-
PDF
- Split View
-
Views
-
Cite
Cite
Augusto M Carvalho, Luiz H Guimarães, Rúbia Costa, Maíra G Saldanha, Iana Prates, Lucas P Carvalho, Sérgio Arruda, Edgar M Carvalho, Impaired Th1 Response Is Associated With Therapeutic Failure in Patients With Cutaneous Leishmaniasis Caused by Leishmania braziliensis, The Journal of Infectious Diseases, Volume 223, Issue 3, 1 February 2021, Pages 527–535, https://doi.org/10.1093/infdis/jiaa374
Close - Share Icon Share
Abstract
Leishmania skin test (LST) evaluates the delayed type hypersensitivity to Leishmania antigens (LA) and has been used for diagnosis of cutaneous leishmaniasis (CL). In CL patients LST is usually positive but a small percentage have negative LST. The aim of this study was to determine the clinical and immunologic features and response to antimony therapy in LST-negative CL patients.
We compare the clinical presentation, response to therapy, and immune response of CL patients with negative vs positive LST.
The clinical presentation was similar in both groups but LST-negative patients had a lower cure rate. In the lesions, LST-negative patients displayed less inflammation and necrosis, and higher frequency of CD8+ T cells. Mononuclear cells from LST-negative patients had a poor T helper 1 cell (Th1) response but levels of interleukin-1β (IL-1β), IL-6, IL-17, granzyme B, and metalloproteinase-9 (MMP-9) were similar to the LST-positive group upon stimulation with LA. Leishmania internalization and killing by macrophages were similar in both groups. Cure of disease was associated with restoration of Th1 response.
In LST-negative patients, impaired Th1 response is associated with therapeutic failure. Increased frequency of CD8+ T cells and high production of inflammatory cytokines, granzyme B, and MMP-9 contributes to immunopathology.
Leishmaniasis is a neglected disease caused by intracellular parasites of genus Leishmania that are transmitted to the human host by infected female sand flies. Leishmania parasites replicate inside macrophages in vertebrate host skin and infection control is based on cell-mediated immunity, mainly through macrophage activation by interferon-γ (IFN-γ) [1, 2]. The T helper 1 cell (Th1) response is important to decrease parasite multiplication but a nonmodulated inflammatory response may lead to tissue damage, as observed in cutaneous leishmaniasis (CL) caused by Leishmania braziliensis [2].
Peripheral blood mononuclear cells (PBMCs) from CL patients secrete high levels of IFN-γ and tumor necrosis factor (TNF) but it is not sufficient to eradicate Leishmania infection [3, 4]. Furthermore, pathology in L. braziliensis infection has been strongly linked with innate cells and CD8+ cytotoxic T cells. The inflammatory monocytes are enhanced in CL and are an important source of IL-1β and TNF [5, 6]. Additionally, there is a direct correlation between the frequency of macrophages and areas of necrosis in L. braziliensis lesions [7]. CD8+ T cells have a predominant cytotoxic profile in CL patients and there is a positive correlation between CD8+ T cells expressing granzyme B and the inflammatory reaction in CL lesions [8].
The Leishmania skin test (LST) produced with soluble Leishmania antigen (SLA) has a high sensitivity and high predict value for diagnosis of tegumentary leishmaniasis (TL) caused by L. braziliensis and has also been used to identify subjects exposed to Leishmania but who do not develop disease [9–11]. The LST is a delayed-type hypersensitivity reaction and is associated with lymphocyte proliferation and IFN-γ production in peripheral blood cells stimulated with SLA [12]. A negative LST during L. amazonensis infection is associated with an impaired T-cell response, parasite replication, and nodular lesions with macrophages full of parasites, as observed in diffuse cutaneous leishmaniasis [13, 14]. Furthermore, a negative LST is also observed in visceral leishmaniasis (VL), a disease associated with parasite dissemination [15, 16].
The village of Corte de Pedra is recognized as one of the most important areas of L. braziliensis transmission in Latin America and more than 1000 cases of CL are seen annually at the Corte de Pedra Health Post. Meglumine antimoniate is the drug recommended by the Brazilian government. While the LST is positive in over 97% of these patients, those with typical CL ulcers may have a negative LST. Here, we compare the clinical presentation, response to therapy, and immune response of CL patients who are LST negative with those who are LST positive. We found an impairment on the production of Th1 cytokines and a high rate of treatment failure in LST-negative patients. However, these patients produced similar levels of interleukin-1β (IL-1β), IL-6, IL-17, granzyme B, and matrix metalloproteinase-9 (MMP-9) and had more CD8+ T cells in the lesions than LST-positive patients.
METHODS
Area of Study and Selection of Individuals
This study was conducted in Corte de Pedra, Bahia, Brazil, an area well known to have high L. braziliensis transmission rates. Participants were 18 CL patients with negative LST and 18 with positive LST. The diagnosis of CL was made based on the presence of a typical skin ulcer and documentation of L. braziliensis DNA identification by polymerase chain reaction (PCR) in lesions biopsies [17]. Human immunodeficiency virus (HIV) tests were negative. All patients were treated with meglumine antimoniate (Glucantime; Sanofi Aventis) 20 mg/kg/day for 20 days. Patients were evaluated every 30 days for response to therapy and the final cure rate was assessed 90 days after initiation of therapy. Cure was defined as complete reepithelialization of the lesions, without raised borders. If cure was not achieved, patients received a second course of Meglumine antimoniate (SBV) for 30 days. If cure was not achieved after the second course, patients were treated with amphotericin B. This study was approved by the Ethics Committee of the Federal University of Bahia Medical School and informed consent was obtained from all participants.
Antigen and Intradermal Leishmania Skin Test
SLA was prepared as previously described [18]. For the LST, 25 μg SLA in 0.1 mL was inoculated in the forearm and induration was determined 48 hours post inoculation. A positive LST was considered when the induration was equal to or greater than 5 mm.
Biopsies and Immunohistochemistry
Twenty-five skin biopsies (12 LST-positive, 13 LST-negative) were obtained from the borders of lesions, using a 4-mm diameter punch, after the application of local anesthetic. Skin tissues were fixed in buffered formaldehyde and embedded in paraffin blocks. Deparaffinization and rehydration of 5-μm thick sections were performed using xylene and absolute alcohol, and antigen retrieval was performed in citrate buffer pH 9.0 at 96°C for 20 minutes. Immunohistochemistry reactions were performed as previously described [7]. Briefly, after blockage of peroxidase activity with 3% hydrogen peroxide for 10 minutes and proteins with Protein Block Serum-Free (DAKO) for 15 minutes, the slides were incubated overnight at 4°C with monoclonal mouse anti-CD20, anti-CD68, anti-CD56, anti-CD4, anti-CD8 (DAKO) and anti-Leishmania (in house). Mouse and rabbit peroxidase kit/horseradish peroxidase (Diagnostic BioSystems) were used to perform the detection according to the manufacturer’s recommendations.
Quantitative Analysis
The quantification of the cells was performed using an optical microscope, selecting 5 random fields of each section with the respective antibodies and magnification ×40. In each field the number of positive cells identified by brown coloration produced by reaction with the chromogenic substrate was quantified.
Morphometry of Inflammation and Necrosis Areas
The histological sections stained with hematoxylin and eosin were scanned with an optical microscope. The total area of the sections as well as the areas of inflammatory infiltrate and necrosis were measured by Image J 1.48v (National Institutes of Health). The total length of the biopsy fragment and the sum of the areas of inflammation and necrosis are shown in mm2. The percentage of inflammation and necrosis in the biopsies were calculated by dividing total area of inflammation and necrosis in mm2 by the total area of the biopsy fragment multiplied by 100.
Cell Culture and ELISA for Cytokines, Metalloproteinase-9, and Granzyme B
PBMCs were obtained from heparinized venous blood layered over a Ficoll Hypaque gradient (GE Healthcare). Cells were washed and resuspended in RPMI 1640 medium (GIBCO BRL) supplemented with 10% human AB serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin (all Invitrogen). Cells (3 × 106/mL) were plated in 24-well plates and stimulated with SLA (5 µg/mL) for 72 hours at 37°C and 5% CO2. Cytokine levels in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems). Recombinant IL-12 (10–20 ng/mL; R&D Systems) was used for IFN-γ restoration assay. Cytokine production was evaluated in all 36 patients of the study before therapy and in 14 patients after cure of the disease.
In Vitro Leishmania Killing Assay
PBMCs (3 × 106) were plated onto 4-well Lab-Tek chamber slides (Thermo Scientific) and incubated for 1 hour at 37°C and 5% CO2. Nonadherent cells (autologous T cells) were removed and frozen; adherent cells were cultured for another 6 days. Macrophages were infected with stationary-phase L. braziliensis (5 parasites per 1 macrophage) for 2 hours at 37°C and 5% CO2. Noninternalized parasites were removed and infected macrophages were further cultured alone, with autologous lymphocytes (2 lymphocytes per 1 macrophage), or in the presence of autologous lymphocytes plus recombinant IFN-γ (10 ng/mL; R&D Systems). After 2, 48, and 72 hours, slides were washed, stained with hematoxylin-eosin, and analyzed by light microscopy for determination of the percentage of infected cells and the number of amastigotes per 100 cells.
Statistical Analysis
Categorical data were compared using the Fisher exact test. The Kaplan-Meier method and log-rank test were used for the analysis of healing time. Receiver operator characteristics (ROC) curve analysis was used to evaluate the ability of cytokines levels to distinguish LST-positive from LST-negative patients. Comparisons between 2 groups were performed by Mann-Whitney test and comparisons among 3 or more groups by Kruskal-Wallis test followed by Dunn multiple comparison tests. Unpaired Student t test was used to compare difference in immunohistochemistry. The Wilcoxon paired test was used to assess differences between variables in the same subjects. Analyses were conducted using GraphPad Prism version 8.0 for Windows, and differences were considered significant at P < .05.
RESULTS
Demographic and Epidemiologic Features of Patients with Positive or Negative LST
The natural history of CL is characterized by lymphadenopathy as a first sign, followed by a nonulcerated papular lesion, and finally, after 2 weeks, the appearance of classical well-limited ulcer with raised borders [19, 20]. All patients with a negative LST displayed a classical CL lesion and there was no significant difference between individuals who were LST-positive and LST-negative regarding demographic and clinical features, as shown in Table 1. However, while cure at day 90 was 66.6% in the control group, it was 27.7% in the LST-negative patients (P < .05, Fisher exact test). Ulcers healed slower in the LST-negative patients than in the LST-positive group (P < .01, Mann-Whitney test). Indeed, a Kaplan-Meier curve showed that the proportion of patients who remained with an active ulcer was significantly greater in the LST-negative group than in the LST-positive group (P < .01, log-rank test; Figure 1).
Demographic and Clinical Characteristics of Leishmania skin test (LST)-Positive and LST-Negative Subjects With Cutaneous Leishmaniasis
| Variables . | LST Positive (n = 18) . | LST Negative (n = 18) . | P Value . |
|---|---|---|---|
| Age, y median (range) | 33 (21–66) | 32.5 (15–65) | .94 |
| Male, No. (%) | 12 (66.6) | 11 (61.1) | .99 |
| Illness duration, d median (range) | 45 (28–75) | 52.5 (20–120) | .58 |
| Lymphadenopathy, No. (%) | 16 (88.8) | 15 (83.3) | .99 |
| Lesion site below the waist, No. (%) | 14 (77.7) | 12 (66.6) | .71 |
| Lesion size, mm2 median (range) | 162 (23.6–392.5) | 292 (19.6–3879) | .14 |
| Number of lesions | 1 (1–4) | 1 (1–9) | .059 |
| Cure on day 90, No. (%) | 12 (66.6) | 5 (27.7) | .043 |
| Healing time, d median (range) | 51.5 (20–140) | 120 (35–485) | .0038 |
| Variables . | LST Positive (n = 18) . | LST Negative (n = 18) . | P Value . |
|---|---|---|---|
| Age, y median (range) | 33 (21–66) | 32.5 (15–65) | .94 |
| Male, No. (%) | 12 (66.6) | 11 (61.1) | .99 |
| Illness duration, d median (range) | 45 (28–75) | 52.5 (20–120) | .58 |
| Lymphadenopathy, No. (%) | 16 (88.8) | 15 (83.3) | .99 |
| Lesion site below the waist, No. (%) | 14 (77.7) | 12 (66.6) | .71 |
| Lesion size, mm2 median (range) | 162 (23.6–392.5) | 292 (19.6–3879) | .14 |
| Number of lesions | 1 (1–4) | 1 (1–9) | .059 |
| Cure on day 90, No. (%) | 12 (66.6) | 5 (27.7) | .043 |
| Healing time, d median (range) | 51.5 (20–140) | 120 (35–485) | .0038 |
Demographic and Clinical Characteristics of Leishmania skin test (LST)-Positive and LST-Negative Subjects With Cutaneous Leishmaniasis
| Variables . | LST Positive (n = 18) . | LST Negative (n = 18) . | P Value . |
|---|---|---|---|
| Age, y median (range) | 33 (21–66) | 32.5 (15–65) | .94 |
| Male, No. (%) | 12 (66.6) | 11 (61.1) | .99 |
| Illness duration, d median (range) | 45 (28–75) | 52.5 (20–120) | .58 |
| Lymphadenopathy, No. (%) | 16 (88.8) | 15 (83.3) | .99 |
| Lesion site below the waist, No. (%) | 14 (77.7) | 12 (66.6) | .71 |
| Lesion size, mm2 median (range) | 162 (23.6–392.5) | 292 (19.6–3879) | .14 |
| Number of lesions | 1 (1–4) | 1 (1–9) | .059 |
| Cure on day 90, No. (%) | 12 (66.6) | 5 (27.7) | .043 |
| Healing time, d median (range) | 51.5 (20–140) | 120 (35–485) | .0038 |
| Variables . | LST Positive (n = 18) . | LST Negative (n = 18) . | P Value . |
|---|---|---|---|
| Age, y median (range) | 33 (21–66) | 32.5 (15–65) | .94 |
| Male, No. (%) | 12 (66.6) | 11 (61.1) | .99 |
| Illness duration, d median (range) | 45 (28–75) | 52.5 (20–120) | .58 |
| Lymphadenopathy, No. (%) | 16 (88.8) | 15 (83.3) | .99 |
| Lesion site below the waist, No. (%) | 14 (77.7) | 12 (66.6) | .71 |
| Lesion size, mm2 median (range) | 162 (23.6–392.5) | 292 (19.6–3879) | .14 |
| Number of lesions | 1 (1–4) | 1 (1–9) | .059 |
| Cure on day 90, No. (%) | 12 (66.6) | 5 (27.7) | .043 |
| Healing time, d median (range) | 51.5 (20–140) | 120 (35–485) | .0038 |
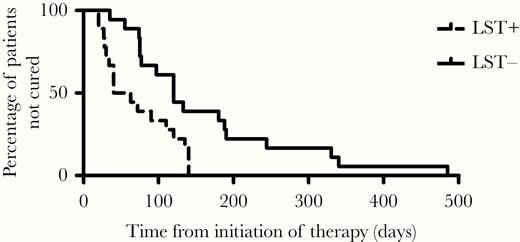
Kaplan-Meier estimates of the proportion of patients not cured in Leishmania skin test (LST)-positive and LST-negative subjects with cutaneous leishmaniasis. LST-positive (n = 18) and LST-negative (n = 18) patients were treated with meglumine antimoniate 20 mg/kg/day for 20 days. P = .0033 by log-rank test and P = .0037 by Wilcoxon test.
Inflammatory Cell Profile of Lesions From CL Patients With Positive or Negative LST
The extension of the inflammatory reaction and the tissue necrosis areas are shown in Figure 2A–C, and the composition of cell infiltrate by immunohistochemistry is shown in Figure 3A and 3B. The area of inflammatory reaction (29.6 vs 41.6, P < .001) and the focus of necrosis (1.2 vs 6.5, P < .001) were lower in biopsies from LST-negative vs LST-positive patients, respectively (Figure 2C). There was no difference in the numbers of CD4+ cells, NK cells, macrophages, B cells, and the number of amastigotes between lesions from LST-positive and LST-negative patients. However, the number of CD8+ cells was significantly higher in biopsies from LST-negative compared to LST-positive patients (5.8 vs 18.7; P ≤ .001; Figure 3A and 3B).
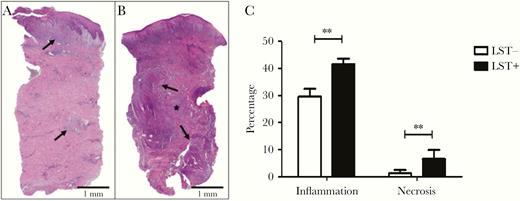
Areas of inflammation and necrosis in biopsies from Leishmania skin test (LST)-negative and LST-positive patients. Histological sections of (A) LST-negative and (B) LST-positive biopsies (hematoxylin and eosin, × 40). Area of inflammation (arrows/purple) and area of necrosis (*) are indicated. C, Percentage of area of inflammation and necrosis in biopsies from LST-negative and LST-positive patients. Statistical analysis was performed using unpaired Student t test; n = 25; ** P < .001. Bars represent mean and standard error.
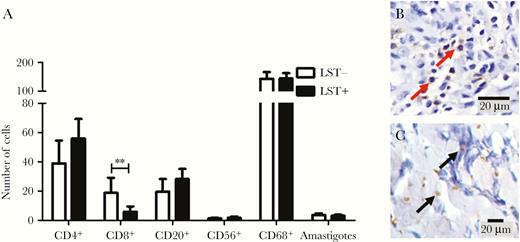
Inflammatory cells and amastigotes in biopsies from Leishmania skin test (LST)-negative and LST-positive patients. A, Number of T lymphocytes (CD4+, CD8+), B cells (CD20+), NK cells (CD56+), macrophages (CD68+), and amastigotes. Statistical analysis was performed using unpaired Student t test; ** P < .001. Bars represent mean and standard error; n = 25. B, CD8+ T cells (red arrows) stained (×40) and (C) Leishmania amastigotes (black arrows; 100×). Anti-CD20, anti-CD68, anti-CD56, anti-CD4, anti-CD8 (DAKO) and anti-Leishmania (in house). Mouse and rabbit peroxidase kit/horseradish peroxidase (Diagnostic BioSystems) were used to perform the detection.
Production of Cytokines, Metalloproteinase-9, and Granzyme B in CL Patients With Positive or Negative LST
The levels of IFN-γ, TNF, and IL-10 were lower in LST-negative than in LST-positive patients (Figure 4A–C). Moreover, in 7 (38%) LST-negative patients IFN-γ levels were not detectable in supernatants of PBMC cultures stimulated with SLA. However, there was no difference in IL-17, IL-1β, IL-6, MMP-9, or granzyme B levels (Figure 4D and Supplementary Figure 1). ROC analysis confirmed that the levels of IFN-γ and TNF distinguished LST-negative from LST-positive patients with high accuracy (Supplementary Figure 2). To determine if the impairment in IFN-γ could be restored by blocking IL-10, exogenous monoclonal antibody anti-IL-10 was added to cultures stimulated with SLA from 7 patients but there was no increase in IFN-γ production (data not shown). However, addition of recombinant IL-12 did restore IFN-γ production in PBMC cultures (Supplementary Figure 3).
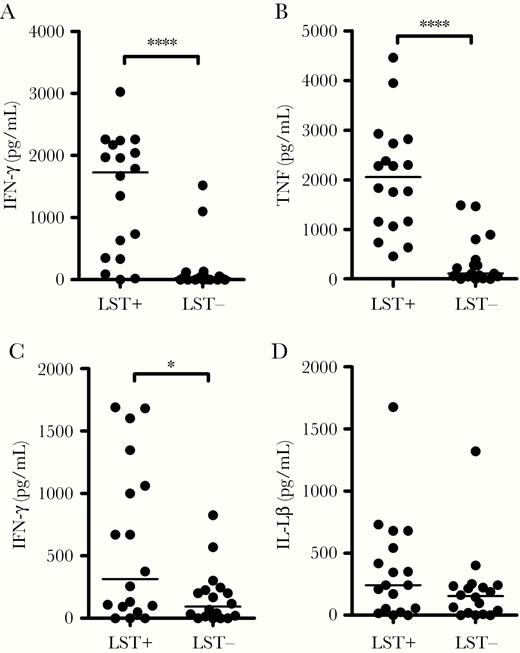
Production of cytokines in LST-positive and LST-negative subjects with cutaneous leishmaniasis. PBMCs from 18 LST-positive and 18 LST-negative CL patients were cultured in the presence of SLA (5 µg/mL) for 72 hours. Cytokine levels in culture supernatants were measured by ELISA: (A) IFN-γ, (B) TNF, (C) IL-10, and (D) IL-1β. Central lines represent median values. Statistical analysis was performed using Mann-Whitney test. *P < .05, ****P < .0001. Abbreviations: CL, cutaneous leishmaniasis; ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon-γ; IL, interleukin; LST, Leishmania skin test; PBMC, peripheral blood mononuclear cell; SLA, soluble Leishmania antigen; TNF, tumor necrosis factor.
Infection Rate Upon Coculture of Autologous Lymphocytes and L. braziliensis–Infected Macrophages
We next asked if macrophages from LST-negative patients were more susceptible to L. braziliensis infection and if coculture of autologous lymphocytes could modify the L. braziliensis infection rate in vitro. Macrophages obtained from LST-negative and LST-positive patients were infected with L. braziliensis. The frequency of infected cells and the number of parasites were similar at different periods of time in LST-positive and LST-negative patients. A “periods of time” means the time of cell culture (2, 48 and 72 hours), and no difference was observed in macrophage infection. However, we observed difference after addition of Lymphocytes in the culture (Figure 5A and 5B), killing of L. braziliensis was not observed in macrophages cocultured with autologous lymphocytes from LST-negative patients (Figure 5C and 5D). This impairment of macrophages from LST-negative patients in their ability to kill Leishmania when cocultured with autologous lymphocytes was restored with addition of IFN-γ. The decreased ability of macrophages from LST negative patients to produce IFN-γ was confirmed by measurement of IFN-γ levels by ELISA in coculture supernatants (Supplementary Figure 4).
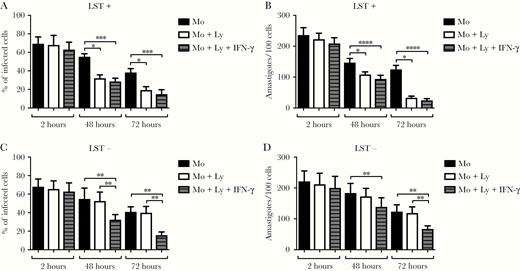
Infection rate of Leishmania braziliensis-infected macrophages after coculture with autologous lymphocytes. Macrophages from 8 CL patients with negative LST and 8 CL patients with positive LST were infected with L. braziliensis promastigotes. Cells were cultured alone, cocultured with autologous lymphocytes, or with lymphocytes plus recombinant IFN-γ. After 2, 48, and 72 hours, glass coverslips were stained with hematoxylin and eosin and assessed by light microscopy for the percentage of infected macrophages (A and C) and the number of amastigotes per 100 macrophages (B and D). Statistical analysis was performed using the Kruskal-Wallis test followed by Dunn multiple comparison tests. *P < .05, **P < .01, ***P < .0005, ****P < .0001. Abbreviations: CL, cutaneous leishmaniasis; IFN-γ, interferon-γ; LST, Leishmania skin test; Ly, lymphocytes; Mo, macrophages.
Restoration of Th1 Immune Response After Therapy in CL Patients With Negative LST
To determine whether therapy could restore in vitro Th1 response, the LST and cytokine production was determined after clinical cure in LST-negative patients. The evaluation of the LST post therapy was performed from 3 to 40 months after the initial evaluation. A positive LST was documented in 11 (78.6%) of the 14 patients tested. Production of IFN-γ, TNF, IL-10, and IL-1β before and after therapy is shown in Figure 6. While IFN-γ and TNF increased after therapy in the majority of the patients, no change in IL-10 and IL-1β levels were observed.
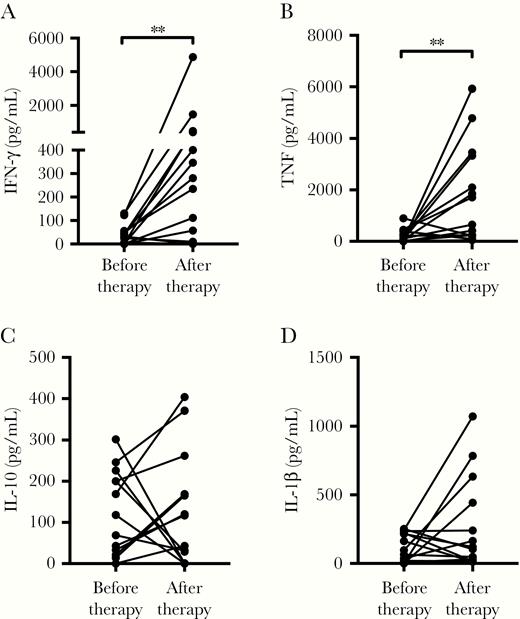
Restoration of Th1 immune response in CL patients with negative LST after therapy. PBMCs from 14 LST-negative CL patients were cultured in the presence of soluble Leishmania antigen (5 µg/mL) for 72 hours. Cytokine levels in culture supernatants were evaluated before and after therapy (3 to 40 months) by ELISA: (A) IFN-γ, (B) TNF, (C) IL-10, and (D) IL-1β. Statistical analysis was performed using the Wilcoxon paired test. **P < .01. Abbreviations: CL, cutaneous leishmaniasis; ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon-γ; IL, interleukin; LST, Leishmania skin test; PBMC, peripheral blood mononuclear cell; Th1, T helper 1 cell; TNF, tumor necrosis factor.
DISCUSSION
The main factors associated with failure of therapy in CL are short duration of illness, number and size of the lesions, and high parasite burden [21–23]. An absence of Th1 immune response, as observed in patients coinfected with HIV, is also associated with failure of therapy [24]. However, CL patients in the early phase of the disease, before ulcer appearance, have a strong proinflammatory response and also present a high rate of failure to cure with SBV [22]. Thus, the relationship between immune response and the pathology of CL, as well as severity of the disease and response to therapy, still need to be clarified. Here, we show that patients with CL due to L. braziliensis and with a negative LST had a low rate of response to therapy. They had a poor production of IFN-γ and TNF upon lymphocyte stimulation with SLA but produced high levels of proinflammatory cytokines IL-1β, IL-6, and IL-17, as well as granzyme B and MMP-9. At the lesion site there was no increase in the number of parasites but less inflammation, necrosis, and higher frequency of CD8+ T cells. In vitro macrophages from LST-negative patients were infected with L. braziliensis similar to cells of LST-positive patients and were able to kill Leishmania after stimulation with IFN-γ. Moreover, an enhancement in IFN-γ production occurred after in vitro addition of IL-12 and the poor Th1 immune response observed before therapy was restored after cure.
The diagnosis of CL should be performed by identification of amastigotes in the lesion or identification of DNA of L. braziliensis in tissue biopsied from the ulcer. Nevertheless, in endemic areas of L. braziliensis, due to the lack of facilities and high cost of these tests, the LST is used to screen patients suspicious for TL and for diagnosis of CL in a patient who has a typical CL ulcer. In areas of L. major transmission, a positive LST is associated with control of the progression of the infection to disease [25]. This association is also observed in endemic areas of L. infantum as subjects with asymptomatic infection have a positive LST and produce IFN-γ in vitro [26]. However, in subjects with asymptomatic L. braziliensis infection the association of a positive LST with protection is not so clear. Actually, the correlation between IFN-γ production and a positive LST is weak in these individuals and protection against CL is linked to the ability to produce IFN-γ rather than a positive LST [27].
The therapeutic response to SbV is quite variable depending upon the species of Leishmania and the region where the study was performed. Patients with CL due to L. guyanensis or L. panamensis have a higher cure rate to SBV than those infected with L. braziliensis [21, 28]. Some patients with disseminated leishmaniasis (DL) caused by L. braziliensis and CL patients coinfected HIV have a negative LST and high treatment failure [24, 29]. DL patients present with up to thousands of acneiforms, and papular and ulcerated lesions, and patients infected with HIV usually present with large or multiple ulcers [24, 30]. In our patients, a negative LST did not modify the clinical presentation of CL. The cutaneous ulcers were similar to that observed in LST-positive patients and the number of lesions did not differ between the 2 groups. However, LST-negative patients had a high rate of treatment failure and the healing time was higher than in those with a positive LST. Usually CL patients who fail to the first course, cure after a second course of SbV [31], but here 3 LST-negative patients needed to receive amphotericin B to achieve cure. This indicates that IFN-γ is required for optimal response to SbV in CL treatment, as less than 30% of LST-negative patients achieved cure after 1 course of SbV therapy. Indeed, the combination of SbV and IFN-γ has been used successfully in treating VL patients refractory to SBV [32].
The histopathologic analysis of patients with CL shows an intense inflammatory reaction mediated by lymphocytes and macrophages with focus of necrosis and paucity of parasites [33]. Biopsies from LST-negative patients have a lower inflammatory reaction and less focus of necrosis than LST-positive patients, but otherwise the histopathology was qualitatively very similar, except for an increase in the frequency of CD8+ T cells. In LST-negative patients, despite the poor IFN-γ production, the number of amastigotes was similar to that observed in patients with a positive LST. Although the use of more sensitive techniques, such as quantification of L. braziliensis DNA, may show different results, we cannot rule out the possibility that the innate immune response mediated by NK cells is controlling parasite multiplication in LST-negative patients.
The pathology in CL has been associated with an exaggerated inflammatory response and tissue damage mediated by macrophages and T cells. The observation here that the clinical aspect of the ulcer in LST-negative patients was similar to that observed in LST-positive patients, even with an impairment in IFN-γ production, argues against the role of CD4+ Th1 cells in the pathology of L. braziliensis. Studies in mice and humans have demonstrated the participation of CD8+ T cells in the pathology of L. braziliensis infection. CD8+ T cells from CL patients express less IFN-γ and have higher cytotoxic activity than cells from subjects with asymptomatic L. braziliensis infection [34]. Additionally, while CD4+ Th1 cells are associated with protection, CD8+ T cells kill L. braziliensis-infected cells and are associated with pathology [35, 36]. In mice infected with L. braziliensis, CD8+ T cells are also associated with severity of the disease [37]. It has been proposed that killing of Leishmania-infected cells by CD8+ T cells leads to the release of damage-associated molecular patterns (DAMPs), promoting inflammasome activation, IL-1β production, and immunopathology [38]. Indeed, IL-1β overproduction has been linked to disease severity in Leishmania infection [39, 40]. The role of IL-17 in mediating tissue damage in human leishmaniasis is less clear, but PBMCs from CL patients stimulated with SLA secret higher levels of IL-17 compared with uninfected controls and IL-17 is also present in CL lesions [41]. Our finding that patients with a negative LST produce high levels of proinflammatory cytokines, such as IL-1β, IL-6, IL-17, and granzyme B, gives support to the role of the CD8+ T cells, Th17, and innate immune response in the pathology of CL.
In addition to proinflammatory cytokines, studies have shown that MMP-9 and granzyme B participate in the pathology of TL. MMP-9 is an enzyme that degrades extracellular matrix proteins and in vitro infection of human macrophages with L. braziliensis increases the secretion of MMP-9, and increased expression of MMP-9 is associated with pathology in CL and mucosal leishmaniasis [42]. Moreover, cells from CL lesions secrete high amounts of MMP-9 compared to healthy subjects [43]. Here, we show that MMP-9 secretion was similar in patients with positive or negative LST. Granzyme B is mainly produced by NK and CD8+ T cells and, together with perforin, induces killing of target cells. The finding that granzyme B levels were as high in cells from LST-negative as LST-positive patients suggests that the cytotoxicity activity of these cells is intact in LST-negative patients, contributing to pathology even in the absence of a Th1 immune response.
IL-10 is the most important regulatory cytokine associated with impairment in the Th1 immune response in leishmaniasis. High IL-10 production is associated with Leishmania proliferation and persistence [44–46]. Others have also found that an increase in IL-10 has an important role in the pathogenesis of CL [47]. Here, IL-10 production was quite variable and neutralization of this cytokine did not restore IFN-γ production. IL-12 is an important IFN-γ–inducing cytokine and is mainly produced by dendritic cells. Here, low levels of IL-12 were observed in LST-negative patients, and addition of exogenous IL-12 restored IFN-γ levels in PBMC cultures. This suggests a possible role for dendritic cells in Th1 impairment in LST-negative patients. Moreover, the persistence of the parasite may have an important role in the impairment of IFN-γ production, as cure of the disease was associated with an enhancement in the Th1 response.
Our study shows a strong association between a negative LST and impairment in the IFN-γ production in CL patients. As the reduction in CD4+ Th1 function did not modify the clinical presentation of CL, our data support the role of CD8+ T cells, proinflammatory cytokines related to innate immunity, and MMP-9 in the pathology of CL. The negative LST was a marker of therapeutic failure of SbV in CL, indicating that other forms of therapy should be used in these patients to increase the cure rate and decrease the time to healing.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. We thank Cristiano Franco and Aline Souza for secretarial assistance.
Financial support. This work was supported by the National Institutes of Health (grant number AI 136032).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




