-
PDF
- Split View
-
Views
-
Cite
Cite
Georg Semmler, Hannah Griebler, Stephan W Aberle, Karin Stiasny, Lukas Richter, Heidemarie Holzmann, Lukas Weseslindtner, Elevated CXCL10 Serum Levels in Measles Virus Primary Infection and Reinfection Correlate With the Serological Stage and Hospitalization Status, The Journal of Infectious Diseases, Volume 222, Issue 12, 15 December 2020, Pages 2030–2034, https://doi.org/10.1093/infdis/jiaa326
Close - Share Icon Share
Abstract
We quantified serum concentrations of chemokine CXCL10 in 288 patients with measles virus (MeV) primary infection and 16 patients with reinfection (vaccine failure). CXCL10 peaked with emergence of IgM antibodies and was elevated in hospitalized patients (3233 vs 1930 pg/mL, P < .0001). CXCL10 differed between primary and reinfection (1958 vs 932 pg/mL, P = .0402). In comparison to other viral infections with rash-like symptoms, CXCL10 was highly elevated in MeV infection (area under the curve = 0.935; 95% confidence interval, .905–.965; P < .0001). CXCL10 is a potential marker for diagnosis, stage, and severity of MeV infection.
Measles virus (MeV) is a highly contagious RNA virus that contributes substantially to global morbidity and mortality [1]. Although safe and efficient vaccines are available, insufficient vaccination coverage has led to measles outbreaks in America and Europe, resulting in the current resurgence of this severe disease [2]. Individuals with an incomplete vaccination series or rare vaccine failure may acquire breakthrough reinfection with the MeV wild type [3]. While this may trigger a secondary rather than a primary immune response, the pathophysiological background of MeV reinfection has not been studied [4].
The chemokine C-X-C ligand 10 (CXCL10) is, among other stimuli, induced by interferon-γ (IFN-γ) and secreted by a variety of cells upon viral infection [5]. It has therefore been proposed as a key driver of inflammation and a marker indicating the strength of viral replication, infection stage, and severity of disease [6–9]. Lin and coworkers reported higher CXCL10 levels in children who subsequently died due to MeV infection, indicating that CXCL10 might serve as marker for the clinical severity of measles [10]. Subacute sclerosing panencephalitis, the most severe complication of MeV, has also been associated with increased CXCL10 levels [11].
Therefore, we assessed CXCL10 serum levels in MeV primary infection and reinfection, in comparison with other viruses causing rash-like symptoms, and in relation to the infection stage and hospitalization status of infected patients.
METHODS
Patients and Samples
The study comprised serum samples from 304 patients with confirmed acute MeV infection and 113 patients with other virus infections causing rash-like symptoms. MeV infection was confirmed either by (1) polymerase chain reaction (PCR) from any material (serum, oral fluid, throat swab, or urine); (2) positive immunoglobulin M (IgM) serum antibodies with concomitant low-avidity immunoglobulin G (IgG) antibodies; or (3) IgG seroconversion in 2 consecutive serum samples.
MeV infection was subgrouped into primary infection (n = 288 patients) and reinfection (ie, vaccine failure; n = 16 patients). Primary infection was defined either as absence of IgG or presence of low-avidity IgG antibodies (avidity index <40%) and neutralizing antibody titers <400. Reinfection cases were defined as PCR positivity from any material in the presence of high-avidity IgG antibodies (>60%) and neutralizing antibody titers ≥1:400 [4]. Patients with (1) MeV infection despite postexposure prophylaxis vaccination, (2) borderline avidity results (40%–60%), and (3) discordant results between avidity and neutralization test were excluded.
Furthermore, we analyzed serum samples from 113 patients with other acute viral infections causing rash-like symptoms: parvovirus B19V (B19V; n = 20), rubella virus (RuV; n = 12), human herpes virus type 6 (HHV6; n = 14), human cytomegalovirus (HCMV; n = 23), and Epstein-Barr virus (EBV; n = 24). We also included 20 samples from patients with acute mumps virus (MuV) infection, as MuV and MeV are both members of the Paramyxoviridae. Detailed information on how these infections were diagnosed is presented in the Supplementary Material. Serum samples of 25 healthy individuals were included as controls.
This study was approved by the ethics committee of the Medical University of Vienna (EK 2156/2019). Because the samples had been acquired for virologic diagnosis and had already been anonymized, no written informed consent from the patients was required.
PCR and Serology
Detailed information on PCR and serological analyses is given in the Supplementary Material.
Quantification of CXCL10
CXCL10 serum levels were quantified using a commercially available enzyme-linked immunosorbent assay (BD Opteia Human IP-10 ELISA Set; Becton Dickinson Biosciences) using a modified protocol, as describe previously [6].
Statistical Analyses
Detailed information on statistical analyses are given in the Supplementary Material.
RESULTS
Patient Characteristics
Of 304 patients with MeV infection, 287 (94.4%) had detectable MeV RNA in either serum, oral fluid, throat swab, or urine, while 17 (5.5%) were diagnosed only by serological methods. Detailed information on the serological test results and further baseline characteristics (eg, clinical manifestation) are presented in the Supplementary Material. Based on serological results, 288 patients (94.7%) were considered to be primarily infected and 16 patients (5.3%) reinfected.
Of all patients, 138 (45.4%) were hospitalized, 116 were explicitly reported to be not hospitalized (38.2%), while hospitalization status could not be determined for 50 patients (16.4%). Notably, 7 patients (2.4%) with primary infections were treated at intensive care units and in 18 patients (6.3%) distinct complications were reported. However, only 3 patients (1.0%) with reinfections were hospitalized, and the only reported complication in reinfected patients was diarrhea in one individual.
CXCL10 in Primary MeV Infection in Comparison to Other Acute Viral Infections
First, we compared CXCL10 levels among primary MeV infection and infections with other viruses causing rash-like symptoms. As shown in Figure 1A, CXCL10 levels in acute MeV infection (median, 3255 pg/mL; interquartile range [IQR], 1939–5054) were significantly elevated compared to B19V (median, 516 pg/mL; IQR, 385–853; P < .0001), HCMV (median, 1316 pg/mL; IQR, 858–1576; P < .0001), RuV (median, 691 pg/mL; IQR, 374–1477; P < .0001), HHV6 (median, 655 pg/mL; IQR, 385–2114; P < .0001), and EBV (median, 864 pg/mL; IQR, 585–1146; P < .0001).
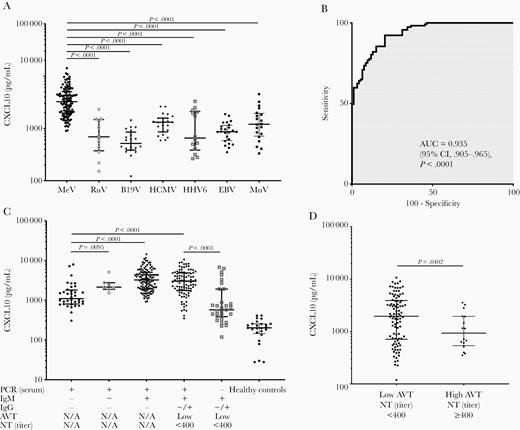
A, Comparison of serum CXCL10 levels among primary MeV infection and other viral infections causing rash-like symptoms. B, ROC analysis to identify the discriminatory ability of CXCL10 to differentiate MeV infection from other viral infections. C, CXCL10 serum levels in patients with different serological stages of acute MeV infection. D, CXCL10 serum levels in patients with primary MeV infection (low IgG avidity and neutralizing antibody titer <400) and serologically confirmed reinfection (secondary infection after vaccine failure; high IgG avidity and neutralizing antibody titer ≥400). Abbreviations: AUC, area under the curve; AVT, IgG avidity; B19V, parvovirus B19V; CI, confidence interval; CXCL10, chemokine C-X-C ligand 10; EBV, Epstein-Barr virus; HCMV, human cytomegalovirus; HHV6, human herpes virus type 6; IgG, immunoglobulin G; IgM, immunoglobulin M; MeV, measles virus; MuV, mumps virus; NT, neutralization test; PCR, polymerase chain reaction; ROC, receiver operator characteristics; RuV, rubella virus. +: positive, ~: borderline, -: negative.
A receiver operator characteristics (ROC) analysis of the discriminatory ability of CXCL10 to differentiate between MeV and other viruses revealed an area under the curve (AUC) of 0.935 (95% confidence interval, .905–.965, P < .0001) with a Youden-optimized cutoff of 1412 pg/mL, giving 92% sensitivity and 80% specificity (Figure 1B). Moreover, CXCL10 levels in acute MeV infection were significantly higher than in acute MuV infection (median, 1204 pg/mL; IQR, 658–2105; P < .0001).
CXCL10 at Different Stages of MeV Infection
Next, we analyzed CXCL10 levels at different serological stages of MeV infection (Figure 1C). Highest CXCL10 levels were observed in patients with detectable MeV RNA in serum, positive IgM, and negative IgG antibodies (median, 3255 pg/mL; IQR, 1939–5054). CXCL10 differed between patients with positive MeV RNA without detectable IgM or IgG antibodies and those with borderline IgM antibodies (median, 1103 vs 2151 pg/mL, respectively; P = .0095), individuals with positive IgM and those with undetectable IgG (median, 1103 vs 3255 pg/mL, respectively; P < .0001) and those with low-avidity IgG antibodies (median, 1103 vs 3030, respectively; P < .0001). Furthermore, CXCL10 was lower when MeV RNA was already undetectable, while IgM and IgG antibodies were still present (median, 575 vs 3030 pg/mL, respectively; P < .0001). All serological stages had significantly higher CXCL10 levels than healthy controls (all P < .0001).
CXCL10 in Primary MeV Infection and Reinfection
Next, we compared CXCL10 serum levels between patients with primary infection (n = 98) and reinfection (n = 16) within the same serological stage (ie, presence of IgG antibodies). Notably, patients with reinfection exhibited significantly lower CXCL10 levels than patients with primary infection (median, 932 vs 1958 pg/mL, respectively; P = .0402; Figure 1D).
CXCL10 in Relation to Onset of Symptoms
We then analyzed CXCL10 levels in relation to days after symptom onset. As shown in Figure 2A, CXCL10 was elevated immediately from the onset of symptoms (median, 2048 pg/mL), remained constantly high with an apparent, but not significant, peak at day 5 (median, 3340 pg/mL), and subsequent declined after day 6.
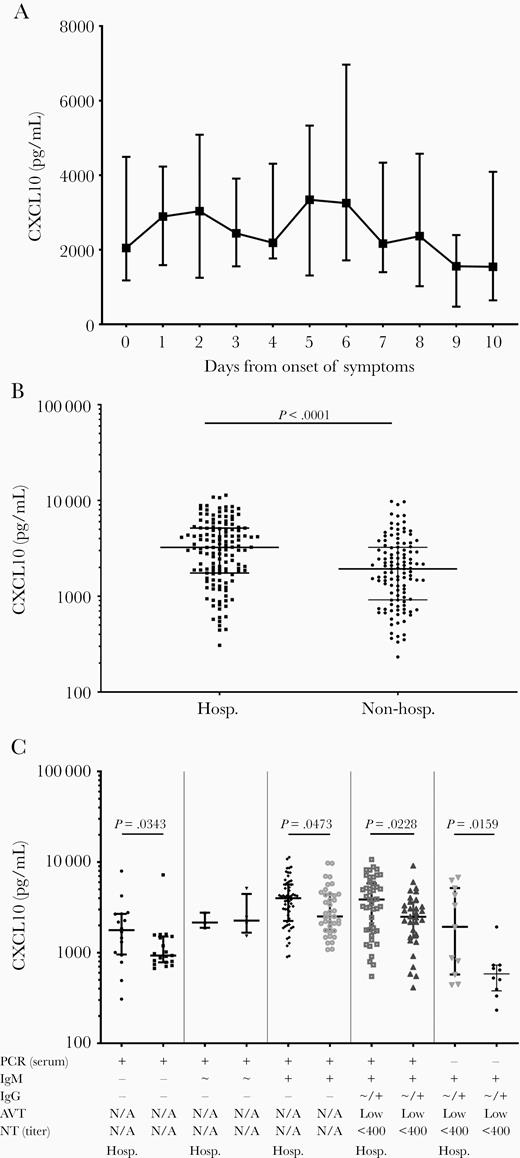
A, CXCL10 serum levels in relation the days since onset of symptoms, displayed as median and IQR. B, CXCL10 serum levels among patients grouped in relation to the hospitalization status. C, CXCL10 serum levels among primary infections in relation to the hospitalization status and the serological profile. Abbreviations: AVT, IgG avidity; CXCL10, chemokine C-X-C ligand 10; Hosp., hospitalized, IgG, immunoglobulin G; IgM, immunoglobulin G; IQR, interquartile range; NT, neutralization test; PCR, polymerase chain reaction. +: positive, ~: borderline, -: negative.
CXCL10 in Relation to Hospitalization Status
Finally, we compared CXCL10 levels in patients who were hospitalized during their MeV infection to those receiving only ambulant medical care and were specifically not hospitalized. CXCL10 levels were significantly higher in hospitalized than in nonhospitalized patients (median, 3233 vs 1930 pg/mL; P < .0001; Figure 2B). This was also evident in subgroup analyses across all serological stages, in early infection before antibody detection (median, 1773 vs 931 pg/mL; P = .0343), when only IgM were detected (median, 3995 vs 2512 pg/mL; P = .0473), after IgG seroconversion (median, 3867 vs 2491 pg/mL; P = .0228), and after disappearance of MeV RNA (median, 1931 vs 584 pg/mL; P = .0159; Figure 2C). When patients were stratified according to genotypes or age, no statistically significant difference in CXCL10 levels was observed (data not shown).
DISCUSSION
In this study, we investigated CXCL10 serum levels in MeV infection, not only in primary infection but for the first time also in reinfection, correlated them to different serological stages, and compared them with levels in other viral infections causing rash-like symptoms. We demonstrate that CXCL10 is significantly elevated during MeV infection, and is able to discriminate MeV from infections with RuV, B19V, HHV6, EBV, and HCMV. Furthermore, we show that CXCL10 kinetics differ among serological stages and peak when MeV-specific IgM emerges. Of note, reinfection is associated with lower CXCL10 levels than primary infection, and significantly higher CXCL10 levels occur in hospitalized individuals, independently of the serological stage.
Our observation that CXCL10 is significantly increased in MeV infection as compared to other viruses causing rash-like symptoms could be related to the fact that the chemokine CXCL10 originates from many different cell types and MeV also has a wide spectrum of tropism [5]. MeV particularly infects epithelial and endothelial cells, as well as the majority of immune cells, causing a systemic infection that is nearly always symptomatic and accompanied by significant malaise [12, 13]. Of note, up to 10% of circulating B cells, CD4+, and CD8+ T cells have been shown to be infected during MeV infection, which apparently triggers a massive CXCL10 response [14].
Although pathophysiological differences between MeV and other viral infections are evident, such a strong CXCL10 increase in MeV-infected patients, even yielding good discriminatory ability in ROC analyses, was surprising. Nonetheless, we acknowledge that in clinical application, CXCL10 could only be used as an unspecific marker, requiring use in combination with specific parameters (eg, IgM and IgG antibodies), and further longitudinal and prospective studies are needed to evaluate its utility.
Moreover, CXCL10 levels were significantly higher in primary infection than reinfection, suggesting that CXCL10 might indicate the cumulative level of viral replication and the extent of dissemination in the host organism. Although the number of reinfected patients we investigated was small, this is of special interest because pathophysiological aspects of reinfection after vaccination have not been studied. Lower CXCL10 levels in reinfection could be mediated by a quicker reaction of memory B and T cells, previously primed during primary infection with the vaccine strain, causing lower levels of viral replication and therefore fewer cells inducing CXCL10.
Of note, we observed a distinct CXCL10 pattern among different serological stages of MeV infection. CXCL10 was induced early after infection, peaked with the appearance of MeV-specific IgM antibodies, and declined subsequently. This finding, which has also been observed in B19V infections, could be related to the fact that CXCL10 is not only triggered by viral replication but can be augmented by the effect of INF-γ [5, 6]. CXCL10 serum levels might thus not only reflect the extent of MeV replication but also the cumulating innate inflammatory immune response. Indeed, we observed only minor changes in CXCL10 kinetics after symptoms occurred. Because MeV infection is typically associated with an incubation period of 8 to 14 days, and onset of rash-like symptoms usually coincides with a peak of viral replication (and the emergence of IgM antibodies), this is not surprising.
Another interesting finding in this study, that CXCL10 levels were higher in hospitalized than in nonhospitalized patients, is in accordance with a previous study, which comprehensively analyzed multiple cytokine levels in MeV infection [10]. The authors demonstrated higher CXCL10 levels in children who subsequently died due to MeV infection, indicating that CXCL10 is associated with clinical severity of MeV infection. Confirming this, our study now shows that CXCL10 levels are elevated in hospitalized cases, but are still related to the serological stage of the infection, a fact that should be taken into account when CXCL10 cutoff levels for clinically severe MeV infections are assessed in the future. Finally, we demonstrate more pronounced CXCL10 differences between hospitalized and nonhospitalized patients in later stages of infection, suggesting that ongoing inflammation and stronger viral replication might aggravate the clinical course of primary infection [1, 15].
This study has several limitations. Despite a precise serological staging in a well-defined cohort with confirmed MeV infections, the assessment of clinical data was based on information reported by the attending physician when samples were submitted. Therefore, these data were not entirely complete, and so we did not carry out subgroup analysis of epidemiological differences. Moreover, we only investigated a single chemokine and in most of the patients only a single sample per patient was available, which did not allow longitudinal analyses.
Nevertheless, we demonstrate that CXCL10 levels are highly elevated in MeV infections, even yielding discriminatory power, and correlate with serological stages and patients’ hospitalization status. CXCL10 levels are, furthermore, higher among patients with MeV primary infection than in reinfection, indicating a lower level of systemic dissemination and viral replication.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Jutta Huttecek for excellent technical assistance and Martin Probst for help with data management.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




