-
PDF
- Split View
-
Views
-
Cite
Cite
Kristine M Erlandson, Melissa P Wilson, Samantha MaWhinney, Eric Rapaport, Jay Liu, Cara C Wilson, Jeremy T Rahkola, Edward N Janoff, Todd T Brown, Thomas B Campbell, Catherine M Jankowski, The Impact of Moderate or High-Intensity Combined Exercise on Systemic Inflammation Among Older Persons With and Without HIV, The Journal of Infectious Diseases, Volume 223, Issue 7, 1 April 2021, Pages 1161–1170, https://doi.org/10.1093/infdis/jiaa494
Close - Share Icon Share
Abstract
We investigated whether higher-intensity exercise provided greater decrease in markers of inflammation, and whether responses differed by HIV serostatus.
People with HIV (PWH; n = 32) and controls (n = 37) aged 50–75 years completed 12 weeks moderate-intensity exercise, then were randomized to moderate- or high-intensity exercise for 12 additional weeks (n = 27 and 29, respectively). Inflammation biomarkers were measured at 0, 12, 24 weeks. Mixed and multiple regression models were adjusted for baseline inflammation, age, and body mass index.
Baseline tumor necrosis factor-α (TNF-α), soluble TNF receptor 2 (sTNFR2), and soluble CD14 (sCD14) were significantly higher among PWH than controls (P < .04). From week 0–12, changes in interleukin-6 (IL-6), TNF-α, and sTNFR1 were not significantly different by HIV serostatus. We found no significant interaction between HIV serostatus/exercise intensity on week 12–24 changes in IL-6, TNF-α, and sTNFR1. Among high-intensity exercisers, PWH and controls had significant increases in sCD14 (P ≤ .003), controls significant increases in IL-10 (P = .01), and PWH nonsignificant decrease in highly sensitive C-reactive protein (P = .07). Other markers were not significantly different by serostatus or intensity.
Moderate and high-intensity exercise elicited similar effects on inflammation among PWH and controls, with additional beneficial effects seen among high-intensity exercisers. Increase in sCD14 and attenuated IL-10 increase (PWH only) merit further study.
NCT02404792.
Many older people with human immunodeficiency virus (PWH) face adverse consequences of aging, including dementia, cancer, cardiovascular disease, diabetes, sarcopenia, and physical function impairments [1–6], often to a greater extent than among persons without human immunodeficiency virus (HIV). The development of many of these conditions in PWH is attributed in part to heightened inflammation and monocyte activation [7, 8]. Several interventions have been tested for anti-inflammatory effects in PWH including statins, aspirin, and low-dose methotrexate [9–11], with variable efficacy in reducing inflammation.
Exercise is associated with numerous health benefits, in addition to improved immune function and anti-inflammatory effects seen in some studies of PWH [12, 13]. Acutely, exercise stimulates production and release of interleukin-6 (IL-6) [14, 15], with greater IL-6 response seen with higher intensity and longer duration of exercise [16, 17]. The rise in IL-6 then appears to contribute to a rise in anti-inflammatory cytokines, including IL-10. Thus, a paradoxical reduction in the resting level of systemic inflammatory cytokines is seen with regular exercise. Under certain conditions (eg, heightened systemic inflammation), higher-intensity exercise may cause muscle damage and a greater acute rise in inflammatory markers, resulting in a maladaptive acute increase in tumor necrosis factor-α (TNF-α) and an increase in chronic systemic inflammatory cytokines [14, 15]. The impact of routine physical activity or an exercise intervention on acute or chronic markers of inflammation among older persons with chronic inflammation, particularly among older PWH, is largely unknown [14]. Furthermore, while exercise can improve general markers of immune function (eg, CD4 count), we do not know if exercise in PWH alters monocyte activation thought to underlie chronic inflammation [8].
In a randomized clinical trial, we recently demonstrated improved physical function among older adults with and without HIV with a 24-week exercise intervention, and additional benefit of higher-intensity exercise on measures of strength among PWH [18]. The present study used data and specimens from this completed trial to investigate whether higher-intensity exercise provided greater overall decrease in key markers of inflammation (IL-6, TNF-α, and soluble TNF receptor-1 [sTNFR-1]) than moderate-intensity exercise, and whether responses to exercise intensity differed by HIV serostatus. We hypothesized that inflammation would decrease among both PWH and controls with 24 weeks of exercise, and that this effect would be greater with higher-intensity exercise. We also compared (1) changes in monocyte activation, (2) change in exploratory biomarkers, and (3) the inflammatory response to a single bout of exercise.
METHODS
The Exercise for Healthy Aging Study enrolled PWH and HIV-uninfected controls from April 2014 to May 2017 (Clinicaltrials.gov NCT02404792). All participants were 50 to 75 years- old, sedentary (<60 minutes/week of self-reported physical activity for 6 months), had a body mass index (BMI) 20–40 kg/m2, and no contraindications to initiating an exercise regimen. Acute inflammatory conditions were excluded until resolution. Use of immunosuppressive or immunomodulatory medications (within 6 months) was not permitted; intranasal/inhaled but not oral or intraarticular corticosteroid use (within 6 months) were allowed. PWH were on stable antiretroviral therapy with an HIV-1 RNA <200 copies/mL for at least 2 years and a CD4 T-cell count ≥200 cells/µL. Study procedures were approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained from all participants.
Study Procedures
As previously described [18], participants attended supervised exercise sessions thrice weekly for 24 weeks at the University of Colorado-Anschutz Medical Campus Exercise Research Laboratory. Following a 2-week low-intensity exercise acclimation (20–30 minutes of treadmill walking and 3 sets of 8 repetitions of bench and leg press, lateral pulldown, and rotating fourth exercise using Cybex machines), cardiovascular exercise intensity increased to 40%–50% of baseline Vo2 max (by monitored heart rate) and duration increased by 5 minutes/week to achieve 50 minutes/session by 12 weeks; resistance exercise increased to 60%–70% of 1-repetition maximum (1RM), with target weight loads adjusted every 3 weeks [19]. At week 12, Vo2 max measurements were repeated. Participants were randomized to continue moderate-intensity exercise or advance to high-intensity (60%–70% of week 12 Vo2 max, >80% 1RM [19]) for an additional 12 weeks. Adherence to the intervention was calculated as attended exercise sessions/expected exercise sessions.
Acute Exercise Sessions
During weeks 2, 12, and 24, participants underwent an acute exercise stimulus conducted between 8:00 and 11:00 am. Non-fasting blood was collected immediately prior (participants were encouraged to have a small breakfast or snack before each session). Participants completed a 5-minute treadmill warm-up, 40 minutes of treadmill exercise at 75% Vo2 max, a brief rest, a warm-up resistance exercise set of 8 repetitions at 10% less than the training load, then 5 sets of 10 repetitions with 2-minute rest between sets. If a participant was unable to complete all 10 repetitions at the target weight, the load was reduced by 10% and the repetitions were completed. Blood was collected immediately after completing the last repetition, and at 60 and 90 minutes during seated rest. Sample processing occurred within 2 hours and plasma was stored at −80oC for analysis.
Measurement of Plasma Soluble Molecules
Commercially available enzyme-linked immunosorbent assays were used to measure baseline and week 24 plasma molecules: IL-6, TNF-α, soluble tumor necrosis factor receptor 1 (sTNFR1), sTNFR2, IL-10, soluble CD14 (sCD14), and intestinal fatty acid binding protein (I-FABP; R&D Systems). Highly sensitive C-reactive protein (hsCRP) was measured by immunoturbidimetrics (Beckman Coulter). All assays were measured in duplicate and followed instructions specified by the manufacturer. IL-6, TNF-α, and sTNFR1 were additionally measured at the 3 acute exercise sessions.
Assessment of Monocyte Subsets and Function
Peripheral blood mononuclear cells (PBMCs) were collected in 9 PWH and 14 controls at week 0 and 12 (PBMC collection was not added until later in the study; see Methods in Supplemental Materials).
Statistical Analysis
We estimated that a sample size of 13/group (by HIV serostatus and intensity) would provide 80% power, at significance level of .05 using 2-sided t tests to detect between-group decreases of 25% (sTNFR1), 28% (TNF-α), or 52% (IL-6) for primary outcomes. Additional analyses were considered exploratory. To allow for log-transformation of inflammation marker concentrations, undetectable inflammation values were set to be half the smallest observed value for IL-10 (1 value) and I-FABP (1 value), and half the detectable level for TNF-α (<3% of values). Unadjusted differences by serostatus were calculated via t tests with estimated mean (95% confidence interval [CI]) or nonparametric tests with medians (interquartile range [IQR]) (Table 1). Results are reported as percent differences from baseline (mean [95% CI]), or from week 12 to 24 (for IL-6, TNF-α, and sTNFR1). Multivariable regression models were used for the primary analyses, with all change results adjusted for precision (age) and confounding variables (baseline concentration of the inflammatory marker and BMI).
| Characteristic . | People With HIV (n = 32) . | Uninfected Controls (n = 37) . |
|---|---|---|
| Age, y, median (IQR) | 57 (55–59) | 58 (56–61) |
| Sex, % male | 88 | 95 |
| Race, % white | 63 | 84 |
| Non-Hispanic, % | 88 | 89 |
| Current smoker, % | 16 | 11 |
| Illicit drug use in the past 2 y, % | 19* | 0* |
| BMI, mean (SD) | 27 (4)* | 30 (5)* |
| Statin use, % | 41 | 41 |
| Antihypertensive use, % | 53 | 43 |
| VACS-1 > 20 points, % | 56* | 24* |
| 3 or more comorbidities, % | 72 | 51 |
| Years since HIV diagnosis, median (IQR) | 21 (16–28) | … |
| Nadir CD4 count, cells/µL, median (IQR) | 200 (85–400) | … |
| CD4 count, cells/µL, median (IQR) | 591 (445–836) | … |
| Detectable HIV-1 RNA at baseline, ≥20 copies/mL, % | 9 | … |
| ART duration, y, median (IQR) | 17 (7–19) | … |
| Receipt of an integrase inhibitor, % | 63 | … |
| Receipt of a protease inhibitor, % | 41 | … |
| Receipt of nonnucleoside reverse transcriptase inhibitor, % | 28 | … |
| Receipt of tenofovir alafenamide or disoproxil fumarate, % | 47 | … |
| Receipt of abacavir, % | 38 | … |
| Hepatitis Ca, % | 13 | 0 |
| IL-6, pg/mL, median (IQR) | 2.08 (1.39–2.55) | 1.79 (1.46–2.58) |
| sTNFR1, pg/mL, median (IQR) | 1180 (945–1491) | 1062 (904–1196) |
| TNF-α, pg/mL, median (IQR) | 1.27 (1.09–1.72)* | 0.99 (0.84–1.28)* |
| hsCRP, mg/L, median (IQR) | 1.88 (0.97–3.77) | 1.07 (0.66–2.42) |
| sCD14, ng/mL, median (IQR) | 1810 (1595–1975)* | 1596 (1309–1691)* |
| sTNFR2, pg/mL, median (IQR)) | 2799 (2134–4111)* | 2289 (1863–2865)* |
| I-FABP, ng/mL, median (IQR) | 331 (201–656) | 209 (89–495) |
| IL-10, pg/mL, median (IQR) | 4.49 (3.29–6.07) | 4.07 (3.04–7.35) |
| Characteristic . | People With HIV (n = 32) . | Uninfected Controls (n = 37) . |
|---|---|---|
| Age, y, median (IQR) | 57 (55–59) | 58 (56–61) |
| Sex, % male | 88 | 95 |
| Race, % white | 63 | 84 |
| Non-Hispanic, % | 88 | 89 |
| Current smoker, % | 16 | 11 |
| Illicit drug use in the past 2 y, % | 19* | 0* |
| BMI, mean (SD) | 27 (4)* | 30 (5)* |
| Statin use, % | 41 | 41 |
| Antihypertensive use, % | 53 | 43 |
| VACS-1 > 20 points, % | 56* | 24* |
| 3 or more comorbidities, % | 72 | 51 |
| Years since HIV diagnosis, median (IQR) | 21 (16–28) | … |
| Nadir CD4 count, cells/µL, median (IQR) | 200 (85–400) | … |
| CD4 count, cells/µL, median (IQR) | 591 (445–836) | … |
| Detectable HIV-1 RNA at baseline, ≥20 copies/mL, % | 9 | … |
| ART duration, y, median (IQR) | 17 (7–19) | … |
| Receipt of an integrase inhibitor, % | 63 | … |
| Receipt of a protease inhibitor, % | 41 | … |
| Receipt of nonnucleoside reverse transcriptase inhibitor, % | 28 | … |
| Receipt of tenofovir alafenamide or disoproxil fumarate, % | 47 | … |
| Receipt of abacavir, % | 38 | … |
| Hepatitis Ca, % | 13 | 0 |
| IL-6, pg/mL, median (IQR) | 2.08 (1.39–2.55) | 1.79 (1.46–2.58) |
| sTNFR1, pg/mL, median (IQR) | 1180 (945–1491) | 1062 (904–1196) |
| TNF-α, pg/mL, median (IQR) | 1.27 (1.09–1.72)* | 0.99 (0.84–1.28)* |
| hsCRP, mg/L, median (IQR) | 1.88 (0.97–3.77) | 1.07 (0.66–2.42) |
| sCD14, ng/mL, median (IQR) | 1810 (1595–1975)* | 1596 (1309–1691)* |
| sTNFR2, pg/mL, median (IQR)) | 2799 (2134–4111)* | 2289 (1863–2865)* |
| I-FABP, ng/mL, median (IQR) | 331 (201–656) | 209 (89–495) |
| IL-10, pg/mL, median (IQR) | 4.49 (3.29–6.07) | 4.07 (3.04–7.35) |
All included participants had baseline inflammatory markers and completed at least 1 acute exercise visit:
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; hsCRP, highly sensitive C-reactive protein; I-FABP, intestinal fatty acid binding protein; IL, interleukin; IQR, interquartile range; sCD14, soluble CD14; sTNFR, soluble tumor necrosis factor receptor; TNF-α, tumor necrosis factor-α; VACS-1, Veterans Aging Comorbidity Score-1.
*P < .05 for between group difference.
aAll participants with hepatitis C had an undetectable viral load; if hepatitis C was treated, treatment had been completed ≥6 months prior to study initiation.
| Characteristic . | People With HIV (n = 32) . | Uninfected Controls (n = 37) . |
|---|---|---|
| Age, y, median (IQR) | 57 (55–59) | 58 (56–61) |
| Sex, % male | 88 | 95 |
| Race, % white | 63 | 84 |
| Non-Hispanic, % | 88 | 89 |
| Current smoker, % | 16 | 11 |
| Illicit drug use in the past 2 y, % | 19* | 0* |
| BMI, mean (SD) | 27 (4)* | 30 (5)* |
| Statin use, % | 41 | 41 |
| Antihypertensive use, % | 53 | 43 |
| VACS-1 > 20 points, % | 56* | 24* |
| 3 or more comorbidities, % | 72 | 51 |
| Years since HIV diagnosis, median (IQR) | 21 (16–28) | … |
| Nadir CD4 count, cells/µL, median (IQR) | 200 (85–400) | … |
| CD4 count, cells/µL, median (IQR) | 591 (445–836) | … |
| Detectable HIV-1 RNA at baseline, ≥20 copies/mL, % | 9 | … |
| ART duration, y, median (IQR) | 17 (7–19) | … |
| Receipt of an integrase inhibitor, % | 63 | … |
| Receipt of a protease inhibitor, % | 41 | … |
| Receipt of nonnucleoside reverse transcriptase inhibitor, % | 28 | … |
| Receipt of tenofovir alafenamide or disoproxil fumarate, % | 47 | … |
| Receipt of abacavir, % | 38 | … |
| Hepatitis Ca, % | 13 | 0 |
| IL-6, pg/mL, median (IQR) | 2.08 (1.39–2.55) | 1.79 (1.46–2.58) |
| sTNFR1, pg/mL, median (IQR) | 1180 (945–1491) | 1062 (904–1196) |
| TNF-α, pg/mL, median (IQR) | 1.27 (1.09–1.72)* | 0.99 (0.84–1.28)* |
| hsCRP, mg/L, median (IQR) | 1.88 (0.97–3.77) | 1.07 (0.66–2.42) |
| sCD14, ng/mL, median (IQR) | 1810 (1595–1975)* | 1596 (1309–1691)* |
| sTNFR2, pg/mL, median (IQR)) | 2799 (2134–4111)* | 2289 (1863–2865)* |
| I-FABP, ng/mL, median (IQR) | 331 (201–656) | 209 (89–495) |
| IL-10, pg/mL, median (IQR) | 4.49 (3.29–6.07) | 4.07 (3.04–7.35) |
| Characteristic . | People With HIV (n = 32) . | Uninfected Controls (n = 37) . |
|---|---|---|
| Age, y, median (IQR) | 57 (55–59) | 58 (56–61) |
| Sex, % male | 88 | 95 |
| Race, % white | 63 | 84 |
| Non-Hispanic, % | 88 | 89 |
| Current smoker, % | 16 | 11 |
| Illicit drug use in the past 2 y, % | 19* | 0* |
| BMI, mean (SD) | 27 (4)* | 30 (5)* |
| Statin use, % | 41 | 41 |
| Antihypertensive use, % | 53 | 43 |
| VACS-1 > 20 points, % | 56* | 24* |
| 3 or more comorbidities, % | 72 | 51 |
| Years since HIV diagnosis, median (IQR) | 21 (16–28) | … |
| Nadir CD4 count, cells/µL, median (IQR) | 200 (85–400) | … |
| CD4 count, cells/µL, median (IQR) | 591 (445–836) | … |
| Detectable HIV-1 RNA at baseline, ≥20 copies/mL, % | 9 | … |
| ART duration, y, median (IQR) | 17 (7–19) | … |
| Receipt of an integrase inhibitor, % | 63 | … |
| Receipt of a protease inhibitor, % | 41 | … |
| Receipt of nonnucleoside reverse transcriptase inhibitor, % | 28 | … |
| Receipt of tenofovir alafenamide or disoproxil fumarate, % | 47 | … |
| Receipt of abacavir, % | 38 | … |
| Hepatitis Ca, % | 13 | 0 |
| IL-6, pg/mL, median (IQR) | 2.08 (1.39–2.55) | 1.79 (1.46–2.58) |
| sTNFR1, pg/mL, median (IQR) | 1180 (945–1491) | 1062 (904–1196) |
| TNF-α, pg/mL, median (IQR) | 1.27 (1.09–1.72)* | 0.99 (0.84–1.28)* |
| hsCRP, mg/L, median (IQR) | 1.88 (0.97–3.77) | 1.07 (0.66–2.42) |
| sCD14, ng/mL, median (IQR) | 1810 (1595–1975)* | 1596 (1309–1691)* |
| sTNFR2, pg/mL, median (IQR)) | 2799 (2134–4111)* | 2289 (1863–2865)* |
| I-FABP, ng/mL, median (IQR) | 331 (201–656) | 209 (89–495) |
| IL-10, pg/mL, median (IQR) | 4.49 (3.29–6.07) | 4.07 (3.04–7.35) |
All included participants had baseline inflammatory markers and completed at least 1 acute exercise visit:
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; hsCRP, highly sensitive C-reactive protein; I-FABP, intestinal fatty acid binding protein; IL, interleukin; IQR, interquartile range; sCD14, soluble CD14; sTNFR, soluble tumor necrosis factor receptor; TNF-α, tumor necrosis factor-α; VACS-1, Veterans Aging Comorbidity Score-1.
*P < .05 for between group difference.
aAll participants with hepatitis C had an undetectable viral load; if hepatitis C was treated, treatment had been completed ≥6 months prior to study initiation.
For acute exercise sessions, area under the curve (AUC) was calculated using the trapezoidal method over postacute exercise session time points (0–90 minutes), using preexercise level as baseline. The initial and maximum rise, and 90-minute inflammatory levels were calculated as log transformed differences from the preexercise inflammatory level. Mixed models were adjusted for preexercise inflammation, age, and BMI. Monocyte analyses were unadjusted and conducted with Wilcoxon signed rank tests (see Supplemental Materials). Associations between physical function change and inflammatory biomarker change were explored using Spearman correlations. All outcomes were considered exploratory, with no adjustment for multiple comparisons.
RESULTS
Thirty-two PWH and 37 uninfected controls contributed baseline data, and 27 PWH (12 moderate- and 15 high-intensity exercise) and 29 controls (15 moderate- and 14 high-intensity exercise) completed the 24 week intervention. The majority of participants were male. PWH had well-controlled HIV with a median CD4 count of 591 cells/µL; 91% had HIV-1 load <20 copies/mL, with a median HIV-1 RNA of 46 (IQR, 39–54) copies/mL for n = 3 with detectable viral load (Table 1). PWH tended to have a higher estimated mortality, as predicted by the Veterans Aging Cohort Study Index [20] and more comorbidities, but a lower BMI than uninfected controls.
Baseline Comparison by HIV Serostatus
At baseline, TNF-α, sTNFR2, and sCD14 were significantly higher among PWH compared to controls (Table 1; P < .04); other markers did not significantly differ. The proportions of classical, intermediate, or nonclassical monocytes were comparable at baseline (Figure 1A; P > .09).
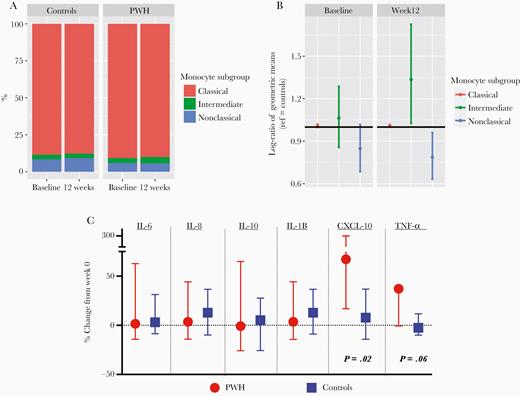
A, Monocyte subgroups by HIV serostatus at baseline and 12 weeks are shown with compositional monocyte data. B, Ratio of monocyte components among PWH compared to controls (reference). As shown, the intermediate monocytes increased and nonclassical decreased among PWH compared to uninfected controls. C, Median (interquartile range) percent change from week 0 to 12 in cytokines following low-dose LPS stimulation, among PWH (red circles) or controls (blue squares), using Wilcoxon signed rank tests. Abbreviations: CXCL-10, C-X-C motif chemokine ligand 10; HIV, human immunodeficiency virus; IL, interleukin; LPS, lipopolysaccharide; PWH, people with HIV; TNF-α, tumor necrosis factor-α.
Effects of 12 Weeks of Moderate-Intensity Exercise on Inflammatory Cytokines and Monocytes
We first compared the percent change in our primary biomarkers IL-6, TNF-α, and sTNFR1 among PWH and controls with the first 12 weeks of moderate-intensity exercise. PWH had a significant decrease in sTNFR1 (mean, −5%; 95% CI, −10% to 0%; P = .04) and controls had a significant decrease in IL-6 from baseline (mean, −15%; 95% CI, −28% to 0%; P = .049; Figure 2). The week 0 to 12 changes were not significantly different between groups.
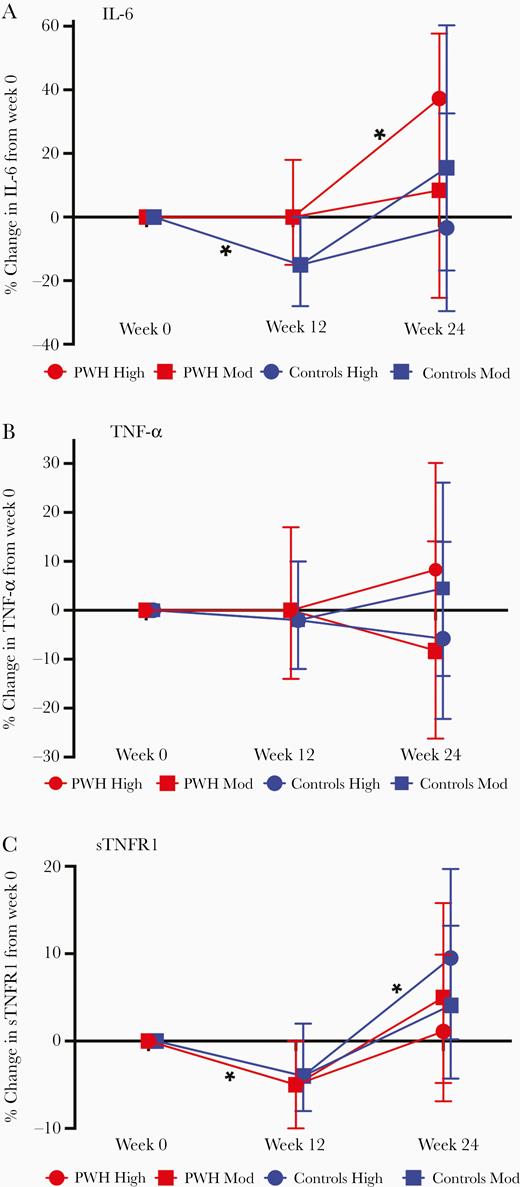
Mean (95% confidence interval) percent change in IL-6 (A), TNF-α (B), and sTNFR1 (C) from week 0 to week 12 by HIV serostatus, and week 12 to 24 by HIV serostatus and exercise intensity (by multivariable regression models, adjusted for age, body mass index, and baseline inflammatory marker). Abbreviations: HIV, human immunodeficiency virus; IL-6, interleukin-6; PWH, people with HIV; sTNFR1, soluble tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-α.
The total number of monocytes remained stable from week 0 to 12 among 9 PWH (mean difference from baseline, 1.7%; 95% CI, −2.8% to 3.7%) and 14 controls (mean, 0.6%; 95% CI, −1.0 to 2.1%). As shown in Figure 1, PWH had significantly higher proportion of intermediate monocytes, fewer nonclassical, but similar proportion of classical monocytes compared to controls at week 12 (Figure 1B). In Supplemental Table 1, we show that exercise was associated with modest differences from baseline in expression of selected monocyte markers previously associated with exercise or inflammation among both PWH and controls. The largest between-group differences with exercise were seen with expression (mean fluorescence intensity) of CD11b and HLA-DR on nonclassical monocytes, with increases among PWH of median 11.4% (IQR, 6.5% to 12.4%) and 14.4% (IQR, −6.9% to 23.2%), respectively, compared to controls (median −0.9% [IQR,−5.7% to 3.7%] and 2.5% [IQR, −12.5% to 7.8%], respectively). As comparisons within monocyte subsets were limited by small sample size, differences were not tested for statistical significance (Supplemental Table 1).
With in vitro monocyte stimulation studies, CXCL-10 (IP-10) and TNF-α increased after 12 weeks of moderate-intensity exercise among PWH (P = .02 and P = .06, respectively) but not controls (Figure 1C).
Effects of Moderate Versus High-Intensity Exercise on Inflammatory Cytokines Among PWH and Controls (Weeks 12–24)
PWH randomized to high-intensity exercise had the greatest increase in both IL-6 and TNF-α and the least increase in sTNFR1 (Figure 2). These week 12 to 24 changes were only significant for IL-6 (P = .04); serostatus-exercise intensity interaction terms did not reach statistical significance. Only controls randomized to high-intensity exercise had a significant increase in sTNFR1 from week 12 to 24, but minimal change in TNF-α and IL-6. To further investigate the effect of either HIV serostatus or exercise intensity, we compared markers by HIV serostatus (moderate and high intensity combined) or exercise intensity (PWH and controls combined). There were no significant differences in the percent change in IL-6, TNF-α, or sTNFR1 by HIV serostatus (Figure 3) or by exercise intensity from week 12 to 24 (Figure 4).
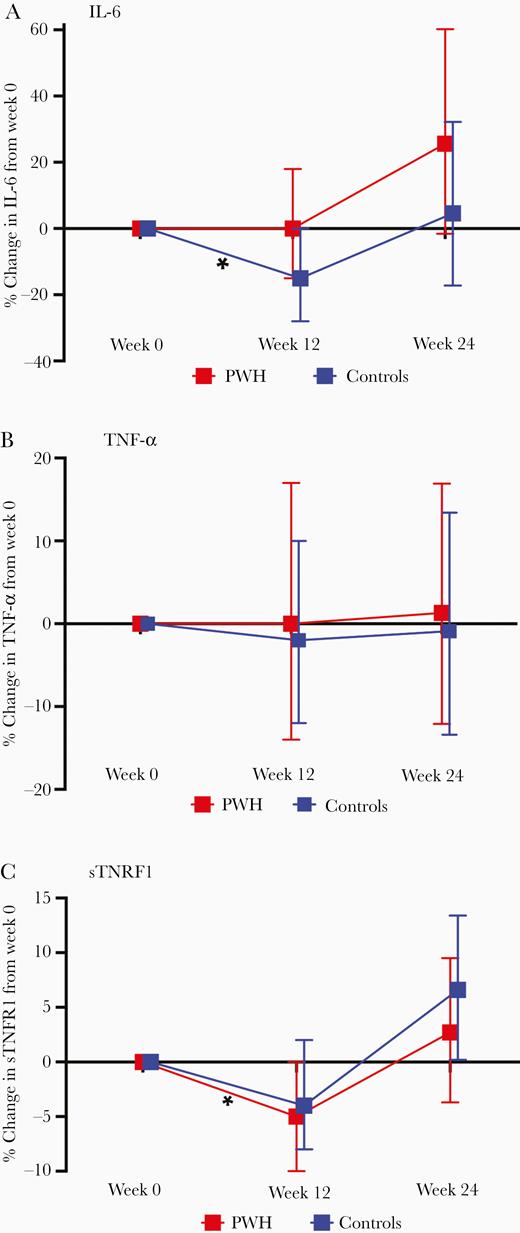
Mean (95% confidence interval) percent change in IL-6 (A), TNF-α (B), and sTNFR1 (C) from week 0 to week 12 by HIV serostatus, and week 12 to 24 by HIV serostatus (moderate and high-intensity combined). Results from multivariable regression models, adjusted for age, body mass index, and baseline inflammatory marker. Abbreviations: HIV, human immunodeficiency virus; IL-6, interleukin-6; sTNFR1, soluble tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-α.
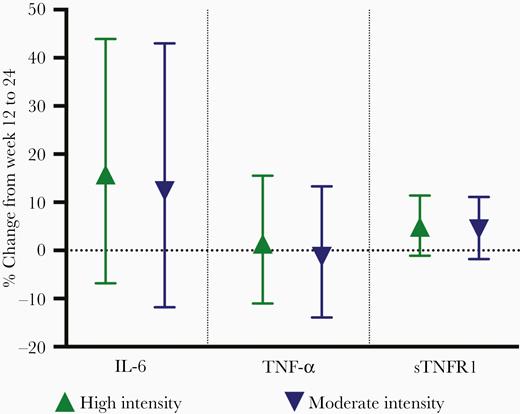
Mean (95% confidence interval) percent change in IL-6, TNF-α, and sTNFR1 from week 12 to 24 by exercise intensity (HIV serostatus groups combined). Results from multivariable regression models, adjusted for age, body mass index, and baseline inflammatory marker. Abbreviations: HIV, human immunodeficiency virus; IL-6, interleukin-6; sTNFR1, soluble tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-α.
Adherence ranged from 83% in controls randomized to high-intensity exercise to 90% in PWH randomized to moderate-intensity exercise (see Supplementary Materials) [18]. In sensitivity analyses, adherence was significantly associated with change in IL-6 (P = .03). When % adherence was added to the models, increases in IL-6 for a participant with average adherence (87.5%) were attenuated among both PWH (mean, 15.5%; 95% CI, −9.7% to 47.7% vs mean, 25.6%; 95% CI, −1.6% to 60.2%) and among uninfected controls (mean, 0.6%; 95% CI, −19.9% to 26.4% vs mean, 4.6%; 95% CI, −17.2 to 32.2%).
Exploratory Comparison of Effects of 24 Weeks of Exercise on Additional Biomarkers
Both PWH and controls randomized to high-intensity exercise had significant increases in sCD14 (P ≤ .003), controls had significant increases in IL-10 (P = .01), and PWH had a nonsignificant decrease in hsCRP (P = .07). Other markers were not significantly different from baseline (Figure 5A) and interaction terms were nonsignificant for all models (P > .27).
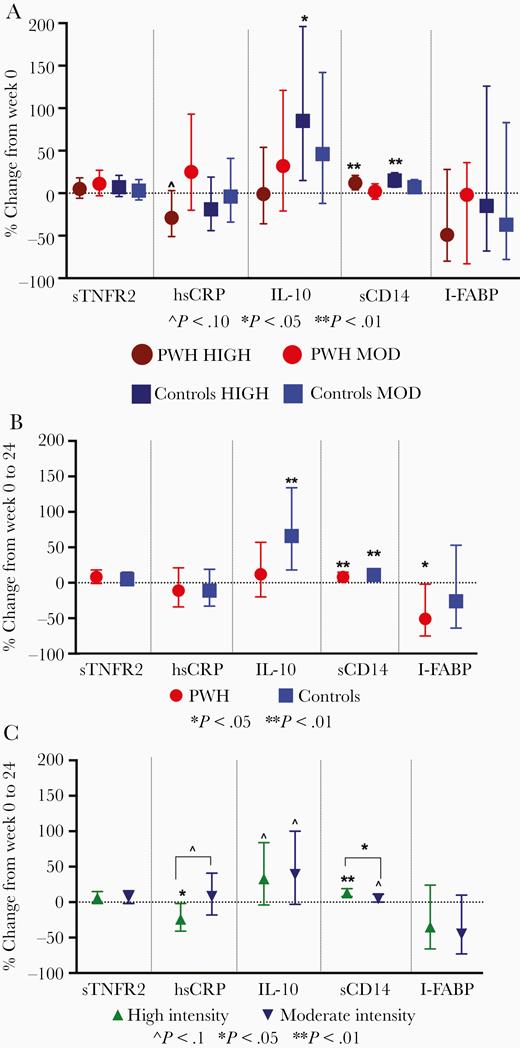
Mean (95% confidence interval) percent change in exploratory cytokines from week 0 to 24 by HIV serostatus and exercise intensity (A), HIV serostatus with exercise intensity combined (B), and exercise intensity with HIV serostatus (C). Results from multivariable regression models, adjusted for age, body mass index, and baseline inflammatory marker. Abbreviations: HIV, human immunodeficiency virus; hsCRP, highly sensitive C-reactive protein; I-FABP, intestinal fatty acid binding protein; IL-10, interleukin-10; MOD, moderate; PWH, people with HIV; sCD14, soluble CD14; sTNFR1, soluble tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-α.
To further investigate the effect of either HIV serostatus or exercise intensity, we compared markers by HIV serostatus (moderate and high intensity combined) or exercise intensity (PWH and controls combined). With intensity groups combined, both PWH and controls had significant increases in sCD14, PWH also had a significant decrease in I-FABP, and controls had a significant increase in IL-10 (Figure 5B). In analyses combined by HIV serostatus, the high-intensity exercise arm had significantly greater increases in sCD14 than the moderate-intensity arm (P = .04); high-intensity exercisers also tended to have greater decreases in hsCRP (P = .06) (Figure 5C).
In sensitivity analyses, 5% greater adherence to exercise (regardless of intensity) was associated with significantly greater decreases in hsCRP (mean, −27.9%; 95% CI, −44.6% to −6.1% vs mean −16.9%; 95% CI, −31.2% to 0.34%; P = .03).
We also investigated the relationship between the 24-week change in physical function measures and change in inflammatory markers, but found no strong correlations (all correlation coefficients < 0.5; Supplementary Figure 2).
Inflammatory Responses to an Acute Exercise Stimulus at 2, 12, and 24 Weeks
Lastly, we explored the response to an acute exercise stimulus without training, with 12 weeks of moderate-intensity exercise and 12 additional weeks of moderate or high-intensity exercise. The AUC, initial rate of rise, maximum change from baseline, and value at 90 minutes postacute exercise were similar for IL-6, sTNFR1, or TNF-α, both by serostatus and by exercise intensity (Supplementary Figure 3).
DISCUSSION
In our 24-week randomized intervention of moderate or high-intensity cardiovascular and resistance exercise among older, sedentary adults, high-intensity exercise was associated with significant increases in IL-6 among PWH and sTNFR1 among controls. Other markers did not appear to have an exaggerated inflammatory response to exercise, either acutely or over the duration of the study, in comparison to controls. Furthermore, although differences did not reach statistical significance (P = .07), PWH randomized to high-intensity had a decrease in a key cardiovascular marker, hsCRP. Thus, overall, exercise did not appear to exacerbate inflammation among PWH compared to controls, and greater intensity and adherence to exercise may be associated with more beneficial changes in inflammation. Several of our findings merit further discussion, including the unexpected increase in sCD14 among both PWH and controls.
How do our inflammatory changes compare to that seen in other exercise interventions? As we have previously reviewed [21], both exercise interventions and regular physical activity are associated with generally beneficial effects on inflammation in the general population and among PWH. To our knowledge, no prior studies have compared the changes in inflammation with an exercise intervention between people with and without HIV to determine whether the inflammatory response differs by HIV serostatus, particularly among older adults with greater comorbid (and hence inflammatory) burden. Furthermore, many prior studies of exercise interventions in HIV were conducted in the early AIDS era, or targeted participants specifically with lipodystrophy. In the era of more contemporary antiretroviral therapy (ART), the effects of exercise are generally similar to our results, with differences likely attributable to differences in the intervention (cardiovascular vs resistance exercise), intensity, frequency, duration, and population (mean age all <50, men only vs men and women). Among men with HIV (n = 16), a short intervention (6 weeks) of twice-weekly cardiovascular exercise had no significant effect on IL-6 or sTNFR2 [22]. In contrast, 12 weeks of thrice-weekly resistance training only (n = 15 PWH) resulted in significant decreases in IL-6 and TNF-α and a significant increase in IL-10 [23]. Among men with HIV randomized to 16 weeks of resistance (n = 10) or cardiovascular exercise (n = 8), small but significant decreases in IL-6, hsCRP, and TNF-α were seen with cardiovascular but not resistance exercise [24]. Lastly, among PWH completing 12 weeks of cardiovascular with (n = 14) or without (n = 21) optional resistance training, the cardiovascular training group experienced significant decreases in IL-6, hsCRP, and D-dimer; cardiovascular with resistance training had significant declines in hsCRP [25]. Overall, these studies indicate that the responses to cardiovascular, resistance, or combined exercise vary widely by participant characteristics, and type and duration of exercise. The responses in our participants may also reflect an older, sedentary population with significant comorbid burden.
Adherence likely contributes to variability within and between studies. As we have shown, those with greater adherence to the intervention had a greater decrease in some key inflammatory markers (IL-6 and hsCRP). In a community-based intervention of PWH, participants were encouraged to attend at least 4 weekly sessions of supervised exercise: only 25% attended at least 2 sessions per week. Although physical function measures improved, hsCRP increased over the 3-month intervention [26]. Similarly, another intervention where only 55% of PWH completed the 16-week cardiovascular and resistance exercise, no significant changes were seen in IL-6, TNF-α, or IL-10, despite improvements in strength [27].
We observed a significant increase in IL-10 among controls, but a blunted increase among PWH. IL-10 is released during acute exercise and elicits anti-inflammatory effects; higher levels have been associated with cardioprotective effects, including among PWH [28]. IL-10 levels are typically greater among persons who regularly partake in physical activity [29, 30], and increases with exercise are seen in other studies of PWH [31]. Although our assessment of anti-inflammatory cytokines was limited to IL-10, our findings suggest that, among older PWH, additional stimulus or interventions may be needed to gain the same anti-inflammatory benefits seen among controls.
In contrast to our hypothesized effect of exercise, we found small but statistically significant increases in sCD14 among both PWH and controls. sCD14 is a marker of microbial translocation and monocyte activation that has been associated with mortality in the general population and among PWH, but relatively little is known of the impact of acute or chronic exercise on sCD14. Intense acute exercise can cause splanchnic hypoperfusion and significant increases in I-FABP [32, 33]. In contrast, routine physical activity may have a beneficial effect on the gut, increasing motility and altering the diversity and content of the microbiome [34]. In uninfected adults, both sCD14 and I-FABP increased significantly immediately following completion of a marathon; I-FABP returned to near normal levels after 1 hour, but sCD14 was not reassessed [35]. Neither a cycling intervention in chronic heart failure patients nor a walking intervention among PWH had significant effects on sCD14 [25, 36]. Lastly, among children with HIV, sCD14 decreased over a 2-year period among persons who routinely exercised versus those who did not exercise [29]. It is possible that sCD14 has a long half-life, and our measurements are capturing acute exercise effects rather than longer-term resting effects. The decrease in I-FABP among PWH and lack of exacerbation of other systemic inflammatory markers does not suggest a detrimental effect on barrier function. Future studies are needed to explore the effects of physical activity or exercise interventions on other markers of gut barrier function and changes in the microbiome among PWH.
Although our sample size was limited, we did find that PWH had significantly more intermediate monocytes and significantly fewer nonclassical monocytes compared to controls, and an increase in monocyte response to submaximal lipopolysaccharide (LPS) stimulation (CXCL-10 [IP-10] and TNF-α) after 12 weeks of moderate-intensity exercise. The increase in intermediate and nonclassical monocytes is in contrast to some findings in other exercise studies: nonclassical and intermediate monocytes were reduced with 10 weeks of high-intensity walking among 12 older adults with rheumatoid arthritis, as was expression of Toll-like receptor 2 (TLR2), TLR4, and HLA-DR [37]. Among 25 younger males, the percentage of classical monocytes decreased, intermediate and nonclassical monocytes increased, and TLR4 expression decreased on classical and intermediate monocytes immediately following an exercise session. Furthermore, LPS-stimulated cytokine production of IL-6 and IL-10 were both lower and TNF-α was higher after the exercise than prior [38]. Similar to the cytokine responses, differences from our study findings are likely explained by acute versus chronic responses, the intensity of the intervention, and the immune function of the hosts. The increase in monocyte response may be a unique effect with exercise among PWH. Among 16 older participants with heart failure, a messaging health intervention to reduce sedentary time was associated with a reduction in the percentage of total circulating monocytes and significant decrease in several cytokines (IL-6, TNF-α, IL-10, IL-8, and IL-1B) after LPS stimulation [39], but no change in monocyte distribution or cell surface expression of CCR2, CD14, or CD11b.
Strengths of our study included the randomized assignment of exercise intensity, high participant adherence to the intervention, measurement of acute and chronic inflammatory markers, an HIV-uninfected control group, and the relatively long duration of the intervention in a standardized, supervised setting. Several limitations should be acknowledged, including the wide variability in many of markers that occurred over the course of 24 weeks. Participants were typically not fasting due to the timing of blood draws with exercise sessions; this may have further contributed to variability. Changes in body composition with the intervention could also contribute to changes in inflammation and are discussed separately [40]. The monocyte studies were limited to a subset of participants and the first 12 weeks of the intervention (to avoid additional confounding by intensity). The cell stimulation studies did not isolate monocytes, so other cell populations may have contributed to the low-dose LPS response, albeit to a lesser extent.
In summary, moderate and high-intensity exercise elicited heterogenous effects among older adults with or without HIV. The attenuated increase in IL-10 among PWH may suggest an impairment in anti-inflammatory signaling and merits further study as to mechanisms, clinical implication, and alternative interventions, such as high-intensity interval training or statin therapy. With the growing population of older adults with HIV experiencing a concomitant increase in obesity and cardiovascular risk in the current era of ART, we have importantly shown that high-intensity exercise improves physical function [18] and does not globally exacerbate inflammation. Furthermore, higher-intensity exercise may add additional benefit in decreasing key markers of cardiovascular risk including hsCRP. Thus, consistent with the physical activity guidelines, providers should encourage older adults with HIV to partake in any physical activity, with the greatest benefits likely seen with higher-intensity exercise.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding sources had no role in data collection, analysis, or interpretation; trial design; or patient recruitment. No payments were made in the writing of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the Gilead Sciences Research Scholars Program in HIV (to K. M. E.); the National Institutes of Health (NIH) National Institute on Aging (grant numbers K23AG050260 to K. M. E. and T32 AG279-15 to J. L.); the NIH National Institute of Allergy and Infectious Diseases (grant numbers K24 AI120834 to T. T. B. and R01 AI108479 to E. J.); NIH National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award (grant number UL1TR002535); and Veterans Affairs Research Service (Merit Review grant number I01CX001464 to E. J.).
Potential conflicts of interest. K. M. E. has received funding to the University of Colorado from Gilead Sciences, and has consulted for ViiV and Gilead Sciences. T. B. C. and T. T. B. have served as consultants to Gilead Sciences, Merck, ViiV, and Theratechnologies.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Workshop on HIV and Aging, New York, NY, September 13 and 14, 2018.




