-
PDF
- Split View
-
Views
-
Cite
Cite
Beth Catlett, Sahar Bajis, Mitchell Starr, Gregory J Dore, Behzad Hajarizadeh, Philip H Cunningham, Tanya L Applegate, Jason Grebely, Evaluation of the Aptima HCV Quant Dx Assay for Hepatitis C Virus RNA Detection from Fingerstick Capillary Dried Blood Spot and Venepuncture-Collected Samples, The Journal of Infectious Diseases, Volume 223, Issue 5, 1 March 2021, Pages 818–826, https://doi.org/10.1093/infdis/jiaa442
Close - Share Icon Share
Abstract
Simplified diagnostic strategies are needed increase hepatitis C virus (HCV) testing to determine active infection and link people into treatment. Collection methods such as dried blood spots (DBS) have advantages over standard phlebotomy, especially within marginalized populations.
We evaluated the diagnostic performance of the Aptima HCV Quant assay for the quantification and detection of HCV RNA from paired DBS and venepuncture samples. Specimens were collected from participants enrolled in an Australian observational study. We compared HCV RNA detection from DBS against venepuncture samples (gold standard).
One hundred sixty-four participants had paired samples and HCV RNA was detected in 45 (27% [95% confidence interval, 21%–35%]) by the Aptima assay in venepuncture samples. Sensitivity of the Aptima assay for HCV RNA quantification from DBS (≥10 IU/mL in plasma) was 100% and specificity was 100%. Sensitivity for HCV RNA detection from DBS was 95.6% and specificity was 94.1%. A small bias in plasma over DBS was observed with good agreement (R2 = 0.96).
The Aptima HCV Quant assay detects active infection from DBS samples with acceptable diagnostic performance and is clinically comparable to plasma. These data will strengthen the case for the registration of a DBS kit insert claim, enabling future clinical utility.
Globally, 71 million people are living with hepatitis C virus (HCV) infection [1]. Despite the availability of effective direct-acting antivirals (DAAs), HCV testing and diagnosis remains low globally [2]. The World Health Organization’s (WHO) goal of eliminating HCV as a public health threat by 2030 includes increasing HCV diagnoses from <20% in 2015 to 90% and the proportion of eligible persons receiving HCV treatment from <10% in 2015 to 80% [3]. However, gaps remain in the availability of simple, reliable, and affordable testing strategies [4]. Simplified strategies to enhance testing can facilitate linkage to care and potentially increase DAA treatment uptake.
The current HCV testing paradigm dictates detection of HCV antibodies to diagnose previous exposure, with confirmatory HCV RNA or core antigen testing to confirm active infection. This 2-step diagnosis pathway requires multiple visits to a practitioner (and perhaps off-site phlebotomists), leading to considerable drop-off in the proportion of people who are HCV antibody positive and receive confirmatory HCV RNA testing [5–10]. Other barriers to testing include stigma and poor HCV knowledge and awareness about testing among providers and patients [11–13]. Difficult venous access is a major barrier to obtaining blood samples by venepuncture among some populations, including people who inject drugs (PWID) [14, 15], and a reason why some people may not present for testing [16, 17].
Dried blood spot (DBS) testing has been shown to enhance HCV testing [18–20] and linkage to care [21]. The benefits of fingerstick capillary DBS testing are that (1) capillary blood sampling avoids the need for phlebotomy, a major advantage where venous access is difficult (eg, in PWID) or where phlebotomy services are unavailable; (2) sample collection can be performed by peers or other community workers; (3) serological testing can be linked to reflex virologic testing for HCV RNA confirmation using additional spots from the same filter paper, thus enabling a definitive diagnosis without the need for the person to return for resampling; (4) DBS samples are stable once dried and easy to transport and store, thus providing a convenient and affordable sampling solution in resource-limited settings; and (5) DBS samples can be used for other purposes, such as HCV sequencing and testing for other viruses (eg, human immunodeficiency virus, hepatitis B virus).
The Aptima HCV Quant Dx (hereafter “Aptima”) assay allows for high throughput testing; is fully automated, sensitive, and specific; and provides reproducible quantification of HCV RNA in serum and plasma samples [22]. The Aptima assay is CE-marked and has received regulatory approval to detect and quantify HCV RNA in clinical practice. However, there are limited field-based studies evaluating HCV RNA with the Aptima assay from paired samples collected via DBS and venepuncture.
In this study, we aimed to evaluate the sensitivity and specificity of the Aptima assay for detection of HCV RNA by capillary-blood DBS samples collected by fingerstick compared with plasma samples collected by venepuncture in a field-base study in Australia.
MATERIALS AND METHODS
Study Participants
LiveRLife is an observational study evaluating the effectiveness of an intervention integrating noninvasive liver disease screening on HCV assessment and treatment uptake [23]. Participants were enrolled at 6 sites in Australia (4 drug treatment clinics, 1 homelessness service, and 1 needle syringe program) between 8 February and 1 December 2016. Inclusion criteria were age of 18 years or older and history of injecting drug use (participants recruited from the homelessness service were exempt from this criterion). Participants received an educational resource package at enrollment (eg, LiveRLife campaign coffee mug, liver health promotion and education booklet) and an AUD$20 voucher at study completion. All participants provided written informed consent before study procedures began. The study was approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee.
Procedures
Participants were provided information about the study while accessing services and were consecutively enrolled into the study. Each clinic site had 4 campaign days with typically 1 campaign day per week over 4 weeks. On each LiveRLife campaign day, participants provided informed consent to be enrolled in the study, provided fingerstick samples for DBS sample collection, completed a self-administered survey on tablet computer, received transient elastography assessment (by FibroScan), attended a clinical nurse visit for HCV assessment, and provided venepuncture blood samples (for standard-of-care clinical testing and storage for future research including HCV RNA testing).
DBS collection was performed by collecting a capillary whole-blood sample from participants via a fingerstick (Blue BD Microtainer contact-activated lancet [order number 366594], BD, Scoresby, Australia) using procedures recommended by WHO [24]. Once the finger was pricked, up to 5 full circles (estimated 70 µL of whole blood per circle) of a Protein Saver card (Munktell, TNF paper [order number 323046], Ahlstrom-Munksjö, Helsinki, Finland) were filled. DBS cards were air-dried overnight and stored in foil bags (Whatman Foil Barrier Reusable bags [order number 10534321]) containing 2 desiccant packs (1 g silica gel [order number SG23401], Desicco, Sydney, Australia). They were then stored at –80°C prior to testing.
For each DBS test, an eluate was prepared. This entailed manually punching a 1cm disk using a stainless steel stationary punch and the use of tweezers (both flame-sterilized for 5 seconds between samples in a Bunsen burner to prevent contamination) to drop the disk into 1 mL of sample transport media (Aptima Specimen Transfer Kit [order number 301154C] within an aliquot tube (specimen aliquot tube [order number 503762], Hologic, San Diego, California). Capped tubes (Transport Tube Cap [order number 504415], Hologic) were then rocked for 30 minutes at room temperature at a speed of 24 rpm (BioMixer [order number B3D1320], Labpro Scientific, Narambga, Australia), prior to centrifugation at 2000 rpm for 10 minutes.
Venepuncture was performed by clinically trained personnel at each site. Whole venous blood collected in an ethylenediaminetetraacetic acid–treated 6-mL primary tube (lavender top, BD Vacutainer [order number 13-680-61], Fisher Scientific, Waltham, Massachusetts), was stored and transported to the central biorepository laboratory (Kirby Institute, UNSW, Sydney, Australia) at ambient temperature and processed within 24 hours, by centrifugation for 10 minutes at 2000g at 4°C. The plasma was aliquoted into two 1-mL aliquots and stored in 2-mL cryovials (Nunc [order number 374513], Thermo Fisher, Scoresby, Australia) at –80°C prior to testing.
Study Assessments
Capillary DBS samples were quantified for HCV RNA by the Aptima assay performed on the Hologic Panther platform (Hologic) alongside paired plasma on the Aptima assay and Cepheid Xpert HCV Viral Load assay (hereafter “Xpert”; Cepheid, Sunnyvale, California) following the manufacturer’s instructions [25, 26]. Testing was performed at St Vincent’s Centre for Applied Medical Research, St Vincent’s Hospital, between 20 November and 4 December 2018. Run and sample validity was determined through onboard software analysis of the quality controls and calibrators. Both the Aptima and Xpert assays have a linear range of quantitation in plasma from the lower limit of quantitation (LOQ) 10 IU/mL (the limit of detection [LOD] in plasma is 3.9 IU/mL for Aptima and 4.0 IU/mL for Xpert) to upper LOQ 1 × 108 IU/mL. All samples that fell below 10 IU/mL were unquantifiable and were considered initially indeterminant and repeated once (if sample was available) using the same procedure described in the manufacturer’s instructions, which indicates repeat testing in plasma samples and local laboratory procedures for HCV testing from DBS samples. Where sample was not available, the original result was retained.
Statistical Analysis
Sensitivity and specificity of the Aptima assay in DBS testing compared with the Aptima assay and Xpert assay in plasma were determined at different thresholds (HCV RNA levels), including all quantifiable samples and detectable samples.
To ensure quantitative compatibility with plasma, due to the significantly lower input volume with DBS samples, we corrected HCV RNA results for hematocrit. Therefore, all post-run DBS HCV RNA results on the Aptima assay were multiplied by 25.97 [26]. This calculation was undertaken to correct for volumetric differences of plasma within whole blood and was based on the following assumptions: 45% hematocrit average in whole blood [27], 70 µL DBS volume, 1000 µL Aptima transfer media volume using the following equation: volume of Aptima transfer media (ATM) (µL) / [DBS volume (µL) × (1 – hematocrit average)] [26, 28]. We used log10 transformed values in IU/mL to represent HCV RNA levels (in paired DBS and plasma). For any samples that were detected but below the limit of quantification, a midpoint of 0.7 log10 (5 IU/mL) was chosen for analysis purposes.
Among samples that were detectable and quantifiable for HCV RNA in both plasma and DBS, the distribution of HCV RNA levels was assessed using a paired Wilcoxon signed-rank test. Correlation of HCV RNA levels between DBS and plasma samples was assessed among all HCV RNA detectable samples (in either plasma or DBS), using Pearson correlation coefficient analysis. Bias and agreement were ascertained using Bland–Altman analysis.
All analyses were performed using Stata 14.2 (StataCorp, College Station, Texas) and GraphPad Prism 6 (GraphPad, San Diego, California).
RESULTS
Participant Characteristics
Overall, 175 participants had available paired samples for HCV RNA testing from plasma and capillary DBS. Participants receiving HCV therapy were excluded from analyses (n = 11).
Among the 164 participants included, the median age was 46 (interquartile range [IQR], 36–52) years, 76% were male, 60% had a history of injecting drug use, 51% were currently receiving opioid agonist therapy, and 23% had significant liver fibrosis (F2–F4) (Table 1). HCV RNA was detected in 27% (45 of 164; 95% confidence interval [CI], 21%–35%]) of participants when using the Aptima HCV Quant Dx assay on plasma samples.
| Characteristic . | No. (%) . |
|---|---|
| Age, y, median (25th, 75th percentile) | 46 (36, 52) |
| Gender | |
| Male | 124 (76) |
| Female | 25 (15) |
| Transgender | 1 (1) |
| Unknownb | 14 (8) |
| History of ever injecting drugs | |
| No | 52 (32) |
| Yes | 98 (60) |
| Unknownb | 14 (8) |
| Injecting drug use in the last monthc | |
| No | 34 (35) |
| Yes | 63 (64) |
| Unknownb | 1 (1) |
| Frequency of drug use in the last monthc | |
| None | 21 (22) |
| Less than daily | 46 (47) |
| Daily or more | 17 (17) |
| Unknownb | 14 (14) |
| Opioid agonist therapyc | |
| No | 29 (30) |
| Yes, previously | 18 (18) |
| Yes, currently | 50 (51) |
| Unknownb | 1 (1) |
| FibroScan liver disease staged | |
| F0–F1 | 106 (65) |
| F2 | 22 (13) |
| F3 | 5 (3) |
| F4 | 11 (7) |
| Invalid score | 20 (12) |
| Characteristic . | No. (%) . |
|---|---|
| Age, y, median (25th, 75th percentile) | 46 (36, 52) |
| Gender | |
| Male | 124 (76) |
| Female | 25 (15) |
| Transgender | 1 (1) |
| Unknownb | 14 (8) |
| History of ever injecting drugs | |
| No | 52 (32) |
| Yes | 98 (60) |
| Unknownb | 14 (8) |
| Injecting drug use in the last monthc | |
| No | 34 (35) |
| Yes | 63 (64) |
| Unknownb | 1 (1) |
| Frequency of drug use in the last monthc | |
| None | 21 (22) |
| Less than daily | 46 (47) |
| Daily or more | 17 (17) |
| Unknownb | 14 (14) |
| Opioid agonist therapyc | |
| No | 29 (30) |
| Yes, previously | 18 (18) |
| Yes, currently | 50 (51) |
| Unknownb | 1 (1) |
| FibroScan liver disease staged | |
| F0–F1 | 106 (65) |
| F2 | 22 (13) |
| F3 | 5 (3) |
| F4 | 11 (7) |
| Invalid score | 20 (12) |
aEleven individuals receiving hepatitis C virus treatment at enrollment were excluded from the analysis.
bMissing due to loss of data during data transfer from tablet computer and 1 stolen tablet.
cAmong participants with a history of injection drug use (n = 98).
dF0–F1, absent or mild fibrosis; F2, significant fibrosis; F3, severe fibrosis; F4, cirrhosis.
| Characteristic . | No. (%) . |
|---|---|
| Age, y, median (25th, 75th percentile) | 46 (36, 52) |
| Gender | |
| Male | 124 (76) |
| Female | 25 (15) |
| Transgender | 1 (1) |
| Unknownb | 14 (8) |
| History of ever injecting drugs | |
| No | 52 (32) |
| Yes | 98 (60) |
| Unknownb | 14 (8) |
| Injecting drug use in the last monthc | |
| No | 34 (35) |
| Yes | 63 (64) |
| Unknownb | 1 (1) |
| Frequency of drug use in the last monthc | |
| None | 21 (22) |
| Less than daily | 46 (47) |
| Daily or more | 17 (17) |
| Unknownb | 14 (14) |
| Opioid agonist therapyc | |
| No | 29 (30) |
| Yes, previously | 18 (18) |
| Yes, currently | 50 (51) |
| Unknownb | 1 (1) |
| FibroScan liver disease staged | |
| F0–F1 | 106 (65) |
| F2 | 22 (13) |
| F3 | 5 (3) |
| F4 | 11 (7) |
| Invalid score | 20 (12) |
| Characteristic . | No. (%) . |
|---|---|
| Age, y, median (25th, 75th percentile) | 46 (36, 52) |
| Gender | |
| Male | 124 (76) |
| Female | 25 (15) |
| Transgender | 1 (1) |
| Unknownb | 14 (8) |
| History of ever injecting drugs | |
| No | 52 (32) |
| Yes | 98 (60) |
| Unknownb | 14 (8) |
| Injecting drug use in the last monthc | |
| No | 34 (35) |
| Yes | 63 (64) |
| Unknownb | 1 (1) |
| Frequency of drug use in the last monthc | |
| None | 21 (22) |
| Less than daily | 46 (47) |
| Daily or more | 17 (17) |
| Unknownb | 14 (14) |
| Opioid agonist therapyc | |
| No | 29 (30) |
| Yes, previously | 18 (18) |
| Yes, currently | 50 (51) |
| Unknownb | 1 (1) |
| FibroScan liver disease staged | |
| F0–F1 | 106 (65) |
| F2 | 22 (13) |
| F3 | 5 (3) |
| F4 | 11 (7) |
| Invalid score | 20 (12) |
aEleven individuals receiving hepatitis C virus treatment at enrollment were excluded from the analysis.
bMissing due to loss of data during data transfer from tablet computer and 1 stolen tablet.
cAmong participants with a history of injection drug use (n = 98).
dF0–F1, absent or mild fibrosis; F2, significant fibrosis; F3, severe fibrosis; F4, cirrhosis.
Repeat Testing
Among the 164 DBS samples tested using the Aptima assay, 23% (n = 38) were detectable but not quantifiable and were considered as indeterminate (below the limit of quantification). Among these 38 samples, 31 had available samples for retesting and were all undetectable. As such, the 7 samples with insufficient sample for retesting retained their original result of detected but unquantifiable for all subsequent analyses.
Sensitivity and Specificity of HCV RNA Quantification and Detection Using DBS Samples
Sensitivity of the Aptima assay for HCV RNA quantification in DBS testing (≥10 IU/mL) compared to plasma (Aptima and Xpert assays) was 100% (95% CI, 91.8%–100% and 91.6%–100%, respectively) (positive predictive value [PPV], 100% for both). For both comparisons, specificity was 100% (95% CI, 97%–100%) (negative predictive value [NPV], 100%) (Table 2).
Sensitivity and Specificity of Quantifiable Hepatitis C Virus RNA (≥10 IU/mL)
| . | . | Aptima Plasma (≥10 IU/mL) . | Total . | |
|---|---|---|---|---|
| Assay . | . | Quantified . | Unquantified . | . |
| Aptima DBS (≥10 IU/mL) | Quantified | 43 | 0 | 43 |
| Unquantified | 0 | 121 | 121 | |
| Total | 43 | 121 | 164 | |
| Xpert Plasma (≥10 IU/mL)a | Total | |||
| Quantified | Unquantified | |||
| Aptima DBS (≥10 IU/mL) | Quantified | 42 | 0 | 42 |
| Unquantified | 0 | 121 | 121 | |
| Total | 42 | 121 | 163 | |
| . | . | Aptima Plasma (≥10 IU/mL) . | Total . | |
|---|---|---|---|---|
| Assay . | . | Quantified . | Unquantified . | . |
| Aptima DBS (≥10 IU/mL) | Quantified | 43 | 0 | 43 |
| Unquantified | 0 | 121 | 121 | |
| Total | 43 | 121 | 164 | |
| Xpert Plasma (≥10 IU/mL)a | Total | |||
| Quantified | Unquantified | |||
| Aptima DBS (≥10 IU/mL) | Quantified | 42 | 0 | 42 |
| Unquantified | 0 | 121 | 121 | |
| Total | 42 | 121 | 163 | |
Abbreviation: DBS, dried blood spot.
aOne sample invalid on the Xpert Plasma cartridge.
Sensitivity and Specificity of Quantifiable Hepatitis C Virus RNA (≥10 IU/mL)
| . | . | Aptima Plasma (≥10 IU/mL) . | Total . | |
|---|---|---|---|---|
| Assay . | . | Quantified . | Unquantified . | . |
| Aptima DBS (≥10 IU/mL) | Quantified | 43 | 0 | 43 |
| Unquantified | 0 | 121 | 121 | |
| Total | 43 | 121 | 164 | |
| Xpert Plasma (≥10 IU/mL)a | Total | |||
| Quantified | Unquantified | |||
| Aptima DBS (≥10 IU/mL) | Quantified | 42 | 0 | 42 |
| Unquantified | 0 | 121 | 121 | |
| Total | 42 | 121 | 163 | |
| . | . | Aptima Plasma (≥10 IU/mL) . | Total . | |
|---|---|---|---|---|
| Assay . | . | Quantified . | Unquantified . | . |
| Aptima DBS (≥10 IU/mL) | Quantified | 43 | 0 | 43 |
| Unquantified | 0 | 121 | 121 | |
| Total | 43 | 121 | 164 | |
| Xpert Plasma (≥10 IU/mL)a | Total | |||
| Quantified | Unquantified | |||
| Aptima DBS (≥10 IU/mL) | Quantified | 42 | 0 | 42 |
| Unquantified | 0 | 121 | 121 | |
| Total | 42 | 121 | 163 | |
Abbreviation: DBS, dried blood spot.
aOne sample invalid on the Xpert Plasma cartridge.
Sensitivity for HCV detection in DBS testing compared to plasma (Aptima and Xpert assays) was 95.6% (95% CI, 84.9%–99.5% [PPV, 86%]) and 97.7% (95% CI, 87.7%–99.9% [PPV, 84%]), respectively. Specificity (obtained after retesting of indeterminant samples) was 94.1% (95% CI, 88.3%–97.6% [NPV, 98.3%]) and 93.3% (95% CI, 88.3%–97.3% [NPV, 99.2%]), respectively (Table 3).
| . | . | Aptima Plasma IU/mL . | Total . | |
|---|---|---|---|---|
| Assay . | . | Detected . | Undetected . | . |
| Aptima DBS IU/mL | Detected | 43 | 7a | 50 |
| Undetected | 2 | 112 | 114 | |
| Total | 45 | 119 | 164 | |
| Xpert Plasma IU/mL | Total | |||
| Detected | Undetected | |||
| Aptima DBS IU/mL | Detected | 42 | 8b | 50 |
| Undetected | 1 | 112 | 113 | |
| Total | 43 | 120 | 163 | |
| . | . | Aptima Plasma IU/mL . | Total . | |
|---|---|---|---|---|
| Assay . | . | Detected . | Undetected . | . |
| Aptima DBS IU/mL | Detected | 43 | 7a | 50 |
| Undetected | 2 | 112 | 114 | |
| Total | 45 | 119 | 164 | |
| Xpert Plasma IU/mL | Total | |||
| Detected | Undetected | |||
| Aptima DBS IU/mL | Detected | 42 | 8b | 50 |
| Undetected | 1 | 112 | 113 | |
| Total | 43 | 120 | 163 | |
Abbreviation: DBS, dried blood spot.
aSeven samples insufficient for retesting (<10 detected on DBS, not detected for plasma).
bSeven samples insufficient for retesting (<10 detected on DBS, not detected for plasma), 1 invalid.
| . | . | Aptima Plasma IU/mL . | Total . | |
|---|---|---|---|---|
| Assay . | . | Detected . | Undetected . | . |
| Aptima DBS IU/mL | Detected | 43 | 7a | 50 |
| Undetected | 2 | 112 | 114 | |
| Total | 45 | 119 | 164 | |
| Xpert Plasma IU/mL | Total | |||
| Detected | Undetected | |||
| Aptima DBS IU/mL | Detected | 42 | 8b | 50 |
| Undetected | 1 | 112 | 113 | |
| Total | 43 | 120 | 163 | |
| . | . | Aptima Plasma IU/mL . | Total . | |
|---|---|---|---|---|
| Assay . | . | Detected . | Undetected . | . |
| Aptima DBS IU/mL | Detected | 43 | 7a | 50 |
| Undetected | 2 | 112 | 114 | |
| Total | 45 | 119 | 164 | |
| Xpert Plasma IU/mL | Total | |||
| Detected | Undetected | |||
| Aptima DBS IU/mL | Detected | 42 | 8b | 50 |
| Undetected | 1 | 112 | 113 | |
| Total | 43 | 120 | 163 | |
Abbreviation: DBS, dried blood spot.
aSeven samples insufficient for retesting (<10 detected on DBS, not detected for plasma).
bSeven samples insufficient for retesting (<10 detected on DBS, not detected for plasma), 1 invalid.
Distribution of HCV RNA in the Paired Plasma and DBS Sample Population
The detection of HCV RNA using the Aptima assay in DBS testing (≥LOD [4.3 IU/mL]) was in agreement for 155 of 164 (94.5%) when compared to HCV RNA in plasma (112 undetectable, 43 detected), with 9 discrepant results (7 samples were detected ≤LOQ [10 IU/mL] in DBS but were not detected in plasma, and 2 samples were detected in plasma less than or equal to the LOQ, but not detected in DBS). The distribution of HCV RNA levels (detectable and quantifiable results) in paired plasma and capillary DBS samples using the Aptima assay is detailed in Figure 1. The median DBS HCV RNA was 5.83 (IQR, 5.2–6.2) log10 IU/mL, and median plasma HCV RNA was 6.49 (IQR, 5.8–6.78) log10 IU/mL from samples tested using the Aptima assay.
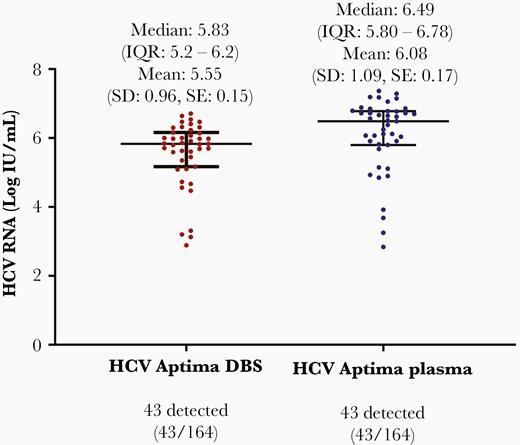
The distribution of paired plasma and capillary dried blood spot samples (among paired specimens with detectable and quantifiable hepatitis C virus RNA) (n = 43). P value <.00001. Abbreviations: DBS, dried blood spot; HCV, hepatitis C virus; IQR, interquartile range; SD, standard deviation; SE, standard error.
Bias and Agreement Measurements
Correlation between paired plasma and DBS tested on the Aptima assay (if HCV RNA was detected in either sample) (n = 52) was assessed using Pearson correlation coefficient analysis (Figure 2), which demonstrated a strong, positive correlation (R2 = 0.96 [95% CI, .97–.99]). Bland–Altman plot analysis (Figure 3) demonstrated a bias of +0.366 log10 between plasma and DBS HCV RNA concentrations (n = 52). The limits of agreement indicate that 95% of the difference between plasma and DBS on the Aptima assay is between –0.779 and 1.551 log10.
![Correlation analysis (among specimens with detectable hepatitis C virus [HCV] RNA in either plasma or dried blood spots [DBS]) (n = 52), R2 = 0.96 (95% confidence interval, .97–.99); the diagonal line represents identical results.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/223/5/10.1093_infdis_jiaa442/1/m_jiaa442f0002.jpeg?Expires=1750256060&Signature=YH7zAqi2p-kmKqZA6rgSBfFH7H-R5s3Ayq4~O63~Nz3Ozpu3Ke0V~UzL4uzgmc85pEAg8iI5ks44CVhFhvjVJghJaHq6SRfRd4MDn3q5YWy5Bkprrc49ic672dUkwI4FC5v2MXyIxP5fBk7tOWdeFzt-7GnOKY-Gm-sJf7rRuHMsDZQAQdIfF~81pmL48rnB8RhsSg7v2-1aV2OGlJkp6fgrmJMPGeOhNvZWhtEoYxFlgNaojV0XM2lAvq76ByCC8-BZLK55q~Uyp9NScjDXbVIh6-A6qjRYzZ16hJf2SZPxH6AgfCDZ05kAIiUqE9S9yYKDr-aVCBjG2Aq2KtJzlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation analysis (among specimens with detectable hepatitis C virus [HCV] RNA in either plasma or dried blood spots [DBS]) (n = 52), R2 = 0.96 (95% confidence interval, .97–.99); the diagonal line represents identical results.
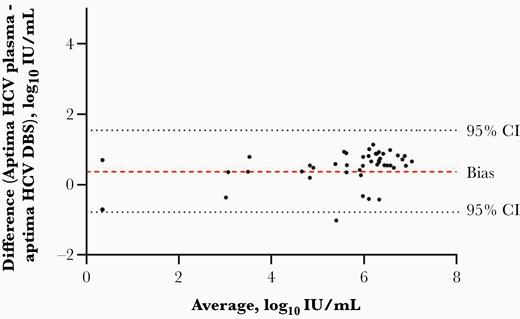
Bland–Altman analysis to show bias and agreement in detectable hepatitis C virus (HCV) RNA in either dried blood spots (DBS) or plasma specimens (n = 52); the dashed line represents bias (0.366 log10 IU/mL); the 2 dotted lines represent the 95% confidence interval (CI, –.779 to 1.551).
DISCUSSION
This study demonstrates that the Aptima HCV Quant Dx assay provides good sensitivity and specificity for HCV RNA quantification and detection in capillary whole blood collected by fingerstick DBS samples when compared with plasma collected by venepuncture in a real-world cohort of people attending drug health and homelessness services in Australia. This study provides important data to strengthen the case for registering DBS as a sample type for HCV RNA quantification and detection with the Aptima assay, allowing for enhanced clinical utility of DBS testing. These findings support further evaluation of HCV RNA detection by DBS collection as a strategy to improve and simplify testing and linkage to care.
Excellent sensitivity (100%) and specificity (100%) for quantifiable RNA (>10 IU/mL) was observed with the Aptima assay when comparing DBS testing to plasma (both Aptima and Xpert HCV assays), consistent with previous studies [26, 29]. However, previous studies were limited in using stored venous samples spotted onto DBS cards in the laboratory, not completely reflecting real-world utility. The major strength of this study is that DBS samples were collected via fingerstick from a cohort of people who inject drugs, providing greater certainty about the performance of this assay in the real world.
A decreased sensitivity (95.6% [Aptima], 97.7% [Xpert]) and specificity (94.1% [Aptima], 93.3% [Xpert]) for detection of HCV RNA in DBS (including samples that were detectable, but not quantifiable) was observed with the Aptima assay. These results were obtained following retesting of 31 samples that were classified as detected but unquantifiable (indeterminate). Aptima performance with DBS samples is consistent with other real-world assay studies, including Xpert (100% sensitivity, 90% specificity) [30], Norvatis dHCV Transcription-mediated Amplification assay (90% sensitivity, 100% specificity) [31], and Roche Cobas HCV viral load assay [32]. However, these assays were originally designed for venepuncture sample types, which may be too sensitive for HCV RNA DBS detection, given the lower plasma equivalency input volume from a DBS sample. Furthermore, the discrepant samples were detected but below the limit of quantification (<10 IU/mL) by the Aptima assay in DBS samples, but undetectable in plasma and therefore not likely to be clinically meaningful. Given that the sensitivity and specificity above the limit of quantification (>10 IU/mL) was 100%, the limit of quantification is probably a more clinically meaningful threshold compared to the LOD.
It is important to note that 23% (38 of 164; all undetectable in plasma) of DBS samples were indeterminant (HCV RNA detectable, but below the limit of quantification) when first tested and required repeat testing (31 of 38 had available samples for retesting and were all undetectable). It is possible that the false-positive results in DBS samples could have been caused by cross-contamination between DBS samples. However, it is likely that samples with cross-contamination would have much higher levels of HCV RNA detected from DBS samples. As such, we hypothesize that the indeterminant results are likely due to low-level noise below the LOQ due to artifacts in the assay. We do not think that the low-level noise was due to storage conditions, given that previous studies have demonstrated that DBS samples are stable when stored and retested at 1 year following collection (particularly when stored at –20° or –80°) [33, 34]. Also, many samples on retesting provided a valid result. As such, we hypothesize that the higher retesting frequency was due to the real-world collection of these samples. The clinical implications of these findings are that HCV RNA DBS samples that are detected but below the limit of quantitation need repeat testing. However, an adapted DBS-specific cutoff among quantified results would provide excellent sensitivity and specificity and remove indeterminant results.
There were some limitations in this study. First, the sample size was relatively small, although the CIs for the estimates of sensitivity and specificity are reasonable. As such, the smaller sample size did not preclude our ability to evaluate the performance of this assay for HCV RNA quantification. Furthermore, the distribution of HCV RNA concentration among this cohort was slightly higher than expected based on previous literature [35, 36], with a median viral load of 6.49 log10 IU/mL in plasma (5.83 log10 IU/mL in DBS) and the lowest quantifiable result was 3.25 log10 IU/mL. It should be noted that HCV RNA detected in DBS were approximately 0.7 log10 IU/mL lower than plasma and so quantitative results from DBS should be interpreted with caution. Therefore, this study was unable to fully characterize assay performance at the lower assay range, and future studies would benefit from determining the lower LOD and quantification in DBS; However, low viral load results (<4 log10) were reported in <5% among people with HCV chronic HCV infection in a real-world sample, so a less sensitive (>3 log10) assay that utilises DBS could be acceptable if this is a means to provide greater global access to testing [37]. Third, we did not perform genotyping as it was outside the scope of this study due to pan-genotypic DAA availability in Australia. Not all DBS samples considered indeterminant (n = 7) were able to be repeated due to low sample availability. We would recommend that any future testing allows for sample recollection in this scenario and that this is built into the testing algorithm, alongside repeating any samples that are detected but below the limit of quantification. Further, health economic analyses should be considered against setting to determine whether centralized DBS testing is a cost-effective approach compared to centralized and decentralized plasma testing.
Although this study provides new evidence of accurate HCV RNA detection from fingerstick capillary DBS samples on the HCV Aptima assay, DBS testing is not yet universally approved for the diagnosis of HCV. However, the WHO recommends DBS as an alternative sample type where phlebotomy is unavailable [38]. There is a public health need for simplified diagnostic strategies to improve testing and linkage to care. Off-site phlebotomy and lack of point-of-care diagnostic options have been highlighted by practitioners as major barriers to testing, causing delays in the receipt of results and reduced HCV RNA confirmation through loss to follow-up, leading to delayed or decreased treatment uptake [16, 17].
Evidence demonstrates that both DBS testing and point-of-care testing can improve testing uptake, awareness of infection, and linkage to treatment [18–20], especially in marginalized populations, including people who inject drugs [39–41]. DBS sampling may also be helpful in other settings where access to venepuncture or sample transport is difficult (in order to cold-chain ship to centralized laboratories for testing), such as regional/rural and lower- and middle-income countries [42, 43]. Prisons offer another opportunity for expanded DBS testing, given the considerable resource and logistical requirements to bring incarcerated persons into prison-based health clinics (eg, additional guards) [19, 44, 45]. For example, in the state of New South Wales, Australia, there has been a rapid uptake in HCV testing in prisons as part of a statewide assisted and self-collected DBS project [46, 47]. This is consistent with data from Scotland demonstrating a rise in HCV testing and treatment across a variety of settings, including prisons and drug treatment clinics, following the introduction of DBS testing [21, 48].
For DBS testing to be included in future HCV clinical testing, accelerated clinical evaluation and approval of diagnostic tests, including new sample types or collection methods for existing assays are needed. This can be challenging for diagnostic companies given the often-limited resources, access to populations, access to well-characterized samples, and multiple competing priorities [49]. WHO prequalification may be helpful in initiating in-country approval [49]. However, this can be costly and time and resource heavy, and should be considered on a case-to-case basis.
The approval pathway in many countries is unclear, and differences in funding, quality assurance and training programs may further hinder scale-up. These hurdles could be overcome through streamlined collaborations with other stakeholders (researchers, health economists, practitioners, government, policy makers, and the affected community), particularly with respect to supporting implementation and evaluation research to translate research outcomes and drive changes in health policy and practice. Results are eagerly awaited from one such initiative from the Foundation for Innovative New Diagnostics (FIND), which is performing a study from paired plasma and DBS samples from several countries to evaluate the performance of the Hologic Aptima, Roche Cobas, and Abbott RealTime assays for detection of HCV RNA from DBS [50]. Study results will be critical to inform WHO prequalification for the suitability of DBS testing and will assist the manufacturers in making product insert claims to support DBS testing globally.
In conclusion, this study demonstrated that active HCV infection from DBS samples can be determined with acceptable diagnostic performance and clinical comparability to plasma using the Aptima HCV Quant Dx assay. However, detectable results below the limit of quantification should be viewed with caution and repeated. These results from a real-world cohort will help strengthen the case for the registration of a DBS kit insert claim and certainly provide evidence of feasibility. Further work is required to evaluate the implementation of HCV RNA DBS testing in different populations and settings, including cost-effectiveness analyses and modeling to determine the potential impact of testing scale-up on HCV elimination efforts [51].
Notes
Acknowledgments. The authors thank the study participants for their contribution to the research, as well as researchers and staff who have participated in the project. The authors also acknowledge Hologic, including Karen Harrington, Nadika Atapattu, Philipp Mueller, Tiffany Clarke, and Michelle Blackwell. The evaluation of the Aptima HCV Dx Quant assay was undertaken at St Vincent’s Centre for Applied Medical Research. The authors gratefully acknowledge the pivotal role played by the following partner organizations and key stakeholders in study planning and implementation: New South Wales (NSW) Health; Hepatitis NSW; NSW Users and AIDS Association; and Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine. We also acknowledge the contributions of members of the LiveRLife Study Group: Coordinating Centre (Kirby Institute, University of New South Wales [UNSW] Sydney): Gregory J. Dore (principal investigator); Jason Grebely (co-investigator); Yasmin Mowat, David Silk, and Michelle Micallef (study coordinators); Mahshid Tamaddoni (data manager); Pip Marks (clinic trials coordinator); Tanya Applegate (co-investigator); Francois Lamoury and Indika Jayasinghe (laboratory technicians/support); Hannah Reid, Sahar Bajis, Alison Marshall, Marianne Martinello, Behzad Hajarizadeh, Evan B. Cunningham, Sofia Bartlett, and Brendan Jacka (participant enrollment). Site Principal Investigators: Michael Edwards (Campbelltown Drug Health Services, NSW); Carla Gorton (Cairns Sexual Health Service, Queensland [QLD]); Jeremy Hayllar (Metro North Hospital and Health Service, QLD); Victoria Cock (Drug and Alcohol Services of South Australia); and Julie Smith (Ozanam Learning Centre, NSW). Site Coordinators: Carina Burns (Campbelltown Drug Health Services, NSW); Rhondda Lewis (Cairns Sexual Health Service, QLD); Daniel Morris, Kathy Donohue, Astrid Carthew, and Nadine Horasak (Youthlink Needle and Syringe Program, QLD); Sue Shin (Metro North Hospital and Health Service, QLD); and Arlene Everson (Ozanam Learning Centre, NSW). The NSW Users and AIDS Association: Sara Adey (participant recruitment) and Cairns Hepatitis Action Team: Kathy Clark (participant recruitment).
Disclaimer. The sponsors had no role in the analysis and interpretation of the study results. The contents of the published material are solely the responsibility of the individual authors and not do not reflect the views of the National Health and Medical Research Council (NHMRC) or NSW Health. The Kirby Institute is funded by the Australian government’s Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian government.
Financial support. The research was supported by the Australian government’s Department of Health and Ageing and NSW Health through an NHMRC Partnership Project Grant (APP1103165); Hologic (provided Aptima HCV Dx assay reagents); NSW Health; and South Eastern Sydney Local Health District. B. C. is supported by an Australian Postgraduate Award from UNSW Sydney and an Australian Government Research Training Program Scholarship. G. J. D. is supported through an NHMRC Practitioner Fellowship. J. G. is supported through an NHMRC Career Development Fellowship. B. H. is supported by an NHMRC Early Career Fellowship.
Potential conflicts of interest. J. G. reports grants from Cepheid, during the conduct of the study; and grants from AbbVie, Cepheid, and Hologic and grants and personal fees from Gilead Sciences and Merck, outside the submitted work. G. J. D. reports grants from Cepheid, during the conduct of the study; grants, personal fees, and nonfinancial support from Gilead, AbbVie, Merck, Bristol-Myers Squibb, Janssen, and Roche; personal fees from GlaxoSmithKline and Abbott Diagnostics; and grants from Cepheid, outside the submitted work. T. L. A. reports grants and personal fees from Cepheid, during the conduct of the study; and grants from Abbott Diagnostics, outside the submitted work. All other authors report no potential conflicts of interest..
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Network on Hepatitis in Substance Users Eighth International Conference on Hepatitis Care in Substance Users, Montreal, Canada, 2019; and the Australasian Viral Hepatitis Elimination Conference, Sydney, Australia, 2019.
References
Author notes
T. L. A. and J. G. contributed equally to this work as joint senior authors.




