-
PDF
- Split View
-
Views
-
Cite
Cite
Kimberly F Breglio, Caian L Vinhaes, María B Arriaga, Martha Nason, Gregg Roby, Joseph Adelsberger, Bruno B Andrade, Virginia Sheikh, Irini Sereti, Clinical and Immunologic Predictors of Mycobacterium avium Complex Immune Reconstitution Inflammatory Syndrome in a Contemporary Cohort of Patients With Human Immunodeficiency Virus, The Journal of Infectious Diseases, Volume 223, Issue 12, 15 June 2021, Pages 2124–2135, https://doi.org/10.1093/infdis/jiaa669
Close - Share Icon Share
Abstract
People with human immunodeficiency virus (HIV) can present with new or worsening symptoms associated with Mycobacterium avium complex (MAC) infection shortly after antiretroviral therapy (ART) initiation as MAC immune reconstitution inflammatory syndrome (MAC-IRIS). In this study, we assessed the utility of several laboratory tests as predictors of MAC-IRIS.
People with HIV with clinical and histologic and/or microbiologic evidence of MAC-IRIS were identified and followed up to 96 weeks post–ART initiation within a prospective study of 206 ART-naive patients with CD4 <100 cells/µL.
Fifteen (7.3%) patients presented with MAC-IRIS within a median interval of 26 days after ART initiation. Patients who developed MAC-IRIS had lower body mass index, lower hemoglobin levels, higher alkaline phosphatase (ALP), and increased CD38 frequency and mean fluorescence intensity on CD8+ T cells at the time of ART initiation compared with non-MAC IRIS patients. A decision tree inference model revealed that stratifying patients based on levels of ALP and D-dimer could predict the likelihood of MAC-IRIS. A binary logistic regression demonstrated that higher levels of ALP at baseline were associated with increased risk of MAC-IRIS development.
High ALP levels and increased CD8+ T-cell activation with low CD4 counts at ART initiation should warrant suspicion for subsequent development of MAC-IRIS.
Mycobacterium avium complex (MAC) is comprised of slow-growing mycobacteria that include both Mycobacterium avium and Mycobacterium intracellulare, as well as lesser-known species. These environmental pathogens are present in soil, animals, and water, and infections have been described after exposure to infected water systems [1, 2]. Before the onset of the human immunodeficiency virus (HIV) epidemic, MAC infections were rare and typically manifested with significant structural lung disease in immunocompetent persons [3].
Beginning in 1981, reports of MAC infections in what would later be known as HIV/AIDS emerged [4]. In contrast to MAC infections in immunocompetent individuals, people with HIV (PWH) presented with disseminated infection and nonspecific constitutional symptoms including fever, weight loss, and night sweats [5, 6]. In the following years, MAC infections contributed significantly to morbidity and mortality in PWH [7], particularly in patients with CD4 counts ≤50 cells/µL [6, 8]. Although the widespread use of combination antiretroviral therapy (ART) has been associated with a 2.4- to 25.8-fold decrease in MAC incidence [9], MAC infection remains a significant pathogen for PWH with severe immunosuppression. Unfortunately, despite tremendous improvements in access to ART worldwide, a significant proportion of PWH continue to be diagnosed late in the course of HIV infection, after having already developed HIV/AIDS [10–12].
Initiation of ART is essential for the survival of severely immunosuppressed PWH; however, patients with underlying MAC infection are at risk for an abrupt onset or worsening of MAC manifestations after initiation of ART called immune reconstitution inflammatory syndrome (IRIS) [3, 13–15]. Approximately 10%–25% of PWH who initiate ART develop IRIS [16, 17], which is called unmasking when occurring in the context of a previously unrecognized opportunistic infection and paradoxical when the opportunistic infection was previously recognized and on treatment.
MAC-IRIS frequently causes significant morbidity, commonly presenting with fever, lymphadenitis, pulmonary, and/or intra-abdominal disease [3, 13–15, 18, 19]. Because the differential diagnosis for these clinical presentations is broad, including other serious conditions such as lymphoma and tuberculosis, the diagnostic workup to establish a diagnosis of MAC-IRIS can be time-consuming, challenging, and burdensome for the patient [3]. To improve the clinical management of PWH with severe immune suppression, clinicians need tools to suspect and efficiently recognize MAC-IRIS. In this study, we describe the clinical and immunologic characteristics of patients with MAC-IRIS and assess the utility of several laboratory tests as predictors of MAC-IRIS.
METHODS
Study Design and Population
Within a previously described prospective observational 96-week study of ART-naive PWH with CD4 <100 cells/µL initiating ART (ClinicalTrials.gov identifier NCT00286767) [20], we identified MAC-IRIS events that occurred in the 206 patients at the National Institutes of Health Clinical Center site (Bethesda, Maryland). All participants signed informed consent before any study procedures. MAC infection was diagnosed prospectively on the basis of clinical and histologic and/or microbiologic evidence of MAC. Clinical signs and symptoms considered suspicious for MAC included persistent fever, weight loss, cough, lymphadenopathy, and radiologic evidence of disease. Microbial evidence of MAC included growth of a MAC species in culture from a bronchoalveolar lavage, sputum sample, blood sample, or fine needle aspirate from a lymph node. Histologic evidence of MAC included granulomas on biopsy.
The clinical team prospectively identified MAC-IRIS events and presented clinical and diagnostic data to an endpoint review committee that adjudicated whether the events were consistent with IRIS using the AIDS Clinical Trials Group IRIS definition criteria, as previously described [20, 21]. One patient developed an IRIS event due to either cytomegalovirus or Strongyloides stercoralis after 10 days post–ART initiation but had a positive sputum culture for MAC at week 28. This patient was determined not to have had MAC-IRIS by an independent committee and was excluded from analysis.
MAC Treatment
Patients diagnosed with MAC were treated as per standard of care, which generally included azithromycin, ethambutol, and moxifloxacin (if CD4+ <50 T cells/µL) [3, 22]. For patients with pulmonary MAC, antimicrobial treatment was continued until the participant was culture negative for 1 year [3]. Participants with disseminated MAC infections were continued on antimicrobial treatment for at least 12 months and until symptoms abated with sustained immune recovery (CD4+ >100 T cells/µL) [3].
Laboratory Methods
C-reactive protein (CRP), D-dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase were measured in a Clinical Laboratory Improvement Act (CLIA)–certified laboratory. Peripheral blood immunophenotyping was performed according to the manufacturer’s instructions, with a modified version of the Centers for Disease Control and Prevention guidelines in a CLIA-certified laboratory. Using whole blood, phenotyping was performed with monoclonal antibodies from BD Biosciences (San Jose, California), then the cells were lysed after staining with Optilyse C (Beckman Coulter, Hialeah, Florida), washed twice, and resuspended in 500 µL of phosphate-buffered saline (Cambrex, Walkersville, Maryland). Sample analysis occurred immediately on a Becton Dickinson FacsCanto flow cytometer (BD Biosciences, San Jose, California). The monoclonal antibodies used were PD-1 (MIH4), HLA-DR (L243), CD38 (HB7), CD3 (UCHT1), CD8 (SK1), CD4 (RPA-T4) and Ki-67 (B56). Data were analyzed with Facs Diva software version 6.1.3.
Statistical Analysis
The median values, with interquartile range (IQR) or 95% confidence interval (CI), were used as measures of central tendency and dispersion, respectively. Pearson χ 2 test was used to compare frequencies between the study groups whereas continuous variables were compared using the Mann-Whitney U test (unpaired comparisons) or Wilcoxon-signed rank test (paired comparisons). Two models of binary multivariable logistic regressions were performed as indicated in the Results, with the odds ratio (OR) and 95% CI of the association between values of indicated parameters and MAC-IRIS occurrence. The variables included in the models were the biomarkers levels at indicated timepoints that displayed a P value <.3 in univariate comparisons. A machine-learning based conditional tree including the values of all the biomarkers was designed to identify the best biomarker or combination of markers that were able to discriminate MAC-IRIS from controls. All analyses were prespecified. A P value < .05 was considered statistically significant. The analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, California), JMP 14 (SAS Institute, Cary, North Carolina), and the party library in R software.
RESULTS
MAC-IRIS Incidence and Clinical Presentation
Fifteen of the 206 (7.3%) participants experienced MAC-IRIS, representing 31% of all IRIS events [20]. Among those 191 participants who did not develop MAC-IRIS, 17% (33 patients) presented with IRIS due to other opportunistic pathogens, mainly varicella zoster virus (Supplementary Table 1). Additionally, 7 participants were diagnosed with MAC but did not develop MAC-IRIS, including 4 who were diagnosed with MAC before and 3 after ART. The median duration from ART initiation to onset of MAC-IRIS symptoms was 26 days. Table 1 provides a detailed summary of the 15 cases (3 paradoxical and 12 unmasking MAC-IRIS).
Case Descriptions of Mycobacterium avium Complex Immune Reconstitution Inflammatory Syndrome
| Age and Sex . | CD4+ T-Cell Count at Baseline (at IRIS) . | ART Regimen . | Other OIs (Before or at the Time of MAC Diagnosis) . | Time to IRIS From ART Initiation, d . | MAC Diagnosis . | MAC-IRIS Presentation . | MAC Therapy, d . | Corticosteroids for MAC Treatment . | Hospitalizations and Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 47 y, M | 7 (97) | ABC + 3TC, ATV | Esophageal candidiasis, oral candidiasis, CMV enterocolitis, cryptosporidiosis | 99 | Liver, lymph node aspirates grew MAC | Lymphadenopathy (unmasking IRIS) | Started 107 d after ART: • Azithromycin (1461) • Ethambutol (1461) | Prednisone | Hospitalized for 12 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, diarrhea, and vomiting). MAC-IRIS resolved after treatment with corticosteroids. |
| 27 y, M | 40 (108) | FTC + TDF, ZDV, ATV + RTV | Pneumocystis carinii pneumonia, HSV (perianal), Kaposi sarcoma, oral candidiasis, oral hairy leukoplakia | 38 | Biopsy of abdominal lymph node positive for MAI | Lymphadenopathy (unmasking IRIS) | Started 60 d after ART: • Clarithromycin (965) • Ethambutol (965) | None | Hospitalized for 3 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fevers, chills, myalgia, and vomiting). MAC-IRIS resolved without corticosteroids. |
| 48 y, F | 1 (48) | EFV + FTC + TDF | Pneumocystis carinii pneumonia, microsporidia, Cryptosporidium, oral candidiasis, genital/oral HSV | 18 | AFB blood culture grew MAC | Disseminated; high fever, hypotension, and tachycardia (unmasking IRIS) | Started 35 d after ART: • Azithromycin (141) • Ethambutol (141) • Moxifloxacin (140) | None | Hospitalized in ICU for 2 d for diagnostic workup of presenting MAC-IRIS symptoms (hypotension, tachycardia, fever). MAC-IRIS resolved without corticosteroids. |
| 25 y, M | 86 (488) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, oral candidiasis | 54 | Sputum positive for MAC by acid-fast stain, culture, and SecA1 PCR/sequencing | Pulmonary (unmasking IRIS) | Started 65 d after ART: • Azithromycin (495) • Ethambutol (495) • Moxifloxacin (495) | None | Briefly hospitalized for diagnostic workup of presenting MAC-IRIS symptoms (cough in the setting of new pulmonary lesions). MAC-IRIS resolved without corticosteroids. |
| 32 y, F | 5 (12) | ATV, FTC + TDF | Disseminated histoplasmosis, Strongyloides, toxoplasmosis, HSV (rectal), CMV viremia, baseline MAC | 44 | AFB blood culture grew MAC | Cervical lymphadenopathy (paradoxical IRIS) | Started 3 d before ART • Azithromycin (172) • Ethambutol (837) • Moxifloxacin (837) | Prednisone | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (cervical adenopathy and fever). MAC-IRIS resolved following approximately 4 mo of corticosteroid treatment. |
| 49 y, M | 2 (13) | FTC + TDF, ATV | CMV retinitis, HSV (perirectal), oral candidiasis, Candida esophagitis, baseline MAC | 93 | AFB blood culture grew MAC | Extensive abdominal lymphadenopathy (paradoxical IRIS) | Started 4 d before ART: • Azithromycin (341) • Ethambutol (808) • Moxifloxacin (467) | None | Briefly hospitalized for MAC-IRIS symptom management (abdominal pain and fever). MAC IRIS resolved without corticosteroids. |
| 45 y, M | 37 (124) | EFV + FTC + TDF | Oral candidiasis, CNS lymphoma | 14 | BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 19 d after ART: • Azithromycin (513) • Ethambutol (513) • Moxifloxacin (513) | Prednisolone | Hospitalized for 13 wk for CNS lymphoma, MAC-IRIS, and failure to thrive. Patient experienced sufficient recovery to allow for hospital discharge but died of sudden cardiac death at home 890 d after ART initiation. |
| 31 y, M | 4 (26) | ATV, FTC + TDF, RTV | Pneumocystis carinii pneumonia, HSV (rectal) | 14 | FNA of lymph node positive by AFB smear, BAL fluid grew MAC | Lymphadenopathy (unmasking IRIS) | Started 42 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (725) | Prednisone | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS recurred >2 y after initial diagnosis despite continuous ART and prolonged MAC treatment. |
| 44 y, M | 5 (119) | EFV + FTC + TDF | Pneumocystis carinii pneumonia | 21 | FNA of paratracheal lymph node positive for AFB by Fite stain, AFB culture of lymph node biopsy material grew MAC | Pulmonary (unmasking IRIS) | Started 48 d after ART: • Azithromycin (599) • Ethambutol (599) • Moxifloxacin (584) | None | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (fever, dry cough, and night sweats). MAC-IRIS resolved without corticosteroids. |
| 47 y, F | 3 (14) | EFV + FTC + TDF | Oral candidiasis, baseline MAC | 14 | AFB blood culture grew MAC | Extensive lymphadenopathy (paradoxical IRIS) | Started 42 d after ART: • Azithromycin (91) • Ethambutol (550) • Moxifloxacin (550) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, adenopathy, and tachycardia) provided through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 50 y, M | 25 (119) | EFV + FTC + TDF | Cryptococcal meningitis | 9 | AFB smear of material collected from FNA of abdominal mass positive; AFB culture of the fluid also grew MAC | Extensive mesenteric lymphadenopathy (unmasking IRIS) | Started 99 d after ART: • Azithromycin (86) • Ethambutol (72) • Rifabutin (86) | None | Extensive workup of MAC-IRIS presenting symptoms (weight loss with new abdominal mass on imaging) conducted primarily in the outpatient setting, with 2 brief hospitalizations. Diagnosis ultimately made following FNA of abdominal mass for AFB culture. MAC-IRIS resolved without corticosteroids. |
| 29 y, M | 29 (157) | RAL, FTC + TDF | None | 56 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 62 d after ART: • Azithromycin (611) • Ethambutol (611) • Moxifloxacin (611) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptom (pleuritic chest pain) provided with frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 39 y, M | 44 (178) | DRV, RAL, FTC + TDF | Strongyloides | 30 | Histologic diagnosis by granulomas on lung biopsy | Pulmonary (unmasking IRIS) | Started 71 d after ART: • Azithromycin (363) • Ethambutol (363) • Moxifloxacin (126) | None | Not hospitalized. Patient did not have clinical symptoms of MAC-IRIS; diagnostic workup revealing unmasking MAC-IRIS was prompted by newly positive PPD 4 wk after ART initiation. |
| 41 y, M | 35 (154) | EFV + FTC + TDF, RAL | Pneumocystis carinii pneumonia, CMV pneumonia | 32 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 42 d after ART: • Azithromycin (579) • Ethambutol (579) | None | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 37 y, F | 8 (11) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, HSV, oral candidiasis | 11 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 17 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (736) | Prednisone | Hospitalized for 10 d for diagnostic workup and management of MAC-IRIS symptoms in the setting of recent cancer treatment and neutropenia. MAC IRIS resolved. |
| Age and Sex . | CD4+ T-Cell Count at Baseline (at IRIS) . | ART Regimen . | Other OIs (Before or at the Time of MAC Diagnosis) . | Time to IRIS From ART Initiation, d . | MAC Diagnosis . | MAC-IRIS Presentation . | MAC Therapy, d . | Corticosteroids for MAC Treatment . | Hospitalizations and Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 47 y, M | 7 (97) | ABC + 3TC, ATV | Esophageal candidiasis, oral candidiasis, CMV enterocolitis, cryptosporidiosis | 99 | Liver, lymph node aspirates grew MAC | Lymphadenopathy (unmasking IRIS) | Started 107 d after ART: • Azithromycin (1461) • Ethambutol (1461) | Prednisone | Hospitalized for 12 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, diarrhea, and vomiting). MAC-IRIS resolved after treatment with corticosteroids. |
| 27 y, M | 40 (108) | FTC + TDF, ZDV, ATV + RTV | Pneumocystis carinii pneumonia, HSV (perianal), Kaposi sarcoma, oral candidiasis, oral hairy leukoplakia | 38 | Biopsy of abdominal lymph node positive for MAI | Lymphadenopathy (unmasking IRIS) | Started 60 d after ART: • Clarithromycin (965) • Ethambutol (965) | None | Hospitalized for 3 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fevers, chills, myalgia, and vomiting). MAC-IRIS resolved without corticosteroids. |
| 48 y, F | 1 (48) | EFV + FTC + TDF | Pneumocystis carinii pneumonia, microsporidia, Cryptosporidium, oral candidiasis, genital/oral HSV | 18 | AFB blood culture grew MAC | Disseminated; high fever, hypotension, and tachycardia (unmasking IRIS) | Started 35 d after ART: • Azithromycin (141) • Ethambutol (141) • Moxifloxacin (140) | None | Hospitalized in ICU for 2 d for diagnostic workup of presenting MAC-IRIS symptoms (hypotension, tachycardia, fever). MAC-IRIS resolved without corticosteroids. |
| 25 y, M | 86 (488) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, oral candidiasis | 54 | Sputum positive for MAC by acid-fast stain, culture, and SecA1 PCR/sequencing | Pulmonary (unmasking IRIS) | Started 65 d after ART: • Azithromycin (495) • Ethambutol (495) • Moxifloxacin (495) | None | Briefly hospitalized for diagnostic workup of presenting MAC-IRIS symptoms (cough in the setting of new pulmonary lesions). MAC-IRIS resolved without corticosteroids. |
| 32 y, F | 5 (12) | ATV, FTC + TDF | Disseminated histoplasmosis, Strongyloides, toxoplasmosis, HSV (rectal), CMV viremia, baseline MAC | 44 | AFB blood culture grew MAC | Cervical lymphadenopathy (paradoxical IRIS) | Started 3 d before ART • Azithromycin (172) • Ethambutol (837) • Moxifloxacin (837) | Prednisone | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (cervical adenopathy and fever). MAC-IRIS resolved following approximately 4 mo of corticosteroid treatment. |
| 49 y, M | 2 (13) | FTC + TDF, ATV | CMV retinitis, HSV (perirectal), oral candidiasis, Candida esophagitis, baseline MAC | 93 | AFB blood culture grew MAC | Extensive abdominal lymphadenopathy (paradoxical IRIS) | Started 4 d before ART: • Azithromycin (341) • Ethambutol (808) • Moxifloxacin (467) | None | Briefly hospitalized for MAC-IRIS symptom management (abdominal pain and fever). MAC IRIS resolved without corticosteroids. |
| 45 y, M | 37 (124) | EFV + FTC + TDF | Oral candidiasis, CNS lymphoma | 14 | BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 19 d after ART: • Azithromycin (513) • Ethambutol (513) • Moxifloxacin (513) | Prednisolone | Hospitalized for 13 wk for CNS lymphoma, MAC-IRIS, and failure to thrive. Patient experienced sufficient recovery to allow for hospital discharge but died of sudden cardiac death at home 890 d after ART initiation. |
| 31 y, M | 4 (26) | ATV, FTC + TDF, RTV | Pneumocystis carinii pneumonia, HSV (rectal) | 14 | FNA of lymph node positive by AFB smear, BAL fluid grew MAC | Lymphadenopathy (unmasking IRIS) | Started 42 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (725) | Prednisone | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS recurred >2 y after initial diagnosis despite continuous ART and prolonged MAC treatment. |
| 44 y, M | 5 (119) | EFV + FTC + TDF | Pneumocystis carinii pneumonia | 21 | FNA of paratracheal lymph node positive for AFB by Fite stain, AFB culture of lymph node biopsy material grew MAC | Pulmonary (unmasking IRIS) | Started 48 d after ART: • Azithromycin (599) • Ethambutol (599) • Moxifloxacin (584) | None | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (fever, dry cough, and night sweats). MAC-IRIS resolved without corticosteroids. |
| 47 y, F | 3 (14) | EFV + FTC + TDF | Oral candidiasis, baseline MAC | 14 | AFB blood culture grew MAC | Extensive lymphadenopathy (paradoxical IRIS) | Started 42 d after ART: • Azithromycin (91) • Ethambutol (550) • Moxifloxacin (550) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, adenopathy, and tachycardia) provided through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 50 y, M | 25 (119) | EFV + FTC + TDF | Cryptococcal meningitis | 9 | AFB smear of material collected from FNA of abdominal mass positive; AFB culture of the fluid also grew MAC | Extensive mesenteric lymphadenopathy (unmasking IRIS) | Started 99 d after ART: • Azithromycin (86) • Ethambutol (72) • Rifabutin (86) | None | Extensive workup of MAC-IRIS presenting symptoms (weight loss with new abdominal mass on imaging) conducted primarily in the outpatient setting, with 2 brief hospitalizations. Diagnosis ultimately made following FNA of abdominal mass for AFB culture. MAC-IRIS resolved without corticosteroids. |
| 29 y, M | 29 (157) | RAL, FTC + TDF | None | 56 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 62 d after ART: • Azithromycin (611) • Ethambutol (611) • Moxifloxacin (611) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptom (pleuritic chest pain) provided with frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 39 y, M | 44 (178) | DRV, RAL, FTC + TDF | Strongyloides | 30 | Histologic diagnosis by granulomas on lung biopsy | Pulmonary (unmasking IRIS) | Started 71 d after ART: • Azithromycin (363) • Ethambutol (363) • Moxifloxacin (126) | None | Not hospitalized. Patient did not have clinical symptoms of MAC-IRIS; diagnostic workup revealing unmasking MAC-IRIS was prompted by newly positive PPD 4 wk after ART initiation. |
| 41 y, M | 35 (154) | EFV + FTC + TDF, RAL | Pneumocystis carinii pneumonia, CMV pneumonia | 32 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 42 d after ART: • Azithromycin (579) • Ethambutol (579) | None | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 37 y, F | 8 (11) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, HSV, oral candidiasis | 11 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 17 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (736) | Prednisone | Hospitalized for 10 d for diagnostic workup and management of MAC-IRIS symptoms in the setting of recent cancer treatment and neutropenia. MAC IRIS resolved. |
Abbreviations: 3TC, lamivudine; ABC, abacavir; AFB, acid-fast bacilli; ART, antiretroviral therapy; ATV, atazanavir; BAL, bronchoalveolar lavage; CMV, cytomegalovirus; CNS, central nervous system; DRV, darunavir; EFV, efavirenz; F, female; FNA, fine needle aspiration; FTC, emtricitabine; HSV, herpes simplex virus; ICU, intensive care unit; IRIS, immune reconstitution inflammatory syndrome; M, male; MAI, Mycobacterium avium intracellulare; MAC, Mycobacterium avium complex; OI, opportunistic infection; PCR, polymerase chain reaction; PPD, purified protein derivative;; RAL, raltegravir; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine.
Case Descriptions of Mycobacterium avium Complex Immune Reconstitution Inflammatory Syndrome
| Age and Sex . | CD4+ T-Cell Count at Baseline (at IRIS) . | ART Regimen . | Other OIs (Before or at the Time of MAC Diagnosis) . | Time to IRIS From ART Initiation, d . | MAC Diagnosis . | MAC-IRIS Presentation . | MAC Therapy, d . | Corticosteroids for MAC Treatment . | Hospitalizations and Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 47 y, M | 7 (97) | ABC + 3TC, ATV | Esophageal candidiasis, oral candidiasis, CMV enterocolitis, cryptosporidiosis | 99 | Liver, lymph node aspirates grew MAC | Lymphadenopathy (unmasking IRIS) | Started 107 d after ART: • Azithromycin (1461) • Ethambutol (1461) | Prednisone | Hospitalized for 12 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, diarrhea, and vomiting). MAC-IRIS resolved after treatment with corticosteroids. |
| 27 y, M | 40 (108) | FTC + TDF, ZDV, ATV + RTV | Pneumocystis carinii pneumonia, HSV (perianal), Kaposi sarcoma, oral candidiasis, oral hairy leukoplakia | 38 | Biopsy of abdominal lymph node positive for MAI | Lymphadenopathy (unmasking IRIS) | Started 60 d after ART: • Clarithromycin (965) • Ethambutol (965) | None | Hospitalized for 3 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fevers, chills, myalgia, and vomiting). MAC-IRIS resolved without corticosteroids. |
| 48 y, F | 1 (48) | EFV + FTC + TDF | Pneumocystis carinii pneumonia, microsporidia, Cryptosporidium, oral candidiasis, genital/oral HSV | 18 | AFB blood culture grew MAC | Disseminated; high fever, hypotension, and tachycardia (unmasking IRIS) | Started 35 d after ART: • Azithromycin (141) • Ethambutol (141) • Moxifloxacin (140) | None | Hospitalized in ICU for 2 d for diagnostic workup of presenting MAC-IRIS symptoms (hypotension, tachycardia, fever). MAC-IRIS resolved without corticosteroids. |
| 25 y, M | 86 (488) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, oral candidiasis | 54 | Sputum positive for MAC by acid-fast stain, culture, and SecA1 PCR/sequencing | Pulmonary (unmasking IRIS) | Started 65 d after ART: • Azithromycin (495) • Ethambutol (495) • Moxifloxacin (495) | None | Briefly hospitalized for diagnostic workup of presenting MAC-IRIS symptoms (cough in the setting of new pulmonary lesions). MAC-IRIS resolved without corticosteroids. |
| 32 y, F | 5 (12) | ATV, FTC + TDF | Disseminated histoplasmosis, Strongyloides, toxoplasmosis, HSV (rectal), CMV viremia, baseline MAC | 44 | AFB blood culture grew MAC | Cervical lymphadenopathy (paradoxical IRIS) | Started 3 d before ART • Azithromycin (172) • Ethambutol (837) • Moxifloxacin (837) | Prednisone | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (cervical adenopathy and fever). MAC-IRIS resolved following approximately 4 mo of corticosteroid treatment. |
| 49 y, M | 2 (13) | FTC + TDF, ATV | CMV retinitis, HSV (perirectal), oral candidiasis, Candida esophagitis, baseline MAC | 93 | AFB blood culture grew MAC | Extensive abdominal lymphadenopathy (paradoxical IRIS) | Started 4 d before ART: • Azithromycin (341) • Ethambutol (808) • Moxifloxacin (467) | None | Briefly hospitalized for MAC-IRIS symptom management (abdominal pain and fever). MAC IRIS resolved without corticosteroids. |
| 45 y, M | 37 (124) | EFV + FTC + TDF | Oral candidiasis, CNS lymphoma | 14 | BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 19 d after ART: • Azithromycin (513) • Ethambutol (513) • Moxifloxacin (513) | Prednisolone | Hospitalized for 13 wk for CNS lymphoma, MAC-IRIS, and failure to thrive. Patient experienced sufficient recovery to allow for hospital discharge but died of sudden cardiac death at home 890 d after ART initiation. |
| 31 y, M | 4 (26) | ATV, FTC + TDF, RTV | Pneumocystis carinii pneumonia, HSV (rectal) | 14 | FNA of lymph node positive by AFB smear, BAL fluid grew MAC | Lymphadenopathy (unmasking IRIS) | Started 42 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (725) | Prednisone | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS recurred >2 y after initial diagnosis despite continuous ART and prolonged MAC treatment. |
| 44 y, M | 5 (119) | EFV + FTC + TDF | Pneumocystis carinii pneumonia | 21 | FNA of paratracheal lymph node positive for AFB by Fite stain, AFB culture of lymph node biopsy material grew MAC | Pulmonary (unmasking IRIS) | Started 48 d after ART: • Azithromycin (599) • Ethambutol (599) • Moxifloxacin (584) | None | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (fever, dry cough, and night sweats). MAC-IRIS resolved without corticosteroids. |
| 47 y, F | 3 (14) | EFV + FTC + TDF | Oral candidiasis, baseline MAC | 14 | AFB blood culture grew MAC | Extensive lymphadenopathy (paradoxical IRIS) | Started 42 d after ART: • Azithromycin (91) • Ethambutol (550) • Moxifloxacin (550) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, adenopathy, and tachycardia) provided through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 50 y, M | 25 (119) | EFV + FTC + TDF | Cryptococcal meningitis | 9 | AFB smear of material collected from FNA of abdominal mass positive; AFB culture of the fluid also grew MAC | Extensive mesenteric lymphadenopathy (unmasking IRIS) | Started 99 d after ART: • Azithromycin (86) • Ethambutol (72) • Rifabutin (86) | None | Extensive workup of MAC-IRIS presenting symptoms (weight loss with new abdominal mass on imaging) conducted primarily in the outpatient setting, with 2 brief hospitalizations. Diagnosis ultimately made following FNA of abdominal mass for AFB culture. MAC-IRIS resolved without corticosteroids. |
| 29 y, M | 29 (157) | RAL, FTC + TDF | None | 56 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 62 d after ART: • Azithromycin (611) • Ethambutol (611) • Moxifloxacin (611) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptom (pleuritic chest pain) provided with frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 39 y, M | 44 (178) | DRV, RAL, FTC + TDF | Strongyloides | 30 | Histologic diagnosis by granulomas on lung biopsy | Pulmonary (unmasking IRIS) | Started 71 d after ART: • Azithromycin (363) • Ethambutol (363) • Moxifloxacin (126) | None | Not hospitalized. Patient did not have clinical symptoms of MAC-IRIS; diagnostic workup revealing unmasking MAC-IRIS was prompted by newly positive PPD 4 wk after ART initiation. |
| 41 y, M | 35 (154) | EFV + FTC + TDF, RAL | Pneumocystis carinii pneumonia, CMV pneumonia | 32 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 42 d after ART: • Azithromycin (579) • Ethambutol (579) | None | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 37 y, F | 8 (11) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, HSV, oral candidiasis | 11 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 17 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (736) | Prednisone | Hospitalized for 10 d for diagnostic workup and management of MAC-IRIS symptoms in the setting of recent cancer treatment and neutropenia. MAC IRIS resolved. |
| Age and Sex . | CD4+ T-Cell Count at Baseline (at IRIS) . | ART Regimen . | Other OIs (Before or at the Time of MAC Diagnosis) . | Time to IRIS From ART Initiation, d . | MAC Diagnosis . | MAC-IRIS Presentation . | MAC Therapy, d . | Corticosteroids for MAC Treatment . | Hospitalizations and Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 47 y, M | 7 (97) | ABC + 3TC, ATV | Esophageal candidiasis, oral candidiasis, CMV enterocolitis, cryptosporidiosis | 99 | Liver, lymph node aspirates grew MAC | Lymphadenopathy (unmasking IRIS) | Started 107 d after ART: • Azithromycin (1461) • Ethambutol (1461) | Prednisone | Hospitalized for 12 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, diarrhea, and vomiting). MAC-IRIS resolved after treatment with corticosteroids. |
| 27 y, M | 40 (108) | FTC + TDF, ZDV, ATV + RTV | Pneumocystis carinii pneumonia, HSV (perianal), Kaposi sarcoma, oral candidiasis, oral hairy leukoplakia | 38 | Biopsy of abdominal lymph node positive for MAI | Lymphadenopathy (unmasking IRIS) | Started 60 d after ART: • Clarithromycin (965) • Ethambutol (965) | None | Hospitalized for 3 d for diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fevers, chills, myalgia, and vomiting). MAC-IRIS resolved without corticosteroids. |
| 48 y, F | 1 (48) | EFV + FTC + TDF | Pneumocystis carinii pneumonia, microsporidia, Cryptosporidium, oral candidiasis, genital/oral HSV | 18 | AFB blood culture grew MAC | Disseminated; high fever, hypotension, and tachycardia (unmasking IRIS) | Started 35 d after ART: • Azithromycin (141) • Ethambutol (141) • Moxifloxacin (140) | None | Hospitalized in ICU for 2 d for diagnostic workup of presenting MAC-IRIS symptoms (hypotension, tachycardia, fever). MAC-IRIS resolved without corticosteroids. |
| 25 y, M | 86 (488) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, oral candidiasis | 54 | Sputum positive for MAC by acid-fast stain, culture, and SecA1 PCR/sequencing | Pulmonary (unmasking IRIS) | Started 65 d after ART: • Azithromycin (495) • Ethambutol (495) • Moxifloxacin (495) | None | Briefly hospitalized for diagnostic workup of presenting MAC-IRIS symptoms (cough in the setting of new pulmonary lesions). MAC-IRIS resolved without corticosteroids. |
| 32 y, F | 5 (12) | ATV, FTC + TDF | Disseminated histoplasmosis, Strongyloides, toxoplasmosis, HSV (rectal), CMV viremia, baseline MAC | 44 | AFB blood culture grew MAC | Cervical lymphadenopathy (paradoxical IRIS) | Started 3 d before ART • Azithromycin (172) • Ethambutol (837) • Moxifloxacin (837) | Prednisone | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (cervical adenopathy and fever). MAC-IRIS resolved following approximately 4 mo of corticosteroid treatment. |
| 49 y, M | 2 (13) | FTC + TDF, ATV | CMV retinitis, HSV (perirectal), oral candidiasis, Candida esophagitis, baseline MAC | 93 | AFB blood culture grew MAC | Extensive abdominal lymphadenopathy (paradoxical IRIS) | Started 4 d before ART: • Azithromycin (341) • Ethambutol (808) • Moxifloxacin (467) | None | Briefly hospitalized for MAC-IRIS symptom management (abdominal pain and fever). MAC IRIS resolved without corticosteroids. |
| 45 y, M | 37 (124) | EFV + FTC + TDF | Oral candidiasis, CNS lymphoma | 14 | BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 19 d after ART: • Azithromycin (513) • Ethambutol (513) • Moxifloxacin (513) | Prednisolone | Hospitalized for 13 wk for CNS lymphoma, MAC-IRIS, and failure to thrive. Patient experienced sufficient recovery to allow for hospital discharge but died of sudden cardiac death at home 890 d after ART initiation. |
| 31 y, M | 4 (26) | ATV, FTC + TDF, RTV | Pneumocystis carinii pneumonia, HSV (rectal) | 14 | FNA of lymph node positive by AFB smear, BAL fluid grew MAC | Lymphadenopathy (unmasking IRIS) | Started 42 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (725) | Prednisone | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS recurred >2 y after initial diagnosis despite continuous ART and prolonged MAC treatment. |
| 44 y, M | 5 (119) | EFV + FTC + TDF | Pneumocystis carinii pneumonia | 21 | FNA of paratracheal lymph node positive for AFB by Fite stain, AFB culture of lymph node biopsy material grew MAC | Pulmonary (unmasking IRIS) | Started 48 d after ART: • Azithromycin (599) • Ethambutol (599) • Moxifloxacin (584) | None | Hospitalized for 3 d for diagnostic workup of presenting MAC-IRIS symptoms (fever, dry cough, and night sweats). MAC-IRIS resolved without corticosteroids. |
| 47 y, F | 3 (14) | EFV + FTC + TDF | Oral candidiasis, baseline MAC | 14 | AFB blood culture grew MAC | Extensive lymphadenopathy (paradoxical IRIS) | Started 42 d after ART: • Azithromycin (91) • Ethambutol (550) • Moxifloxacin (550) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptoms (fever, adenopathy, and tachycardia) provided through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 50 y, M | 25 (119) | EFV + FTC + TDF | Cryptococcal meningitis | 9 | AFB smear of material collected from FNA of abdominal mass positive; AFB culture of the fluid also grew MAC | Extensive mesenteric lymphadenopathy (unmasking IRIS) | Started 99 d after ART: • Azithromycin (86) • Ethambutol (72) • Rifabutin (86) | None | Extensive workup of MAC-IRIS presenting symptoms (weight loss with new abdominal mass on imaging) conducted primarily in the outpatient setting, with 2 brief hospitalizations. Diagnosis ultimately made following FNA of abdominal mass for AFB culture. MAC-IRIS resolved without corticosteroids. |
| 29 y, M | 29 (157) | RAL, FTC + TDF | None | 56 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 62 d after ART: • Azithromycin (611) • Ethambutol (611) • Moxifloxacin (611) | None | Not hospitalized; diagnostic workup and supportive care for presenting MAC-IRIS symptom (pleuritic chest pain) provided with frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 39 y, M | 44 (178) | DRV, RAL, FTC + TDF | Strongyloides | 30 | Histologic diagnosis by granulomas on lung biopsy | Pulmonary (unmasking IRIS) | Started 71 d after ART: • Azithromycin (363) • Ethambutol (363) • Moxifloxacin (126) | None | Not hospitalized. Patient did not have clinical symptoms of MAC-IRIS; diagnostic workup revealing unmasking MAC-IRIS was prompted by newly positive PPD 4 wk after ART initiation. |
| 41 y, M | 35 (154) | EFV + FTC + TDF, RAL | Pneumocystis carinii pneumonia, CMV pneumonia | 32 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 42 d after ART: • Azithromycin (579) • Ethambutol (579) | None | Not hospitalized; diagnostic workup and MAC-IRIS symptoms monitored through frequent outpatient visits. MAC-IRIS resolved without corticosteroids. |
| 37 y, F | 8 (11) | EFV + FTC + TDF | Diffuse large B-cell lymphoma, HSV, oral candidiasis | 11 | AFB culture of BAL fluid grew MAC | Pulmonary (unmasking IRIS) | Started 17 d after ART: • Azithromycin (736) • Ethambutol (736) • Moxifloxacin (736) | Prednisone | Hospitalized for 10 d for diagnostic workup and management of MAC-IRIS symptoms in the setting of recent cancer treatment and neutropenia. MAC IRIS resolved. |
Abbreviations: 3TC, lamivudine; ABC, abacavir; AFB, acid-fast bacilli; ART, antiretroviral therapy; ATV, atazanavir; BAL, bronchoalveolar lavage; CMV, cytomegalovirus; CNS, central nervous system; DRV, darunavir; EFV, efavirenz; F, female; FNA, fine needle aspiration; FTC, emtricitabine; HSV, herpes simplex virus; ICU, intensive care unit; IRIS, immune reconstitution inflammatory syndrome; M, male; MAI, Mycobacterium avium intracellulare; MAC, Mycobacterium avium complex; OI, opportunistic infection; PCR, polymerase chain reaction; PPD, purified protein derivative;; RAL, raltegravir; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine.
The participants who experienced paradoxical MAC-IRIS had been diagnosed with HIV with extremely low CD4+ T-cell counts (3, 2, and 5 cells/µL) and MAC (before ART) after being found to have MAC bacteremia. One participant’s acid-fast bacilli (AFB) blood culture had been prompted by the presence of pancytopenia, high alkaline phosphatase (ALP), and diffuse adenopathy on physical examination, whereas the other 2 participants’ AFB blood cultures were ordered to evaluate weight loss and fever. Antimycobacterial therapy (azithromycin, ethambutol with or without moxifloxacin) was started in all prior to ART. With paradoxical MAC-IRIS (after ART), 1 participant developed cervical lymphadenopathy, 1 participant (with baseline abdominal lymphadenopathy) developed diarrhea, abdominal pain, and weight loss, and 1 participant experienced fever, fatigue, and extensive adenopathy, most notable in the axillary, epicardic, mesenteric, and retroperitoneal regions.
Of the 12 participants who developed unmasking MAC-IRIS, 7 presented with pulmonary MAC-IRIS, 4 presented with adenopathy, and 1 presented with symptoms consistent with sepsis (high fever, tachycardia, and hypotension) and was found to have MAC bacteremia. Median baseline CD4 T-cell count among unmasking MAC-IRIS participants was 27 cells/µL (range, 1–86 cells/µL).
Many of the patients who developed MAC-IRIS were coinfected with other opportunistic pathogens or presented with HIV-related malignancies at the time of IRIS: oral/esophageal candidiasis (n = 7), genital/rectal herpes simplex virus (n = 6), Pneumocystis jirovecii pneumonia (n = 5), cytomegalovirus (n = 4), diarrheal illness associated with HIV (n = 3), HIV-related lymphoma (n = 3), Strongyloides stercoralis (n = 2), human papillomavirus–related malignancy (n = 1), or cryptococcal meningitis (n = 1) (Table 1).
MAC-IRIS Management and Morbidity
Triple antibiotic therapy, with azithromycin, ethambutol, and moxifloxacin, was implemented in the majority of participants (n = 11) because of the low CD4 count and severity of disease. The median duration of treatment for patients who completed therapy was 418 days for azithromycin (n = 10), 599 days for ethambutol (n = 11), and 513 days for moxifloxacin (n = 9).
Ten participants (66%) were hospitalized for the diagnostic workup and/or symptom management of MAC-IRIS events. Three participants required corticosteroids for management of persistent systemic symptoms such as painful adenopathy, respiratory distress, or intractable fevers. The median duration of time between onset of IRIS to resolution of symptoms was 7.6 weeks (range, 1.7–23.6 weeks). One participant who experienced resolution of MAC-IRIS symptoms after a prolonged course of corticosteroids (approximately 4 months) subsequently presented with a MAC-IRIS flare >2 years later despite continued ART and MAC treatment. One participant (6.7%) died of sudden cardiac death 890 days after starting ART following multiple, prolonged hospitalizations secondary to MAC-IRIS, lymphoma, and failure to thrive.
Risk Factors for MAC-IRIS
Participants who developed MAC-IRIS did not differ in terms of sex and age from those who did not (Supplementary Table 1). Laboratory and clinical evaluations, as well as the immunophenotyping of lymphocytes, were performed at study baseline (pre-ART) and after 2 weeks of ART initiation (Figure 1 and Supplementary Table 2). Importantly, plasma HIV viremia (5.32 vs 5.11 log copies/mL; P = .26) and CD4+ T-cell count (8.0 vs 19.5 cells/µL; P = .11) were not different between the clinical groups before ART initiation (Supplementary Table 2). Patients who developed MAC-IRIS during the follow-up had a lower median body mass index (BMI) (21.1 vs 23.4 kg/m2; P = .03) and hemoglobin values (9.9 vs 11.0 g/dL; P = .03) at the time of ART initiation than non-MAC-IRIS patients (Figure 1A). Furthermore, pre-ART levels of ALP (178.0 vs 86.0 units/L; P = .001), AST (41.0 vs 33.0 IU/L; P = .04) and ALT (61.0 vs 41.0 IU/L; P = .021) were higher in MAC-IRIS participants than in non-IRIS controls (Figure 1A).
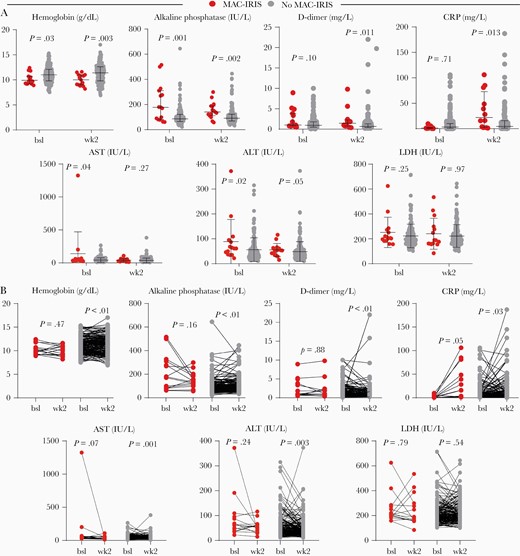
Differences in plasma biomarkers in Mycobacterium avium complex immune reconstitution inflammatory syndrome (MAC-IRIS). A, Circulating levels of indicated laboratory biomarkers pre–antiretroviral therapy (ART) (baseline) and week 2 of ART within each group of patients (red symbols for MAC-IRIS and light gray for no MAC-IRIS). The Mann-Whitney U test was used. B, Distributions of the indicated measurements were compared between MAC-IRIS and no MAC-IRIS controls at each study timepoint. Comparisons were performed using the Wilcoxon-signed rank paired test. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; bsl, baseline; CRP, C-reactive protein; LDH, lactate dehydrogenase; MAC-IRIS, Mycobacterium avium complex immune reconstitution inflammatory syndrome.
After 2 weeks on ART, values of ALP (P = .002), D-dimer (P = .011), and CRP (P = .013) were higher in those with MAC-IRIS, with persistent lower levels of hemoglobin (P = .003) compared to non-IRIS controls (Figure 1A). When variation in values of these biomarkers between pre-ART and week 2 were examined within each clinical group, we observed an overall decrease in levels of all measurements in both groups, except for CRP values, which tended to increase mainly in those who developed MAC-IRIS, with marginal statistical significance (P = .05; Figure 1B).
The results presented above suggested that increased systemic inflammation can characterize MAC-IRIS participants; therefore, we investigated the implications of such scenario in T-cell activation (Figure 2 and Supplementary Table 2). Because other studies have reported that IRIS is closely related to expansion and activation of CD4+ T cells, that is, frequently associated with activation of other cells such as CD8+ T lymphocytes [23], we hypothesized that differential activation profile of these cell types could characterize MAC-IRIS patients. To test this hypothesis, we examined frequencies of CD4+ or CD8+ T cells expressing CD38, Ki-67, and/or PD-1 at pre-ART and 2 weeks after initiation. Although the expression of these activation markers in CD4+ T cells of MAC-IRIS patients was indistinguishable from non-IRIS controls, we observed a higher proportion of CD8+ T cells expressing CD38+ (97% vs 93%; P = .005) and higher mean fluorescence intensity (MFI) of CD8+CD38+ T cells (360 vs 197.2 arbitrary units; P = .009) compared to non-MAC-IRIS controls both at baseline and at week 2 after therapy commencement (Figure 2A and Supplementary Table 2). Additionally, we observed higher proportions of CD8+ T cells expressing Ki67 at 2 weeks on ART in those with MAC-IRIS compared to non-IRIS (16% vs 9.5%; P = .01) (Figure 2A and Supplementary Table 2). Furthermore, we found that the proportion of CD8+ T cells expressing PD-1, and the MFI CD38 on CD8+ T cells substantially decreased at week 2 in non-IRIS patients but did not change significantly in those who developed MAC-IRIS (Figure 2B).

Immunophenotyping of CD8+ T cells in Mycobacterium avium complex immune reconstitution inflammatory syndrome (MAC-IRIS). A, Dynamics of markers associated with CD8+ T-cell activation between pre– antiretroviral therapy (ART) (baseline) and week 2 of ART within each group of patients, stratified according to occurrence of MAC-IRIS. The Mann-Whitney U test was used. B, Distributions of the indicated parameters were compared between MAC-IRIS and no MAC-IRIS controls at each study timepoint. Comparisons were performed using the Wilcoxon signed-rank paired test. Abbreviations: AU, arbitrary unit; bsl, baseline; HLA, human leukocyte antigen; MAC-IRIS, Mycobacterium avium complex immune reconstitution inflammatory syndrome; MFI mean fluorescence intensity.
Predicting MAC-IRIS
We further extended our analyses to test whether biomarkers measured in peripheral blood could be used to predict MAC-IRIS. We first employed a multivariate regression model (Figure 3) using the biomarkers that displayed P values <.3 in the univariate models presented in Supplementary Table 2. After adjustment for baseline levels of BMI, hemoglobin, HIV viral load, and CD4+ and CD8+ T-cell counts, we found that higher pre-ART levels of ALP were independently associated with increased odds ratio of MAC-IRIS occurrence (OR, 1.007 [95% CI, 1.001–1.012]; P = .013), whereas levels of D-dimer (OR, 1.123 [95% CI, .846–1.489]; P = .42), CRP (OR, 0.981 [95% CI, .940–1.025]; P = .39), and CD8+ T-cell activation markers were not independently associated with risk of MAC-IRIS (Figure 3). Of note, applying the same model to values obtained in week 2, we found that higher levels of CRP (OR, 1.015 [95% CI, 1.00–1.030]; P = .043), increased frequency of CD8+CD38+ T cells (OR, 1.159 [95% CI, 1.009–1332]; P = .036), and higher CD38 MFI values on CD8+ T cells (OR, 1.005 [95% CI, 1.001–1.009]; P = .023) were independently associated with MAC-IRIS occurrence.
![Multivariate regression analysis to evaluate association between plasma levels of laboratory parameters and Mycobacterium avium complex immune reconstitution inflammatory syndrome (MAC-IRIS). The multivariate regression model included the variables shown in Table 1, which displayed univariate P ≤ .3. Odds ratios represent the 95% confidence interval increase in concentrations of each biomarker. Target variables for comparisons at study baseline (pre–antiretroviral therapy [ART]) and week 2 on ART: alkaline phosphatase (IU/L), D-dimer (mg/L), C-reactive protein (mg/L), percentage of cells expressing CD38 and HLA-DR, percentage of CD8 expressing CD38, mean fluorescence intensity of CD38, percentage of cells expressing Ki67, and percentage of cells expressing PD-1. Variables used for adjustment in both timepoints: baseline levels of body mass index (kg/m2), CD4+ T-cell count (cells/µL), CD8+ T-cell count, hemoglobin (g/dL), and viral load human immunodeficiency virus RNA (log copies/mL). Significant associations are highlighted in red and significant P values are shown in bold. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CRP, C-reactive protein; HLA, human leukocyte antigen; LDH, lactate dehydrogenase; MAC-IRIS, Mycobacterium avium complex immune reconstitution inflammatory syndrome; MFI, mean fluorescence intensity; OR, odds ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/223/12/10.1093_infdis_jiaa669/1/m_jiaa669f0003.jpeg?Expires=1750156478&Signature=zkfVy61tL~Y52tmXPtBXICj7M4nZx0no1yc6qh~jaxJ5NbQ5rOGvnozNLCOBU-IxB4ZPLdkybnSKcEy2VemanJJmJ4ozcEX62fIv6geM~HK071e9E1gD7JrWHpa7govvAANW6dJjW4ozgwl2t36RL2z8jcfcaCbmKRAM0jT6Rx5oUdDBOc9H8Ln7kpMwtkvkwJyBiWjilsDile34ada5OGZU9C-jzM0~auCyMUOV-X8XzgDJb67GlVuMMDf-OmQaaz2Vz8nt9Ek9MUlCSyGdbwEjg98EHSMlbXiWkDGPfHDkoM1IRoTv0-C3cVa67zwo3LD7lUxsYQy5wqXmxz-rWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Multivariate regression analysis to evaluate association between plasma levels of laboratory parameters and Mycobacterium avium complex immune reconstitution inflammatory syndrome (MAC-IRIS). The multivariate regression model included the variables shown in Table 1, which displayed univariate P ≤ .3. Odds ratios represent the 95% confidence interval increase in concentrations of each biomarker. Target variables for comparisons at study baseline (pre–antiretroviral therapy [ART]) and week 2 on ART: alkaline phosphatase (IU/L), D-dimer (mg/L), C-reactive protein (mg/L), percentage of cells expressing CD38 and HLA-DR, percentage of CD8 expressing CD38, mean fluorescence intensity of CD38, percentage of cells expressing Ki67, and percentage of cells expressing PD-1. Variables used for adjustment in both timepoints: baseline levels of body mass index (kg/m2), CD4+ T-cell count (cells/µL), CD8+ T-cell count, hemoglobin (g/dL), and viral load human immunodeficiency virus RNA (log copies/mL). Significant associations are highlighted in red and significant P values are shown in bold. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CRP, C-reactive protein; HLA, human leukocyte antigen; LDH, lactate dehydrogenase; MAC-IRIS, Mycobacterium avium complex immune reconstitution inflammatory syndrome; MFI, mean fluorescence intensity; OR, odds ratio.
A machine-learning conditional tree inference model incorporating values of all the clinical laboratory parameters assessed at the 2 distinct study time points was designed with 2 main objectives: (1) to identify a combination of biomarkers that could best identify MAC-IRIS cases; and (2) to establish cutoff values of the markers that could be used to differentiate between cases and noncases. This approach identified 3 significant splits in the decision tree, including 2 distinct values of ALP at baseline and of D-dimer at week 2 of ART (Figure 4). To identify MAC-IRIS patients, the first split in the decision tree was composed by pre-ART ALP levels >248 IU/L, which alone could identify the majority of the cases (Figure 4A and 4B). A second split using D-dimer levels at week 2 on ART >3.15 mg/L was useful in prediction of MAC-IRIS. Among those who exhibited D-dimer levels ≤3.15 mg/L at week 2 of ART, a final split using ALP >165 IU/L at baseline could identify the remaining MAC-IRIS cases, except for 4 who were misclassified using this system (Figure 4A and 4B). We next employed a second model of multivariate regression, using the variables identified in the decision tree and categorizing the study participants according to the cutoff values established (Figure 4C). The variables used for adjustment were BMI (kg/m2), CD4+ T-cell count (cells/µL), hemoglobin (g/dL), and CRP (mg/L) at baseline (for adjustment of ALP) or week 2 (for adjustment of D-dimer). This analysis revealed that a patient presenting with ALP levels >248 IU/L pre-ART exhibited 15 times increased odds of developing MAC-IRIS than those who had lower levels (OR, 15.1 [IQR, 1.2–188.1]; P = .035). Using this same approach, levels of D-dimer >3.15 mg/L at week 2 of ART were associated with almost 4 times higher odds of MAC-IRIS than those who had lower levels (P < .01); however, such association was lost in the adjusted model (P = .107). These observations demonstrate that increased risk of MAC-IRIS is strongly associated with higher concentrations of ALP in plasma.
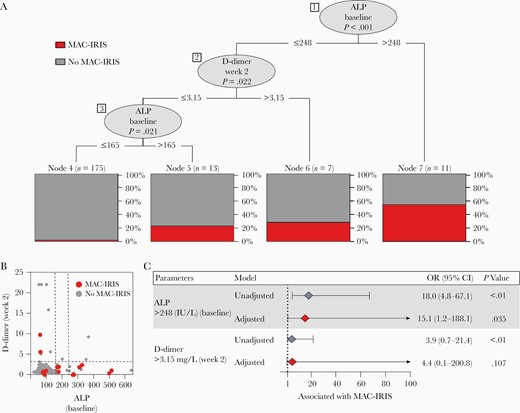
Machine learning decision tree model to predict Mycobacterium avium complex immune reconstitution inflammatory syndrome (MAC-IRIS). All variables included in Table 1 were included in the model. Variables included body mass index (BMI; kg/m2), plasma human immunodeficiency virus RNA load (log copies/mL), CD4+ and CD8+ T-cell counts (cells/µL), hemoglobin (g/dL), alkaline phosphatase (ALP; IU/L), D-dimer (mg/L), and C-reactive protein (CRP; mg/L), from pre–antiretroviral therapy (ART) and week 2 when indicated. Only ALP and D-dimer were shown to be informative in the system to segregate MAC-IRIS cases from no MAC-IRIS patients. A, Three splits were identified in the decision tree. The first split marks ALP at the time of ART initiation (week 0). The second split is comprised of D-dimer levels at week 2. The final split is ALP at the time of ART initiation. B, Box plot of distribution of individual according to levels of markers used in (A). Dashed lines represent the cutoff values used in the conditional model. C, Multivariable regression model of variables (categorized by cutoff values) shown to be significant in the decision tree model. The variables used for adjustment were BMI (kg/m2), CD4+ T-cell count (cells/µL), hemoglobin (g/dL), and CRP (mg/L) at baseline (for adjustment of ALP) or week 2 (for adjustment of D-dimer). Abbreviation: ALP, alkaline phosphatase; CI, confidence interval; CRP, C-reactive protein; OR, odds ratio.
DISCUSSION
Although the increased availability of ART has led to a steep decline in MAC incidence, PWH who are diagnosed with HIV after having already developed severe immunosuppression remain at risk of MAC and MAC-IRIS in the weeks following ART initiation. Given the significant morbidity associated with MAC-IRIS and the diagnostic challenges it poses, clinicians need tools to predict and efficiently recognize MAC-IRIS. Prompt initiation of antimycobacterial therapy, which could be started empirically in patients with a high clinical suspicion of MAC-IRIS even before results of cultures are available, could abrogate the morbidity associated with MAC-IRIS. With the goal of identifying higher clinical risk persons, we conducted this investigation focused on the incidence, clinical presentation, risks factors, and predictive markers for MAC-IRIS.
The incidence of MAC-IRIS in immunosuppressed PWH is 2.6%–3.5% [14, 24]. Of note in our study, 7.3% of ART-naive patients with CD4+ T-cell counts <100 cells/µL starting ART developed MAC-IRIS, demonstrating that MAC-IRIS remains an important concern for severely immunosuppressed patients initiating ART. Apart from 3 participants with paradoxical IRIS, the majority of patients experienced unmasking MAC-IRIS, developing signs and symptoms of MAC infection only after initiating ART. These data suggest that clinicians should have a high clinical suspicion for MAC infection in PWH with severe immunosuppression, both before and immediately after the initiation of ART. Our results also show that MAC-IRIS causes a significant burden to patients, often necessitating hospitalization and the administration of corticosteroids for symptomatic management. In some cases, MAC-IRIS can persist for months and/or recur despite continued ART, HIV viral suppression, and continued MAC therapy.
Previous studies of MAC-IRIS in PWH have described lymphadenopathy as the primary manifestation of this syndrome [3]. Importantly, despite a high incidence of lymph node disease in our cohort, pulmonary symptoms represented the most common manifestation of MAC-IRIS overall in our study. This important finding highlights that clinicians should consider the possibility of pulmonary MAC-IRIS in the differential diagnosis of patients who present with fever and respiratory symptoms soon after initiating HIV therapy.
In univariate analysis, we found that lower BMI, lower hemoglobin, higher ALP, and increased expression of CD38 (frequency of cells or MFI) by CD8+ T cells at the time of ART initiation were risk factors for the development of MAC-IRIS. In adjusted analysis, elevated ALP levels before ART initiation remained an independent predictor of MAC-IRIS infection.
Lower BMI and hemoglobin may indicate more severe immunosuppression and, thus, higher risk for opportunistic infections like MAC, whereas elevations in ALP may be due to MAC infiltration into the liver and possibly the bone marrow prior to immune reconstitution. CD38 expression on CD8+ T cells may be a marker of baseline inflammation and has importantly been described as an independent predictor or mortality in the pre-ART era in advanced disease [25, 26].
We sought to create a clinical predictive tool using circulating levels of laboratory parameters at the time of ART initiation. We found that elevated ALP (>248 IU/L at ART initiation) and D-dimer (>3.15 mg/L at week 2 of ART) supported the diagnosis of MAC-IRIS in this cohort. Our data suggest that in the absence of these elevated biomarkers, development of MAC-IRIS is unlikely. Further studies exploring prospectively the use of these markers as predictors of MAC-IRIS should be considered.
Improved understanding of the pathogenesis of MAC-IRIS is necessary to create targeted strategies to control the inflammatory response in these patients. Although the baseline immunophenotypes of CD4+ T cells of patients who develop MAC-IRIS were similar to those patients who would not develop disease, the CD8+ T-cell profiles were different. CD38 is a marker of cell activation and CD8+CD38+ lymphocytes have been shown to be an independent predictor of mortality in HIV patients in the pre–highly active antiretroviral therapy (HAART) era [27]. Given that the high mortality associated with HIV infection in the pre-HAART era was largely due to opportunistic infections including MAC, the activation of CD8+ T cells previously associated with mortality could have been due to underlying opportunistic infections. Ki67 is a marker of cell cycling and, therefore, an increase in CD8+Ki67+ T cells in patients who would develop MAC-IRIS could indicate that these cells are highly activated due to underlying infections or a higher HIV antigen load. Importantly, here we demonstrated that higher expression of CD38 by CD8+ T cells after 2 weeks on ART was associated with MAC-IRIS occurrence after adjustment for confounding variables. Nevertheless, T-cell activation measurements were not identified as relevant to distinguish MAC-IRIS from controls in our machine learning model.
Our study has 2 important limitations. First, the focus was on PWH starting ART with low CD4 counts and may not be generalizable to persons starting ART at higher CD4 counts. Additionally, our study included only 15 MAC-IRIS events, which likely does not reflect the entire breadth of potential MAC-IRIS presentations.
MAC infection and MAC-IRIS continue to cause significant morbidity among PWH with severe immunosuppression initiating ART, and our findings indicate that clinicians should suspect MAC-IRIS in patients with low BMI and hemoglobin who present with lymphadenopathy or respiratory symptoms in the weeks that follow ART initiation, especially in those with a high ALP value at ART initiation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants; the clinical staff; and Jeannette Higgins, who helped in performing the flow panel for the study.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported in part by the Intramural Research program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH). This research was also made possible through the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, the Doris Duke Charitable Foundation, the Alexandria Real Estate Equities, Inc. Mr and Mrs Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. The work of B. B. A. is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; senior fellow) and by the Intramural Research Program of the Fundação Oswaldo Cruz, Brazil. C. L. V. received a research fellowship from CNPq. M. B. A. received a research fellowship from the Fundação de Amparo à Pesquisa do Estado da Bahia. This project has also been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
K. F. B. and C. L. V. contributed equally to this work.
B. B. A., V. S., and I. S. contributed equally to this work.




