-
PDF
- Split View
-
Views
-
Cite
Cite
Wei-Yuan Chang, Yen-Cheng Chiu, Fang-Wei Chiu, Yao-Chun Hsu, Tai-Chung Tseng, Pin-Nan Cheng, Sheng-Shun Yang, Chun-Jen Liu, Tung-Hung Su, Hung-Chih Yang, Chen-Hua Liu, Pei-Jer Chen, Ding-Shinn Chen, Jia-Horng Kao, High Risk of Clinical Relapse in Patients With Chronic Hepatitis B Virus Infection After Cessation of Prophylactic Antiviral Therapy for Rituximab-Containing Chemotherapy, The Journal of Infectious Diseases, Volume 222, Issue 8, 15 October 2020, Pages 1345–1352, https://doi.org/10.1093/infdis/jiaa256
Close - Share Icon Share
Abstract
Prophylaxis with nucleos(t)ide analogue (NA) is recommended to prevent hepatitis B virus (HBV) reactivation in hepatitis B surface antigen (HBsAg)–positive patients receiving rituximab-based B-cell depletion therapy. However, little is known about the risk of clinical relapse after withdrawal of NA.
We retrospectively analyzed 77 noncirrhotic HBsAg carriers with hematological cancer who received rituximab-containing chemotherapy. All of them received either prophylactic entecavir or tenofovir therapy. The risk of clinical relapse and hepatic decompensation after cessation of NA was explored.
Clinical relapse and hepatic decompensation developed in 25 (32.5 %) and 11 (14.3 %) of the patients, respectively, and 2 patients died of hepatic decompensation. Most of the hepatic events occurred within 1 year (20 of 25; 80.0%) after stopping NA. A higher pretreatment viral load (≥2000 vs <2000 IU/mL) was associated with increased risks of clinical relapse (hazard ratio, 3.47; 95% confidence interval, 1.56–7.73) and hepatic decompensation (9.91; 2.14–45.92). Of 51 patients with pretreatment viral load <2000 IU/mL, clinical relapse occurred in 10 (19.6 %) and hepatic decompensation in 2 (3.9%).
Pretreatment HBV DNA ≥2000 IU/mL is associated with increased risk of liver-related disease after cessation of prophylactic NA therapy in patients who received rituximab-containing chemotherapy.
An estimated 2 billion people worldwide are infected with the hepatitis B virus (HBV), and nearly 400 million are chronic hepatitis B surface antigen (HBsAg) carriers [1–3]. HBV reactivation is common in HBsAg carriers who take cytotoxic or immunosuppressive agents for cancer; incidences vary from 14% to 72% and are particularly high in patients with lymphoma [4–7]. The mortality rate for these patients after HBV reactivation is between 23% and 71% [6, 8, 9]. In addition to liver-related disease and death, HBV reactivation may cause a delay or premature discontinuation of anticancer therapy in 50%–70% of patients with HBV infection and cancer [5]. Hence, the clinical guidelines recommend prophylactic nucleos(t)ide analogue (NA) therapy for HBV carriers before anticancer treatment [10–12].
Rituximab is a chimeric murine/human monoclonal antibody targeting CD20 antigen present on the surface of normal and malignant B lymphocytes. In combination with other cytotoxic therapy, it has become the standard treatment for CD20-positive B-cell cancer. For example, R-CHOP, treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), has become the first-line treatment for patients with CD20-positive diffuse large B-cell lymphoma [13–15].
The HBV reactivation rate during cytotoxic therapy containing rituximab could be as high as 20%–55%, considerably higher than during therapy without rituximab [7, 8, 16–18]. Even among patients with resolved HBV infection (HBsAg negative but positive for antibody to hepatitis B core antigen), who rarely experience HBV reactivation during traditional chemotherapy, approximately 4.1%–23.8% may experience HBV reactivation during cytotoxic therapy with rituximab [19, 20]. Taking these lines of evidence together, we infer that patients with HBV infection are highly susceptible to HBV reactivation during rituximab-containing cytotoxic therapy. Thus, antiviral prophylaxis for ≥6 months after chemotherapy is strongly recommended to prevent subsequent complications [10–12].
However, whether the risk of clinical relapse remains after cessation of prophylactic NA treatment is unclear. To address this question, we retrospectively analyzed 77 noncirrhotic HBsAg carriers with hematological cancer who received rituximab-containing chemotherapy.
PATIENTS AND METHODS
Study Design and Patient Cohort
Noncirrhotic HBsAg carriers with hematological cancers treated with rituximab-containing cytotoxic therapy and prophylactic NA, including entecavir or tenofovir, were retrospectively enrolled from National Taiwan University Hospital, National Cheng Kong University Hospital, E-Da Hospital, and Taichung Veterans General Hospital. Of these 300 patients, a number were excluded, for the following reasons: 131 because they did not stop NA therapy, 26 because NA was prescribed later than chemotherapy, 8 because of lamivudine use, 7 because of lack of pretreatment HBV load data, 4 because of hepatitis C virus (HCV) and 1 because of hepatitis D virus coinfection, and 3 because of unclear NA prescription history. We also excluded 29 patients for inadequate length of extended NA therapy (<24 weeks) and 14 patients with pretreatment alanine aminotransferase (ALT) levels >40 U/L accompanied by HBV loads ≥2000 IU/mL, because they needed prolonged antiviral therapy. Finally, a total of 77 patients were included. (Figure 1)
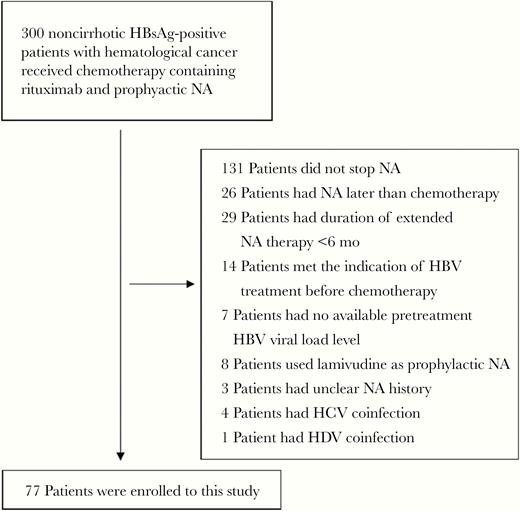
Enrollment flow of patients from 4 hospitals. Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; NA, nucleos(t)ide analogue.
Data Collection
Clinical and histological data, including age, sex, pathological data based on immunohistochemical staining (germinal center and nongerminal center B cell), laboratory test results (including ALT, hepatitis B e antigen, HBsAg, and HBV DNA levels), antiviral drug use and duration of use, and cytotoxic therapy regimens before the start of antiviral therapy were retrieved from the medical records of each participating hospital.
The follow-up period started at the end of NA therapy. Viral and liver function values were periodically determined, including aspartate aminotransferase , ALT, and bilirubin levels and prothrombin time. NA therapy was promptly readministered for all cases with HBV clinical relapse or, in some cases, solely viral relapse.
The study protocol has been approved by the Institutional Review Boards of National Taiwan University Hospital, National Cheng Kong University Hospital, E-Da Hospital, and Taichung Veterans General Hospital, respectively.
Definition of Hepatic Events
Virological relapse was defined as HBV DNA levels ≥2000 IU/mL after withdrawal of prophylactic NA therapy. Clinical relapse was defined as serum ALT level > 2 × the upper limit of normal (40 U/L) accompanied by viral relapse [21]. HBV-associated hepatic decompensation was defined as impaired synthetic function (total bilirubin >3 mg/dL or international normalized ratio >1.5) [22], and HBV-associated liver failure was defined as the development of ascites, encephalopathy, or hepatorenal syndrome.
Serological Assays
Serum HBsAg, anti-HCV, and anti–hepatitis D virus were tested using commercial assays (Abbott Laboratories).
Quantification of HBV DNA Level
HBV DNA level was quantified using the Abbott Real-time HBV assay (Abbott Laboratories): a 0.2-mL protocol with a detection limit of 15 IU/mL in the National Taiwan University Hospital and a 0.5-mL protocol with a detection limit of 10 IU/mL in the E-Da Hospital. The Roche COBAS TaqMan 48 real-time polymerase chain reaction system (Roche Molecular Systems) with a detection limit of 20 to 1.7 × 108 IU/mL was used in Taichung Veterans General Hospital and National Cheng Kung University Hospital.
Statistical Analyses
Clinical relapse was defined as the primary end point. Virological relapse and HBV-associated liver decompensation were defined as 2 separate secondary end points. Clinical follow-up started from the time of withdrawal of prophylactic NA. Person-years were calculated according to the date of developing each end point, death, the last date of follow up, or 31 January 2019, whichever came first. The cumulative incidence of each end point stratified by different variables was derived using Kaplan-Meier curve analysis, and log-rank tests were used to determine statistical difference.
Continuous variables were compared using t or Mann-Whitney rank sum tests. The Cox proportional hazards regression model was used to calculate crude and multivariable-adjusted hazard ratios (HR) in predicting different end points. Statistical significance for all tests was defined as P < .05 (2 tailed). The analyses were performed using Stata statistical software (version 10.0; StataCorp).
RESULTS
Patient Characteristics
The baseline characteristics of 77 patients are summarized in Table 1. In brief, the median age was 58 years (range, 22–83 years) and 47 men (61.0%) were included. The most common diagnosis of hematological disease was diffused large B-cell lymphoma (50 of 77), followed by follicular lymphoma (12 of 77) and mantle cell lymphoma (5 of 77). Twenty-six patients (33.8%) had a high HBV DNA level (≥2000 IU/mL) before the administration of prophylactic NA. The median ALT level before administration of prophylactic NA was 21 U/L (range 3–70 U/L), and 10 patients had an abnormal ALT level (≥40 U/L). All of these 10 patients had HBV DNA levels <2000 IU/mL.
Pretreatment Clinical and Virological Characteristics of the 77 Patients Receiving Chemotherapy Containing Anti-CD20 Antibody
| Characteristic . | Patients, No. (%) . |
|---|---|
| Sex | |
| Female | 30 (39.0) |
| Male | 47 (61.0) |
| Age at administration of NA, y | |
| 18–39 | 8 (10.4) |
| 40–49 | 14 (18.2) |
| 50–59 | 21 (27.3) |
| ≥60 | 34 (44.2) |
| Serum ALT level, U/L | |
| <20 | 34 (44.2) |
| 20–39 | 33 (42.9) |
| ≥40a | 10 (12.9) |
| Serum HBV load, IU/mL | |
| <2000 | 51(66.2) |
| ≥2000 | 26(33.8) |
| Diagnosis | |
| DLBCL | 50 (64.9) |
| Follicular cell lymphoma | 12 (15.6) |
| Mantle cell lymphoma | 5 (6.5) |
| Other | 10 (13.0) |
| NA regimen | |
| Entecavir | 72 (93.5) |
| TDF | 5 (6.5) |
| Duration of extended NA therapy, mo | |
| 6–12 | 55 (71.4) |
| 12–18 | 11 (14.3) |
| >18 | 11 (14.3) |
| Characteristic . | Patients, No. (%) . |
|---|---|
| Sex | |
| Female | 30 (39.0) |
| Male | 47 (61.0) |
| Age at administration of NA, y | |
| 18–39 | 8 (10.4) |
| 40–49 | 14 (18.2) |
| 50–59 | 21 (27.3) |
| ≥60 | 34 (44.2) |
| Serum ALT level, U/L | |
| <20 | 34 (44.2) |
| 20–39 | 33 (42.9) |
| ≥40a | 10 (12.9) |
| Serum HBV load, IU/mL | |
| <2000 | 51(66.2) |
| ≥2000 | 26(33.8) |
| Diagnosis | |
| DLBCL | 50 (64.9) |
| Follicular cell lymphoma | 12 (15.6) |
| Mantle cell lymphoma | 5 (6.5) |
| Other | 10 (13.0) |
| NA regimen | |
| Entecavir | 72 (93.5) |
| TDF | 5 (6.5) |
| Duration of extended NA therapy, mo | |
| 6–12 | 55 (71.4) |
| 12–18 | 11 (14.3) |
| >18 | 11 (14.3) |
aThe HBV loads in patients with elevated ALT levels were all <2000 IU/mL
Abbreviations: ALT, alanine aminotransferase; DLBCL, diffuse large B-cell lymphoma; HBV, hepatitis B virus; NA, nucleos(t)ide analogue; TDF, tenofovir disoproxil fumarate.
Pretreatment Clinical and Virological Characteristics of the 77 Patients Receiving Chemotherapy Containing Anti-CD20 Antibody
| Characteristic . | Patients, No. (%) . |
|---|---|
| Sex | |
| Female | 30 (39.0) |
| Male | 47 (61.0) |
| Age at administration of NA, y | |
| 18–39 | 8 (10.4) |
| 40–49 | 14 (18.2) |
| 50–59 | 21 (27.3) |
| ≥60 | 34 (44.2) |
| Serum ALT level, U/L | |
| <20 | 34 (44.2) |
| 20–39 | 33 (42.9) |
| ≥40a | 10 (12.9) |
| Serum HBV load, IU/mL | |
| <2000 | 51(66.2) |
| ≥2000 | 26(33.8) |
| Diagnosis | |
| DLBCL | 50 (64.9) |
| Follicular cell lymphoma | 12 (15.6) |
| Mantle cell lymphoma | 5 (6.5) |
| Other | 10 (13.0) |
| NA regimen | |
| Entecavir | 72 (93.5) |
| TDF | 5 (6.5) |
| Duration of extended NA therapy, mo | |
| 6–12 | 55 (71.4) |
| 12–18 | 11 (14.3) |
| >18 | 11 (14.3) |
| Characteristic . | Patients, No. (%) . |
|---|---|
| Sex | |
| Female | 30 (39.0) |
| Male | 47 (61.0) |
| Age at administration of NA, y | |
| 18–39 | 8 (10.4) |
| 40–49 | 14 (18.2) |
| 50–59 | 21 (27.3) |
| ≥60 | 34 (44.2) |
| Serum ALT level, U/L | |
| <20 | 34 (44.2) |
| 20–39 | 33 (42.9) |
| ≥40a | 10 (12.9) |
| Serum HBV load, IU/mL | |
| <2000 | 51(66.2) |
| ≥2000 | 26(33.8) |
| Diagnosis | |
| DLBCL | 50 (64.9) |
| Follicular cell lymphoma | 12 (15.6) |
| Mantle cell lymphoma | 5 (6.5) |
| Other | 10 (13.0) |
| NA regimen | |
| Entecavir | 72 (93.5) |
| TDF | 5 (6.5) |
| Duration of extended NA therapy, mo | |
| 6–12 | 55 (71.4) |
| 12–18 | 11 (14.3) |
| >18 | 11 (14.3) |
aThe HBV loads in patients with elevated ALT levels were all <2000 IU/mL
Abbreviations: ALT, alanine aminotransferase; DLBCL, diffuse large B-cell lymphoma; HBV, hepatitis B virus; NA, nucleos(t)ide analogue; TDF, tenofovir disoproxil fumarate.
Prophylactic NA was used in all patients not later than the day of anticancer therapy administration; 72 patients used entecavir, and the other 5 used tenofovir. The median duration of the extended NA therapy was 6.96 months (range, 6.11–34.07), which was defined as the time from the cessation of anticancer therapy to the withdrawal of prophylactic NA. Viral loads were available in 61 patients before cessation of NA, and viral clearance was achieved in all but 1 patient. That patient received entecavir and had an HBV DNA level of 295 IU/mL 6 days before stopping NA, but he did not experience virological or clinical relapse.
Virological Relapse After Withdrawal of Prophylactic NA Therapy
Every patient in our cohort underwent a liver function test, but not an HBV DNA test, periodically after completion of prophylactic NA therapy. HBV load data were available in 54 patients after withdrawal of prophylactic NA. Among these patients, 32 (59.3%) experienced viral relapse, 22 in the first year after the withdrawal of prophylactic NA therapy.
Clinical Relapse and Subsequent Hepatic Decompensation After Withdrawal of Prophylactic NA Therapy
During prophylactic NA therapy, no patient experienced clinical relapse. The cumulative incidence of clinical relapse is presented in Figure 2A. In total, 25 patients (32.5%) experienced clinical relapse during the follow-up period, and the 2-year cumulative incidence of relapse was 31.2% (24 of 77), most (20 of 25 [80.0%]) occurring within the first year. Among patients with clinical relapse, 11 (44.0%) experienced hepatic decompensation. Although all patients with clinical relapse were immediately prescribed NA, 3 patients progressed into hepatic failure, of whom 2 died. The cumulative incidence of HBV-associated hepatic decompensation is shown in Figure 2B. The baseline characteristics of 11 patients with HBV-associated hepatic decompensation are summarized in Supplementary Table 1.
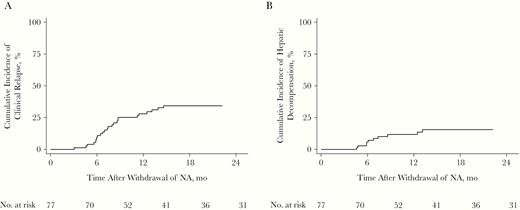
Cumulative incidence of clinical relapse (A) and hepatitis B virus–associated hepatic decompensation (B) after withdrawal of prophylactic nucleos(t)ide analogue (NA) in 77 patients.
Factors Associated With Clinical Relapse and Hepatic Decompensation
We explored the factors associated with clinical relapse and hepatic decompensation. We found that a pretreatment HBV DNA level ≥2000 IU/mL was associated with an increased risk of HBV clinical relapse (P = .002) (Figure 3A). No difference in the risk was observed between patients receiving short (6–12 months) versus longer (>12 months) extended NA therapy (Figure 3B). Multivariable analysis indicated that a pretreatment HBV DNA level ≥2000 IU/mL remained a risk factor for clinical relapse, with an HR of 4.12 (95% confidence interval [CI], 1.71–9.95; P = .002), compared with those with low viral load (Table 2).
Univariable and Multivariable Analysis of Factors Associated With Clinical Relapse in 77 Patients With Chronic Hepatitis B Virus Infection After Withdrawal of Prophylactic Nucleos(t)ide Analogue
| Factor . | Patients, No. . | Duration of Follow-up, mo . | Patients With Clinical Relapse, No. . | Crude HR (95% CI) . | Adjusted HR (95% CI) . |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 30 | 961.57 | 7 | 1.0 | 1.0 |
| Male | 47 | 1062.63 | 18 | 2.00 (.84–4.80) | 1.65 (.68–4.00) |
| Age (per 1-y increase) | 0.99 (.97–1.03) | 1.02 (.99–1.06) | |||
| Pretreatment serum ALT level, U/L | |||||
| <20 | 34 | 887.2 | 8 | 1.0 | 1.0 |
| ≥20 | 43 | 1121.27 | 17 | 1.81 (.78–4.21) | 1.98 (.82–4.78) |
| HBV load, IU/mL | |||||
| <2000 | 51 | 1447.7 | 10 | 1.0 | 1.0 |
| ≥2000 | 26 | 573.43 | 15 | 3.47 (1.56–7.73)a | 4.12 (1.71–9.95)a |
| Duration of extended therapy, mo | |||||
| 6–12 | 56 | 1518.93 | 17 | 1.0 | 1.0 |
| >12 | 21 | 502.2 | 8 | 1.23 (.53–2.86) | 1.03 (.41–2.55) |
| Factor . | Patients, No. . | Duration of Follow-up, mo . | Patients With Clinical Relapse, No. . | Crude HR (95% CI) . | Adjusted HR (95% CI) . |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 30 | 961.57 | 7 | 1.0 | 1.0 |
| Male | 47 | 1062.63 | 18 | 2.00 (.84–4.80) | 1.65 (.68–4.00) |
| Age (per 1-y increase) | 0.99 (.97–1.03) | 1.02 (.99–1.06) | |||
| Pretreatment serum ALT level, U/L | |||||
| <20 | 34 | 887.2 | 8 | 1.0 | 1.0 |
| ≥20 | 43 | 1121.27 | 17 | 1.81 (.78–4.21) | 1.98 (.82–4.78) |
| HBV load, IU/mL | |||||
| <2000 | 51 | 1447.7 | 10 | 1.0 | 1.0 |
| ≥2000 | 26 | 573.43 | 15 | 3.47 (1.56–7.73)a | 4.12 (1.71–9.95)a |
| Duration of extended therapy, mo | |||||
| 6–12 | 56 | 1518.93 | 17 | 1.0 | 1.0 |
| >12 | 21 | 502.2 | 8 | 1.23 (.53–2.86) | 1.03 (.41–2.55) |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HR, hazard ratio.
aSignificant at P < .05.
Univariable and Multivariable Analysis of Factors Associated With Clinical Relapse in 77 Patients With Chronic Hepatitis B Virus Infection After Withdrawal of Prophylactic Nucleos(t)ide Analogue
| Factor . | Patients, No. . | Duration of Follow-up, mo . | Patients With Clinical Relapse, No. . | Crude HR (95% CI) . | Adjusted HR (95% CI) . |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 30 | 961.57 | 7 | 1.0 | 1.0 |
| Male | 47 | 1062.63 | 18 | 2.00 (.84–4.80) | 1.65 (.68–4.00) |
| Age (per 1-y increase) | 0.99 (.97–1.03) | 1.02 (.99–1.06) | |||
| Pretreatment serum ALT level, U/L | |||||
| <20 | 34 | 887.2 | 8 | 1.0 | 1.0 |
| ≥20 | 43 | 1121.27 | 17 | 1.81 (.78–4.21) | 1.98 (.82–4.78) |
| HBV load, IU/mL | |||||
| <2000 | 51 | 1447.7 | 10 | 1.0 | 1.0 |
| ≥2000 | 26 | 573.43 | 15 | 3.47 (1.56–7.73)a | 4.12 (1.71–9.95)a |
| Duration of extended therapy, mo | |||||
| 6–12 | 56 | 1518.93 | 17 | 1.0 | 1.0 |
| >12 | 21 | 502.2 | 8 | 1.23 (.53–2.86) | 1.03 (.41–2.55) |
| Factor . | Patients, No. . | Duration of Follow-up, mo . | Patients With Clinical Relapse, No. . | Crude HR (95% CI) . | Adjusted HR (95% CI) . |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 30 | 961.57 | 7 | 1.0 | 1.0 |
| Male | 47 | 1062.63 | 18 | 2.00 (.84–4.80) | 1.65 (.68–4.00) |
| Age (per 1-y increase) | 0.99 (.97–1.03) | 1.02 (.99–1.06) | |||
| Pretreatment serum ALT level, U/L | |||||
| <20 | 34 | 887.2 | 8 | 1.0 | 1.0 |
| ≥20 | 43 | 1121.27 | 17 | 1.81 (.78–4.21) | 1.98 (.82–4.78) |
| HBV load, IU/mL | |||||
| <2000 | 51 | 1447.7 | 10 | 1.0 | 1.0 |
| ≥2000 | 26 | 573.43 | 15 | 3.47 (1.56–7.73)a | 4.12 (1.71–9.95)a |
| Duration of extended therapy, mo | |||||
| 6–12 | 56 | 1518.93 | 17 | 1.0 | 1.0 |
| >12 | 21 | 502.2 | 8 | 1.23 (.53–2.86) | 1.03 (.41–2.55) |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HR, hazard ratio.
aSignificant at P < .05.
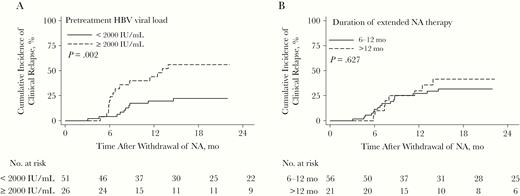
Cumulative incidence of clinical relapse after withdrawal of prophylactic nucleos(t)ide analogue (NA) stratified according to pretreatment viral load (<2000 vs ≥2000 IU/mL) (A) and duration of extended NA therapy (6–12 vs >12 months) (B).
In addition to clinical relapse, pretreatment HBV DNA level ≥2000 IU/mL was consistently associated with the risk of HBV-associated hepatic decompensation (Figure 4A) (HR, 9.9;1 95% CI, 2.14–45.92; P = .003). Longer extended NA therapy was not associated with lower risk of liver decompensation. (Figure 4B)
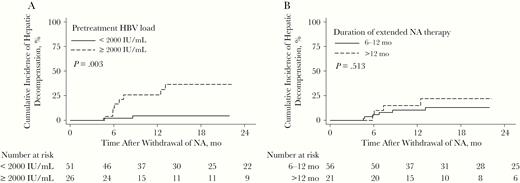
Cumulative incidence of hepatitis B virus (HBV)–associated hepatic decompensation after withdrawal of prophylactic nucleos(t)ide analogue (NA) stratified by pretreatment viral load of 2000 IU/mL load (<2000 vs ≥2000 IU/mL) (A) and duration of extended NA therapy (6–12 vs >12 months) (B).
Factors Associated With Virological Relapse
From the 54 patients with available HBV load profiles during the follow-up period, risk factors of virological relapse were explored. Pretreatment viral load was the only risk factor, and multivariable analysis showed that a pretreatment HBV DNA level ≥2000 IU/mL was the only risk factor with an HR of 3.91 (95% CI, 1.91–7.97; P < .005), compared with those with lower viral load.
Risk of Clinical Relapse and Hepatic Decompensation Remains in Patients With Low HBV DNA Levels
A total of 51 patients presented with a low pretreatment HBV load (<2000 IU/mL) before administration of prophylactic NA therapy. After withdrawal of prophylactic NA, clinical relapse occurred in 10 patients (19.6%). Among them, 8 patients received 6–7 months of extended NA therapy, and the other 2 patients received >24 months of therapy. The viral and host factors were comparable between those with and those without clinical relapse (Table 3). Notably, 2 patients who received 6–7 months of extended NA therapy experienced hepatic decompensation after withdrawal of NA and 1 eventually died from hepatic failure.
Host and Viral Factors in 51 Patients With Low Pretreatment Viral Load, With or Without Clinical Relapse After Withdrawal of Nucleos(t)ide Analogue
| . | Patients, No. (%)a . | . | |
|---|---|---|---|
| Factor . | Without Clinical Relapse (n = 41) . | With Clinical Relapse (n = 10) . | P Value . |
| Age, mean (SD), y | 58 (13) | 63 (11) | .81 |
| Sex | |||
| Male | 23 (56.1) | 7 (70) | .42 |
| Female | 18 (43.9) | 3 (30) | |
| HBeAg status before NAb | |||
| Positive | 0/33 (0) | 0/8 (0) | 1.00 |
| Negative | 33/33 (100) | 8/8 (100) | |
| Fibrosis 4 index before NA, mean (SD) | 2.11 (2.4) | 1.75 (0.9) | .64 |
| Undetectable HBV DNA level before cessation of NAc | |||
| Achieved | 36/37 (97.3) | 9/9 (100) | .62 |
| Not achieved | 1/37 (2.7) | 0/9 (0) | |
| Duration of extended NA therapy, mo | |||
| 6–12 | 33 (80.5) | 8 (80) | .97 |
| >12 | 8 (19.5) | 2 (20) | |
| NA regimen | |||
| Entecavir | 39 (95.1) | 9 (90) | .53 |
| TDF | 2 (4.9) | 1 (10) | |
| Hematological disease | |||
| DLBCL | 29 (70.7) | 3 (30) | .09 |
| Follicular cell lymphoma | 5 (12.2) | 3 (30) | |
| Mantle cell lymphoma | 2 (4.9) | 2 (20) | |
| Others | 5 (12.2) | 2 (20) | |
| . | Patients, No. (%)a . | . | |
|---|---|---|---|
| Factor . | Without Clinical Relapse (n = 41) . | With Clinical Relapse (n = 10) . | P Value . |
| Age, mean (SD), y | 58 (13) | 63 (11) | .81 |
| Sex | |||
| Male | 23 (56.1) | 7 (70) | .42 |
| Female | 18 (43.9) | 3 (30) | |
| HBeAg status before NAb | |||
| Positive | 0/33 (0) | 0/8 (0) | 1.00 |
| Negative | 33/33 (100) | 8/8 (100) | |
| Fibrosis 4 index before NA, mean (SD) | 2.11 (2.4) | 1.75 (0.9) | .64 |
| Undetectable HBV DNA level before cessation of NAc | |||
| Achieved | 36/37 (97.3) | 9/9 (100) | .62 |
| Not achieved | 1/37 (2.7) | 0/9 (0) | |
| Duration of extended NA therapy, mo | |||
| 6–12 | 33 (80.5) | 8 (80) | .97 |
| >12 | 8 (19.5) | 2 (20) | |
| NA regimen | |||
| Entecavir | 39 (95.1) | 9 (90) | .53 |
| TDF | 2 (4.9) | 1 (10) | |
| Hematological disease | |||
| DLBCL | 29 (70.7) | 3 (30) | .09 |
| Follicular cell lymphoma | 5 (12.2) | 3 (30) | |
| Mantle cell lymphoma | 2 (4.9) | 2 (20) | |
| Others | 5 (12.2) | 2 (20) | |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; NA, nucleos(t)ide analogue; SD standard deviation; TDF, tenofovir disoproxil fumarate.
aData represent no. (%) of patients unless otherwise specified.
bData available in 41 patients.
cData available in 46 patients.
Host and Viral Factors in 51 Patients With Low Pretreatment Viral Load, With or Without Clinical Relapse After Withdrawal of Nucleos(t)ide Analogue
| . | Patients, No. (%)a . | . | |
|---|---|---|---|
| Factor . | Without Clinical Relapse (n = 41) . | With Clinical Relapse (n = 10) . | P Value . |
| Age, mean (SD), y | 58 (13) | 63 (11) | .81 |
| Sex | |||
| Male | 23 (56.1) | 7 (70) | .42 |
| Female | 18 (43.9) | 3 (30) | |
| HBeAg status before NAb | |||
| Positive | 0/33 (0) | 0/8 (0) | 1.00 |
| Negative | 33/33 (100) | 8/8 (100) | |
| Fibrosis 4 index before NA, mean (SD) | 2.11 (2.4) | 1.75 (0.9) | .64 |
| Undetectable HBV DNA level before cessation of NAc | |||
| Achieved | 36/37 (97.3) | 9/9 (100) | .62 |
| Not achieved | 1/37 (2.7) | 0/9 (0) | |
| Duration of extended NA therapy, mo | |||
| 6–12 | 33 (80.5) | 8 (80) | .97 |
| >12 | 8 (19.5) | 2 (20) | |
| NA regimen | |||
| Entecavir | 39 (95.1) | 9 (90) | .53 |
| TDF | 2 (4.9) | 1 (10) | |
| Hematological disease | |||
| DLBCL | 29 (70.7) | 3 (30) | .09 |
| Follicular cell lymphoma | 5 (12.2) | 3 (30) | |
| Mantle cell lymphoma | 2 (4.9) | 2 (20) | |
| Others | 5 (12.2) | 2 (20) | |
| . | Patients, No. (%)a . | . | |
|---|---|---|---|
| Factor . | Without Clinical Relapse (n = 41) . | With Clinical Relapse (n = 10) . | P Value . |
| Age, mean (SD), y | 58 (13) | 63 (11) | .81 |
| Sex | |||
| Male | 23 (56.1) | 7 (70) | .42 |
| Female | 18 (43.9) | 3 (30) | |
| HBeAg status before NAb | |||
| Positive | 0/33 (0) | 0/8 (0) | 1.00 |
| Negative | 33/33 (100) | 8/8 (100) | |
| Fibrosis 4 index before NA, mean (SD) | 2.11 (2.4) | 1.75 (0.9) | .64 |
| Undetectable HBV DNA level before cessation of NAc | |||
| Achieved | 36/37 (97.3) | 9/9 (100) | .62 |
| Not achieved | 1/37 (2.7) | 0/9 (0) | |
| Duration of extended NA therapy, mo | |||
| 6–12 | 33 (80.5) | 8 (80) | .97 |
| >12 | 8 (19.5) | 2 (20) | |
| NA regimen | |||
| Entecavir | 39 (95.1) | 9 (90) | .53 |
| TDF | 2 (4.9) | 1 (10) | |
| Hematological disease | |||
| DLBCL | 29 (70.7) | 3 (30) | .09 |
| Follicular cell lymphoma | 5 (12.2) | 3 (30) | |
| Mantle cell lymphoma | 2 (4.9) | 2 (20) | |
| Others | 5 (12.2) | 2 (20) | |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; NA, nucleos(t)ide analogue; SD standard deviation; TDF, tenofovir disoproxil fumarate.
aData represent no. (%) of patients unless otherwise specified.
bData available in 41 patients.
cData available in 46 patients.
DISCUSSION
Prophylactic NA therapy reduces HBV reactivation in HBV carriers receiving rituximab-containing cytotoxic therapy. Although an extended NA treatment is recommended, the optimal duration has not yet been defined. The present study demonstrated a high risk of clinical relapse (32.5% [25 of 77]) and hepatic decompensation (14.3% [11 of 77]) after cessation of prophylactic NA therapy in noncirrhotic HBV carriers receiving rituximab-containing cytotoxic therapy. Most complications occurred within 1 year after the end of NA therapy. The pretreatment HBV DNA level was associated with development of clinical relapse and hepatic decompensation. Even in patients with a pretreatment HBV DNA level <2000 IU/mL, the risk of clinical relapse (19.6% [10 of 51]) or even hepatic decompensation (3.9% [2 of 51]) remained. Such a risk profile suggests that indefinite NA treatment should be considered in this special clinical setting if our findings are validated.
The risk of clinical relapse or development of hepatic decompensation is usually minimal after cessation of prophylactic NA treatment in HBV carriers receiving cytotoxic therapy without rituximab [21, 22]. However, our data revealed alarming consequences for HBV carriers who received rituximab-containing cytotoxic therapy against B-cell cancer. Although our cohort enrolled noncirrhotic patients only and none of them meet the indication of HBV treatment before chemotherapy, the cumulative incidence of subsequent HBV clinical relapse and hepatic decompensation after withdrawal of prophylactic NA was still high. Whether the high risk was attributable to rituximab or other cytotoxic drugs was unclear.
We thus compared our data with data from studies enrolling HBV carriers with hematological cancer who received cytotoxic treatment without rituximab. Hsu et al [23] reported that in 26 HBV carriers in Taiwan receiving lamivudine as prophylaxis for CHOP therapy, the 1-year incidence of clinical relapse and hepatic decompensation was 15.4% and 11.5%, respectively, after cessation of prophylactic lamivudine. A similar result was reported by Hui et al [24], who explored post-NA risks in 46 patients in Hong Kong with hematological cancer receiving chemotherapy without rituximab. After an extended lamivudine treatment with a median duration of 3.1 month (range, 3.0–3.4 months), incidence rates of clinical HBV reactivation and subsequent hepatic failure were 23.9% and 6.5%, respectively. Lamivudine is a less potent NA with a higher risk of resistant mutant variants, and it is thus associated with a higher risk of clinical relapse [25–28]. Even so, both studies reported a lower risk of clinical relapse and hepatic decompensation than in our cohort using rituximab. Taken together, these findings indicate that rituximab may play a crucial role in inducing HBV relapse.
Our analysis revealed that a pretreatment HBV DNA level ≥2000 IU/mL was associated with an increased risk of clinical relapse in patients receiving chemotherapy containing anti-CD20 antibody. An association between high pretreatment HBV load and HBV reactivation during cytotoxic therapy has been reported [29–31]. The association between high viral load and increased clinical relapse after withdrawal of prophylactic antiviral therapy was reported only by Hui et al [24], concerning patients receiving cytotoxic therapy without rituximab. Their data also suggested that a pretreatment serum HBV DNA level ≥2000 IU/mL stratified the risk of clinical relapse after withdrawal of prophylactic lamivudine therapy (10% vs 50%; P = .001). Combining the data from Hui et al [24] and our findings, pretreatment viral load is an important predictor for post-NA clinical relapse in chronic hepatitis B patients receiving cytotoxic therapy with or without anti-CD20 antibody to treat hematological cancer.
How the post-NA risk of clinical relapse is enhanced by rituximab-containing therapy is unclear. HBV relapse after rituximab treatment is probably caused by the depletion of B cells. However, the risk of clinical relapse remains in patients receiving extended NA therapy for >12 months, a period expected to be adequate for B-cell recovery. A possible explanation is that B cells against HBV may not return to pretreatment levels. Studies are required to determine the underlying mechanisms.
Our study had several limitations. First, because it was retrospective and observational, it was inevitable that some data would be missing, including the pretreatment fibrosis severity, HBsAg level before cessation of NA, and HBV DNA levels during follow-up. A prospective cohort study will provide more comprehensive data and more accurate estimations of virological relapse. Second, our study cohort involved different hematological diseases and anticancer regimens. The effect of underlying hematological diseases and the influence of different cytotoxic therapy regimens on a given patient’s immunological control of HBV should be considered. Third, potential predictors of HBV clinical relapse and hepatic failure in this population remain to be explored. For example, in a search for predictive factors for post-NA HBV reactivation in HBV-infected patients without cancer, the HBV DNA levels at 1 month after NA discontinuation and end-of-therapy HBsAg levels both showed a significant association with post-NA hepatic flares [32]. Therefore, a prospective study is required to explore the relationship between these factors and the hepatic outcome, which can help enhance risk stratification for these high-risk patients.
In summary, our data indicate a high risk of clinical HBV relapse or hepatic decompensation after withdrawal of prophylactic NA treatment in with patients HBV infection receiving rituximab-containing cytotoxic therapy. A higher pretreatment HBV viral load is associated with increased risk of clinical relapse. Vigilant management is strongly recommended after cessation of NA therapy in these at-risk patients with HBV.
Notes
Acknowledgments. This manuscript was edited by Wallace Academic Editing. We thank the staff of the Department of Medical Research, National Taiwan University Hospital for providing the integrated medical database (NTUH-iMD).
Author contributions. Study concept and design: T. C. T. and J. H. K. Data acquisition: Y. C. C., F. W. C., T. C. T., P. N. C., S. S. Y., C. J. L., T. H. S., H. C. Y., C. H. L., P. J. C., D. S. C., and J. H. K. Manuscript drafting: W. Y. C. and T. C. T. Critical review of the manuscript: J. H. K. Statistical analysis: W. Y. C. and T. C. T. Funding acquisition: T. C. T. and J. H. K. Technical and material support: T. C. T. Study supervision: D. S. C. and J. H. K. All authors approved the final version of the manuscript.
Financial support. This work was supported by the National Taiwan University Hospital (grants 107-N4041 and 108-N4157), the Ministry of Science and Technology, Executive Yuan, Taiwan (grant 106-2314-B-002-136), and the National Health Research Institutes (NHRI-EX108-10807BC).
Potential conflicts of interest. Y. C. H. has received lecture fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and Novartis, served as an advisory committee member for Gilead Sciences, and received funding from Gilead Sciences for research unrelated to the present study. T. C. T. has received speaker fees from AbbVie, Bristol-Myers Squibb, and Gilead Sciences. S. S. Y. has received speaking fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Ipsen, and Merck Sharp & Dohme and has served as an advisory board member for AbbVie. P. J. C. has received grants from Pharmaessentia outside the submitted work and honoraria from Gilead Sciences and Evotec. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
W. Y. C. and Y. C. C. contributed equally to this work.




