-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas D Knudsen, Andreas Ronit, Thomas Kristensen, Magda Teresa Thomsen, Anne-Mette Lebech, Michael Huy Cuong, Per Ejlstrup Sigvardsen, Jørgen Tobias Kühl, Andreas Fuchs, Lars Køber, Jens Lundgren, Jørgen Vestbo, Klaus F Kofoed, Susanne D Nielsen, Pulmonary Arterial Enlargement in Well-Treated Persons With Human Immunodeficiency Virus, The Journal of Infectious Diseases, Volume 223, Issue 1, 1 January 2021, Pages 94–100, https://doi.org/10.1093/infdis/jiaa339
Close - Share Icon Share
Abstract
Pulmonary artery enlargement is a marker of pulmonary hypertension. We aimed to determine the proportion with pulmonary artery enlargement among well-treated persons with human immunodeficiency virus HIV (PWH) and uninfected controls.
PWH with a chest computed tomography were included from the ongoing Copenhagen Comorbidity in HIV Infection (COCOMO) study. Age and sex-matched uninfected controls were recruited from the Copenhagen General Population Study. Pulmonary artery enlargement was defined as a ratio of >1 between the diameter of the main pulmonary artery (at the level of its bifurcation) and the diameter of the ascending aorta.
In total, 900 PWH were included, and 44 (5%) had a pulmonary artery–aorta ratio (PA:A) >1. After adjustment for age, sex, and body mass index, obesity (adjusted odds ratio, 4.33; 95% confidence interval, 1.76–10.65; P = .001) and injection drug use (IDU) (4.90; 1.00–18.46; P = .03) were associated with higher odds of having a PA:A >1, and pulmonary indices and smoking status were not. HIV seropositivity was borderline associated with a PA:A >1 (adjusted odds ratio, 1.89; 95% confidence interval, .92–3.85; P = .08).
A PA:A >1 was common in PWH. Obesity and IDU were independently associated with this finding and HIV serostatus was borderline associated with it, but HIV-related factors were not. Increased awareness may be appropriate in obese PWH and those with IDU.
Pulmonary hypertension (PH) has been associated with human immunodeficiency virus (HIV) infection and may contribute to disease and death among persons with HIV (PWH) [1, 2]. PH is currently defined as a mean pulmonary arterial pressure of ≥25 mm Hg at rest, as assessed by right heart catheterization [3, 4]. Although right heart catheterization remains the reference standard, PH can be assessed noninvasively. As determined using chest computed tomography (CT), the ratio of the diameter of the main pulmonary artery (at the level of its bifurcation) to the diameter of the ascending aorta (PA:A) is correlated with pulmonary artery pressure, and a PA:A >1 signifies pulmonary enlargement, which has been suggested as a marker of PH [5–11]. In the general population, larger PA:A values have been associated with higher mortality rates among persons with chronic obstructive pulmonary disease (COPD), and a PA:A >1 predicts a higher risk of COPD exacerbations [12].
Several factors may be directly or indirectly associated with PH in PWH. These include hepatitis C virus (HCV) coinfection, injection drug use (IDU), cocaine-methamphetamine use, and chronic inflammation [1, 3], features overrepresented in some populations of PWH. In addition, factors unique to HIV infection, such as ongoing HIV replication and proteins (envelope glycoprotein 120 (gp120), Nef, and Tat) may directly influence pulmonary arterial pressure [13]; gp120 induces release of endothelin-1, a potent constrictor, from lung endothelial cells and monocytes, and both Nef and Tat have been associated with remodeling of the pulmonary vasculature [1]. In persons with HCV infection, interferon-based therapies have been associated with increased risk of PH [14], and newer direct-acting antivirals (DAAs), in particular sofosbuvir, have also been linked to PH development [15].
Because histories of IDU and use of other recreational drugs are common among persons with HCV infection, discerning a culprit factor may not always be possible. Moreover, with the prolonged life expectancy of PWH and projected increase in age-associated comorbid conditions, the PH burden due to cardiac disease and COPD may also increase. With improved oral and intravenous medical therapies for PH, identifying at-risk persons and initiating timely therapy are clearly important. We aimed to determine the prevalence of having a PA:A >1 in a large cohort of well-treated PWH and to explore the association with HIV-related and HIV-unrelated risk factors. Furthermore, we determined whether HIV was independently associated with having a PA:A >1 by comparing prevalence in PWH with that in sex- and age-matched uninfected controls.
METHODS
Study Populations
Participants were included from the ongoing Copenhagen Comorbidity in HIV Infection (COCOMO) study (NCT02382822). The COCOMO study is an observational, longitudinal study with the aim of assessing non-AIDS comorbid conditions in PWH. The objectives and methods of the COCOMO study have been published elsewhere [16, 17]. In brief, all PWH attending the outpatient HIV clinics at University Hospital Rigshospitalet and University Hospital Hvidovre in Copenhagen were invited to participate. Participants completed a detailed questionnaire regarding symptoms and lifestyle. A physical examination, including dynamic spirometry, was performed. Participants were also invited to undergo high-resolution CT of the chest. The inclusion criterion for this study was a noncontrast chest CT scan. A sex- and age-matched sample of uninfected controls was recruited from the Copenhagen General Population Study (CGPS) [18]. Because only individuals >40 years of age underwent CT imaging in the CGPS, we restricted matching to PWH >40 years of age (n = 752) to make the 2 groups comparable. Ethical approvals were acquired from the Regional Ethics Committee of Copenhagen (COCOMO: H-15017350; CGPS: H-KF-01-144/01). Oral and written informed consent were collected from all participants.
Spirometry
Spirometry was performed as described in detail elsewhere [16]. In brief, we used an ultrasonic spirometer (ndd Medical Technologies), in accordance with American Thoracic Society/European Respiratory Society guidelines, to measure forced expiratory volume in 1 second and forced vital capacity. Predicted values were calculated using multiethnic prediction equations and R software macros provided by the Global Lung Function Initiative. We defined airflow limitation as a value <0.7 for the ratio of prebronchodilator forced expiratory volume in 1 second to forced vital capacity, with concomitant forced expiratory volume in 1 second <80% of predicted [19]. Spirometric data were available only for PWH.
CT Image Acquisition and Analyses
Chest CT scans were obtained as part of COCOMO and CGPS, using the same 320-multidetector CT Aquilion ONE Vision Edition scanner (Canon Medical Systems) and identical protocols [16]. In brief, images were acquired at full inspiration and at 120 kV and with tube current modulation (SureExposure; standard deviation, 15 kV). Reconstruction was done using an iterative reconstruction algorithm (adaptive iterative dose reduction; Canon Medical Systems) with 3-mm sections, 3-mm increments, and a soft-tissue kernel for evaluation of great vessels. For the quantitative measurements of emphysema, images were reconstructed with 1-mm sections, 1-mm increments, a soft-tissue kernel (FC08, Canon Medical Systems), and filtered back-projection.
CT scans were analyzed by 2 operators (T. K. and M. H. C.) blinded to participants’ clinical characteristics. Using axial CT images, they measured the diameters of the main pulmonary artery at the level of its bifurcation and the ascending aorta in its maximum dimension on the same image (Figure 1) [6, 12]. The PA:A was calculated as the diameter of the pulmonary artery divided by the diameter of the aorta.
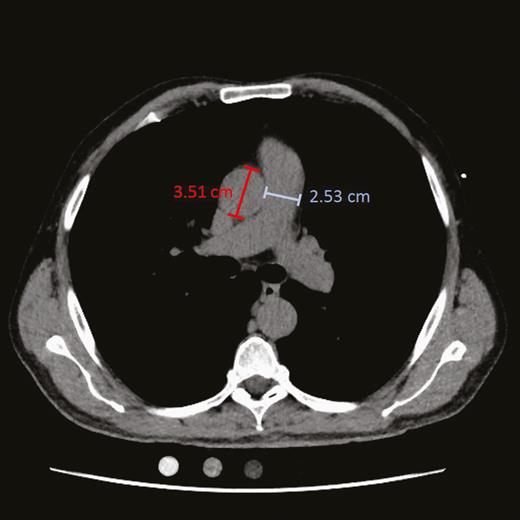
Chest computed tomographic scan of a study participant. The diameters of the main pulmonary artery (blue) at the level of its bifurcation and of the ascending aorta (red) in its maximum dimension were measured on the same image.
Emphysema measurements were performed using a dedicated lung density measurement program (Vitrea Vital Images), as described elsewhere [16]. Briefly, the proportion of lung voxels with an attenuation density below –950 Hounsfield units was calculated for each participant, and a proportion >10% was the criterion for CT-defined emphysema [16]. Emphysema measurements were available only for PWH.
Variable Definitions
We categorized body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) according to World Health Organization definitions; obesity was defined as a BMI >30 [17]. Hypertension was defined as current antihypertensive treatment, systolic blood pressure ≥140 mm Hg, and/or diastolic blood pressure ≥90 mm Hg. Smoking was characterized as never, former, or current smoking, and pulmonary function was defined as a composite of airflow limitation by spirometry and/or CT-defined emphysema. Current HCV coinfection was defined as a positive HCV RNA test result, and former HCV coinfection as a positive HCV antibody result but a negative HCV RNA, results. Recreational drug use was defined as an affirmative answer to the question: “Do you use stimulants other than alcohol and/or cannabis such as cocaine, MDMA, benzodiazepines and amphetamine?” IDU was defined as current or past use of intravenous drugs. HCV status and questionnaires regarding recreational drug use were available only for PWH.
Statistical Analysis
The distribution and fit of models were evaluated graphically. We assessed whether HIV-related and HIV-unrelated variables were associated with having a PA:A >1, using simple and multiple logistic regression analyses adjusted for age, sex, and BMI. We fitted each considered independent variable into the model one at a time and computed crude odds ratios (ORs), adjusted ORs (aORs), and 95% (profile likelihood) confidence intervals [CIs]. We performed additional sensitivity analyses with >1.08 as the cutoff for PA:A, because this cutoff has been found to have a specificity of 95% [8]. All analyses were conducted using R software, version 3.6.1, with the package ggplot2 [20].
RESULTS
In total, 900 PWH were included, and 44 (5%) had a PA:A >1 (Table 1 and Figure 2A). Their mean age (standard deviation) was 51 (11) years, and 119 (13%) of them were female. Almost two-thirds (n = 571 [63%]) were current or former smokers, and emphysema and/or airflow limitation was present in 106 (12%) (Table 1). Participants were predominantly well treated, with 887 (99%) currently using combination antiretroviral therapy and 847 (94%) having undetectable viral replication.
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | PA:A ≤1 (n = 856) . | PA:A >1 (n = 44) . |
| PA:A, median (IQR) | 0.78 (0.85–0.91) | 1.07 (1.04–1.12) |
| Age, mean (SD), y | 52 (11) | 40 (9) |
| Female sex, N (%) | 110 (13) | 9 (20) |
| BMI, mean (SD)b | 24.8 (3.6) | 25.7 (4.8) |
| BMI category | ||
| Underweight | 22 (2.6) | 1 (2.3) |
| Normal | 459 (54) | 21(48) |
| Overweight | 306 (67) | 13 (30) |
| Obese | 63 (14) | 9 (21) |
| Hypertension | 370 (43) | 9 (21) |
| Smoking status | ||
| Current smoker | 233 (27) | 17 (39) |
| Former smoker | 311 (36) | 10 (23) |
| Cumulative pack-years,c median (IQR) | 19 (7–33) | 12 (4–23) |
| Airflow limitation (spirometry)d | 76 (9) | 0 (0) |
| FEV1/FVC <0.7 | 128 (15) | 1 (2) |
| FEV1, % of predicted | ||
| ≥80% | 52 (6) | 1 (100) |
| 50%–79% | 64 (8) | 0 (0) |
| 30%–49% | 9 (1) | 0 (0) |
| <30% | 3 (0.4) | 0 (0) |
| CT-defined emphysemae | 38 (4.4) | 0 (0) |
| Recreational drug use | 177 (21) | 13 (30) |
| Injection drug usef | 14 (1.6) | 3 (6.8) |
| HCV infection | ||
| Former | 41 (4.8) | 4 (9.1) |
| Current | 46 (5.4) | 1 (2.3) |
| Previous AIDS-defining disease | 154 (18) | 8 (18) |
| Current cART use | 846 (98.8) | 41 (93.2) |
| Virological suppressiong | 811 (95) | 38 (86) |
| CD4+ T-cell count nadir <200/µL | 362 (43) | 12 (27) |
| DAA exposure | 11 (1.3) | 1 (2.3) |
| Sofosbuvir exposure | 5 (0.6) | 0 (0) |
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | PA:A ≤1 (n = 856) . | PA:A >1 (n = 44) . |
| PA:A, median (IQR) | 0.78 (0.85–0.91) | 1.07 (1.04–1.12) |
| Age, mean (SD), y | 52 (11) | 40 (9) |
| Female sex, N (%) | 110 (13) | 9 (20) |
| BMI, mean (SD)b | 24.8 (3.6) | 25.7 (4.8) |
| BMI category | ||
| Underweight | 22 (2.6) | 1 (2.3) |
| Normal | 459 (54) | 21(48) |
| Overweight | 306 (67) | 13 (30) |
| Obese | 63 (14) | 9 (21) |
| Hypertension | 370 (43) | 9 (21) |
| Smoking status | ||
| Current smoker | 233 (27) | 17 (39) |
| Former smoker | 311 (36) | 10 (23) |
| Cumulative pack-years,c median (IQR) | 19 (7–33) | 12 (4–23) |
| Airflow limitation (spirometry)d | 76 (9) | 0 (0) |
| FEV1/FVC <0.7 | 128 (15) | 1 (2) |
| FEV1, % of predicted | ||
| ≥80% | 52 (6) | 1 (100) |
| 50%–79% | 64 (8) | 0 (0) |
| 30%–49% | 9 (1) | 0 (0) |
| <30% | 3 (0.4) | 0 (0) |
| CT-defined emphysemae | 38 (4.4) | 0 (0) |
| Recreational drug use | 177 (21) | 13 (30) |
| Injection drug usef | 14 (1.6) | 3 (6.8) |
| HCV infection | ||
| Former | 41 (4.8) | 4 (9.1) |
| Current | 46 (5.4) | 1 (2.3) |
| Previous AIDS-defining disease | 154 (18) | 8 (18) |
| Current cART use | 846 (98.8) | 41 (93.2) |
| Virological suppressiong | 811 (95) | 38 (86) |
| CD4+ T-cell count nadir <200/µL | 362 (43) | 12 (27) |
| DAA exposure | 11 (1.3) | 1 (2.3) |
| Sofosbuvir exposure | 5 (0.6) | 0 (0) |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; CT, computed tomography; DAA, direct-acting antiviral; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; PA:A, pulmonary artery:aorta-ratio; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified. Where percentages do not add up to 100%, this is owing to missing information.
bBMI calculated as weight in kilograms divided by height in meters squared.
cPack-years were defined as the number of packs of cigarettes smoked per day multiplied by the number of years the person has smoked (among both current and former smokers).
dAirflow limitation was defined as FEV1/FVC <0.7 with concomitant predicted FEV1 <80% of predicted.
eCT-defined emphysema was defined as >10% of lung voxels with an attenuation density below –950 Hounsfield units.
fAll participants who reported injection drug use also reported oral recreational drug use.
gVirological suppression was defined as a viral load <50 copies/mL.
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | PA:A ≤1 (n = 856) . | PA:A >1 (n = 44) . |
| PA:A, median (IQR) | 0.78 (0.85–0.91) | 1.07 (1.04–1.12) |
| Age, mean (SD), y | 52 (11) | 40 (9) |
| Female sex, N (%) | 110 (13) | 9 (20) |
| BMI, mean (SD)b | 24.8 (3.6) | 25.7 (4.8) |
| BMI category | ||
| Underweight | 22 (2.6) | 1 (2.3) |
| Normal | 459 (54) | 21(48) |
| Overweight | 306 (67) | 13 (30) |
| Obese | 63 (14) | 9 (21) |
| Hypertension | 370 (43) | 9 (21) |
| Smoking status | ||
| Current smoker | 233 (27) | 17 (39) |
| Former smoker | 311 (36) | 10 (23) |
| Cumulative pack-years,c median (IQR) | 19 (7–33) | 12 (4–23) |
| Airflow limitation (spirometry)d | 76 (9) | 0 (0) |
| FEV1/FVC <0.7 | 128 (15) | 1 (2) |
| FEV1, % of predicted | ||
| ≥80% | 52 (6) | 1 (100) |
| 50%–79% | 64 (8) | 0 (0) |
| 30%–49% | 9 (1) | 0 (0) |
| <30% | 3 (0.4) | 0 (0) |
| CT-defined emphysemae | 38 (4.4) | 0 (0) |
| Recreational drug use | 177 (21) | 13 (30) |
| Injection drug usef | 14 (1.6) | 3 (6.8) |
| HCV infection | ||
| Former | 41 (4.8) | 4 (9.1) |
| Current | 46 (5.4) | 1 (2.3) |
| Previous AIDS-defining disease | 154 (18) | 8 (18) |
| Current cART use | 846 (98.8) | 41 (93.2) |
| Virological suppressiong | 811 (95) | 38 (86) |
| CD4+ T-cell count nadir <200/µL | 362 (43) | 12 (27) |
| DAA exposure | 11 (1.3) | 1 (2.3) |
| Sofosbuvir exposure | 5 (0.6) | 0 (0) |
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | PA:A ≤1 (n = 856) . | PA:A >1 (n = 44) . |
| PA:A, median (IQR) | 0.78 (0.85–0.91) | 1.07 (1.04–1.12) |
| Age, mean (SD), y | 52 (11) | 40 (9) |
| Female sex, N (%) | 110 (13) | 9 (20) |
| BMI, mean (SD)b | 24.8 (3.6) | 25.7 (4.8) |
| BMI category | ||
| Underweight | 22 (2.6) | 1 (2.3) |
| Normal | 459 (54) | 21(48) |
| Overweight | 306 (67) | 13 (30) |
| Obese | 63 (14) | 9 (21) |
| Hypertension | 370 (43) | 9 (21) |
| Smoking status | ||
| Current smoker | 233 (27) | 17 (39) |
| Former smoker | 311 (36) | 10 (23) |
| Cumulative pack-years,c median (IQR) | 19 (7–33) | 12 (4–23) |
| Airflow limitation (spirometry)d | 76 (9) | 0 (0) |
| FEV1/FVC <0.7 | 128 (15) | 1 (2) |
| FEV1, % of predicted | ||
| ≥80% | 52 (6) | 1 (100) |
| 50%–79% | 64 (8) | 0 (0) |
| 30%–49% | 9 (1) | 0 (0) |
| <30% | 3 (0.4) | 0 (0) |
| CT-defined emphysemae | 38 (4.4) | 0 (0) |
| Recreational drug use | 177 (21) | 13 (30) |
| Injection drug usef | 14 (1.6) | 3 (6.8) |
| HCV infection | ||
| Former | 41 (4.8) | 4 (9.1) |
| Current | 46 (5.4) | 1 (2.3) |
| Previous AIDS-defining disease | 154 (18) | 8 (18) |
| Current cART use | 846 (98.8) | 41 (93.2) |
| Virological suppressiong | 811 (95) | 38 (86) |
| CD4+ T-cell count nadir <200/µL | 362 (43) | 12 (27) |
| DAA exposure | 11 (1.3) | 1 (2.3) |
| Sofosbuvir exposure | 5 (0.6) | 0 (0) |
Abbreviations: BMI, body mass index; cART, combination antiretroviral therapy; CT, computed tomography; DAA, direct-acting antiviral; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; PA:A, pulmonary artery:aorta-ratio; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified. Where percentages do not add up to 100%, this is owing to missing information.
bBMI calculated as weight in kilograms divided by height in meters squared.
cPack-years were defined as the number of packs of cigarettes smoked per day multiplied by the number of years the person has smoked (among both current and former smokers).
dAirflow limitation was defined as FEV1/FVC <0.7 with concomitant predicted FEV1 <80% of predicted.
eCT-defined emphysema was defined as >10% of lung voxels with an attenuation density below –950 Hounsfield units.
fAll participants who reported injection drug use also reported oral recreational drug use.
gVirological suppression was defined as a viral load <50 copies/mL.
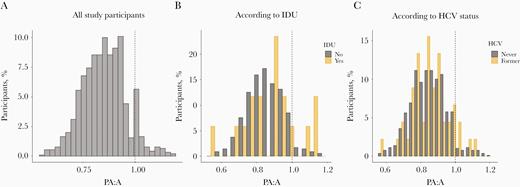
Distribution of the ratio of pulmonary artery diameter to ascending aorta diameter (PA:A) in study participants, without stratification (A) or stratified according to injection drug use (IDU) (B) or former hepatitis C virus (HCV) infection (C). Dashed lines represents cutoff for pulmonary artery enlargement (PA:A = 1).
Prior or current use of recreational drugs was reported by 190 (21%) of participants, and 17 (2%) reported IDU. All participants who reported IDU also reported oral recreational drug use. HCV coinfection was present in 47 (5%) of participants, and 45 (5%) had former HCV infection. Of those with former or current HCV coinfection, 11 (12%) reported IDU, 25 (26%) had been treated with interferon, and 12 (13%) had DAA exposure, including 5 (42%) who had been treated with sofosbuvir.
Variables Associated With a PA:A >1
In unadjusted analyses, having a PA:A >1 was associated with younger age (OR per decade older, 0.34; 95% CI, .24–.47; P < .001), obesity (3.12; 1.37–7.12; P = .007), hypertension (0.33; .15–.69; P = .004), IDU (4.40; 1.22–15.92; P = .02), CD4+ T-cell count nadir <200/µL (0.51; .26–1.00; P = .050), and ongoing viral replication (3.37; 1.34–8.46; P = .01). In unadjusted analyses, having a PA:A >1 was not associated with female sex (P = .15), current smoking (P = .42), former smoking (P = .20), airflow limitation by spirometry and/or CT-defined emphysema (P = .09), use of recreational drugs aside from IDU (P = .12), or HCV infection (P = .26). Values for PA:A stratified by IDU or by HCV infection are shown in Figure 2B and 2C, respectively. There were not enough cases with exposure to interferon and DAA, including sofosbuvir, to investigate associations between these variables and having a PA:A >1.
In adjusted regression analyses, younger age (aOR per decade older, 0.29; 95% CI, .20–.43; P < .001), obesity (4.33; 1.76–10.65; P = .001), former HCV infection (3.84; 1.03–11.66; P = .03), and IDU (4.90; 1.00–18.46; P = .03) were associated with higher odds of having a PA:A >1. Female sex, smoking, hypertension, CT-defined emphysema, airflow limitation, airflow limitation by spirometry and/or CT-defined emphysema, CD4+ T-cell count nadir <200/µL, and prior AIDS-defining disease were not associated with having a PA:A >1.
In sensitivity analyses, we used >1.08 as the PA:A cutoff. With this cutoff, age, hypertension, and IDU remained associated with pulmonary artery enlargement. Obesity, CD4+ T-cell count nadir <200, and ongoing viral replication were not associated with having a PA:A >1.08. In adjusted analyses with >1.08 as the PA:A cutoff, age (aOR per decade older, 0.21; 95% CI, .12–.39; P < .001) and IDU (7.71; 1.06–37.10; P = .02) remained associated with pulmonary artery enlargement. Obesity (aOR, 1.72; 95% CI, .34–8.56; P = .51) and former HCV infection (2.58; .13–15.88 P = .39) were not associated with having a PA:A >1.08. When both IDU and HCV were included in the model, together with age, sex, and BMI, IDU was associated with pulmonary enlargement, with an OR of 8.96 (95% CI, 1.04–76.97; P = .047), whereas HCV no longer was (aOR, 0.68; 95% CI, .04–11.04; P = .79).
Pulmonary Artery Enlargement in PWH Versus Uninfected Controls
Of 900 PWH, 752 were >40 years old and the prevalence of having a PA:A >1 in this group was 22 (2.9%). In the 752 sex- and age-matched, uninfected controls, the prevalence of having a PA:A >1 was 13 (1.7%) (P = .17). The characteristics of the PWH >40 years of age and of the uninfected controls are presented in Table 2. In unadjusted analyses, HIV was associated with having a PA:A >1, with an OR of 1.71 (95% CI, .86–3.43; P = .17). After adjustment for age, sex, and BMI, HIV was borderline associated, with an aOR of 1.89 (95% CI, .92–3.85; P = .08), and further adjustment for smoking did not change this estimate (1.88; .92–3.86; P = .09).
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | Persons With HIV (n = 752) . | Uninfected Controls (n = 752) . |
| PA:A, median (IQR) | 0.84 (0.77–0.91) | 0.76 (0.69–0.82) |
| PA:A >1 | 22 (2.9) | 13 (1.7) |
| Age, median (IQR), y | 52.3 (47–61) | 52.4 (47–61) |
| Female sex | 106 (14) | 102 (14) |
| BMI, mean (SD)b | 25.0 (3.7) | 26.5 (3.5) |
| BMI category | ||
| Underweight | 18 (2.4) | 1 (0.1) |
| Normal | 387 (52) | 259 (34) |
| Overweight | 277 (37) | 375 (50) |
| Obese | 65 (8.6) | 117 (16) |
| Hypertension | 349 (48) | 450 (60) |
| Smoking status | ||
| Current smoker | 198 (27) | 93 (12) |
| Former smoker | 290 (39) | 299 (40) |
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | Persons With HIV (n = 752) . | Uninfected Controls (n = 752) . |
| PA:A, median (IQR) | 0.84 (0.77–0.91) | 0.76 (0.69–0.82) |
| PA:A >1 | 22 (2.9) | 13 (1.7) |
| Age, median (IQR), y | 52.3 (47–61) | 52.4 (47–61) |
| Female sex | 106 (14) | 102 (14) |
| BMI, mean (SD)b | 25.0 (3.7) | 26.5 (3.5) |
| BMI category | ||
| Underweight | 18 (2.4) | 1 (0.1) |
| Normal | 387 (52) | 259 (34) |
| Overweight | 277 (37) | 375 (50) |
| Obese | 65 (8.6) | 117 (16) |
| Hypertension | 349 (48) | 450 (60) |
| Smoking status | ||
| Current smoker | 198 (27) | 93 (12) |
| Former smoker | 290 (39) | 299 (40) |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; PA:A, pulmonary artery–aorta ratio; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified. Where percentages do not add up to 100%, this is owing to missing information.
bBMI calculated as weight in kilograms divided by height in meters squared.
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | Persons With HIV (n = 752) . | Uninfected Controls (n = 752) . |
| PA:A, median (IQR) | 0.84 (0.77–0.91) | 0.76 (0.69–0.82) |
| PA:A >1 | 22 (2.9) | 13 (1.7) |
| Age, median (IQR), y | 52.3 (47–61) | 52.4 (47–61) |
| Female sex | 106 (14) | 102 (14) |
| BMI, mean (SD)b | 25.0 (3.7) | 26.5 (3.5) |
| BMI category | ||
| Underweight | 18 (2.4) | 1 (0.1) |
| Normal | 387 (52) | 259 (34) |
| Overweight | 277 (37) | 375 (50) |
| Obese | 65 (8.6) | 117 (16) |
| Hypertension | 349 (48) | 450 (60) |
| Smoking status | ||
| Current smoker | 198 (27) | 93 (12) |
| Former smoker | 290 (39) | 299 (40) |
| . | Participants, No. (%)a . | |
|---|---|---|
| Characteristic . | Persons With HIV (n = 752) . | Uninfected Controls (n = 752) . |
| PA:A, median (IQR) | 0.84 (0.77–0.91) | 0.76 (0.69–0.82) |
| PA:A >1 | 22 (2.9) | 13 (1.7) |
| Age, median (IQR), y | 52.3 (47–61) | 52.4 (47–61) |
| Female sex | 106 (14) | 102 (14) |
| BMI, mean (SD)b | 25.0 (3.7) | 26.5 (3.5) |
| BMI category | ||
| Underweight | 18 (2.4) | 1 (0.1) |
| Normal | 387 (52) | 259 (34) |
| Overweight | 277 (37) | 375 (50) |
| Obese | 65 (8.6) | 117 (16) |
| Hypertension | 349 (48) | 450 (60) |
| Smoking status | ||
| Current smoker | 198 (27) | 93 (12) |
| Former smoker | 290 (39) | 299 (40) |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; PA:A, pulmonary artery–aorta ratio; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified. Where percentages do not add up to 100%, this is owing to missing information.
bBMI calculated as weight in kilograms divided by height in meters squared.
Discussion
The prevalence of having a PA:A >1 was 5% in this large group of well-treated PWH. Older age was associated with lower odds and obesity, IDU, and former HCV infection with higher odds of a PA:A >1. Airflow limitation by spirometry and/or CT-defined emphysema or smoking were not associated with higher odds of having a PA:A >1. HIV serostatus was associated with almost twice the odds of having a PA:A >1 after adjustment for age, sex, and BMI, although this association was only borderline significant.
The European Society of Cardiology and the European Respiratory Society classify PH into 5 groups [3]. Pulmonary arterial hypertension (PAH) constitutes group 1 and is an established complication of HIV infection [3]. The reported prevalence of PAH, verified by right heart catheterization, is 0.5% among PWH, many-fold higher than the estimated 26–52 cases per million in the general European population [3]. Of note, pulmonary enlargement, defined as a PA:A >1, is a marker of increased pulmonary pressures, and our point prevalence, which is 10 times that of the recognized estimate for PAH, encompasses all 5 groups of PH. Obesity is a previously described risk factor for PH [3], and obese PWH had 4-fold higher odds of having a PA:A >1 than PWH of normal weight. The underlying pathophysiological mechanism is likely multifactorial, with increased obesity-related inflammation, left heart disease [21], obstructive sleep apnea, and hypoventilation syndrome (Pickwickian syndrome). However, in sensitivity analyses using a PA:A >1.08 as the cutoff point, obesity was not associated with pulmonary artery enlargement. As such, this result should be interpreting with caution
As described by others, older age was associated with lower odds of having a PA:A >1 [22], which could be explained by a lower sensitivity among older populations with aortic enlargement caused by long-standing hypertension [22, 23]. Smoking and airflow limitation by spirometry and/or CT-defined emphysema were not associated with having a PA:A >1, although pulmonary disease is a recognized risk factor and constitutes a distinct group of PH [3]. Indeed, a PA:A >1 is a predictor of poor prognosis in persons with COPD [12]. Our data, which included both radiographic and spirometric assessments of pulmonary function, indicate that smoking and pulmonary comorbid conditions may not be important drivers of PH in PWH, and other risk factors contribute to a greater extent. Thus, odds of having a PA:A >1 were 4 times higher among PWH with IDU or HCV infection than among those without IDU or HCV, respectively. However, the association between HCV and pulmonary artery enlargement was not present in sensitivity analyses where 1.08 was used as the cutoff and in analyses where IDU and HCV were adjusted for each other. This may indicate that the associations between HCV and PA:A represent confounding by IDU, or other factors related to IDU. IDU has previously been associated with PH development in animal models [1, 13], and it is often a feature in patients with HIV-associated PH, although the exact pathophysiological mechanisms are incompletely characterized [24].
Methamphetamine use is currently regarded as a definite cause of PH, but we did not find use of recreational drugs besides IDU to be associated with having a PA:A >1. However, because only some recreational drugs are considered definite inducers of PH [3], this could be explained by our broad definition of recreational drugs. Participants were not asked what specific drugs they used, but methamphetamine use is prevalent among Danish PWH, especially among men who have sex with men [25]. HCV coinfection has previously been associated with PH in PWH [1, 2, 13], and though the pathogenesis is incompletely understood, PH could arise from viral effects on the pulmonary vasculature, could result from portal hypertension, or may represent adverse treatment effects [1, 3, 14, 15].
Studies have associated interferon therapy with PH since the early 90s [14], but more recently, severe cases of PH have also been described in patients treated with new DAAs, including sofosbuvir [15]. There were, however, too few cases to reliably access the association between interferon therapy or DAA treatment and having a PA:A >1. As such, we cannot say whether the described effects are merely reversible [14] or whether other, concurrent, mechanisms are involved in PAH development in HCV.
Although this finding was only borderline significant, the odds of having a PA:A >1 in PWH was almost twice that in the uninfected controls, after adjustment for age, sex, and BMI. This analysis was conducted as a sensitivity analysis, and data on pulmonary function and presence of emphysema were not available for the uninfected controls. However, adjustment for smoking status, which is highly associated with pulmonary disease, did not change the size of the association, suggesting that differences in smoking-related pulmonary diseases were not of great importance. A strength of the present study was the size of the study population. Because PH is an infrequent disease with few expected cases, a large and well-characterized population sample is needed to explore associations between potential associated factors and disease. Regarding variables of interest, including pulmonary function, tobacco use, demographic characteristics, and HIV-related variables, information was obtained by medical professionals in a standardized research setting, reducing errors from measurements.
We did not have an invasive measurement of the pulmonary pressures and used a PA:A >1 as a proxy for PH. Although right heart catheterization is the reference standard for diagnosing PH, this is an invasive procedure that cannot be used as a screening tool. Like CT, transthoracic echocardiography may noninvasively estimate the pulmonary pressures, but direct measurements from echocardiography are not correlated well with those obtained through invasive procedures [10, 26–29]. Our estimate of 5%, which would include all PH groups, is comparable to the estimates from echocardiographic studies in European PWH [2], but because there are several causes of pulmonary artery enlargement, including infectious and connective tissue diseases, we likely overestimate the prevalence of PH. Thus, estimates should be interpreted with caution. A PA:A >1.08 has 95% specificity for PH [8], and with this cutoff, the prevalence among well-treated PWH was 2.2%. However, we may inadvertently fail to identify cases of PH with this higher cutoff point. We did not have data on which intravenous drugs were used, and thus we cannot say whether a PA:A >1 is related to specific drugs or other features of IDU, such as talc. In addition, the nature of cross-sectional studies does not allow for conclusions regarding causal relationships.
In conclusion, 1 in 20 participants had a PA:A >1 in this cohort of well-treated PWH. After adjustment for age, sex, and BMI, HIV-unrelated risk factors, including obesity and IDU, were associated with having a PA:A >1, but HIV-related factors were not. HIV serostatus was borderline associated with a PA:A >1, and increased awareness may be appropriate among PWH who are obese or use PWH with IDU.
Notes
Acknowledgments. We extend our gratitude to all participants in the Copenhagen Comorbidity in HIV Infection Study and to the nurses and staff of the departments of Infectious Diseases and Radiology at Rigshospitalet, Copenhagen.
Financial support. This work was supported by the Danish Heart Foundation, the Novo Nordisk Foundation, the Augustinus Foundation, the Lundbeck Foundation, the Rigshospitalet Research Council, and the NIHR Manchester Biomedical Research Centre (J. V.).
Potential conflicts of interest. A. D. K. has received a grant from the Danish Heart Foundation and a travelling grant from Gilead, unrelated to this work. A. M. L. has received traveling grants from Gilead and Merck Sharp & Dohme and has served on advisory boards for Gilead and GlaxoSmithKline (GSK)/ViiV. L. K. has received personal fees from AstraZeneca and Novartis as a speaker at symposia, unrelated to this work. J. V. has received personal fees for advising and/or presenting from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, and Novartis, all unrelated to this work. S. D. N. has received unrestricted research grants from the Novo Nordisk Foundation, the Lundbeck Foundation, the Augustinus Foundation, and the Rigshospitalet Research Council and traveling grants from Gilead, and she has served on advisory boards for Gilead and GSK/ViiV, all unrelated to this work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Bigna JJ, Nansseu JR, Noubiap JJ. Pulmonary hypertension in the global population of adolescents and adults living with HIV: a systematic review and meta-analysis. Sci Rep
Galie N, Humbert M, Simon G, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension—web addenda. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and of the European Respiratory. Eur Heart J
Hoeper MM, Bogaard HJ, Condliffe R, et al. . Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol
Kiely DG, Levin DL, Hassoun PM, et al. . Statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ
Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology
Shen Y, Wan C, Tian P, et al. . CT-base pulmonary artery measurement in the detection of pulmonary hypertension. Medicine (Baltimore)
Corson N, Armato SG 3rd, Labby ZE, Straus C, Starkey A, Gomberg-Maitland M. CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol
Mahammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging
Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest
Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging
Terzikhan N, Bos D, Lahousse L, et al. . Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J
Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev
Savale L, Chaumais MC, O’Connell C, Humbert M, Sitbon O. Interferon-induced pulmonary hypertension: an update. Curr Opin Pulm Med
Renard S, Borentain P, Salaun E, et al. . Severe pulmonary arterial hypertension in patients treated for hepatitis C with sofosbuvir. Chest
Ronit A, Kristensen T, Hoseth VS, et al. . Computed tomography quantification of emphysema in people living with HIV and uninfected controls. Eur Respir J
Ronit A, Haissman J, Kirkegaard-Klitbo DM, et al. . Copenhagen Comorbidity in HIV Infection (COCOMO) study: a study protocol for a longitudinal, non-interventional assessment of non-AIDS comorbidity in HIV infection in Denmark. BMC Infect Dis
Knudsen AD, Fuchs A, Kühl JT, et al. . Coronary artery calcium assessed with calibrated mass scoring in asymptomatic individuals: results from the Copenhagen General Population Study. Eur Radiol
Ronit A, Lundgren J, Afzal S, et al; Copenhagen Co-morbidity in HIV infection (COCOMO) study group. Airflow limitation in people living with HIV and matched uninfected controls. Thorax
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing,
Vachiéry JL, Tedford RJ, Rosenkranz S, et al. . Pulmonary hypertension due to left heart disease. Eur Respir J
Truong QA, Massaro JM, Rogers IS, et al. . Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging
Pham MHC, Ballegaard C, de Knegt MC, et al. . Normal values of aortic dimensions assessed by multidetector computed tomography in the Copenhagen General Population Study. Eur Heart J Cardiovasc Imaging
Harter ZJ, Agarwal S, Dalvi P, Voelkel NF, Dhillon NK. Drug abuse and HIV-related pulmonary hypertension: double hit injury. AIDS
Dittfeld T. Sex på stoffer blandt homoseksuelle, biseksuelle og andre mænd der har sex med mænd i Danmark 2017.
Greiner S, Jud A, Aurich M, et al. . Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc
Lafitte S, Pillois X, Reant P, et al. . Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: a retrospective comparison of routine echocardiography and invasive hemodynamics. J Am Soc Echocardiogr
Fisher MR, Criner GJ, Fishman AP, et al; NETT Research Group. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J




