-
PDF
- Split View
-
Views
-
Cite
Cite
Shelly Bolotin, Stephanie L Hughes, Nazish Gul, Sumaiya Khan, Paul A Rota, Alberto Severini, Susan Hahné, Andrea Tricco, William J Moss, Walter Orenstein, Nikki Turner, David Durrheim, Jane M Heffernan, Natasha Crowcroft, What Is the Evidence to Support a Correlate of Protection for Measles? A Systematic Review, The Journal of Infectious Diseases, Volume 221, Issue 10, 15 May 2020, Pages 1576–1583, https://doi.org/10.1093/infdis/jiz380
Close - Share Icon Share
Abstract
Many studies assume that the serologic correlate of protection from measles disease is 120 mIU/mL. We systematically reviewed the literature to examine the evidence supporting this correlate of protection.
We searched peer-reviewed and gray literature for articles reporting a measles correlate of protection. We excluded studies focusing on special populations, infants aged <9 months, and those using animal models or nonstandard vaccines or administration routes. We extracted and synthesized data from full-text articles that met inclusion criteria.
We screened 14 778 articles and included 5 studies in our review. The studies reported either preexposure antibody concentrations of individuals along with a description of symptoms postexposure, or the proportion of measles cases that had preexposure antibody concentrations above a threshold of immunity specified by the authors. Some studies also described secondary antibody responses upon exposure. The variation in laboratory methods between studies made comparisons difficult. Some of the studies that assumed 120 mIU/mL as a correlate of protection identified symptomatic individuals with preexposure titers exceeding this threshold.
Our findings underscore the scant data upon which the commonly used 120 mIU/mL measles threshold of protection is based, suggesting that further work is required to characterize the measles immunity threshold.
(See the Editorial Commentary by Plotkin on pages 1598–606.)
A correlate of protection, defined as a specific immune marker that is associated with protection against infection or disease [1], is important for vaccine development [1, 2], evaluating individual susceptibility to disease, and evaluating population immunity [3]. Many serologic studies assume that the correlate of protection against measles is 120 mIU/mL, as measured with the standard plaque reduction neutralization test (PRNT) [4]. This correlate was derived from a seminal study authored by Chen et al in 1990 [5], which has been cited in the literature >500 times. The study was well-designed, using available preexposure sera from a blood donation drive that occurred a few weeks prior to a measles outbreak at a university. However, as is often the case when research studies originate from outbreaks, the sample size was limited, with only 8 preexposure serum samples available from the blood donation drive from individuals who became measles cases. Furthermore, the study was conducted in the United States at a time when measles was still endemic.
We performed a systematic review to examine the evidence to support a serologic correlate of protection for measles, to analyze and interpret the available data, and to describe knowledge gaps.
METHODS
We used the Population Intervention Comparison Outcome Study Design framework to refine the research question. Our population was individuals ≥9 months of age; our intervention was exposure to wild-type measles virus; our comparison was preexposure antibody titers and/or measures of cell-mediated immunity in exposed individuals who developed measles infection compared to those who did not; and our outcome was laboratory-confirmed measles virus infection, with or without clinical symptoms. We focused on observational study designs. We registered our protocol on the International Prospective Register of Systematic Reviews (PROSPERO; identifier CRD42018109248).
Search Strategy
We searched the Medline (Ovid), Embase, Global Health (EBSCOhost), BIOSIS Previews (Web of Science), and Science Citation Index (Web of Science) databases (Supplementary File 1). We included studies published from the time of inception of each database to 24 January 2018. We excluded animal studies, case studies, letters, conference abstracts and posters, editorials, news items, commentaries, and non-English studies. We searched gray literature using online catalogues, gray literature repositories and web search engines, and by requesting recommendations of pertinent studies from experts in the field. We scanned the reference lists of all included studies (ie, snowballing) to capture articles not identified through the above means.
Title and Abstract Screening
We used Distiller SR systematic review software (Ottawa, Ontario, Canada) for study screening. Before screening, we performed a calibration exercise where we screened an initial 500 abstracts in duplicate to ensure consistency between reviewers. One of 2 reviewers then screened each of the remaining titles/abstracts. We included any study if its title or abstract indicated it may report a correlate of protection to measles. In addition to continuing to apply the exclusion criteria from the search strategy, we also excluded studies in special populations (eg, human immunodeficiency virus infected, immunocompromised), modeling studies, findings related to vaccination in infants <9 months of age, studies using experimental or nonlicensed vaccines, high-titer vaccines (defined as vaccines with a Median tissue culture infective dose (TCID50) of ≥4.7 log10/dose), vaccines that are combined with immunoglobulin or vitamin A, and studies reporting vaccination through any administration route other than subcutaneous.
Full-Text Screening
We performed full-text screening in duplicate on studies that met the inclusion criteria for title/abstract screening. Discrepancies were resolved through discussion between the 2 reviewers. In cases of disagreement, the study in question was screened by a third reviewer. We calculated Cohen κ using GraphPad software (La Jolla, California). Studies passed full-text screening if they reported data on (1) preexposure measures of secondary immune responses (generated through previous vaccination or wild-type measles virus infection) in individuals recently exposed to measles AND description of presence/absence of clinical symptoms, if the exposure occurred ≥14 days postvaccination (for vaccinated individuals only); or (2) studies reporting a quantitative correlate of protection against measles.
We continued to apply the exclusion criteria from the search strategy and title/abstract screening, using the following hierarchy for exclusion: improper format of article (eg, commentary) > nongeneralizable population type (ie, infants, special populations) > nongeneralizable vaccine conditions (eg, high titer, experimental) > unrelated to research question > excluded language. If articles met >1 exclusion criteria, we recorded the highest criterion in the hierarchy as the reason for exclusion.
Quality Appraisal
We performed quality appraisal in duplicate, using the Public Health Ontario Meta Quality Appraisal Tool (MetaQAT) to identify methodological issues that may affect the relevancy, reliability, validity, or applicability of the findings [6].
Data Extraction
We extracted data from each eligible study in duplicate, including individual-level immunity measure data along with measles virus infection status, and aggregate immunity measure data reported for groups of cases or noncases. In instances where individual-level data were represented in graphical form without accompanying text, we utilized the Plot Digitizer tool (Austin, Texas) to extract data points [7].
Data Synthesis
We synthesized extracted data from the included studies and stratified the synthesis by laboratory method and whether the reported correlate of protection was against clinical disease or a secondary immune response.
Ethical Approval
Research ethics board approval was not required for this study. Systematic reviews are exempt as per the Government of Canada Panel on Research Ethics, Tri-Council Policy Statement 2 (2018), Article 2.2b.
RESULTS
Study Screening
Our literature search retrieved 32 832 titles/abstracts and 21 gray-literature documents (Figure 1). An additional article was identified through snowballing and expert consultations. After removing duplicates (n = 18 076), we screened 14 778 titles and abstracts in search of studies that may report a correlate of protection for measles. Of these, we selected 43 papers for full-text review, 5 of which met inclusion criteria for data extraction and quality appraisal. We excluded the remaining 38 studies because they did not contain data pertinent to our research question (n = 32), they were reviews with no primary data (n = 5), or the full-text article was not available for retrieval (n = 1). Our duplicate screening process resulted in a Cohen κ of 93%.
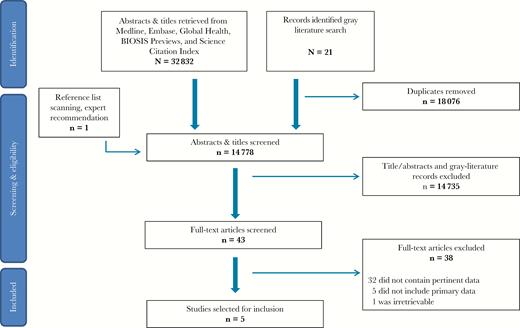
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram outlining the steps of this systematic review.
Data Extraction and Synthesis
Included studies were conducted in Canada [8], the United States [5], Luxembourg [9, 10], and the Netherlands [11], with dates ranging from 1980 to 2014 (Supplementary File 2). Only 1 study, Hahné et al, took place in an elimination setting, as the Netherlands eliminated measles between 2012 and 2014 [11, 12]. All investigated a serologic correlate of protection, with none reporting measures of cell-mediated immunity. Laboratory methods varied and included PRNT [5, 11], neutralization test (NT) [8,10], hemagglutinin inhibition assay (HI) [8,10], enzyme-linked immunosorbent assay (ELISA) [8, 9], and antihemolysin testing [8]. All included studies passed quality appraisal.
Chen et al’s study [5] was published in 1990 and is the landmark paper describing a measles correlate of protection. The study describes a measles outbreak in a dormitory at Boston University that occurred a few weeks after a blood donation drive, providing an opportunity to test preexposure sera.
Muller et al and Huiss et al, 2 studies of the same set of serum samples collected in Luxembourg, were published in 1996 and 1997, respectively. The studies describe antibody titers of parents who cared for their children infected with measles and were therefore exposed [9, 10]. Banked sera from as far back as almost 6 years before the outbreak allowed for assessment of preexposure titers. Muller et al presented pre- and postexposure titers measured by NT and HI. Huiss et al repeated these assays generating highly similar results (which we did not include in our review as they duplicated the work by Muller et al), and added ELISA test results (which we included as they were novel data).
Neumann et al collected sera from high-school students in Prince Edward Island, Canada in 1980, 1 month before a measles outbreak [8]. The authors stated the outbreak was anticipated but do not explain how. However, another article identified through our literature search [13] explained that measles-containing vaccine coverage in the same school was only 82%.
Hahné and colleagues investigated a 2014 measles outbreak in a hospital in the Netherlands [11], which eliminated measles between 2012 and 2014 [12]. Eight healthcare workers were infected, including 6 twice-vaccinated cases and 1 who received 1 dose of measles-containing vaccine. Banked preexposure sera, collected between 3.9 months to 8 years before measles exposure, were available for analysis for 5 infected healthcare workers.
Determination of a Measles Correlate of Protection
Two main types of analyses were pertinent to this systematic review: (1) studies that reported preexposure antibody titers of exposed individuals, along with whether or not they experienced clinical measles symptoms or met a measles case definition; and (2) studies reporting the proportion of measles cases that met an antibody threshold of immunity specified by the authors [5, 8].
Threshold of Immunity Estimated Using Preexposure Antibody Titers and Measles Status of Exposed Individuals (Table 1)
Threshold of Immunity Derived From Preexposure Antibody Titers and Measles Status of Exposed Individuals
| Study . | Study Year . | Preexposure Titers, by Laboratory Method and Measles Status . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA (mOD) . | ||||||||
| . | . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . |
| Chen et al, 1990 [5] | 1985 | a | <16–120 | 56–20 658 | … | … | … | … | … | … | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | … | … | >1:32 | … | 1:33–1:2126 | >1:22 | … | 1:22–1:8343 | … | … | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | … | … | … | … | … | … | ΔA <100 = susceptible; ΔA >200 = immune | … | 437–2383 |
| Hahné et al, 2016 [11] | 2014 | ≥120 | 51–525 | … | … | … | … | … | … | … | … | … | … |
| Study . | Study Year . | Preexposure Titers, by Laboratory Method and Measles Status . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA (mOD) . | ||||||||
| . | . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . |
| Chen et al, 1990 [5] | 1985 | a | <16–120 | 56–20 658 | … | … | … | … | … | … | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | … | … | >1:32 | … | 1:33–1:2126 | >1:22 | … | 1:22–1:8343 | … | … | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | … | … | … | … | … | … | ΔA <100 = susceptible; ΔA >200 = immune | … | 437–2383 |
| Hahné et al, 2016 [11] | 2014 | ≥120 | 51–525 | … | … | … | … | … | … | … | … | … | … |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; mOD, milli–optical density; NT, neutralization test; PRNT, plaque reduction neutralization test.
aCutoff not reported; 50% neutralization dose (ND50) measured.
Threshold of Immunity Derived From Preexposure Antibody Titers and Measles Status of Exposed Individuals
| Study . | Study Year . | Preexposure Titers, by Laboratory Method and Measles Status . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA (mOD) . | ||||||||
| . | . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . |
| Chen et al, 1990 [5] | 1985 | a | <16–120 | 56–20 658 | … | … | … | … | … | … | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | … | … | >1:32 | … | 1:33–1:2126 | >1:22 | … | 1:22–1:8343 | … | … | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | … | … | … | … | … | … | ΔA <100 = susceptible; ΔA >200 = immune | … | 437–2383 |
| Hahné et al, 2016 [11] | 2014 | ≥120 | 51–525 | … | … | … | … | … | … | … | … | … | … |
| Study . | Study Year . | Preexposure Titers, by Laboratory Method and Measles Status . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA (mOD) . | ||||||||
| . | . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . | Cutoff . | Cases . | Noncases . |
| Chen et al, 1990 [5] | 1985 | a | <16–120 | 56–20 658 | … | … | … | … | … | … | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | … | … | >1:32 | … | 1:33–1:2126 | >1:22 | … | 1:22–1:8343 | … | … | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | … | … | … | … | … | … | ΔA <100 = susceptible; ΔA >200 = immune | … | 437–2383 |
| Hahné et al, 2016 [11] | 2014 | ≥120 | 51–525 | … | … | … | … | … | … | … | … | … | … |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; mOD, milli–optical density; NT, neutralization test; PRNT, plaque reduction neutralization test.
aCutoff not reported; 50% neutralization dose (ND50) measured.
Chen et al used PRNT to measure antibody titers to measles, but the test was not performed with World Health Organization International Serum Standards (IS) for measles and the titer was reported as the reciprocal of the dilution of serum that neutralized at least 50% of input virus, as per the Karber formula [14]. A standard protocol for the measles PRNT was published in 2007 [4]. A titer of 120 in the paper by Chen et al was extrapolated to be 200 mIU/mL using the first IS and 120 mIU/mL using the second IS [4]. Chen et al reported that the preexposure antibody titer of cases (n = 8) did not exceed 120. In contrast, 99% (71/72) who did not meet a clinical case definition for measles had PRN antibody titers >120. The exception was 1 student with a measured PRN titer of 56, whose symptoms or exposure details are not characterized further in the study [5]. Similar to Chen et al, Hahné and colleagues used preexposure antibody titers of measles cases (n = 5) and observed that 3 of the 5 cases had titers <120 mIU/mL, including a titer of 115 mIU/mL in serum collected approximately 8 months before exposure; a titer of 51 mIU/mL from serum collected approximately 7 months before exposure; and a titer of 85 mIU/mL from serum collected 8 years before exposure. The remaining 2 cases had titers of 146 mIU/mL, at approximately 4 months prior to rash onset and 525 mIU/mL, in serum banked for 8 years prior to the outbreak [11]. All patients but 1 (the unvaccinated individual with an antibody titer of 51 mIU/mL) were previously vaccinated with 2 doses of measles-containing vaccine and had high avidity postexposure antibody.
Muller et al [10] reported on measles NT and HI titers of preexposure sera from parents exposed to their measles-infected children (N = 45) collected from 2 to 66 months before exposure. None of the parents were immunized and most reported being previously infected, and none reported symptoms. Using Plot Digitizer, we generated 44 data points in the figure presented in this study (we were not able to generate 1 value due to point overlap). The lowest measles antibody titers were 1:33 and 1:22, by NT and HI, respectively [10]. Huiss et al [9] tested 44 of 45 of the same sera using ELISA, and reported that the lowest measles antibody titer of exposed non–case parents was 437 milli–optical density units (mOD). Using a third publication from the same group [15], which did not meet inclusion criteria in the review but provided immunoglobulin G (IgG) titers correlated to mOD values for the same Enzygnost commercial ELISA kit used in Huiss et al, we generated a polynomial calibration curve (Figure 2) and estimated that 437 mOD correlates to approximately 900 mIU/mL.
![Relationship between enzyme immunoassay (EIA) milli–optical density (mOD) units and net immunoglobulin G (IgG) titer. Data adapted from: Damien et al [15].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/221/10/10.1093_infdis_jiz380/1/m_jiz380f0002.jpeg?Expires=1749811188&Signature=KbRWSldR9ZWASrGeN2vSwOyRISfhvSltWdask3pIz4gFuaV~LqznOlZ9qzvzQOIRvbXpt8wj2-6hPQwirl2bcW9x1Q5~WBm3W3BkWFvE2B1dILGulR1M-qXJ8JR~u8jU83tAxK3HK6gppVWW8aUmOHLlLHMaqAcFrYpWElFfabbzan9Pjv8bvWjBKH97Jivq5dfAkCKBjKUYHJjIj3u2UGaWFqBaN06HeQRoiMw~wQE5p9brplZC~4RvvDO31tybLeAZ5G4ffhRHmzBdISXIKXWzhyZYa3QNTmBjlyJtddWvXYbPytMg31sJyKD0DBoVtgPH2ChoR0qOldYEQw0X~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Relationship between enzyme immunoassay (EIA) milli–optical density (mOD) units and net immunoglobulin G (IgG) titer. Data adapted from: Damien et al [15].
Threshold of Immunity Estimated by What Proportion of Cases and Noncases Met a Specified Antibody Titer (Table 2)
Threshold of Immunity Evaluated by the Proportion of Cases and Noncases That Met a Study-Specific Antibody Titer
| Study . | Study Year . | Laboratory-Determined Threshold of Immunity and Measles Case Status . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA Titer . | AH . | ||||||||||
| . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . |
| . | . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . |
| Neumann et al, 1985 [8] | 1980 | … | … | … | ≥1:4 | 0/28 (0) | 203/210 (97) | ≥1:4 | 0/28 (0) | 134/210 (64) | >1:6 | 0/28 (0) | 201/210 (96) | ≥5 mm | 0/28 (0) | 202/210 (96) |
| Chen et al, 1990 [5] | 1985 | ≥1052 | … | 35/72 (49) | … | … | … | … | … | … | … | … | … | … | … | … |
| >120 | 0/8 (0) | 71/72 (99) | ||||||||||||||
| Study . | Study Year . | Laboratory-Determined Threshold of Immunity and Measles Case Status . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA Titer . | AH . | ||||||||||
| . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . |
| . | . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . |
| Neumann et al, 1985 [8] | 1980 | … | … | … | ≥1:4 | 0/28 (0) | 203/210 (97) | ≥1:4 | 0/28 (0) | 134/210 (64) | >1:6 | 0/28 (0) | 201/210 (96) | ≥5 mm | 0/28 (0) | 202/210 (96) |
| Chen et al, 1990 [5] | 1985 | ≥1052 | … | 35/72 (49) | … | … | … | … | … | … | … | … | … | … | … | … |
| >120 | 0/8 (0) | 71/72 (99) | ||||||||||||||
Abbreviations: AH, antihemolysin test; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; NT, neutralization test; PRNT, plaque reduction neutralization test.
Threshold of Immunity Evaluated by the Proportion of Cases and Noncases That Met a Study-Specific Antibody Titer
| Study . | Study Year . | Laboratory-Determined Threshold of Immunity and Measles Case Status . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA Titer . | AH . | ||||||||||
| . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . |
| . | . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . |
| Neumann et al, 1985 [8] | 1980 | … | … | … | ≥1:4 | 0/28 (0) | 203/210 (97) | ≥1:4 | 0/28 (0) | 134/210 (64) | >1:6 | 0/28 (0) | 201/210 (96) | ≥5 mm | 0/28 (0) | 202/210 (96) |
| Chen et al, 1990 [5] | 1985 | ≥1052 | … | 35/72 (49) | … | … | … | … | … | … | … | … | … | … | … | … |
| >120 | 0/8 (0) | 71/72 (99) | ||||||||||||||
| Study . | Study Year . | Laboratory-Determined Threshold of Immunity and Measles Case Status . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | PRNT Titer . | NT Titer . | HI Titer . | ELISA Titer . | AH . | ||||||||||
| . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . | Threshold . | Proportion Immune (%) . | . |
| . | . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . | . | Cases . | Noncases . |
| Neumann et al, 1985 [8] | 1980 | … | … | … | ≥1:4 | 0/28 (0) | 203/210 (97) | ≥1:4 | 0/28 (0) | 134/210 (64) | >1:6 | 0/28 (0) | 201/210 (96) | ≥5 mm | 0/28 (0) | 202/210 (96) |
| Chen et al, 1990 [5] | 1985 | ≥1052 | … | 35/72 (49) | … | … | … | … | … | … | … | … | … | … | … | … |
| >120 | 0/8 (0) | 71/72 (99) | ||||||||||||||
Abbreviations: AH, antihemolysin test; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; NT, neutralization test; PRNT, plaque reduction neutralization test.
Chen et al noted that 0 of 71 (0%) exposed individuals with a PRN titer of >120 met the Centers for Disease Control and Prevention’s (CDC) clinical case definition for measles, whereas 8 of 9 blood donors with a PRN titer of ≤120 did meet the clinical measles case definition. Of the noncases, 35 of 72 (49%) had a measles antibody preexposure titer of ≥1052. However, many of the noncases in the study reported measles-compatible symptoms, regardless of whether their PRN antibody titer was greater than or less than 1052. Chen et al also performed ELISA testing on cases and noncases but did not report a threshold of protection, only noting that 0 of 8 cases (0/8 or 0%) and 69 of 72 (96%) noncases had detectable levels of antibodies [5]. Although we extracted these data, we did not include them in our data synthesis tables as a threshold of protection (or even detection) was not provided. Neumann and colleagues reported that 0/28 (0%) of measles cases had preexposure titers of ≥1:4, ≥1:4, >1:6 and ≥5 mm using NT, HI, ELISA, and antihemolysin testing, respectively [8].
Determination of a Correlate of Protection Against a Secondary Immune Response
Three of the 5 studies reported an antibody titer range within which exposed but nonsymptomatic individuals mounted a secondary immune response to measles (Table 3). Chen et al noted that 7 of 11 (64%) of those with PRNT preexposure titers between 1:216 and 1:874 had a 4-fold boost in antibody levels, compared with 0 of 7 (0%) individuals with PRNT titers ≥1:1052 [5]. Muller and colleagues determined thresholds for secondary immune response of 1:64 and 1:88 by NT and HI, respectively, above which no antibody boosting was detected [10]. Four of 45 (9%) participants experienced a secondary immune response. Huiss et al tested the same group of sera using ELISA and found that with the exception of 1 sample, parents with a preexposure ELISA titer >870 mOD experienced either no boost or only a slight boost of antibody titers [9]. In contrast, those with preexposure titers < 780 mOD experienced an antibody boost of 750–1400 mOD [9]. A third publication from the same group [15], which did not meet our inclusion criteria, correlated the mOD range of 780–870 mIU/mL to 2147–2559 mIU/mL using the same Enzygnost commercial kit that was used in the study by Huiss and colleagues [9].
Antibody Titer Range for Secondary Immune Response, by Study and Laboratory Method
| . | . | Antibody Titer Range for Secondary Immune Response, by Laboratory Method . | |||
|---|---|---|---|---|---|
| Study . | Study Year . | PRNT Titer . | NT Titer . | HI Titer . | ELISA mOD . |
| Chen et al, 1990 [5] | 1985 | 1:216–1:874 | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | 1:32–1:64 | 1:22–1:88 | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | 780–870 mOD by ELISAa |
| . | . | Antibody Titer Range for Secondary Immune Response, by Laboratory Method . | |||
|---|---|---|---|---|---|
| Study . | Study Year . | PRNT Titer . | NT Titer . | HI Titer . | ELISA mOD . |
| Chen et al, 1990 [5] | 1985 | 1:216–1:874 | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | 1:32–1:64 | 1:22–1:88 | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | 780–870 mOD by ELISAa |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; mOD, milli–optical density; NT, neutralization test; PRNT, plaque reduction neutralization test.
aCorrelates to 2147–2559 mIU/mL, based on Damien et al [15].
Antibody Titer Range for Secondary Immune Response, by Study and Laboratory Method
| . | . | Antibody Titer Range for Secondary Immune Response, by Laboratory Method . | |||
|---|---|---|---|---|---|
| Study . | Study Year . | PRNT Titer . | NT Titer . | HI Titer . | ELISA mOD . |
| Chen et al, 1990 [5] | 1985 | 1:216–1:874 | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | 1:32–1:64 | 1:22–1:88 | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | 780–870 mOD by ELISAa |
| . | . | Antibody Titer Range for Secondary Immune Response, by Laboratory Method . | |||
|---|---|---|---|---|---|
| Study . | Study Year . | PRNT Titer . | NT Titer . | HI Titer . | ELISA mOD . |
| Chen et al, 1990 [5] | 1985 | 1:216–1:874 | … | … | … |
| Muller et al, 1996 [10] | 1996 | … | 1:32–1:64 | 1:22–1:88 | … |
| Huiss et al, 1997 [9] | 1996 | … | … | … | 780–870 mOD by ELISAa |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; mOD, milli–optical density; NT, neutralization test; PRNT, plaque reduction neutralization test.
aCorrelates to 2147–2559 mIU/mL, based on Damien et al [15].
DISCUSSION
After screening nearly 15 000 publications, our systematic review identified only 5 studies meeting our inclusion criteria. All 5 studies investigated a serologic correlate of protection, using recently collected or banked preexposure sera. To date, Chen et al is the most methodologically robust study describing a correlate of protection due to the short duration between collection of preexposure sera and symptom development in cases. It is the only study to have a statistically significant finding that those with antibody titers >120 were protected against measles virus infection that met a clinical case definition.
Of the 4 studies that estimated a threshold of immunity using preexposure antibody titers and measles status of exposed individuals, the most comparable were those by Chen et al and Hahné and colleagues. Both studies utilized PRNT; however, their findings varied. While Chen et al found that all cases meeting the CDC measles clinical case definition of fever, rash, and ≥1 of cough, coryza, or conjunctivitis had a preexposure titer ≤120 [5], 2 of the 5 cases in Hahné et al had preexposure sera antibody levels >120 mIU/mL [11]. Of these, one met the European Union measles case definition of fever, rash, and ≥1 of cough, coryza, or conjunctivitis, whereas the other presented with milder symptoms. Additionally, although Chen and colleagues were able to set a threshold for protection of >120 against measles meeting the CDC case definition, many noncases described by Chen et al with high antibody titers using PRNT reported symptoms compatible with measles despite not meeting the CDC case definition. Of noncases with antibody titers <1052 mIU/mL, 26/37 (70%) reported at least 1 symptom. In comparison, of noncases with titers ≥1052, 11 of 35 (31%) reported at least 1 symptom [5]. The significance of these findings remains unclear, but they demonstrate that the accepted serological threshold of protection of 120 mIU/mL is not an absolute indicator of protection against disease. Chen and colleagues posit that these scenarios suggest that measles immunity is a continuum rather than a binary state [5], with the probability of infection decreasing as antibody levels rise. While there is evidence of this being true in other studies, in the absence of laboratory testing to isolate measles virus or detect viral RNA, it remains unclear whether these individuals represent mild cases. In addition, as the combination of fever and rash is a hallmark of many viral illnesses, it is possible that the etiology of such symptoms is not measles. In contrast to Chen and colleagues, Huiss et al, who published 7 years later, were of the opinion that a secondary immune response is not a continuum, but a binary “all or none” response [9] where a secondary immune response occurs if preexposure antibody levels are below a certain threshold.
Only 2 of our 5 studies reported a threshold of immunity based on what proportion of cases and noncases met a specified antibody titer [5, 8]. Between these 2 studies, 5 laboratory methods were used to report results, making comparisons problematic. Three of the 5 studies included in our review investigated the immunity threshold that protects against clinical disease but results in a secondary immune response, characterized by a rise in antibody levels. Despite varying laboratory methods and potential inter- and intra-assay variability, in each study the range of values for a secondary immune response was well-defined and was characterized in a considerable minority of noncases [5, 9, 10]; however, some individuals with titers within the defined ranges did not experience a boost in antibody levels. A secondary immune response may indicate transient infection [9], even in individuals lacking clinical symptoms or with mild disease. This is concerning because these infected individuals may transmit virus to susceptible individuals [9, 16]. However, to our knowledge, none of the studies attempted to isolate virus from these individuals, making these results difficult to interpret conclusively.
All but 1 of the studies in our review was conducted in an endemic setting, in which immunity is often derived from infection with wild-type measles virus. However, in elimination settings vaccine-derived immunity is prevalent, which manifests lower antibody levels [17] and may be less durable than immunity from natural infection [18–21]. Furthermore, waning immunity may occur due to a lack of boosting from circulating wild-type measles virus [21, 22], potentially leaving a proportion of the population in eliminated settings with antibody titers below the threshold of protection. However, it has been hypothesized that even if humoral immunity induced by vaccination is low or undetectable, immunological memory and cell-mediated immunity exists, and vaccinated individuals might be protected if exposed [20]. Studies from several jurisdictions suggest that the threshold of 120 mIU/mL should be reevaluated in elimination settings. Population serosurveys in Australia [23], the Republic of Korea [24], Finland [25], and Canada [26] demonstrate waning antibody levels in vaccinated cohorts in elimination settings, but no large outbreaks. The findings from Hahné et al [11], which characterized secondary vaccine failure in healthcare workers in an elimination setting, further suggest that the measles threshold of protection would benefit from being reexamined. Of 5 cases with preexposure sera, 2 twice-vaccinated cases had titers >120 mIU/mL [11]. Accounting for the fact that neutralization titers can vary over a 2-fold range, the significance of these findings is unclear. However, Hahné and colleagues comment that, even assuming a mean decline of measles IgG of 7.1% annually [27], antibody titers of both cases should have remained >120 mIU/mL at the time of infection [11].
There are several limitations to our review. Only 5 studies passed full-text screening and quality assessment, resulting in limited available data. It was difficult to compare results between studies due to varying laboratory methods and the lack of standardized units such as mIU/mL. The sample sizes for several of the studies were small as they were limited by the number of measles cases associated with each outbreak. Last, our findings are limited to serum antibody as a marker of immunity, as no studies examining cell-mediated immunity met our inclusion criteria.
Although the small number of studies that were included in our review is disappointing, this underscores the scant data on which the commonly used measles threshold of protection 120 mIU/mL is based. The data in our included studies suggest that further work is required to characterize the measles immunity threshold, including cellular immunity. Characterization of secondary immune responses to measles is also important to assess whether individuals who mount a secondary immune response are infectious and to differentiate this group from mildly symptomatic cases, sometimes called “modified measles,” which are increasingly being reported [16, 28–31], some of which have transmitted measles to others [32]. Opportunities to design an experiment to adequately characterize the measles immunity threshold may include a large outbreak that serendipitously includes individuals with prebanked sera, which is unlikely, or a human challenge study, which has ethical and logistical considerations. In some settings, there may be opportunity to assess a measles correlate of protection during outbreaks. These include institutional outbreaks (ie, universities, military), where there may be the opportunity to draw serum from very recently exposed individuals and then follow them to determine who has developed clinical symptoms. Ready research protocols and preemptive ethical approval may facilitate these types of studies. In the interim, mathematical modeling of populations [33] or in-host models [34], as well as further in vitro studies, may be required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge Allison McArthur, Sarah Morgan, and Erin Spicer from Public Health Ontario Library Services for developing and running search strategies; Allison Crehore for coordination; Dr Lennon Li, from Public Health Ontario, for providing statistical support; and the members of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization Measles and Rubella Working Group for providing expert consultation.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the WHO (contract number APW201970235).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: World Health Organization Strategic Advisory Group of Experts on Immunization Measles and Rubella Working Group Meeting, Geneva, Switzerland, 24 July 2018.
References
World Health Organization. RVC conclusions on measles and rubella elimination status in Member States in 2014 and for the period 2012-2014. In:
Author notes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.




