Trust India Alliance (S. C.); DBT (Ramalingaswami Fellowship R. P.); CSIR (P. B. and A. L.); and University Grants Commission, India (R. D. and J. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
P. B., R. D. R., A. L., J. S., Y. R., and S. R. P. contributed equally.




