-
PDF
- Split View
-
Views
-
Cite
Cite
Barbara Knust, Shelley Brown, Annabelle de St. Maurice, Shannon Whitmer, Sarah E Koske, Elizabeth Ervin, Ketan Patel, James Graziano, Maria E Morales-Betoulle, Jennifer House, Deborah Cannon, Janna Kerins, Stacy Holzbauer, Connie Austin, Suzanne Gibbons-Burgener, Leah Colton, John Dunn, Sara Zufan, Mary Joung Choi, William R Davis, Cheng-Feng Chiang, Craig R Manning, Linda Roesch, Trevor Shoemaker, Lawrence Purpura, Jennifer McQuiston, Dallin Peterson, Rachel Radcliffe, Ann Garvey, Ellen Christel, Laura Morgan, Joni Scheftel, James Kazmierczak, John D Klena, Stuart T Nichol, Pierre E Rollin, the Multistate Seoul Virus Outbreak Investigation Team , Seoul Virus Infection and Spread in United States Home-Based Ratteries: Rat and Human Testing Results From a Multistate Outbreak Investigation, The Journal of Infectious Diseases, Volume 222, Issue 8, 15 October 2020, Pages 1311–1319, https://doi.org/10.1093/infdis/jiaa307
Close - Share Icon Share
Abstract
During 2017, a multistate outbreak investigation occurred after the confirmation of Seoul virus (SEOV) infections in people and pet rats. A total of 147 humans and 897 rats were tested.
In addition to immunoglobulin (Ig)G and IgM serology and traditional reverse-transcription polymerase chain reaction (RT-PCR), novel quantitative RT-PCR primers/probe were developed, and whole genome sequencing was performed.
Seventeen people had SEOV IgM, indicating recent infection; 7 reported symptoms and 3 were hospitalized. All patients recovered. Thirty-one facilities in 11 US states had SEOV infection, and among those with ≥10 rats tested, rat IgG prevalence ranged 2%–70% and SEOV RT-PCR positivity ranged 0%–70%. Human laboratory-confirmed cases were significantly associated with rat IgG positivity and RT-PCR positivity (P = .03 and P = .006, respectively). Genomic sequencing identified >99.5% homology between SEOV sequences in this outbreak, and these were >99% identical to SEOV associated with previous pet rat infections in England, the Netherlands, and France. Frequent trade of rats between home-based ratteries contributed to transmission of SEOV between facilities.
Pet rat owners, breeders, and the healthcare and public health community should be aware and take steps to prevent SEOV transmission in pet rats and to humans. Biosecurity measures and diagnostic testing can prevent further infections.
(See the Editorial Commentary by Fill, on pages 1247–9.)
Seoul virus (SEOV), an Old World Orthohantavirus, is globally endemic due to the worldwide distribution of its primary rodent hosts: Rattus norvegicus, the Norway rat [1], and Rattus rattus, the black rat [2]. Trapping studies of wild rats in many parts of the world have found SEOV ribonucleic acid (RNA) [3, 4], infectious virus [5], and antibodies [6, 7]. Infected rats shed virus in urine, saliva, and feces for periods from 1 month to >4 months [8], and they can develop antibodies while they are actively infected and shedding virus [9]. Rats become infected through bites or from exposure to infectious urine and feces of other rats [10, 11]. There is no known clinical disease exhibited by rats infected with SEOV.
Humans can become infected with SEOV through aerosol exposure to virus shed in rodent urine, saliva, or feces or via direct inoculation (eg, rodent bites or scratches, or contact with mucous membranes) [12]. Human infections with SEOV and other Old World hantaviruses are classically characterized as hemorrhagic fever with renal syndrome (HFRS). The HFRS symptoms include fever, headache, muscle aches, and abdominal pain. Clinical manifestations can include acute kidney injury that may progress to oliguric renal failure, conjunctivitis, and hemorrhage in severe cases [13]. Mortality for SEOV patients with HFRS is approximately 2%. Mild or unapparent illness with SEOV infection is also reported [14].
Hantavirus infections from exposure to wild rodents are most common in Asia, with more than 10 000 cases per year reported in China alone [15]. Numerous SEOV outbreaks in several countries have been reported among laboratory workers who directly handled or were in the vicinity of infected laboratory rats, or those who worked with infected tissue culture lines [16–18]. Effective biosecurity and test-and-cull strategies were developed to eradicate infection from research rat colonies, and, currently, diagnostic testing for SEOV is recommended for routine rodent health monitoring [19]. In the United States, SEOV infections are uncommonly reported [20]. In previous studies, SEOV cases were reported in patients with occupational exposure to wild rats in Texas [21] and Maryland [22] and recently in Washington DC [23].
In 2013, SEOV infections were reported in “fancy rat” breeders and colonies in the United Kingdom [24] and Sweden [25]. Animal trade between breeding colonies contributed to SEOV spread. In 2017, the US public health community investigated a multistate outbreak of SEOV in pet rats. After initial laboratory confirmation of 2 symptomatic case-patients from a Wisconsin home-based rattery [26], trace-back and trace-forward testing of rats and humans with rat contact found a total of 31 rat-owning households or ratteries (“facilities”) in 11 states with human and/or rat SEOV infection [26]. In this report, we summarize the results of the epidemiologic investigation and laboratory testing of humans and rats during the outbreak.
MATERIALS AND METHODS
Epidemiologic Investigation
Many details of the epidemiologic investigation have been described previously [26]. In brief, facilities that had either received rats from (trace-forward) or sent rats to (trace-backward) a facility with laboratory-confirmed SEOV infections in people or rats (confirmed facility) were suspected of having rats with SEOV infection (suspected facility) (Supplementary Appendix Figure 1). Testing of rats or exposed persons was offered to establish whether SEOV infection was present (Supplementary Appendix), and exposed persons were interviewed about recent illness. If serology or reverse-transcription polymerase chain reaction (RT-PCR) testing found evidence of SEOV infection in either rats or humans, the facility was classified as confirmed, and additional trace-forward and trace-backward investigations occurred. If all tests were negative, the facility was no longer suspected and was considered “cleared.” Infection control measures included quarantine of rats from confirmed and suspected facilities and euthanasia of infected rats. Guidance was provided to rat owners on handling and cleaning methods to prevent virus transmission (Supplementary Appendix).
Recent or current SEOV infection in a human was confirmed through detection of specific anti-SEOV immunoglobulin (Ig)M or SEOV RNA. Acute SEOV infection was defined as a person having contact with rats from a confirmed or suspected facility who had either fever or other symptoms compatible with SEOV infection and confirmed laboratory test results.
Serology and Reverse-Transcription Polymerase Chain Reaction
Human and rat anti-SEOV IgG and human IgM enzyme-linked immunosorbent assay (ELISA) was performed at the Centers for Disease Control and Prevention (CDC)’s Viral Special Pathogens diagnostic laboratory as previously described [27] (Supplementary Appendix). A specimen was IgM positive when the adjusted optical density (OD) of at least one 400-fold dilution was greater than 0.1 and the sum of the adjusted OD for the specimen was greater than 0.45. A specimen was IgG positive when the adjusted OD of at least one 400-fold dilution was greater than 0.2 and the sum of the adjusted OD for the specimen was greater than 0.95. A specimen was negative if it failed to meet either criterion.
Ribonucleic acid was extracted from blood and tissue using an automated magnetic bead separation system (MagMax). A pan-hantavirus L segment-nested RT-PCR assay was performed on extracted RNA. Sequences obtained from the second-round deoxyribonucleic acid fragments were used to develop a strain-specific, real-time RT-PCR (Supplementary Appendix).
Ribonucleic Acid Sequencing and Phylogenetic Analysis
Extracted SEOV RNA from multiple facilities and states were selected for next-generation sequencing (NGS) using MiSeq and MiniSeq systems (Illumina), and reads were assembled to multiple full-length hantavirus reference genomes using Geneious mapper. Consensus sequences, excluding reference sequences, were generated in Geneious 9.1.4 using the highest quality threshold (Supplementary Appendix).
In June 2018, 15 months after the last positive rat was detected in the 2017 outbreak investigation, a rat carcass suspected of SEOV infection was submitted to the CDC for testing and was RT-PCR positive. The RNA from this rat was included in NGS analysis. Viral genomes were assembled using viral-ngs (Broad Institute) with a custom database consisting of pan-hantavirus genomes. The SEOV genomes were deposited into GenBank (MK360773-98). Nearly full-length genomes for each segment were aligned using MUSCLE in Geneious/v11.1.2, and phylogenetic trees were constructed (Supplementary Appendix).
Statistical Analysis
All human and rodent data, specimen type, and related test results were compiled in Microsoft Excel. Data were analyzed using SAS version 9.3. Statistical analysis included χ 2 test for categorical comparisons and Wilcoxon rank-sum and Spearman correlation tests for continuous variable comparisons. P < .05 was considered statistically significant. Rat facilities that had ≥10 rats tested were included for analyses comparing seroprevalence and RT-PCR-positive prevalence and associations with human SEOV infections. Rat movement networks were constructed retrospectively by entering trace-back information using UCINET 6.636 and visualized using NetDraw 1.161 (Harvard, MA).
RESULTS
Human Testing
A total of 209 blood specimens from 176 individuals were received for SEOV testing from December 2016 to May 2017. Of all 176 people tested, 65% were female, and the median age was 27 years (range = 1–66). Forty-five persons were associated with confirmed rat facilities, whereas the remaining 131 persons were associated with facilities that did not have confirmed SEOV infection. Four persons (9%) had only IgG detected and no symptoms, indicating previous exposure to SEOV. Of these 4, 1 was associated with a facility that had positive rats and the remaining 3 persons were associated with 2 facilities that did not have rats available for testing at the time of the investigation.
Seventeen of the 45 (38%) exposed persons had anti-SEOV IgM, indicating recent infection—all other people tested were IgM negative. Seven of these 17 individuals (41%) reported illness that met the case definition for acute SEOV infection, 4 sought healthcare when ill, and 3 (18%) were hospitalized. Three persons with symptoms did not seek healthcare, and the remaining 10 individuals with IgM reported no symptoms.
Seoul virus RT-PCR was performed on 3 laboratory-confirmed patients who were acutely ill at the time of specimen collection, and 2 were both IgM positive and RT-PCR positive, at 2 and 4 days postonset. For 2 case-patients with serial ELISAs performed, IgM persisted beyond 83 and 115 days postonset, respectively, but was undetectable at 145 days (Supplementary Appendix Table 3). Sin Nombre virus (SNV) ELISA was performed on 25 specimens from 21 SEOV antibody-positive patients; although cross-reactivity was observed for some individuals that had high IgG or IgM titers, the SNV ELISA did not meet the criteria for positivity in 18 of 23 (78%) IgM-positive specimens and 18 of 23 (78%) IgG-positive specimens, with an estimated sensitivity of 22%.
Among symptomatic case-patients, illness onsets occurred between early December 2016 and late April 2017 (Figure 1). Commonly reported symptoms included fever, headache, and muscle aches (Table 1). Medical records were available for 3 case-patients who sought healthcare during their illness; all 3 had proteinuria detected, and 2 had microhematuria. Additional physical exam and laboratory abnormalities included tachycardia (n = 2; 133 beats per minute), tachypnea (n = 1; 25 breaths per minute), thrombocytopenia (n = 2; nadir platelet values 143, 115; reference range 150–400), leukopenia (n = 2; nadir value 3.5; reference range 4.5–10.8), hyperglycemia (n = 3; peak values 118, 124, 125; reference range 70–99), elevated creatinine (n = 2; peak values 1.13; reference range 0.5–1.1), and mild elevations in aspartate aminotransferase (n = 3; peak values 64, 50, 41; reference range 10–40), alanine aminotransferase (n = 2; peak values 62; reference range 10–50), and prothrombin time (n = 2; peak values 15.4 and 12.4; reference ranges 11.6–15.2 and 9.7–11.8, respectively). In addition, partial thromboplastin time was elevated in 1 patient where it was measured (value = 31; reference range 22–30). Detailed medical records were not available for 1 additional hospitalized patient who had pulmonary infiltrates noted on chest x-ray and also experienced renal failure. All case-patients recovered from their illnesses with supportive care.
Reported Symptoms by 7 Case-Patients With Laboratory-Confirmed Recent SEOV Infection (IgM Detected)
| Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . |
|---|---|---|---|---|---|
| Muscle aches | 6 (86) | Joint pain | 3 (43) | Hematuria | 1 (14) |
| Headache | 6 (86) | Chills | 3 (43) | Ocular hyperemia | 1 (14) |
| Fever | 5 (71) | Diarrhea | 3 (43) | Blurred vision | 1 (14) |
| Decreased appetite | 4 (57) | Sore throat | 2 (29) | Back pain | 1 (14) |
| Nausea | 4 (57) | Dizziness | 2 (29) | Chest pain | 1 (14) |
| Abdominal pain | 3 (43) | Shortness of breath | 2 (29) | Sweating | 1 (14) |
| Cough | 3 (43) | Weight loss | 2 (29) | Drowsiness | 1 (14) |
| Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . |
|---|---|---|---|---|---|
| Muscle aches | 6 (86) | Joint pain | 3 (43) | Hematuria | 1 (14) |
| Headache | 6 (86) | Chills | 3 (43) | Ocular hyperemia | 1 (14) |
| Fever | 5 (71) | Diarrhea | 3 (43) | Blurred vision | 1 (14) |
| Decreased appetite | 4 (57) | Sore throat | 2 (29) | Back pain | 1 (14) |
| Nausea | 4 (57) | Dizziness | 2 (29) | Chest pain | 1 (14) |
| Abdominal pain | 3 (43) | Shortness of breath | 2 (29) | Sweating | 1 (14) |
| Cough | 3 (43) | Weight loss | 2 (29) | Drowsiness | 1 (14) |
Abbreviations: IgM, immunoglobulin M; SEOV, Seoul virus.
Reported Symptoms by 7 Case-Patients With Laboratory-Confirmed Recent SEOV Infection (IgM Detected)
| Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . |
|---|---|---|---|---|---|
| Muscle aches | 6 (86) | Joint pain | 3 (43) | Hematuria | 1 (14) |
| Headache | 6 (86) | Chills | 3 (43) | Ocular hyperemia | 1 (14) |
| Fever | 5 (71) | Diarrhea | 3 (43) | Blurred vision | 1 (14) |
| Decreased appetite | 4 (57) | Sore throat | 2 (29) | Back pain | 1 (14) |
| Nausea | 4 (57) | Dizziness | 2 (29) | Chest pain | 1 (14) |
| Abdominal pain | 3 (43) | Shortness of breath | 2 (29) | Sweating | 1 (14) |
| Cough | 3 (43) | Weight loss | 2 (29) | Drowsiness | 1 (14) |
| Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . | Symptom . | Cases Reporting (%) . |
|---|---|---|---|---|---|
| Muscle aches | 6 (86) | Joint pain | 3 (43) | Hematuria | 1 (14) |
| Headache | 6 (86) | Chills | 3 (43) | Ocular hyperemia | 1 (14) |
| Fever | 5 (71) | Diarrhea | 3 (43) | Blurred vision | 1 (14) |
| Decreased appetite | 4 (57) | Sore throat | 2 (29) | Back pain | 1 (14) |
| Nausea | 4 (57) | Dizziness | 2 (29) | Chest pain | 1 (14) |
| Abdominal pain | 3 (43) | Shortness of breath | 2 (29) | Sweating | 1 (14) |
| Cough | 3 (43) | Weight loss | 2 (29) | Drowsiness | 1 (14) |
Abbreviations: IgM, immunoglobulin M; SEOV, Seoul virus.
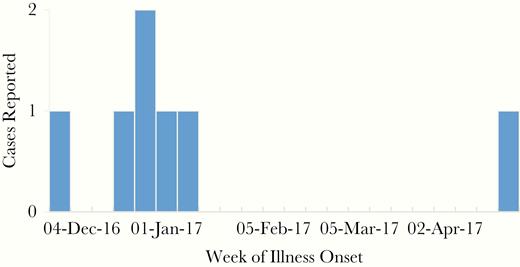
Count of symptomatic laboratory-confirmed human Seoul virus case-patients by week of illness onset (n = 7).
Rat Testing
Overall, 1947 rat specimens were tested by the CDC, which included 1377 blood specimens from 91 rat facilities. Tests performed varied, depending on the specimen types submitted and the blood volume available. Serology was performed on all blood specimens received, whereas RT-PCR was only performed if adequate volume was available. Evidence of rats with SEOV infection was found in 24 facilities (26%), and the number of rats tested ranged from 1 to 127. Among 897 total rats tested from confirmed facilities, 243 (25%) had evidence of SEOV infection either by IgG or RT-PCR positivity or both (Table 2). Four facilities confirmed rats’ SEOV infection by testing at a commercial laboratory, and 3 facilities had humans test positive but no rats were tested, so the total number of confirmed facilities in the US outbreak was 31. Prevalences of ELISA-positive and RT-PCR-positive rats varied considerably by facility: 2%–70% IgG positivity and 0%–70% RT-PCR positivity in facilities with at least 10 rats tested (n = 18 facilities) (Table 2). Seoul virus was not isolated in any virus culture attempts (Supplementary Appendix).
SEOV ELISA and RT-PCR Results for Humans and Rats Tested at 31 Confirmed Facilities
| Facility ID . | Rats IgG Tested Totala . | Rats IgG Positive (%) . | Rats RT-PCR Tested Total . | Rats RT-PCR Positive (%) . | Humans IgM/IgG Tested Total . | Humans IgM Positive (%) . |
|---|---|---|---|---|---|---|
| 1 | 127 | 65 (51%) | 129 | 48 (37%) | 2 | 2 (100%) |
| 2 | 107 | 4 (4%) | 65 | 0 | 0 | |
| 3 | 101 | 2 (2%) | 9 | 0 | 0 | |
| 4 | 98 | 27 (28%) | 98 | 23 (23%) | 5 | 1 (20%) |
| 5 | 94 | 29 (31%) | 94 | 42 (45%) | 8 | 2 (25%) |
| 6 | 50 | 2 (4%) | 0 | 0 | ||
| 7 | 43 | 2 (5%) | 43 | 4 (9%) | 1 | 0 |
| 8 | 35 | 5 (14%) | 7 | 2 (29%) | 2 | 1 (50%) |
| 9 | 34 | 7 (21%) | 34 | 1 (3%) | 1 | 0 |
| 10 | 29 | 11 (38%) | 29 | 9 (31%) | 2 | 1 (50%) |
| 11 | 26 | 15 (58%) | 26 | 16 (62%) | 4 | 4 (100%) |
| 12 | 26 | 17 (65%) | 11 | 4 (36%) | 0 | |
| 13 | 26 | 1 (4%) | 24 | 0 | 1 | 0 |
| 14 | 25 | 2 (8%) | 16 | 1 (6%) | 0 | |
| 15 | 19 | 6 (32%) | 19 | 1 (5%) | 1 | 0 |
| 16 | 16 | 1 (6%) | 1 | 0 | 0 | |
| 17 | 15 | 1 (7%) | 0 | 2 | 0 | |
| 18 | 10 | 7 (70%) | 10 | 7 (70%) | 2 | 1 (50%) |
| 19 | 6 | 2 (33%) | 6 | 3 (50%) | 0 | |
| 20 | 3 | 2 (67%) | 0 | 0 | ||
| 21 | 2 | 2 (100%) | 2 | 2 (100%) | 2 | 1 (50%) |
| 22 | 2 | 1 (50%) | 2 | 1 (50%) | 5 | 0 |
| 23 | 2 | 1 (50%) | 2 | 1 (50%) | 3 | 0 |
| 24 | 1 | 1 (100%) | 1 | 1 (100%) | 1 | 0 |
| 25b | 0 | 0 | 3 | 0 | ||
| 26 | 0 | 0 | 1 | 1 (100%) | ||
| 27b | 0 | 0 | 2 | 0 | ||
| 28b | 0 | 0 | 2 | 0 | ||
| 29b | 0 | 0 | 3 | 0 | ||
| 30 | 0 | 0 | 2 | 2 (100%) | ||
| 31 | 0 | 0 | 1 | 1 (100%) | ||
| Total | 897 | 213 (24%) | 628 | 166 (26%) | 56 | 17 (30%) |
| Facility ID . | Rats IgG Tested Totala . | Rats IgG Positive (%) . | Rats RT-PCR Tested Total . | Rats RT-PCR Positive (%) . | Humans IgM/IgG Tested Total . | Humans IgM Positive (%) . |
|---|---|---|---|---|---|---|
| 1 | 127 | 65 (51%) | 129 | 48 (37%) | 2 | 2 (100%) |
| 2 | 107 | 4 (4%) | 65 | 0 | 0 | |
| 3 | 101 | 2 (2%) | 9 | 0 | 0 | |
| 4 | 98 | 27 (28%) | 98 | 23 (23%) | 5 | 1 (20%) |
| 5 | 94 | 29 (31%) | 94 | 42 (45%) | 8 | 2 (25%) |
| 6 | 50 | 2 (4%) | 0 | 0 | ||
| 7 | 43 | 2 (5%) | 43 | 4 (9%) | 1 | 0 |
| 8 | 35 | 5 (14%) | 7 | 2 (29%) | 2 | 1 (50%) |
| 9 | 34 | 7 (21%) | 34 | 1 (3%) | 1 | 0 |
| 10 | 29 | 11 (38%) | 29 | 9 (31%) | 2 | 1 (50%) |
| 11 | 26 | 15 (58%) | 26 | 16 (62%) | 4 | 4 (100%) |
| 12 | 26 | 17 (65%) | 11 | 4 (36%) | 0 | |
| 13 | 26 | 1 (4%) | 24 | 0 | 1 | 0 |
| 14 | 25 | 2 (8%) | 16 | 1 (6%) | 0 | |
| 15 | 19 | 6 (32%) | 19 | 1 (5%) | 1 | 0 |
| 16 | 16 | 1 (6%) | 1 | 0 | 0 | |
| 17 | 15 | 1 (7%) | 0 | 2 | 0 | |
| 18 | 10 | 7 (70%) | 10 | 7 (70%) | 2 | 1 (50%) |
| 19 | 6 | 2 (33%) | 6 | 3 (50%) | 0 | |
| 20 | 3 | 2 (67%) | 0 | 0 | ||
| 21 | 2 | 2 (100%) | 2 | 2 (100%) | 2 | 1 (50%) |
| 22 | 2 | 1 (50%) | 2 | 1 (50%) | 5 | 0 |
| 23 | 2 | 1 (50%) | 2 | 1 (50%) | 3 | 0 |
| 24 | 1 | 1 (100%) | 1 | 1 (100%) | 1 | 0 |
| 25b | 0 | 0 | 3 | 0 | ||
| 26 | 0 | 0 | 1 | 1 (100%) | ||
| 27b | 0 | 0 | 2 | 0 | ||
| 28b | 0 | 0 | 2 | 0 | ||
| 29b | 0 | 0 | 3 | 0 | ||
| 30 | 0 | 0 | 2 | 2 (100%) | ||
| 31 | 0 | 0 | 1 | 1 (100%) | ||
| Total | 897 | 213 (24%) | 628 | 166 (26%) | 56 | 17 (30%) |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; ID, identification; Ig, immunoglobulin; RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
aRepresents total number of individual animals tested per facility.
bSEOV infection was confirmed in rats via testing at a commercial laboratory.
SEOV ELISA and RT-PCR Results for Humans and Rats Tested at 31 Confirmed Facilities
| Facility ID . | Rats IgG Tested Totala . | Rats IgG Positive (%) . | Rats RT-PCR Tested Total . | Rats RT-PCR Positive (%) . | Humans IgM/IgG Tested Total . | Humans IgM Positive (%) . |
|---|---|---|---|---|---|---|
| 1 | 127 | 65 (51%) | 129 | 48 (37%) | 2 | 2 (100%) |
| 2 | 107 | 4 (4%) | 65 | 0 | 0 | |
| 3 | 101 | 2 (2%) | 9 | 0 | 0 | |
| 4 | 98 | 27 (28%) | 98 | 23 (23%) | 5 | 1 (20%) |
| 5 | 94 | 29 (31%) | 94 | 42 (45%) | 8 | 2 (25%) |
| 6 | 50 | 2 (4%) | 0 | 0 | ||
| 7 | 43 | 2 (5%) | 43 | 4 (9%) | 1 | 0 |
| 8 | 35 | 5 (14%) | 7 | 2 (29%) | 2 | 1 (50%) |
| 9 | 34 | 7 (21%) | 34 | 1 (3%) | 1 | 0 |
| 10 | 29 | 11 (38%) | 29 | 9 (31%) | 2 | 1 (50%) |
| 11 | 26 | 15 (58%) | 26 | 16 (62%) | 4 | 4 (100%) |
| 12 | 26 | 17 (65%) | 11 | 4 (36%) | 0 | |
| 13 | 26 | 1 (4%) | 24 | 0 | 1 | 0 |
| 14 | 25 | 2 (8%) | 16 | 1 (6%) | 0 | |
| 15 | 19 | 6 (32%) | 19 | 1 (5%) | 1 | 0 |
| 16 | 16 | 1 (6%) | 1 | 0 | 0 | |
| 17 | 15 | 1 (7%) | 0 | 2 | 0 | |
| 18 | 10 | 7 (70%) | 10 | 7 (70%) | 2 | 1 (50%) |
| 19 | 6 | 2 (33%) | 6 | 3 (50%) | 0 | |
| 20 | 3 | 2 (67%) | 0 | 0 | ||
| 21 | 2 | 2 (100%) | 2 | 2 (100%) | 2 | 1 (50%) |
| 22 | 2 | 1 (50%) | 2 | 1 (50%) | 5 | 0 |
| 23 | 2 | 1 (50%) | 2 | 1 (50%) | 3 | 0 |
| 24 | 1 | 1 (100%) | 1 | 1 (100%) | 1 | 0 |
| 25b | 0 | 0 | 3 | 0 | ||
| 26 | 0 | 0 | 1 | 1 (100%) | ||
| 27b | 0 | 0 | 2 | 0 | ||
| 28b | 0 | 0 | 2 | 0 | ||
| 29b | 0 | 0 | 3 | 0 | ||
| 30 | 0 | 0 | 2 | 2 (100%) | ||
| 31 | 0 | 0 | 1 | 1 (100%) | ||
| Total | 897 | 213 (24%) | 628 | 166 (26%) | 56 | 17 (30%) |
| Facility ID . | Rats IgG Tested Totala . | Rats IgG Positive (%) . | Rats RT-PCR Tested Total . | Rats RT-PCR Positive (%) . | Humans IgM/IgG Tested Total . | Humans IgM Positive (%) . |
|---|---|---|---|---|---|---|
| 1 | 127 | 65 (51%) | 129 | 48 (37%) | 2 | 2 (100%) |
| 2 | 107 | 4 (4%) | 65 | 0 | 0 | |
| 3 | 101 | 2 (2%) | 9 | 0 | 0 | |
| 4 | 98 | 27 (28%) | 98 | 23 (23%) | 5 | 1 (20%) |
| 5 | 94 | 29 (31%) | 94 | 42 (45%) | 8 | 2 (25%) |
| 6 | 50 | 2 (4%) | 0 | 0 | ||
| 7 | 43 | 2 (5%) | 43 | 4 (9%) | 1 | 0 |
| 8 | 35 | 5 (14%) | 7 | 2 (29%) | 2 | 1 (50%) |
| 9 | 34 | 7 (21%) | 34 | 1 (3%) | 1 | 0 |
| 10 | 29 | 11 (38%) | 29 | 9 (31%) | 2 | 1 (50%) |
| 11 | 26 | 15 (58%) | 26 | 16 (62%) | 4 | 4 (100%) |
| 12 | 26 | 17 (65%) | 11 | 4 (36%) | 0 | |
| 13 | 26 | 1 (4%) | 24 | 0 | 1 | 0 |
| 14 | 25 | 2 (8%) | 16 | 1 (6%) | 0 | |
| 15 | 19 | 6 (32%) | 19 | 1 (5%) | 1 | 0 |
| 16 | 16 | 1 (6%) | 1 | 0 | 0 | |
| 17 | 15 | 1 (7%) | 0 | 2 | 0 | |
| 18 | 10 | 7 (70%) | 10 | 7 (70%) | 2 | 1 (50%) |
| 19 | 6 | 2 (33%) | 6 | 3 (50%) | 0 | |
| 20 | 3 | 2 (67%) | 0 | 0 | ||
| 21 | 2 | 2 (100%) | 2 | 2 (100%) | 2 | 1 (50%) |
| 22 | 2 | 1 (50%) | 2 | 1 (50%) | 5 | 0 |
| 23 | 2 | 1 (50%) | 2 | 1 (50%) | 3 | 0 |
| 24 | 1 | 1 (100%) | 1 | 1 (100%) | 1 | 0 |
| 25b | 0 | 0 | 3 | 0 | ||
| 26 | 0 | 0 | 1 | 1 (100%) | ||
| 27b | 0 | 0 | 2 | 0 | ||
| 28b | 0 | 0 | 2 | 0 | ||
| 29b | 0 | 0 | 3 | 0 | ||
| 30 | 0 | 0 | 2 | 2 (100%) | ||
| 31 | 0 | 0 | 1 | 1 (100%) | ||
| Total | 897 | 213 (24%) | 628 | 166 (26%) | 56 | 17 (30%) |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; ID, identification; Ig, immunoglobulin; RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
aRepresents total number of individual animals tested per facility.
bSEOV infection was confirmed in rats via testing at a commercial laboratory.
Of 547 rat carcasses tested by both RT-PCR and ELISA, 29% were RT-PCR positive and 30% were IgG positive; 23% had both IgG and RNA, whereas 6% were RT-PCR positive only and 7% were IgG positive only (Table 3). Reverse-transcription polymerase chain reaction was performed on antemortem venous blood specimens and postmortem lung tissue from 41 rats at 2 confirmed facilities. A total of 28 rats (68%) were RT-PCR positive in lung tissue, whereas only 12 (29%) were also positive in venous blood. The sensitivity of venous blood in detecting SEOV RNA compared with lung tissue was 43% (Table 4). There was no relationship between facility size (approximated by number of rats tested) and seroprevalence or RT-PCR prevalence (IgG Spearman coefficient = −0.29, P = .24; RT-PCR Spearman coefficient = 0.21, P = .44).
Comparison of SEOV blood IgG ELISA and Carcass RT-PCR Results Performed in Parallel on 547 Rats
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| IgG-positive blood | 128 (23%) | 36 (7%) | 164 (30%) |
| IgG-negative blood | 31 (6%) | 352 (64%) | 383 (70%) |
| Total | 159 (29%) | 388 (71%) | 547 |
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| IgG-positive blood | 128 (23%) | 36 (7%) | 164 (30%) |
| IgG-negative blood | 31 (6%) | 352 (64%) | 383 (70%) |
| Total | 159 (29%) | 388 (71%) | 547 |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
Comparison of SEOV blood IgG ELISA and Carcass RT-PCR Results Performed in Parallel on 547 Rats
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| IgG-positive blood | 128 (23%) | 36 (7%) | 164 (30%) |
| IgG-negative blood | 31 (6%) | 352 (64%) | 383 (70%) |
| Total | 159 (29%) | 388 (71%) | 547 |
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| IgG-positive blood | 128 (23%) | 36 (7%) | 164 (30%) |
| IgG-negative blood | 31 (6%) | 352 (64%) | 383 (70%) |
| Total | 159 (29%) | 388 (71%) | 547 |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
Comparison of SEOV Blood and Carcass RT-PCR Results Performed in Parallel on 41 Rats
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| RT-PCR-positive blood | 12 (29%) | 0 | 12 (29%) |
| RT-PCR-negative blood | 16 (39%) | 13 (31%) | 29 (71%) |
| Total | 28 (68%) | 13 (31%) | 41 |
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| RT-PCR-positive blood | 12 (29%) | 0 | 12 (29%) |
| RT-PCR-negative blood | 16 (39%) | 13 (31%) | 29 (71%) |
| Total | 28 (68%) | 13 (31%) | 41 |
Abbreviations: RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
Comparison of SEOV Blood and Carcass RT-PCR Results Performed in Parallel on 41 Rats
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| RT-PCR-positive blood | 12 (29%) | 0 | 12 (29%) |
| RT-PCR-negative blood | 16 (39%) | 13 (31%) | 29 (71%) |
| Total | 28 (68%) | 13 (31%) | 41 |
| Test Result . | RT-PCR-Positive Lung Tissue . | RT-PCR-Negative Lung Tissue . | Total . |
|---|---|---|---|
| RT-PCR-positive blood | 12 (29%) | 0 | 12 (29%) |
| RT-PCR-negative blood | 16 (39%) | 13 (31%) | 29 (71%) |
| Total | 28 (68%) | 13 (31%) | 41 |
Abbreviations: RT-PCR, reverse-transcription polymerase chain reaction; SEOV, Seoul virus.
A total of 12 confirmed facilities had both humans and ≥10 rats tested by IgG and 10 by RT-PCR. Among these, the median rat SEOV IgG seroprevalence in facilities with at least 1 IgM positive human was 38%, whereas median rat SEOV IgG seroprevalence in facilities where humans tested negative was 13%. This difference was significant by Wilcoxon rank sum (P = .03). The median prevalence of RT-PCR-positive rats in facilities with IgM-positive humans was 42%, whereas median RT-PCR-positive prevalence in facilities where humans tested negative was 4%, a significant association (Wilcoxon rank sum, P = .01).
Ribonucleic Acid Sequencing
In agreement with Kim et al [28], most SEOV genome sequences analyzed clustered together by geographic origin. L, M, and S segment sequences from the 2017 outbreak were nearly identical (99.565% L, 99.793% M, 99.509% S minimum pairwise identity) (L phylogenetic tree [Figure 2]; M phylogenetic tree and S phylogenetic tree [Supplemental Figures 2and 3]) and clustered on the same clade as a sequence from a pet rat in Cherwell, England in 2013 (99.436% L, 99.586% M, 99.167% S minimum pairwise identity) [29]. Sequences on the Cherwell clade are distinct from SEOV sequences collected from wild rats in the United Kingdom (2012, Humber strain), wild rats in the United States (2002, Northeast Baltimore), and rats collected in the Netherlands in 2013 (Rn84). The inferred phylogenetic relationships support a rapid and broad geographic expansion of the Cherwell clade that occurred internationally and within the United States in a short time frame. Furthermore, a sequence derived from a pet rat in Illinois in June 2018 (Specimen 201802480) suggests that the outbreak strain continues to circulate in the United States.
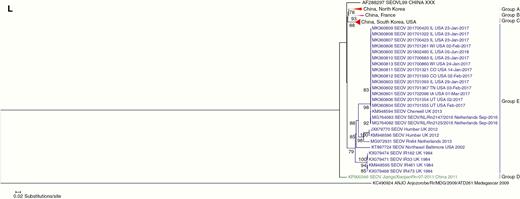
Phylogenetic tree of L segment of Seoul virus (SEOV) sequences from 2017 US outbreak and select reference SEOV strains.
Trace-Back Findings and Rat Facilities
Thirty-one facilities in 11 states were confirmed to have SEOV infection through testing of rats and/or humans. Affected facilities were located throughout the United States, with the greatest number of confirmed facilities in Illinois (n = 12) and Wisconsin (n = 5). Exchange of rats with Canadian facilities was noted, and the Public Health Agency of Canada’s investigation identified additional confirmed ratteries and 1 recent human case [26]. Confirmed US facilities included home-based ratteries, local pet stores, and homes. Rats were sold and traded as household pets, breeding animals, and for feeding to carnivores (snakes or raptors). No commercial ratteries supplying national or regional chain pet stores were apparently involved. Trace-back investigations found that rats were commonly exchanged between facilities, mainly for breeding purposes (Figure 3).
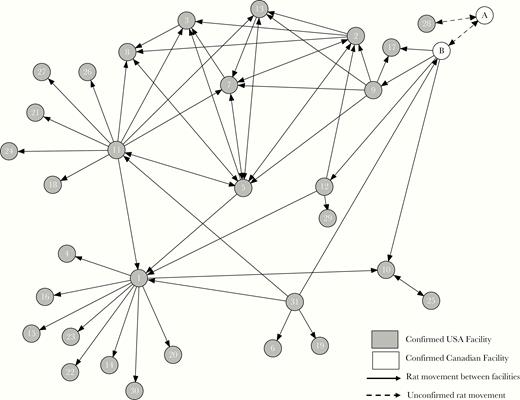
Transmission chain of Seoul virus-infected rats between confirmed facilities.
Given the complex movement of infected rats between facilities, we were unable to determine a single source of infected animals. However, the close match of sequence to the Cherwell strain as previously described suggests possible importation to the United States and Canada from the United Kingdom or Europe. Some confirmed facilities did not have available or complete records of rat transactions with other facilities, and so ascertainment of all infected rats was incomplete. Details of public health measures, education, and outbreak investigation coordination are described further in the Supplementary Appendix.
DISCUSSION
Altogether, the 2017 US SEOV outbreak investigation identified 31 facilities with infected rats or people, and 17 people with evidence of recent infection. Although SEOV was described in wild Norway rats in the United States [7, 20, 21, 30–33], it was not reported previously in pet rats. It is likely that SEOV was recently introduced into this pet rat population, considering that 81% of antibody-positive people in this outbreak had IgM detected, indicating recent exposure to SEOV (<6 months) despite long-term rat contact. Many case-patients with evidence of recent infection were asymptomatic. Fewer than half (7) had acute illness symptoms, and 3 were hospitalized and recovered after supportive care. Among case-patients with medical records available, proteinuria was universally detected, as was previously reported in 51 SEOV patients in China [34]. Proteinuria is a highly sensitive and universal clinical indicator for HFRS [13]. One patient had acute renal failure, a frequent feature of severe HFRS, and also pulmonary infiltrates, which has been described in both New World and Old World hantavirus infections [13, 34, 35]. The relatively mild clinical course of illness in these patients stands in contrast to most New World hantavirus infections, where intubation and treatment for hypotension are a frequent feature [1].
Gathering a patient’s history of animal exposures and travel is important in the diagnosis of rare infectious diseases, and this is especially crucial for identifying hantavirus disease. The nonspecific clinical symptoms of the first patient and many others in the outbreak could have easily been overlooked by clinicians; fortunately, history of rodent exposures was noted, which led to hantavirus testing [26]. Hantavirus infections are nationally notifiable in the United States. The CDC, several state laboratories, and a commercial diagnostic laboratory provide diagnostic serological testing for hantavirus infection, and the assays used are primarily directed toward detection of antibodies to New World orthohantaviruses (eg, SNV and other related viruses). Although antibody cross-reactivity was observed between Old World and New World orthohantavirus assays, the SNV IgM and IgG ELISAs most frequently used are poorly sensitive. If SEOV infection is suspected, the clinician should specifically request SEOV antibody testing, which is available from the CDC. The CDC routinely performs both SEOV and SNV antibody ELISA on all specimens submitted for hantavirus testing.
There was strong bootstrap support for inferred relatedness of US pet rat SEOV strains to the Cherwell strain [29], which points to international trade of pet rats as the likely source of this outbreak in the United States and Canada. The Cherwell strain has also appeared in France and the Netherlands associated with pet or feeder rats in 2014 and 2016 [36, 37]. The molecular evidence supports the spread of the Cherwell strain to rats in 5 countries to date.
Currently, the only regulation governing the international trade of rodents into the United States is a ban on rodents originating from Africa, which was developed after the 2003 monkeypox outbreak that affected 41 people in 5 states [38, 39]. Parallels between the monkeypox and SEOV outbreak can be found: both outbreaks could be linked to imported infected pet rodents, and both outbreaks were focused in the upper Midwestern United States, with spread through trade of animals across state lines. Aside from SEOV and Monkeypox, frozen rodents imported to the United Kingdom and the United States have also been linked to outbreaks of Salmonella [40, 41], and other zoonotic diseases may be spread by rodents across international borders, such as plague, tularemia, and lymphocytic choriomeningitis virus. After the 2003 US ban on importation of African rodents, rodent importations to the United States from other international regions continue to rise, particularly rodents originating from Europe [42].
Seoul virus antibody testing for rat blood specimens is available from commercial diagnostic laboratories, as is RT-PCR for blood, tissue, and environmental swab specimens. We found that IgG positivity is a moderately sensitive (80.5%) indicator of an individual animal’s current SEOV infection status. Blood RT-PCR had relatively low diagnostic sensitivity (43%). We did not test any environmental swab specimens during the investigation, and we are not aware of any validation studies to measure the suitability of environmental swabs to detect SEOV shed by infected rats. Until evidence can be provided, we would not recommend testing environmental swab specimens as a reliable indicator of SEOV infection. Currently, serology remains the best antemortem diagnostic test to determine whether a rat has or had SEOV infection.
Rat seroprevalence and RT-PCR positivity varied widely among facilities with ≥10 rats tested; the highest IgG prevalence observed was 70%, and the highest RT-PCR prevalence was 62%. Comparably high prevalences have been previously observed in United Kingdom pet rat facilities [29] and in wild rat capture studies [7, 11]. There were associations between increasing rat IgG prevalence and RT-PCR tissue prevalence and the occurrence of human SEOV infections. This could be due to more rodents with active infections and viral shedding in the environment, leading to increased exposure risk for rat handlers.
Outbreak facilities were largely home-based pet rat breeding operations, and they included households with pet rats and small pet stores. An important factor in the virus spread was the frequent practice of trading rats between ratteries for breeding purposes. Rattery owners can reduce the risk of SEOV infection in both their animals and in people by observing biosecurity measures that will reduce infection transmission between animals. Biosecurity methods include a 4-week quarantine and serological testing of new animals before commingling with the animals in the colony, cohorting animal groups, regular cleaning and disinfection of enclosures, and recordkeeping of animal acquisitions. Resources regarding SEOV testing, prevention, and best management practices are available from several sources [30, 44, 45]. Previous interviews with pet rat owners in the United Kingdom found that education and communication about SEOV should center on protecting the health and purity of the pet rat colony through biosecurity measures [46].
Observational results reported here have limitations because different specimens were available for testing from different ratteries, and rats were not sampled randomly. Furthermore, we had limited information of the timeframe during which SEOV infection was introduced into a given facility or the number of infected animals introduced.
CONCLUSIONS
Despite extensive efforts to identify all facilities with SEOV-infected rats, we were not able to trace the outbreak to its origins, nor completely trace all potentially infected rats from all confirmed facilities, which contributed to gaps in identifying and controlling further spread of SEOV. Since this outbreak investigation concluded in May 2017, no further cases of pet rat-associated SEOV were identified in humans. However, in June 2018, an infection was detected in a pet rat from Illinois (Specimen 201802480; Figure 2), confirming the Cherwell strain’s ongoing presence. As a result, we expect that SEOV is still circulating in US pet rats. Pet rat owners and breeders, veterinarians, and public health officials should be prepared for future cases. Use of biosecurity practices and testing can prevent further transmission in pet rats, whereas clinical awareness of symptoms and gathering of pertinent animal exposure history can help identify human cases. Prompt reporting of suspected human cases and laboratory confirmation will facilitate identification and response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. Funding for the work described in this manuscript was provided by federal and state emergency response funds.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Multistate Seoul Virus Investigation Team
Dee Jones, Alabama Department of Public Health; Susan Weinstein, Arkansas Department of Health; Peter Buck, Public Health Agency of Canada; Casey Barton Behravesh, Sarah Genzer, Eddie Jackson, M. Harley Jenks, Gregory Langham, George Lathrop, Nishi Patel, Nathaniel Powell, Anne Straily, and Ute Ströher, Centers for Disease Control and Prevention; Natalie Marzec, Colorado Department of Public Health and Environment; Nhiem Luong, Delaware Health and Social Services; Danielle Stanek, Florida Department of Health; Julie Gabel, Georgia Department of Public Health; Kris Carter, Idaho Department of Health and Welfare; Jodi Lovejoy, Indiana Board of Animal Health; Jennifer Brown and Betsy Schroeder, Indiana State Department of Health; Jennifer Layden, Illinois Department of Public Health; Gary Balsamo, Louisiana Department of Health; David Blythe, Maryland Department of Health; Caroline Castillo, Jennifer Sidge, and Mary Grace Stobierski, Michigan Department of Health and Human Services; Victoria Hall, Malia Ireland, and Kimberly Signs, Minnesota Department of Health; Howard Pue, Missouri Department of Health and Senior Services; Colin Campbell, New Jersey Department of Health; Jill Baber, Laura Cronquist, Michelle Feist, and Susan Keller, North Dakota Department of Health; Amber Singh, Ohio Department of Health; Karen Gowdy and Dean Middleton, Ontario Ministry of Public Health and Long Term Care; Jan Achenbach, Drew D. Dycus, Aaron Smee, and Andre Weltman, Pennsylvania Department of Health; Mary Margaret Fill, Heather Henderson, Timothy Jones, Andrew Stephen May, and Heather Mullins, Tennessee Department of Health; Tom Sidwa, Texas Department of State Health Services; Allyn Nakashima, Utah Department of Health; Dennis Foelker, Wisconsin Department of Agriculture Trade and Consumer Protection; Jordan Dieckman, Rachel F. Klos, and Anna Kocharian, Wisconsin Department of Health Services.




