-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshikazu Yuki, Shiho Kurokawa, Shintaro Sato, Ai Sasou, Naomi Matsumoto, Akio Suzuki, Naomi Sakon, Yuki Goda, Natsumi Takeyama, Tatsuya Miyoshi, Harold Marcotte, Tomoyuki Tanaka, Lennart Hammarstrom, Hiroshi Kiyono, A Heterodimeric Antibody Fragment for Passive Immunotherapy Against Norovirus Infection, The Journal of Infectious Diseases, Volume 222, Issue 3, 1 August 2020, Pages 470–478, https://doi.org/10.1093/infdis/jiaa115
Close - Share Icon Share
Abstract
Human noroviruses cause an estimated 685 million infections and 200 000 deaths annually worldwide. Although vaccines against GII.4 and GI.1 genotypes are under development, no information is available regarding vaccines or monoclonal antibodies to other noroviral genotypes. Here, we developed 2 variable-domain llama heavy-chain antibody fragment (VHHs) clones, 7C6 and 1E4, against GII.4 and GII.17 human noroviruses, respectively. Although 7C6 cross-reacted with virus-like particles (VLPs) of GII.17, GII.6, GII.3, and GII.4, it neutralized only GII.4 norovirus. In contrast, 1E4 reacted with and neutralized only GII.17 VLPs. Both VHHs blocked VLP binding to human induced pluripotent stem cell-derived intestinal epithelial cells and carbohydrate attachment factors. Using these 2 VHHs, we produced a heterodimeric VHH fragment that neutralized both GII.4 and GII.17 noroviruses. Because VHH fragments are heat- and acid-stable recombinant monoclonal antibodies, the heterodimer likely will be useful for oral immunotherapy and prophylaxis against GII.4 and GII.17 noroviruses in young, elderly, or immunocompromised persons.
Human noroviruses are responsible for about 20% of global gastroenteritis [1]. Human norovirus infection is common both in developed and developing countries and is associated with severe complications in children younger than 5 years, the elderly, and immunocompromised persons [1, 2]. In addition, human norovirus infection presents a significant economic burden, accounting for an estimated $4.2 billion in direct health system costs and $60.3 billion in social costs worldwide each year [3, 4].
Noroviruses are highly diverse and are divided into 7 genogroups according to their capsid sequence, with viruses in genogroups GI, GII, and GIV able to infect humans [5]. Although the GII.4 genotype remains the predominant genotype in human norovirus epidemics, other genotypes—including GII.2, GII.6, and GII.17—are becoming more frequent [6]. The norovirus capsid comprises 180 copies of the major capsid protein VP1 (virus protein 1), and the virus infects humans via interaction of the protruding (P) domain of VP1 with human histo-blood group antigens (HBGAs). Indeed, the induction of HBGA-blocking antibodies (Abs) has been shown to correlate with protection from gastroenteritis in humans infected with norovirus [7].
Currently, no licensed human norovirus vaccine, pharmaceutical drug, or therapy is available. Two vaccine candidates are in phase 1–2 trials: one involves intramuscular injection with virus-like particles (VLPs) of GI.1 and GII.4 [8]; the other is an oral recombinant adenovirus expressing GI.1 VP1 [9]. Although vaccination with the GII.4 VLP vaccine induces a broad blocking Ab response to several GII.4 strains, including nonvaccine GII.4 strains such as GII.4 Sydney_2012, there are currently no Abs available that block GII noroviruses other than those within the GII.4 genotype [8, 10].
A recent breakthrough in human norovirus research is the development of an in vitro culture system using human intestinal enteroid culture derived from biopsies [11]. More recently, we have developed a human norovirus propagation system in human induced pluripotent stem cell (iPSC)-derived intestinal epithelial cells (IECs) [12]. We thus applied a human norovirus culture system using iPSC-derived human organoids to show that immunization of rabbits with GII.4 VLPs induces cross-reacting Abs to GII genogroup VLPs but not neutralizing Abs to non-GII.4 genotypes [12]. However, during a human noroviruses outbreak, a vaccine raised against a specific genotype will most likely also need to be effective against many other variant genotypes.
Immunocompromised hosts do not achieve protective immunity through natural infectious processes and often develop a complicated disease course after norovirus infection that is characterized by prolonged diarrhea, malnutrition, dehydration, and occasionally death [13]. In addition, vaccines are often associated with poor efficacy in immunocompromised or immunodeficient individuals, premature infants, and elderly persons. Whether current vaccine candidates can induce effective immune responses to human norovirus GI.1 or GII.4 in children younger than 5 years or elderly people older than 65 years is unknown [14]. Thus, alternative strategies are needed that can be used when available vaccines are contraindicated or as a complementary strategy when vaccination alone may not be sufficiently effective.
The variable domain of llama heavy-chain Abs comprises only a single-chain domain, called VHH. With a molecular weight of 15 kDa [15], VHH is the smallest, naturally occurring antigen-binding unit reported to date. Because of its small size, VHH enables the construction of engineered multivalent formats with higher avidity than monoclonal antibodies (mAbs). In addition, VHH has several attractive features including resistance to pepsin, acidic environments, and heat up to 90°C [16, 17]. Given these unique characteristics, VHH is potentially useful for the development of oral passive immunotherapy for use in selected groups, such as hospitalized children, elderly adults, and immunocompromised persons. Indeed, oral administration of yeast-expressed VHH against rotavirus has been shown to safely and effectively reduce the severity of diarrhea among children in a phase 2 clinical trial in Bangladesh [18]. Furthermore, the first VHH-based medicine, Cablivi (caplacizumab), has recently been approved in Europe and the United States for treatment as a subcutaneous injection in adults with acquired thrombotic thrombocytopenic purpura (European Medicines Evaluation Agency product number, EMEA/H/C/004426, 31 August 2018; Food and Drug Administration Biologic License Application no. 761112, 8 February 2019).
In the current study, we immunized llamas with GII.4 or GII.17 VLPs and subsequently established 2 monomeric VHHs that neutralize either GII.4 or GII.17 human norovirus. We then established a heterodimer of the 2 VHHs that neutralizes both GII.4 and GII.17 human noroviruses for use in oral passive immunotherapy to control human norovirus infections.
METHODS
Virus-Like Particles
The nucleotides encoding VP1 of GII.3 (OsakaFB1650), GII.4 Sakai08-403_2006b, GII.4 Sydney_2012 (OsakaFB1616), and GII.17 Kawasaki308 (OsakaFB16421) were sequenced and then VLPs of these strains were produced as described in Supplementary Methods. The VLPs of GII.4 Saga1_2006, GII.3 SaitamaU201_1998, GII.6 Ueno7k_1994, GII.2 TokyoMK04_2004, GI.6 WUG1_2000, and GI.4 Chiba407_1987 for enzyme-linked immunosorbent assays (ELISAs) were kindly provided by Professor Kazuhiko Katayama (Kitasato University, Japan).
Production of VHHs
The production of VHHs to the VLPs of GII.4 Sakai08-403_2006b and GII.17 Kawasaki308 was outsourced to QVQ BV (Utrecht, The Netherlands). The VHH and recombinant heterodimeric VHH production methods are described in Supplementary Methods. All VHHs were concentrated to 1 mg/mL in phosphate-buffered saline (PBS) and stored at −80°C. The 4 VHHs used in the current study are shown in Supplementary Table 1.
Virus-Like Particle ELISA
The binding affinity of the purified VHHs was analyzed by ELISA using immobilized GI and GII genogroup human norovirus VLPs (Supplementary Table 2). The VLP-ELISA procedure is described in Supplementary Methods.
Porcine Gastric Mucin Inhibition Assay and Immunofluorescence Microscopy of VLP-Cell Binding
A porcine gastric mucin (PGM) inhibition assay was used to examine the binding of the VLPs to HBGAs. Immunofluorescent microscopy was used to examine VLP-cell binding. The methods for these experiments are described in Supplementary Methods.
Preparation and Western Blot Analysis of Recombinant P Domains and P Subdomains of GII.4 and GII.17 VLPs
The preparation of the P domains and subdomains, and the western blotting procedure, are described in Supplementary Methods.
IEC Culture as Organoids or Monolayered Cells
IECs were prepared from human iPSC line TkDN4-M [19], which was supplied by the University of Tokyo (Japan), as described previously [20]. The culture and passage of the human iPSC-derived IECs in Matrigel (Corning) were performed as described previously. For human norovirus inoculation, dissociated IECs were seeded on 2.5% Matrigel-coated 96-well plates or Transwell membranes (Corning 3470) at 2 × 104/well in 100 μL of organoid culture medium (Advanced Dulbecco’s modified Eagle medium/F12 [Thermo Fisher Scientific] supplemented with 10 mM HEPES, pH 7.3 [Thermo Fisher Scientific]; 2 mM Glutamax [Thermo Fisher Scientific]; 100 units/mL penicillin plus 100 μg/mL streptomycin; 25% mouse Wnt3a, human R-spondin1, and human Noggin-conditioned medium [20]; 1 × B-27 [Thermo Fisher Scientific]; 50 ng/mL mouse epidermal growth factor [EGF, Peprotech], 50 ng/mL human hepatocyte growth factor [R&D Systems]; 10 μM SB202190 [Sigma-Aldrich]; and 500 nM A83-01 [Tocris], plus 10-μM Y-27632 [Wako]). After 2 days of culture in a 5% CO2 incubator at 37°C, the medium was changed to differentiation medium (Advanced Dulbecco’s modified Eagle medium/F12 supplemented with 10 mM HEPES [pH 7.3], 2 mM Glutamax, 100 units/mL penicillin plus 100 μg/mL streptomycin, 1 × B-27 base medium, 1.25% fetal bovine serum [Biosera], 50 ng/mL mouse EGF, 375 ng/mL mouse R-Spondin1 [R&D Systems], 50 ng/mL mouse Noggin [Peprotech], and 500 nM A83-01), and after another 2 days, the medium was changed to differentiation medium with or without 0.03% porcine bile (Sigma-Aldrich). The cells were incubated for another 1 to 2 days and then used.
Neutralization Assay of Human Noroviruses
Human norovirus-positive stool samples collected in Osaka, Japan (Supplementary Table 3), were suspended in PBS at 10% (w/v) by vigorous vortexing. The suspensions were centrifuged at 9100g for 15 minutes, and the supernatants were serially filtered over 0.45- and 0.22-µM filters. The filtered samples were aliquoted and stored at −80°C as undiluted virus solutions. Just before use, each virus solution was diluted to 1.5 × 107 genome equivalents per 100 µL with and without VHH (5 µg or indicated amount) in base medium and then incubated for 2 hours. Prepared IECs (3 to 6 wells per sample) were inoculated with 100 µL (5 × 106 genome equivalents) of diluted virus solutions and then left for 3 hours in a 5% CO2 incubator at 37°C. The inoculum was then removed and the cells were washed twice with 150 µL of base medium. We then added 100 μL of differentiation medium—with or without 0.03% bile—to the cells, gently pipetted them up and down twice, and collected them. This step was repeated and the suspensions were pooled and collected as 3-hour infection reference samples (total, 200 µL). Another 100 µL of differentiation medium with or without 0.03% bile was added into each well and the mixtures were then cultured for 48–72 hours in a 5% CO2 incubator at 37°C. The supernatants were then collected with 1 wash, in the same way as the reference samples (total, 200 µL). The concentration 50% inhibitory to human norovirus replication was calculated for each VHH dilution by using a 5-parameter dose-response curve in Prism 7.
Statistical Analysis
Results were compared by using unpaired 2-tailed Student t tests. All statistical analyses were performed in Prism 7.
Ethics Statement
The llama immunization experiment at QVQ BV to produce VHH was performed in accordance with the Guidelines for Use and Care of Experimental Animals, the Federation of European Laboratory Animal Science Associations, and the Welfare Legislation for Laboratory Animals (2010/63/EU), and were approved by the Institutional Animal Care and Use Committee of Service Publique Federal of Belgium (approval number LA1800104). Female New Zealand white rabbits (9 weeks old) to produce Abs raised against GII.4 and GII.17 VLPs were purchased from Oriental Yeast Co. Ltd. The rabbit immunization experiment was performed in accordance with the Guidelines for Use and Care of Experimental Animals and was approved by the Institutional Animal Care and Use Committee of the University of Tokyo (approval number A18-35). Under informed consent, all stool samples were collected and provided by the Osaka Institute of Public Health. The study was approved by the human ethical committee of the University of Tokyo (approval number 28-40) and Osaka University (approval number 28-3).
RESULTS
Binding Specificity of VHHs According to VLP-ELISAs
We obtained 12 VHHs from libraries generated from peripheral blood mononuclear cells of llamas immunized with VLPs of GII.4 Sakai08-403_2006b and used human norovirus genotype VLP-ELISAs to confirm their binding ability (Supplementary Table 2 and Supplementary Methods). Eight of the VHHs, including 6A2, showed GII.4-specific binding, and the remaining 4 VHHs, including 7C6, showed broad binding to all of the GII VLPs tested (Table 1).
Reactivity of the VHHs Against VLPs of Representative Strains from Different Genotypes
| VLP Genotype . | Strain . | VHHs . | ||||
|---|---|---|---|---|---|---|
| . | . | 6A2 . | 7C6 . | 1A6 . | 1E4 . | Heterodimer 7C6-1E4 . |
| GI.4 | Chiba407_1987 | − | − | − | − | − |
| GI.6 | WUG1_2000 | − | − | − | − | − |
| GII.2 | TokyoMK04_2004 | − | +++ | +++ | − | +++ |
| GII.3 | SaitamaU201_1998 | − | +++ | +++ | − | +++ |
| Osaka (FB1650) | − | ++ | ++ | − | +++ | |
| GII.4 | Saga1_2006 | +++ | ++ | ± | − | +++ |
| Sakai08-403_2006b | +++ | +++ | ± | − | +++ | |
| Sydney_2012 | +++ | ++ | ± | − | +++ | |
| GII.6 | Ueno7K_1994 | − | ++ | − | − | ++ |
| GII.17 | Kawasaki308 | − | ++ | +++ | +++ | +++ |
| VLP Genotype . | Strain . | VHHs . | ||||
|---|---|---|---|---|---|---|
| . | . | 6A2 . | 7C6 . | 1A6 . | 1E4 . | Heterodimer 7C6-1E4 . |
| GI.4 | Chiba407_1987 | − | − | − | − | − |
| GI.6 | WUG1_2000 | − | − | − | − | − |
| GII.2 | TokyoMK04_2004 | − | +++ | +++ | − | +++ |
| GII.3 | SaitamaU201_1998 | − | +++ | +++ | − | +++ |
| Osaka (FB1650) | − | ++ | ++ | − | +++ | |
| GII.4 | Saga1_2006 | +++ | ++ | ± | − | +++ |
| Sakai08-403_2006b | +++ | +++ | ± | − | +++ | |
| Sydney_2012 | +++ | ++ | ± | − | +++ | |
| GII.6 | Ueno7K_1994 | − | ++ | − | − | ++ |
| GII.17 | Kawasaki308 | − | ++ | +++ | +++ | +++ |
Abbreviations: VHH, variable-domain heavy-chain antibody fragment; VLP, virus-like particle.
−, absence of reaction (100 nM VHH, absorbance at 450 nm < 0.2); ±, detectable reaction (100 nM VHH, absorbance at 450 nm > 0.2); +, low reaction (100 nM VHH, absorbance at 450nm > 1.0); ++, moderate reaction (10 nM VHH, absorbance at 450 nm > 1.0); +++, high reaction (1 nM VHH, absorbance at 450 nm > 1.0).
Reactivity of the VHHs Against VLPs of Representative Strains from Different Genotypes
| VLP Genotype . | Strain . | VHHs . | ||||
|---|---|---|---|---|---|---|
| . | . | 6A2 . | 7C6 . | 1A6 . | 1E4 . | Heterodimer 7C6-1E4 . |
| GI.4 | Chiba407_1987 | − | − | − | − | − |
| GI.6 | WUG1_2000 | − | − | − | − | − |
| GII.2 | TokyoMK04_2004 | − | +++ | +++ | − | +++ |
| GII.3 | SaitamaU201_1998 | − | +++ | +++ | − | +++ |
| Osaka (FB1650) | − | ++ | ++ | − | +++ | |
| GII.4 | Saga1_2006 | +++ | ++ | ± | − | +++ |
| Sakai08-403_2006b | +++ | +++ | ± | − | +++ | |
| Sydney_2012 | +++ | ++ | ± | − | +++ | |
| GII.6 | Ueno7K_1994 | − | ++ | − | − | ++ |
| GII.17 | Kawasaki308 | − | ++ | +++ | +++ | +++ |
| VLP Genotype . | Strain . | VHHs . | ||||
|---|---|---|---|---|---|---|
| . | . | 6A2 . | 7C6 . | 1A6 . | 1E4 . | Heterodimer 7C6-1E4 . |
| GI.4 | Chiba407_1987 | − | − | − | − | − |
| GI.6 | WUG1_2000 | − | − | − | − | − |
| GII.2 | TokyoMK04_2004 | − | +++ | +++ | − | +++ |
| GII.3 | SaitamaU201_1998 | − | +++ | +++ | − | +++ |
| Osaka (FB1650) | − | ++ | ++ | − | +++ | |
| GII.4 | Saga1_2006 | +++ | ++ | ± | − | +++ |
| Sakai08-403_2006b | +++ | +++ | ± | − | +++ | |
| Sydney_2012 | +++ | ++ | ± | − | +++ | |
| GII.6 | Ueno7K_1994 | − | ++ | − | − | ++ |
| GII.17 | Kawasaki308 | − | ++ | +++ | +++ | +++ |
Abbreviations: VHH, variable-domain heavy-chain antibody fragment; VLP, virus-like particle.
−, absence of reaction (100 nM VHH, absorbance at 450 nm < 0.2); ±, detectable reaction (100 nM VHH, absorbance at 450 nm > 0.2); +, low reaction (100 nM VHH, absorbance at 450nm > 1.0); ++, moderate reaction (10 nM VHH, absorbance at 450 nm > 1.0); +++, high reaction (1 nM VHH, absorbance at 450 nm > 1.0).
We also obtained 8 VHHs from libraries generated from peripheral blood mononuclear cells of llamas immunized with VLPs of GII.17 Kawasaki308 and again used VLP-ELISAs to confirm their binding ability. Seven of the VHHs, including 1E4, showed GII.17-specific binding, and the remaining VHH, 1A6, showed broad binding to most of the GII VLPs tested (Table 1). None of the 20 VHHs cross-reacted with GI.4 or GI.6 VLPs.
Neutralizing Specificity of VHHs 7C6 and 1E4 According to Propagation Assay Using Human iPSC-Derived IECs
Next, we used human iPSC-derived IECs to test whether the obtained VHHs could neutralize human norovirus propagation.
First, we examined the VHHs obtained from llamas immunized with VLPs of GII.4 Sakai08-403_2006b. VHH 6A2 in the GII.4-specific binding group failed to neutralize GII.4 Sydney_2012 propagation (Supplementary Figure 1A). Of the 4 VHHs that showed broad binding to all of the GII VLPs tested, 3, including 7C6, neutralized GII.4 Sydney_2012 propagation (Supplementary Figure 1A and Figure 1A). When we further examined 7C6, which had the highest inhibition capacity, we found that it did not neutralize GII.17, GII.3, or GII.6 norovirus propagation (Figure 1B and Figure 2). Although we routinely added bile to the GII.4 Sydney_2012 culture system for better growth, the neutralization ability of 7C6 did not differ in the presence (Figure 1A) or absence (Supplementary Figure 2A) of bile supplementation.
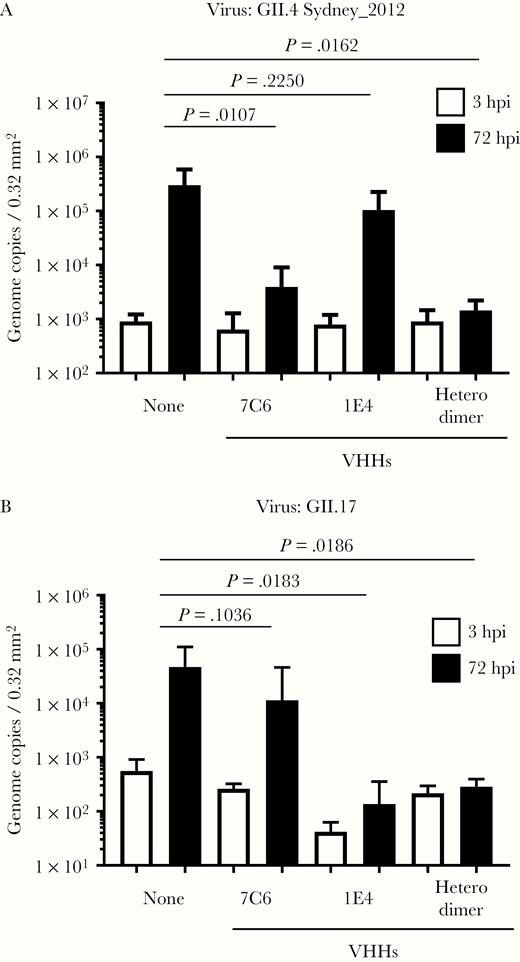
Neutralization of GII.4 and GII.17 human noroviruses by variable-domain heavy-chain antibody fragments (VHHs) 7C6, 1E4, and their heterodimer. We incubated 2 × 106 genome equivalents of GII.4 Sydney_2012 (A) or GII.17_Kawasaki308 (B) human norovirus with 5 µg of the indicated VHH for 2 hours before using the noroviruses to inoculate human intestinal epithelial cells (IECs). After inoculation for 3 hours, the IECs were cultured for 72 hours in differentiation medium with bile supplementation. Viral genome RNA was extracted from the supernatants at 3 and 72 hours postinfection (hpi), and then genome equivalents were quantified with real-time PCR. Each value is representative of at least 3 independent experiments and data are shown as the mean ± 1 SD of 4–6 wells of supernatant from each culture group.
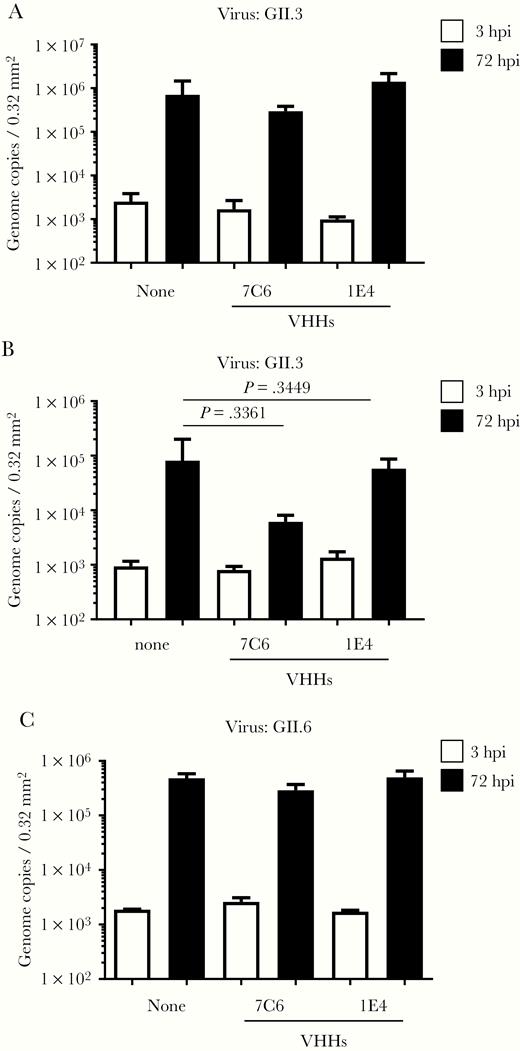
Neutralization of GII.3 and GII.6 human noroviruses by variable-domain heavy-chain antibody fragments (VHHs) 7C6 and 1E4. We incubated 2 × 106 genome equivalents of GII.3_2016 (A, B) or GII.6_2018 (C) human noroviruses with 5 µg of the indicated VHH for 2 hours before using them to inoculate human intestinal epithelial cells (IECs). After inoculation for 3 hours, IECs were cultured for 72 hours in differentiation medium with (A, C) or without (B) bile supplementation. Viral genome RNA was extracted from the supernatants at 3 and 72 hours postinfection (hpi), and then genome equivalents were quantified with real-time PCR. Each value is representative of at least 3 independent experiments and data are shown as the mean ± 1 SD of 4–6 wells of supernatant from each culture group.
Finally, we examined the VHHs obtained from llamas immunized with VLPs of GII.17 Kawasaki308. The single VHH that showed broad binding to most of the GII VLPs tested, 1A6, did not neutralize GII.17 norovirus propagation (Supplementary Figure 1B). Of the 7 VHHs that showed GII.17-specific binding, 4, including 1E4, neutralized GII.17 propagation but not GII.3, GII.6, or GII.4 Sydney_2012 norovirus propagation (Figure 1 and Figure 2). We chose VHH 1E4, which showed the highest inhibition capacity, to test in further experiments. We also added bile to the GII.17 norovirus culture system but this addition had no effect on the neutralization ability of the VHHs (Figure 1B and Supplementary Figure 2B).
VHHs 7C6 and 1E4 Blocked the Binding of VLPs to HBGA Carbohydrate and Human iPSC-Derived IECs
Next, we used PGM to test whether 7C6 and 1E4 blocked VLP binding through carbohydrate attachment factors. Both 7C6 and 1E4 blocked the binding of GII.4 Sydney_2012 and GII.17 VLPs to PGM, respectively, but 1E4 was more effective than 7C6 (Supplementary Figure 3). VHHs 7C6 and 1E4 also blocked the binding of GII.4 Sydney_2012 and GII.17 VLPs, respectively, to human iPSC-derived IECs (Supplementary Figure 4). Together, these results suggest that 7C6 and 1E4 inhibit the binding of norovirus VLPs to human IECs via HBGA carbohydrate binding sites.
Binding Sites for VHHs 7C6, 6A2, 1A6, and 1E4 on VLPs
To elucidate the sites to which 7C6, 6A2, 1A6, and 1E4 bind to the respective VLPs, we used an Escherichia coli system to express the full P domain and the P2 and P1-2 subdomains of VP1 (Figure 3A) derived from GII.4 Sakai08-403_2006b and GII.17. Western blot analysis revealed that 7C6 and 6A2 bound to the P1-2 and P2 subdomains of GII.4 VP1, respectively (Figure 3B), and the 1E4 and 1A6 both bound to the P2 subdomain of GII.17 VP1 (Figure 3C). Because the results show binding at the linear sequence of VP1, we believe that 7C6 interacts conformationally with P1-2 together with the P2 domain, where the carbohydrate-binding site is located.
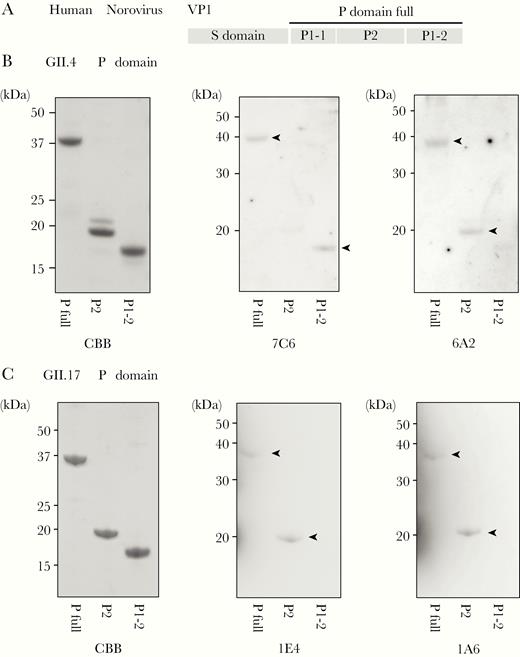
Sites in GII.4 and GII.17 VP1 that are bound by VHHs 7C6, 6A2, 1E4, and 1A6. A, Diagram showing the locations of the various subdomains of VP1. Recombinant proteins containing the full P domain and the P2 and P1-2 subdomains of GII.4_2006b (B) and GII.17_Kawasaki308 VP1 (C) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated recombinant proteins were transferred to a polyvinylidene fluoride membrane and incubated with VHHs 7C6 and 6A2 (B), and 1E4 and 1A6 (C). VHHs 7C6 and 6A2 bound to the full P domain as well as the P1-2 and P2 subdomains, respectively (B, arrow heads). VHH 1E4 and 1A6 bound to the full P domain and the P2 subdomain (C, arrow heads). Abbreviations: CBB, Coomassie brilliant blue; P domain, protruding domain; VHH, variable-domain heavy-chain antibody fragment; VP1, virus protein 1.
A Heterodimeric VHH Neutralizes Both GII.4 and GII.17 Human Noroviruses
Finally, we constructed a 7C6-1E4 heterodimer that showed cross-reactivity with GII genogroup, but not GI genogroup, VLPs, which is similar to the reactivity of the monomeric VHH 7C6 (Table 1). In addition, the heterodimer neutralized GII.4 Sydney_2012 and GII.17 human norovirus propagation (Figure 1). The amount of heterodimer that that was needed to neutralize GII.4 Sydney_2012 norovirus propagation was almost the same on a per-mole basis as that of monomeric VHH 7C6; however, only 1/200 (on a per-mole basis) of the amount of VHH 1E4 was needed for the heterodimer to neutralize GII.17 norovirus propagation (Figure 4). The heterodimer blocked both GII.4 Sydney_2012 and GII.17 VLPs from binding to PGM (Supplementary Figure 3) and human iPSC-derived IECs (Supplementary Figure 4).
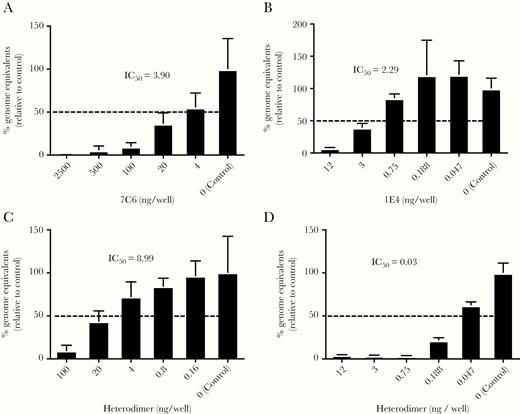
Fifty percent inhibitory concentration (IC50) of in vitro human norovirus replication by variable-domain heavy-chain antibody fragments (VHHs) 7C6, 1E4, and their heterodimer. Inhibition of replication of GII.4 Sydney_2012 (A, C) and GII.17_Kawasaki308 (B, D) noroviruses by using VHH 7C6 (A), 1E4 (B), and their heterodimer (C, D) was tested in a human induced pluripotent stem cell-derived intestinal epithelial cells (IEC) system. An additional control for each experiment was norovirus incubated without VHH. All cultures contained 0.03% bile. The IC50 for each VHH is indicated. Data are representative of 2 independent experiments and are shown as the number (mean ± 1 SD, n = 6) of genome equivalents for each tested concentration of each VHH, including the no-antibody control.
DISCUSSION
Given the lack of an effective vaccine, pharmaceutical drug, or immunotherapy for populations at high risk of severe human norovirus infection, we developed a heterodimeric VHH capable of neutralizing GII.4 and GII.17 human noroviruses for use as oral passive immunotherapy. Specifically, the VHHs used in the heterodimer were 7C6, which bound to GII.4 Sakai08-403_2006b VLPs, and 1E4, which bound to GII.17 VLPs. The unique features of VHHs include ease of high-quantity protein expression, heat stability, and very low immunogenicity, making them an attractive candidate for oral antinoroviral therapy as an alternative to vaccination [21].
In the current study, VHHs 7C6 and 6A2 were cloned from a phage library generated from peripheral blood mononuclear cells of llamas immunized with GII.4 Sakai08-403_2006b VLPs. VHH 7C6 cross-reacted with GII.2, GII.3, GII.6, and GII.17 VLPs as well as GII.4_2006b and GII.4 Sydney_2012 VLPs, whereas 6A2 reacted only with GII.4_2006b and GII.4 Sydney_2012 VLPs. However, using our human iPSC-induced IEC system, we found that 7C6 neutralized GII.4 Sydney_2012 norovirus but not GII.3, GII.6, or GII.17 norovirus propagation, and 6A2 did not neutralize GII.4 Sydney_2012 norovirus propagation. These different findings for 7C6 and 6A2 may be attributed to their different binding locations in the linear sequence of the P domain of the GII.4 Sakai08-403_2006b VLP (P1-2 vs P2 subdomain, respectively).
Two papers have been published on VHHs against human norovirus [22, 23]. The first study examined 2 VHHs, Nano-25 and Nano-85, which were raised against GII.10 Vietnam 026_2005 VLPs and showed cross-reactivity with GII.10 VLPs only and with GII.10, GII.12, and GII.4 VLPs, respectively [22]. The second study examined VHHs that were raised against GII.4 MD_2004 or GI.1 Norwalk_1968 VLPs, and all VHHs were shown to recognize the P domain of each VLP [23]. However, VHHs that recognized GII.4 VLPs did not cross-react with GI.1 VLP and vice versa; these findings are consistent with our results regarding GII.4 VLPs. Neither of these previous VHH studies reported any neutralization data [22, 23].
The mouse mAb 10E9, which was raised against GII.4 Minerva_2006 VLPs, was recently shown to inhibit the replication of GII.4 Sydney_2012 in a human intestinal enteroid system [24]. The authors considered that the binding pocket of mAb 10E9 partially overlaps the HBGA pocket and directly competes for conserved HBGA binding residues [24]. Recently, human IgG and IgA mAbs raised from peripheral blood mononuclear cells of donors who had recovered from natural noroviral infections have been established: 2 of the IgG mAbs and 3 IgA mAbs bind the full P domain to neutralize GII.4 Sydney_2012 human norovirus and block GII.4 Sydney_2012 VLPs from binding to PGM [25]. These results suggest that blockage of the HBGA binding site by mAbs correlates with neutralization of GII.4 norovirus virions in a human enteroid culture system. In the current study, we showed that the ability of VHH 7C6 to neutralize GII.4 Sydney_2012 norovirus propagation correlated with blockage of the HBGA biding site in our human iPSC-derived IEC culture system, suggesting that 7C6 inhibits GII.4 Sydney_2012 norovirus propagation through direct competition for residues in the HBGA binding site or by steric hindrance with the HBGA pocket. A similar mechanism may occur regarding the neutralization of GII.17 norovirus by VHH 1E4.
In the winter of 2014–2015, a novel GII.17 Kawasaki308 human norovirus variant emerged in several countries in Asia [26]. In the current study, we showed that VHH 1E4 neutralized GII.17 human norovirus. VHH 7C6 cross-reacted with GII.17 VLP but did not neutralize GII.17 norovirus. In addition, we showed that GII.17 norovirus neutralization correlated with blockage of the HBGA binding site by VHH 1E4 in a human iPSC-derived IEC culture system because 1E4 bound the P2 subdomain, where the carbohydrate-binding site is located. Surprisingly, the 7C6-1E4 heterodimer was 200-fold more effective on a per-mole basis than monomeric VHH 1E4 in terms of GII.17 norovirus neutralization, but the heterodimer and monomeric 7C6 showed almost the same level of neutralization of GII.4 Sydney_2012 norovirus.
GI.1 and GII.4 consensus VLPs have been developed as candidate norovirus vaccines [27]. In particular, the GII.4 consensus VLP is an engineered VLP composed of the consensus sequence of 3 human norovirus GII.4 strains that circulated in 2002 and 2006; vaccination with GII.4 consensus VLP induces a broad blocking Ab response to nonvaccine human norovirus strains including GII.4 Sydney_2012 [10, 27]. However, it may be difficult to achieve a vaccine that is effective against human noroviruses beyond the GII.4 genotype because we showed recently that a rabbit Ab to GII.4 Sydney_2012 VLPs neutralized GII.4 but not GII.3, GII.6, or GII.17 noroviruses [12]. Together, these results suggest that the need for GII-based VLP vaccines to control infections due to GII noroviruses is increasing. In particular, vaccines capable of inducing neutralizing Abs to human noroviruses in young children and elderly persons are needed. Our heterodimeric VHH neutralized not only GII.4 but also GII.17 human norovirus, suggesting its potential for use as oral passive immunotherapy in immunocompromised hosts, young children, and elderly people.
Currently there is no animal model for human norovirus infection, which is an issue for preclinical testing. Although a replication system of human norovirus in zebrafish has been developed [28], this method cannot be used as an in vivo animal model for human norovirus. Therefore, we may need to confirm the efficacy of our heterodimer in a human clinical study. As a high-yield expression system of VHH for a clinical study, we may use a yeast-based expression system (eg, 100–200 mg/L culture) [29] to produce the heterodimeric VHH. In fact, a minimum of 50 mg of purified VHH against rotavirus (ARP1) from yeast per oral dose has been used in a clinical study in Bangladesh to control rotaviral disease in children [18]. For the heterodimeric VHH, we may turn to an alternative expression system, such as lactobacilli, which express VHH in a cell-surface-anchored form [30, 31]. Oral inoculation with lactobacilli producing the ARP1 against rotavirus was very effective in a mouse model [30]. Transgenic rice that express VHHs is another alternative oral immunotherapy platform [32, 333cx23]; MucoRice-ARP1 seed that highly expressed (0.5% to 1.0% of rice seed weight) the ARP1 against rotavirus markedly decreased the severity of diarrhea and viral load in a murine model and decreased viral shedding in SCID mice with chronic rotavirus infection [32].
In conclusion, we developed a heterodimeric VHH that simultaneously neutralized GII.4 and GII.17 human noroviruses. This heterodimer may offer a novel approach to preventing and treating disease symptoms (eg, diarrhea) due to human norovirus GII.4 Sydney_2012 or GII.17 infection, may provide an alternative to vaccination, and, most importantly, may complement current vaccination programs by covering situations and patient age groups in which rapid protection to control the spread of infection is required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Professor Kazuhiko Katayama (Laboratory of Viral Infection I, Kitasato Institute for Life Sciences, Kitasato University) for providing many of human norovirus VLPs; and Drs Yohei Uchida, Rika Ouchida-Nakahashi, and Kohtaro Fujihashi for helping to analyze the data.
Author contributions. Y. Y., S. K., and S. S. planned the experimental project. Y. Y., S. K., S. S., H. M., T. T., L. H., and H. K. analyzed the data. S. K., S. S., A. Sa., N. M., A. Su., N. S., Y. G., N. T., T. T., and T. M. assisted with and performed the experiments. Y. Y., S. K., S. S., A. Sa., and H. K. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Agency for Medical Research and Development (Program for Creating STart-ups from Advanced Research and Technology grant to H. K., University of Tokyo); Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (grant numbers JP18H05280 to H. K. and JP19H03702 and JP19K22532 to S.S.); Japan Agency for Medical Research and Development/Japanese International Cooperation Agency (grant number JP19jm0110012h0005 to H. K.); and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to S. S.).
Potential conflicts of interest. S. S. and A. Su. were employed by the Research Foundation for Microbial Diseases, Osaka University. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Y. Y., S. K., and S. S. contributed equally to this work.




