-
PDF
- Split View
-
Views
-
Cite
Cite
Christine H Yang, Malaya K Sahoo, Megan Fitzpatrick, Audrey H Lau, Benjamin A Pinsky, Olivia M Martinez, Evaluating for Human Herpesvirus 6 in the Liver Explants of Children With Liver Failure of Unknown Etiology, The Journal of Infectious Diseases, Volume 220, Issue 3, 1 August 2019, Pages 361–369, https://doi.org/10.1093/infdis/jiy644
Close - Share Icon Share
Abstract
Liver failure of unknown etiology (LFUE) has a transplant-free survival rate <25%. Human herpesvirus 6 (HHV-6) may be associated with LFUE, but studies are limited by small sample size.
We identified all children who underwent liver transplant for LFUE at a single quaternary children’s hospital; 51/65 cases could be age matched with controls (children who underwent liver transplant for metabolic liver disease). Quantitative polymerase chain reaction for HHV-6 was performed on DNA from formalin-fixed paraffin-embedded liver explant tissue.
HHV-6 was detected in 34/51 cases (66.7%) and 19/51 controls (37.3%) (P = .005). Average HHV-6 viral load was 213207 copies/106 cells in positive cases (range: 7293–1102030) and 38115 copies/106 cells in positive controls (range: 1382–122375) (P = .0008). HHV-6 was present significantly more often in cases compared to controls in patients younger than 6 years. In particular, in patients younger than 3 years, HHV-6 was present in 13/27 cases (48.1%) and 2/27 controls (7.4%) (P = .0009).
HHV-6 was detected in liver explants significantly more often and in higher quantities in children transplanted for LFUE compared to controls, suggesting HHV-6 should be evaluated in young children who present with LFUE.
(See the Editorial Commentary by Pellett et al, on pages 343–5.)
Liver failure of unknown etiology (LFUE) is found more frequently in children than in adults. Compared to liver failure from hepatitis B virus, LFUE progresses more slowly, liver regeneration is difficult, and because it does not respond well to medical therapy, transplant is usually required for treatment. Transplant-free survival in cases of LFUE has been documented as less than 25% [1, 2]. While survival in pediatric liver transplantation is generally good, liver transplant is a major surgery with significant risks, and consigns children to a lifetime of immunosuppressive medication, as well as an increased risk of life-threatening infections and cancers as sequelae to immunosuppression. Many children also develop chronic medical conditions, including obesity, dyslipidemia, and chronic kidney disease [3]. It has been suggested that a viral agent is responsible for LFUE, but it is unknown which virus, or viruses, may be responsible. Viruses that are consistently evaluated for in patients presenting with liver failure include hepatitis A, B, and C, along with Epstein-Barr Virus (EBV) and cytomegalovirus (CMV); however, these viruses are negative in LFUE [4].
Human herpesvirus 6 (HHV-6) is a lymphotrophic herpesvirus belonging to the same subfamily as CMV. There are 2 distinct species of HHV-6: HHV-6A and HHV-6B, although the term HHV-6 is used to reference both species [5]. The 2 species have different replication cycles, and subsequent differences in cell tropism, microenvironment interactions, host immune response, and pathogenesis [6, 7]. In industrialized countries such as the United States, seroprevalence of HHV-6 ranges from 72% to 95%, and it is frequently acquired during childhood, with seroconversion rates highest between ages 6–12 months [8]. In immunocompetent children, infection with HHV-6 usually results in benign and self-limited disease; however, severe complications, including febrile seizures, encephalopathy, and fatal disseminated infection, can occur. Furthermore, HHV-6 can reactivate in immunocompromised children [9]. HHV-6 was first associated with LFUE in 1990, when the virus was detected via serological assays in an infant that died of LFUE. Since then, several other similar cases have been reported in neonates, infants, and children [4]. Prospective studies, which have been limited in size, have found HHV-6 to be present significantly more often in fresh frozen liver biopsies from children with LFUE compared to controls [4, 10]. However, despite the ready availability of both serologic and polymerase chain reaction (PCR) assays for HHV-6, evaluation for HHV-6 is rarely done at most institutions as part of the work up for LFUE [11]. Although prospective studies using antivirals to treat HHV-6 have not been reported, case reports and retrospective studies of children with disseminated infections observed a reduction in viral load following treatment with ganciclovir or foscarnet. In addition, cidofovir has been observed to have in vitro activity against HHV-6 [12, 13]. Therefore, if HHV-6 is established to be an etiologic agent for LFUE, early detection and subsequent treatment with specific antivirals may mitigate its clinical course, and potentially prevent a need for transplant.
METHODS
Patient Selection and Samples
Institutional review board approval was obtained from the study institution, which is a quaternary children’s hospital. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All procedure consents at the study institution include consent for the banking and use of human tissue for research purposes. No donor organs were obtained from executed prisoners or other institutionalized persons.
All children who underwent liver transplant at the study institution over the past 20 years were reviewed, and 65 cases of LFUE were identified. LFUE was defined as acute onset of liver failure in the absence of preexisting liver disease, without an identified etiology either before or after transplant. Screening to attempt to identify an etiology for LFUE in cases included the following laboratory tests as appropriate: viral hepatitis PCR and serology for hepatitis A, hepatitis B, hepatitis C, EBV, CMV, and adenovirus; antismooth muscle antibody, antinuclear antibody, and antiliver kidney microsome antibody; ceruloplasmin level; alpha-one-antitrypsin phenotype; acetaminophen level; urine toxin screen; serum amino acids and urine organic acids; and ferritin level. Of the 65 cases of LFUE, 62 had formalin-fixed paraffin-embedded (FFPE) samples from their liver explants available. Of these 62, 51 were able to be age matched with controls to be within 6 months of age of each other at time of transplant, for a total of 51 cases and 51 controls. Of the 11 unmatched cases, 3 of these cases had no age-matched control available, while 8 had age-matched controls identified, but the FFPE blocks of the controls could not be found, and there were no alternate matches available. Age matching was done to prevent the increasing incidence of HHV-6 infection with age from confounding results. Controls were children who underwent liver transplant for metabolic liver disease at the same institution.
DNA Isolation and Quantitative PCR Analysis of HHV-6
Extraction of HHV-6 DNA was performed as previously described [14]. Briefly, 3 to 5 FFPE tissue scrolls of 5–10 μm thickness were cut from each FFPE liver explant block from each patient, with different sterile blades used for each block. The initial scroll cut from each FFPE block was discarded. Extraction was performed with the Qiagen EZ1 DNA Tissue Kit (Qiagen, Valencia, CA). Briefly, buffer G2 (190 μL) was added to the samples and incubated at 75°C for 5 minutes. After incubation, 10 μL of proteinase K was added and incubated at 56°C overnight; 190 μL of each lysate was removed and extracted on the EZ1 workstation using the EZ1 Paraffin Card, and the purified DNA was eluted to a final volume to 200 μL. HHV-6 DNA was detected using a laboratory-developed, quantitative PCR assay targeting a conserved region of the HHV-6 U66 gene [15], which has been validated in plasma as a quantitative assay with a linear range of 1000 to 75000000 copies/mL. This assay detects both HHV-6A and HHV-6B DNA but does not distinguish between them. The HHV-6 assay was performed using the Quantifast Pathogen + internal control (IC) kit on the Rotor-Gene Q instrument (both from Qiagen, Germantown, MD) and reaction set up and cycling conditions were carried out according to the manufacturer’s recommendations. Ten μL of eluate was used in each reaction, with a final reaction volume of 25 μL. Primers (HHV6Q_FWD: GAACACGTGGGTCAGATAGTTGAT; HHV6Q_REV: CATCGCCGTCACCAAACTT) and hydrolysis probe (HHV6Q Probe: FAM-CACGATTGGCTAAAGC-MGB-NFQ) were added at final concentrations of 400 nM and 200 nM, respectively. The Quantifast Pathogen +IC master mix also contained a primer/probe set targeting the IC DNA. Cycling conditions were: hold at 95°C for 5 minutes, then 45 cycles of 95°C for 15 seconds, then at 60°C for 30 seconds. Detection was performed in the green (HHV-6) and yellow (IC) channels; the threshold was set at 0.1 for both channels. β-globin was quantitated as previously described [11]. HHV-6/β-globin ratios were calculated using the following formula: (HHV-6 copies/mL) × 106/(0.5 × β-globin copies/mL).
Statistical Analysis
Fisher exact test was used to evaluate whether HHV-6 was present significantly more often in cases compared to controls, and the Mann-Whitney U test was used to evaluate for significant differences in viral load between cases and controls, with P < .05 being statistically significant. To determine a threshold value for HHV-6 viral load suggestive as the etiology for LFUE, we plotted the receiver operating characteristic (ROC) curve for HHV-6 viral load, using each patient’s case or control status as the disease outcome measure. The ROC curve illustrates the true positive rate (sensitivity) and the false negative rate (1 − specificity) for different HHV-6 viral load cut points. We selected the optimal HHV-6 cut point using the Youden index [16], which indicates the viral load cut point with the highest sensitivity and specificity for LFUE (sensitivity + specificity −1). The area under the ROC curve (AUC) was calculated to determine how well the HHV-6 viral load discriminates LFUE cases. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Power calculations, using reported average incidences of HHV-6 of 57.5% in the target population and 23% in controls [4, 10, 17], indicated that 31 cases and 31 controls were required to achieve 80% power.
RESULTS
Patient Characteristics and LFUE Histology
Demographic information, clinical diagnoses, and documented associated symptoms at time of presentation were obtained from review of clinical history and pathology reports (Table 1). Histologic analysis of LFUE by hematoxylin and eosin stained liver tissue showed cholestasis, lymphocytic parenchymal infiltration, bile duct proliferation, and hepatocellular damage (Figure 1). Average age of cases and controls was 4 years (range: 2 months to 15 years 5 months for cases, 3 months to 15 years 5 months for controls); 21 cases were female and 30 cases were male; 27 controls were female and 24 controls were male. There was no significant difference in the average age or gender distribution between the case and control groups.
Demographics, Diagnoses, and Viral Load of HHV-6 in the Liver Explants of Cases and Controls
| Cases . | Age at Transplant/Sex . | Diagnosis/Clinical Presentation . | HHV-6/106 cells . | Controls . | Age at Transplant/Sex . | Diagnosis . | HHV-6/106 cells . |
|---|---|---|---|---|---|---|---|
| 1A | 2 mo/F | LFUE | 0 | 1B | 8 mo/F | Urea cycle defect | 0 |
| 2A | 4 mo/F | LFUE | 0 | 2B | 6 mo/M | Organic acidemia | 0 |
| 3A | 4 mo/M | LFUE | 0 | 3B | 8.5 mo/F | Urea cycle defect | 0 |
| 4A | 5 mo/M | LFUE | 7293 | 4B | 8 mo/F | Urea cycle defect | 0 |
| 5A | 5.5 mo/M | LFUE | 23357 | 5B | 5.5 mo/F | Urea cycle defect | 0 |
| 6A | 6 mo/F | LFUE | 0 | 6B | 4 mo/M | Urea cycle defect | 0 |
| 7A | 6 mo/M | LFUE | 0 | 7B | 6.5 mo/M | Urea cycle defect | 0 |
| 8A | 7 mo/M | LFUE | 0 | 8B | 3 mo/M | Urea cycle defect | 0 |
| 9A | 7 mo/F | LFUE | 18728 | 9B | 7 mo/M | Urea cycle defect | 0 |
| 10A | 7 mo/F | LFUE w/fatigue | 1102030 | 10B | 10 mo/F | Urea cycle defect | 0 |
| 11A | 7.5 mo/F | LFUE | 123062 | 11B | 7 mo/M | Organic acidemia | 0 |
| 12A | 11 mo/M | LFUE w/diarrhea | 0 | 12B | 13 mo/M | Organic acidemia | 0 |
| 13A | 1 y/M | LFUE after URI | 0 | 13B | 7 mo/M | Aminoacidopathy | 0 |
| 14A | 1 y/M | LFUE | 0 | 14B | 7.5 mo/M | Organic acidemia | 0 |
| 15A | 1 y/M | LFUE after fever, vomiting | 976596 | 15B | 10 mo/F | Urea cycle defect | 0 |
| 16A | 1 y/F | LFUE | 331001 | 16B | 1 y/M | Organic acidemia | 0 |
| 17A | 1 y/F | LFUE after diarrhea | 0 | 17B | 1 y/M | Urea cycle defect | 0 |
| 18A | 1 y/M | LFUE after URI | 0 | 18B | 1 y/F | Organic acidemia | 0 |
| 19A | 1.5 y/F | LFUE | 0 | 19B | 1 y/F | Urea cycle defect | 0 |
| 20A | 1.5 y/F | LFUE after URI | 343515 | 20B | 1 y/F | Urea cycle defect | 0 |
| 21A | 1.5 y/M | LFUE after fatigue | 14937 | 21B | 1 y/F | Oxalosis | 69542 |
| 22A | 2 y/F | LFUE after URI | 0 | 22B | 1.5 y/M | Aminoacidopathy | 0 |
| 23A | 2 y/M | LFUE after vomiting, fatigue | 73723 | 23B | 1.5 y/M | Oxalosis | 0 |
| 24A | 2 y/M | LFUE | 68821 | 24B | 1.5 y/F | Organic acidemia | 0 |
| 25A | 2 y/F | LFUE | 80371 | 25B | 2.5 y/F | Organic acidemia | 0 |
| 26A | 2.5 y/M | LFUE w/vomiting | 95965 | 26B | 2 y/F | Oxalosis | 0 |
| 27A | 2.5 y/M | LFUE after fatigue | 0 | 27B | 2.5 y/M | Glycogen storage disease | 14239 |
| 28A | 3 y/M | LFUE w/abdominal pain | 177382 | 28B | 3 y/F | Organic acidemia | 120359 |
| 29A | 3 y/M | LFUE w/fatigue | 28062 | 29B | 3 y/F | Organic acidemia | 0 |
| 30A | 3 y/M | LFUE w/vomiting, fatigue | 32416 | 30B | 3.5 y/F | Organic acidemia | 4766 |
| 31A | 3.5 y/M | LFUE w/vomiting, fatigue | 465356 | 31B | 3.5 y/F | Urea cycle defect | 0 |
| 32A | 3.5 y/F | LFUE w/vomiting, fatigue | 0 | 32B | 3.5 y/F | Urea cycle defect | 46011 |
| 33A | 4 y/F | LFUE w/constipation, vomiting | 91113 | 33B | 4 y/F | Urea cycle defect | 20725 |
| 34A | 4 y/M | LFUE | 49306 | 34B | 4.5 y/M | Urea cycle defect | 0 |
| 35A | 4.5 y/F | LFUE | 0 | 35B | 4.5 y/F | Organic acidemia | 53075 |
| 36A | 4.5 y/M | LFUE after URI | 330418 | 36B | 5 y/M | Urea cycle defect | 0 |
| 37A | 4.5 y/M | LFUE | 58234 | 37B | 5 y/M | Oxalosis | 5593 |
| 38A | 5.5 y/M | LFUE | 181134 | 38B | 5 y/F | Urea cycle defect | 0 |
| 39A | 6 y/M | LFUE after URI | 156293 | 39B | 6.5 y/F | Urea cycle defect | 58016 |
| 40A | 6.5 y/M | LFUE after URI | 993528 | 40B | 6 y/M | Urea cycle defect | 6875 |
| 41A | 7 y/F | LFUE after URI | 20911 | 41B | 7 y/F | Urea cycle defect | 0 |
| 42A | 7.5 y/F | LFUE w/diarrhea, abdominal pain | 253282 | 42B | 7.5 y/F | Urea cycle defect | 122375 |
| 43A | 8 y/M | LFUE w/diarrhea | 32292 | 43B | 8 y/M | Oxalosis | 5723 |
| 44A | 8.5 y/F | LFUE after rash | 23376 | 44B | 8.5 y/M | Organic acidemia | 18741 |
| 45A | 10 y/F | LFUE w/abdominal pain | 860093 | 45B | 10.5 y/M | Oxalosis | 9811 |
| 46A | 10 y/F | LFUE w/abdominal pain, vomiting | 52587 | 46B | 10 y/F | Urea cycle defect | 1382 |
| 47A | 10.5 y/F | LFUE w/abdominal pain | 0 | 47B | 10 y/F | Urea cycle defect | 11824 |
| 48A | 11 y/M | LFUE w/fatigue | 56385 | 48B | 10.5 y/M | Organic acidemia | 39585 |
| 49A | 11 y/M | LFUE w/fatigue | 62266 | 49B | 11 y/M | Organic acidemia | 23260 |
| 50A | 14 y/M | LFUE after URI | 16399 | 50B | 14 y/M | Organic acidemia | 0 |
| 51A | 15 y/M | LFUE w/diarrhea, abdominal pain | 48791 | 51B | 15 y/F | Organic acidemia | 92289 |
| Cases . | Age at Transplant/Sex . | Diagnosis/Clinical Presentation . | HHV-6/106 cells . | Controls . | Age at Transplant/Sex . | Diagnosis . | HHV-6/106 cells . |
|---|---|---|---|---|---|---|---|
| 1A | 2 mo/F | LFUE | 0 | 1B | 8 mo/F | Urea cycle defect | 0 |
| 2A | 4 mo/F | LFUE | 0 | 2B | 6 mo/M | Organic acidemia | 0 |
| 3A | 4 mo/M | LFUE | 0 | 3B | 8.5 mo/F | Urea cycle defect | 0 |
| 4A | 5 mo/M | LFUE | 7293 | 4B | 8 mo/F | Urea cycle defect | 0 |
| 5A | 5.5 mo/M | LFUE | 23357 | 5B | 5.5 mo/F | Urea cycle defect | 0 |
| 6A | 6 mo/F | LFUE | 0 | 6B | 4 mo/M | Urea cycle defect | 0 |
| 7A | 6 mo/M | LFUE | 0 | 7B | 6.5 mo/M | Urea cycle defect | 0 |
| 8A | 7 mo/M | LFUE | 0 | 8B | 3 mo/M | Urea cycle defect | 0 |
| 9A | 7 mo/F | LFUE | 18728 | 9B | 7 mo/M | Urea cycle defect | 0 |
| 10A | 7 mo/F | LFUE w/fatigue | 1102030 | 10B | 10 mo/F | Urea cycle defect | 0 |
| 11A | 7.5 mo/F | LFUE | 123062 | 11B | 7 mo/M | Organic acidemia | 0 |
| 12A | 11 mo/M | LFUE w/diarrhea | 0 | 12B | 13 mo/M | Organic acidemia | 0 |
| 13A | 1 y/M | LFUE after URI | 0 | 13B | 7 mo/M | Aminoacidopathy | 0 |
| 14A | 1 y/M | LFUE | 0 | 14B | 7.5 mo/M | Organic acidemia | 0 |
| 15A | 1 y/M | LFUE after fever, vomiting | 976596 | 15B | 10 mo/F | Urea cycle defect | 0 |
| 16A | 1 y/F | LFUE | 331001 | 16B | 1 y/M | Organic acidemia | 0 |
| 17A | 1 y/F | LFUE after diarrhea | 0 | 17B | 1 y/M | Urea cycle defect | 0 |
| 18A | 1 y/M | LFUE after URI | 0 | 18B | 1 y/F | Organic acidemia | 0 |
| 19A | 1.5 y/F | LFUE | 0 | 19B | 1 y/F | Urea cycle defect | 0 |
| 20A | 1.5 y/F | LFUE after URI | 343515 | 20B | 1 y/F | Urea cycle defect | 0 |
| 21A | 1.5 y/M | LFUE after fatigue | 14937 | 21B | 1 y/F | Oxalosis | 69542 |
| 22A | 2 y/F | LFUE after URI | 0 | 22B | 1.5 y/M | Aminoacidopathy | 0 |
| 23A | 2 y/M | LFUE after vomiting, fatigue | 73723 | 23B | 1.5 y/M | Oxalosis | 0 |
| 24A | 2 y/M | LFUE | 68821 | 24B | 1.5 y/F | Organic acidemia | 0 |
| 25A | 2 y/F | LFUE | 80371 | 25B | 2.5 y/F | Organic acidemia | 0 |
| 26A | 2.5 y/M | LFUE w/vomiting | 95965 | 26B | 2 y/F | Oxalosis | 0 |
| 27A | 2.5 y/M | LFUE after fatigue | 0 | 27B | 2.5 y/M | Glycogen storage disease | 14239 |
| 28A | 3 y/M | LFUE w/abdominal pain | 177382 | 28B | 3 y/F | Organic acidemia | 120359 |
| 29A | 3 y/M | LFUE w/fatigue | 28062 | 29B | 3 y/F | Organic acidemia | 0 |
| 30A | 3 y/M | LFUE w/vomiting, fatigue | 32416 | 30B | 3.5 y/F | Organic acidemia | 4766 |
| 31A | 3.5 y/M | LFUE w/vomiting, fatigue | 465356 | 31B | 3.5 y/F | Urea cycle defect | 0 |
| 32A | 3.5 y/F | LFUE w/vomiting, fatigue | 0 | 32B | 3.5 y/F | Urea cycle defect | 46011 |
| 33A | 4 y/F | LFUE w/constipation, vomiting | 91113 | 33B | 4 y/F | Urea cycle defect | 20725 |
| 34A | 4 y/M | LFUE | 49306 | 34B | 4.5 y/M | Urea cycle defect | 0 |
| 35A | 4.5 y/F | LFUE | 0 | 35B | 4.5 y/F | Organic acidemia | 53075 |
| 36A | 4.5 y/M | LFUE after URI | 330418 | 36B | 5 y/M | Urea cycle defect | 0 |
| 37A | 4.5 y/M | LFUE | 58234 | 37B | 5 y/M | Oxalosis | 5593 |
| 38A | 5.5 y/M | LFUE | 181134 | 38B | 5 y/F | Urea cycle defect | 0 |
| 39A | 6 y/M | LFUE after URI | 156293 | 39B | 6.5 y/F | Urea cycle defect | 58016 |
| 40A | 6.5 y/M | LFUE after URI | 993528 | 40B | 6 y/M | Urea cycle defect | 6875 |
| 41A | 7 y/F | LFUE after URI | 20911 | 41B | 7 y/F | Urea cycle defect | 0 |
| 42A | 7.5 y/F | LFUE w/diarrhea, abdominal pain | 253282 | 42B | 7.5 y/F | Urea cycle defect | 122375 |
| 43A | 8 y/M | LFUE w/diarrhea | 32292 | 43B | 8 y/M | Oxalosis | 5723 |
| 44A | 8.5 y/F | LFUE after rash | 23376 | 44B | 8.5 y/M | Organic acidemia | 18741 |
| 45A | 10 y/F | LFUE w/abdominal pain | 860093 | 45B | 10.5 y/M | Oxalosis | 9811 |
| 46A | 10 y/F | LFUE w/abdominal pain, vomiting | 52587 | 46B | 10 y/F | Urea cycle defect | 1382 |
| 47A | 10.5 y/F | LFUE w/abdominal pain | 0 | 47B | 10 y/F | Urea cycle defect | 11824 |
| 48A | 11 y/M | LFUE w/fatigue | 56385 | 48B | 10.5 y/M | Organic acidemia | 39585 |
| 49A | 11 y/M | LFUE w/fatigue | 62266 | 49B | 11 y/M | Organic acidemia | 23260 |
| 50A | 14 y/M | LFUE after URI | 16399 | 50B | 14 y/M | Organic acidemia | 0 |
| 51A | 15 y/M | LFUE w/diarrhea, abdominal pain | 48791 | 51B | 15 y/F | Organic acidemia | 92289 |
Abbreviations: F, female; HHV-6, human herpesvirus 6; LFUE, liver failure of unknown etiology; M, male; mo, months; URI, upper respiratory infection; w/, with; y, years.
Demographics, Diagnoses, and Viral Load of HHV-6 in the Liver Explants of Cases and Controls
| Cases . | Age at Transplant/Sex . | Diagnosis/Clinical Presentation . | HHV-6/106 cells . | Controls . | Age at Transplant/Sex . | Diagnosis . | HHV-6/106 cells . |
|---|---|---|---|---|---|---|---|
| 1A | 2 mo/F | LFUE | 0 | 1B | 8 mo/F | Urea cycle defect | 0 |
| 2A | 4 mo/F | LFUE | 0 | 2B | 6 mo/M | Organic acidemia | 0 |
| 3A | 4 mo/M | LFUE | 0 | 3B | 8.5 mo/F | Urea cycle defect | 0 |
| 4A | 5 mo/M | LFUE | 7293 | 4B | 8 mo/F | Urea cycle defect | 0 |
| 5A | 5.5 mo/M | LFUE | 23357 | 5B | 5.5 mo/F | Urea cycle defect | 0 |
| 6A | 6 mo/F | LFUE | 0 | 6B | 4 mo/M | Urea cycle defect | 0 |
| 7A | 6 mo/M | LFUE | 0 | 7B | 6.5 mo/M | Urea cycle defect | 0 |
| 8A | 7 mo/M | LFUE | 0 | 8B | 3 mo/M | Urea cycle defect | 0 |
| 9A | 7 mo/F | LFUE | 18728 | 9B | 7 mo/M | Urea cycle defect | 0 |
| 10A | 7 mo/F | LFUE w/fatigue | 1102030 | 10B | 10 mo/F | Urea cycle defect | 0 |
| 11A | 7.5 mo/F | LFUE | 123062 | 11B | 7 mo/M | Organic acidemia | 0 |
| 12A | 11 mo/M | LFUE w/diarrhea | 0 | 12B | 13 mo/M | Organic acidemia | 0 |
| 13A | 1 y/M | LFUE after URI | 0 | 13B | 7 mo/M | Aminoacidopathy | 0 |
| 14A | 1 y/M | LFUE | 0 | 14B | 7.5 mo/M | Organic acidemia | 0 |
| 15A | 1 y/M | LFUE after fever, vomiting | 976596 | 15B | 10 mo/F | Urea cycle defect | 0 |
| 16A | 1 y/F | LFUE | 331001 | 16B | 1 y/M | Organic acidemia | 0 |
| 17A | 1 y/F | LFUE after diarrhea | 0 | 17B | 1 y/M | Urea cycle defect | 0 |
| 18A | 1 y/M | LFUE after URI | 0 | 18B | 1 y/F | Organic acidemia | 0 |
| 19A | 1.5 y/F | LFUE | 0 | 19B | 1 y/F | Urea cycle defect | 0 |
| 20A | 1.5 y/F | LFUE after URI | 343515 | 20B | 1 y/F | Urea cycle defect | 0 |
| 21A | 1.5 y/M | LFUE after fatigue | 14937 | 21B | 1 y/F | Oxalosis | 69542 |
| 22A | 2 y/F | LFUE after URI | 0 | 22B | 1.5 y/M | Aminoacidopathy | 0 |
| 23A | 2 y/M | LFUE after vomiting, fatigue | 73723 | 23B | 1.5 y/M | Oxalosis | 0 |
| 24A | 2 y/M | LFUE | 68821 | 24B | 1.5 y/F | Organic acidemia | 0 |
| 25A | 2 y/F | LFUE | 80371 | 25B | 2.5 y/F | Organic acidemia | 0 |
| 26A | 2.5 y/M | LFUE w/vomiting | 95965 | 26B | 2 y/F | Oxalosis | 0 |
| 27A | 2.5 y/M | LFUE after fatigue | 0 | 27B | 2.5 y/M | Glycogen storage disease | 14239 |
| 28A | 3 y/M | LFUE w/abdominal pain | 177382 | 28B | 3 y/F | Organic acidemia | 120359 |
| 29A | 3 y/M | LFUE w/fatigue | 28062 | 29B | 3 y/F | Organic acidemia | 0 |
| 30A | 3 y/M | LFUE w/vomiting, fatigue | 32416 | 30B | 3.5 y/F | Organic acidemia | 4766 |
| 31A | 3.5 y/M | LFUE w/vomiting, fatigue | 465356 | 31B | 3.5 y/F | Urea cycle defect | 0 |
| 32A | 3.5 y/F | LFUE w/vomiting, fatigue | 0 | 32B | 3.5 y/F | Urea cycle defect | 46011 |
| 33A | 4 y/F | LFUE w/constipation, vomiting | 91113 | 33B | 4 y/F | Urea cycle defect | 20725 |
| 34A | 4 y/M | LFUE | 49306 | 34B | 4.5 y/M | Urea cycle defect | 0 |
| 35A | 4.5 y/F | LFUE | 0 | 35B | 4.5 y/F | Organic acidemia | 53075 |
| 36A | 4.5 y/M | LFUE after URI | 330418 | 36B | 5 y/M | Urea cycle defect | 0 |
| 37A | 4.5 y/M | LFUE | 58234 | 37B | 5 y/M | Oxalosis | 5593 |
| 38A | 5.5 y/M | LFUE | 181134 | 38B | 5 y/F | Urea cycle defect | 0 |
| 39A | 6 y/M | LFUE after URI | 156293 | 39B | 6.5 y/F | Urea cycle defect | 58016 |
| 40A | 6.5 y/M | LFUE after URI | 993528 | 40B | 6 y/M | Urea cycle defect | 6875 |
| 41A | 7 y/F | LFUE after URI | 20911 | 41B | 7 y/F | Urea cycle defect | 0 |
| 42A | 7.5 y/F | LFUE w/diarrhea, abdominal pain | 253282 | 42B | 7.5 y/F | Urea cycle defect | 122375 |
| 43A | 8 y/M | LFUE w/diarrhea | 32292 | 43B | 8 y/M | Oxalosis | 5723 |
| 44A | 8.5 y/F | LFUE after rash | 23376 | 44B | 8.5 y/M | Organic acidemia | 18741 |
| 45A | 10 y/F | LFUE w/abdominal pain | 860093 | 45B | 10.5 y/M | Oxalosis | 9811 |
| 46A | 10 y/F | LFUE w/abdominal pain, vomiting | 52587 | 46B | 10 y/F | Urea cycle defect | 1382 |
| 47A | 10.5 y/F | LFUE w/abdominal pain | 0 | 47B | 10 y/F | Urea cycle defect | 11824 |
| 48A | 11 y/M | LFUE w/fatigue | 56385 | 48B | 10.5 y/M | Organic acidemia | 39585 |
| 49A | 11 y/M | LFUE w/fatigue | 62266 | 49B | 11 y/M | Organic acidemia | 23260 |
| 50A | 14 y/M | LFUE after URI | 16399 | 50B | 14 y/M | Organic acidemia | 0 |
| 51A | 15 y/M | LFUE w/diarrhea, abdominal pain | 48791 | 51B | 15 y/F | Organic acidemia | 92289 |
| Cases . | Age at Transplant/Sex . | Diagnosis/Clinical Presentation . | HHV-6/106 cells . | Controls . | Age at Transplant/Sex . | Diagnosis . | HHV-6/106 cells . |
|---|---|---|---|---|---|---|---|
| 1A | 2 mo/F | LFUE | 0 | 1B | 8 mo/F | Urea cycle defect | 0 |
| 2A | 4 mo/F | LFUE | 0 | 2B | 6 mo/M | Organic acidemia | 0 |
| 3A | 4 mo/M | LFUE | 0 | 3B | 8.5 mo/F | Urea cycle defect | 0 |
| 4A | 5 mo/M | LFUE | 7293 | 4B | 8 mo/F | Urea cycle defect | 0 |
| 5A | 5.5 mo/M | LFUE | 23357 | 5B | 5.5 mo/F | Urea cycle defect | 0 |
| 6A | 6 mo/F | LFUE | 0 | 6B | 4 mo/M | Urea cycle defect | 0 |
| 7A | 6 mo/M | LFUE | 0 | 7B | 6.5 mo/M | Urea cycle defect | 0 |
| 8A | 7 mo/M | LFUE | 0 | 8B | 3 mo/M | Urea cycle defect | 0 |
| 9A | 7 mo/F | LFUE | 18728 | 9B | 7 mo/M | Urea cycle defect | 0 |
| 10A | 7 mo/F | LFUE w/fatigue | 1102030 | 10B | 10 mo/F | Urea cycle defect | 0 |
| 11A | 7.5 mo/F | LFUE | 123062 | 11B | 7 mo/M | Organic acidemia | 0 |
| 12A | 11 mo/M | LFUE w/diarrhea | 0 | 12B | 13 mo/M | Organic acidemia | 0 |
| 13A | 1 y/M | LFUE after URI | 0 | 13B | 7 mo/M | Aminoacidopathy | 0 |
| 14A | 1 y/M | LFUE | 0 | 14B | 7.5 mo/M | Organic acidemia | 0 |
| 15A | 1 y/M | LFUE after fever, vomiting | 976596 | 15B | 10 mo/F | Urea cycle defect | 0 |
| 16A | 1 y/F | LFUE | 331001 | 16B | 1 y/M | Organic acidemia | 0 |
| 17A | 1 y/F | LFUE after diarrhea | 0 | 17B | 1 y/M | Urea cycle defect | 0 |
| 18A | 1 y/M | LFUE after URI | 0 | 18B | 1 y/F | Organic acidemia | 0 |
| 19A | 1.5 y/F | LFUE | 0 | 19B | 1 y/F | Urea cycle defect | 0 |
| 20A | 1.5 y/F | LFUE after URI | 343515 | 20B | 1 y/F | Urea cycle defect | 0 |
| 21A | 1.5 y/M | LFUE after fatigue | 14937 | 21B | 1 y/F | Oxalosis | 69542 |
| 22A | 2 y/F | LFUE after URI | 0 | 22B | 1.5 y/M | Aminoacidopathy | 0 |
| 23A | 2 y/M | LFUE after vomiting, fatigue | 73723 | 23B | 1.5 y/M | Oxalosis | 0 |
| 24A | 2 y/M | LFUE | 68821 | 24B | 1.5 y/F | Organic acidemia | 0 |
| 25A | 2 y/F | LFUE | 80371 | 25B | 2.5 y/F | Organic acidemia | 0 |
| 26A | 2.5 y/M | LFUE w/vomiting | 95965 | 26B | 2 y/F | Oxalosis | 0 |
| 27A | 2.5 y/M | LFUE after fatigue | 0 | 27B | 2.5 y/M | Glycogen storage disease | 14239 |
| 28A | 3 y/M | LFUE w/abdominal pain | 177382 | 28B | 3 y/F | Organic acidemia | 120359 |
| 29A | 3 y/M | LFUE w/fatigue | 28062 | 29B | 3 y/F | Organic acidemia | 0 |
| 30A | 3 y/M | LFUE w/vomiting, fatigue | 32416 | 30B | 3.5 y/F | Organic acidemia | 4766 |
| 31A | 3.5 y/M | LFUE w/vomiting, fatigue | 465356 | 31B | 3.5 y/F | Urea cycle defect | 0 |
| 32A | 3.5 y/F | LFUE w/vomiting, fatigue | 0 | 32B | 3.5 y/F | Urea cycle defect | 46011 |
| 33A | 4 y/F | LFUE w/constipation, vomiting | 91113 | 33B | 4 y/F | Urea cycle defect | 20725 |
| 34A | 4 y/M | LFUE | 49306 | 34B | 4.5 y/M | Urea cycle defect | 0 |
| 35A | 4.5 y/F | LFUE | 0 | 35B | 4.5 y/F | Organic acidemia | 53075 |
| 36A | 4.5 y/M | LFUE after URI | 330418 | 36B | 5 y/M | Urea cycle defect | 0 |
| 37A | 4.5 y/M | LFUE | 58234 | 37B | 5 y/M | Oxalosis | 5593 |
| 38A | 5.5 y/M | LFUE | 181134 | 38B | 5 y/F | Urea cycle defect | 0 |
| 39A | 6 y/M | LFUE after URI | 156293 | 39B | 6.5 y/F | Urea cycle defect | 58016 |
| 40A | 6.5 y/M | LFUE after URI | 993528 | 40B | 6 y/M | Urea cycle defect | 6875 |
| 41A | 7 y/F | LFUE after URI | 20911 | 41B | 7 y/F | Urea cycle defect | 0 |
| 42A | 7.5 y/F | LFUE w/diarrhea, abdominal pain | 253282 | 42B | 7.5 y/F | Urea cycle defect | 122375 |
| 43A | 8 y/M | LFUE w/diarrhea | 32292 | 43B | 8 y/M | Oxalosis | 5723 |
| 44A | 8.5 y/F | LFUE after rash | 23376 | 44B | 8.5 y/M | Organic acidemia | 18741 |
| 45A | 10 y/F | LFUE w/abdominal pain | 860093 | 45B | 10.5 y/M | Oxalosis | 9811 |
| 46A | 10 y/F | LFUE w/abdominal pain, vomiting | 52587 | 46B | 10 y/F | Urea cycle defect | 1382 |
| 47A | 10.5 y/F | LFUE w/abdominal pain | 0 | 47B | 10 y/F | Urea cycle defect | 11824 |
| 48A | 11 y/M | LFUE w/fatigue | 56385 | 48B | 10.5 y/M | Organic acidemia | 39585 |
| 49A | 11 y/M | LFUE w/fatigue | 62266 | 49B | 11 y/M | Organic acidemia | 23260 |
| 50A | 14 y/M | LFUE after URI | 16399 | 50B | 14 y/M | Organic acidemia | 0 |
| 51A | 15 y/M | LFUE w/diarrhea, abdominal pain | 48791 | 51B | 15 y/F | Organic acidemia | 92289 |
Abbreviations: F, female; HHV-6, human herpesvirus 6; LFUE, liver failure of unknown etiology; M, male; mo, months; URI, upper respiratory infection; w/, with; y, years.
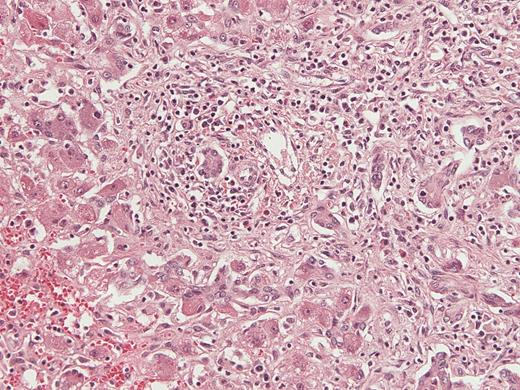
Histologic appearance of liver tissue in a patient who presented with vomiting and fatigue, found to have liver failure of unknown etiology and human herpesvirus 6 detected in the liver explant (case 30A). Hematoxylin and eosin stained sections demonstrate cholestasis, extensive parenchymal infiltration by small lymphocytes, bile duct proliferation, and hepatocellular damage with granulated cytoplasm, consistent with an acute viral infection. Magnification × 200.
Increased Frequency of Detection and Greater HHV-6 Viral Load in Children with LFUE Compared to Age-Matched Controls
To evaluate the frequency of HHV-6 in LFUE, we performed quantitative PCR analysis on DNA isolated from FFPE sections. HHV-6 was detected in 34/51 cases (66.7%), and 19/51 controls (37.3%) (P = .005) (Figure 2). Among patients with detected HHV-6, the mean tissue HHV-6 DNA level was 2.1 × 105 copies/106 cells in LFUE cases (range: 7.3 × 103–1.1 × 106) and 3.8 × 104 copies/106 cells in controls (range: 1.4 × 103–1.2 × 105) (P = .0008) (Figure 3).
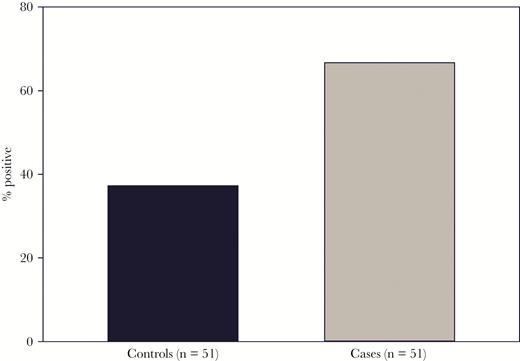
Human herpesvirus 6 (HHV-6) detection rates in cases and controls. HHV-6 DNA was quantitated in formalin-fixed paraffin-embedded sections from patients with liver failure of unknown etiology (cases) and controls. HHV-6 was detected in liver explants of 34 out of 51 cases (66.7%), and 19 out of 51 controls (37.3%), with P = .005.
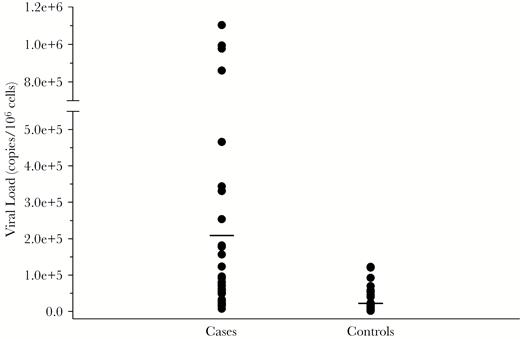
Human herpesvirus 6 (HHV-6) DNA levels in cases and controls. Distribution of viral load as measured by HHV-6 copies/106 cells is shown in liver failure of unknown etiology cases and controls. No HHV-6 DNA was detected in 17 cases and 32 controls. In the 34 cases and 19 controls where HHV-6 DNA was detected, the range in cases was 7293–1102030 copies/106 cells (mean: 213207 copies/106 cells), and 1382–122375 copies/106 cells in controls (mean: 38115 copies/106 cells), with P = .0008. Means in cases and controls are indicated by a horizontal line.
Differences in the Frequency of HHV-6 in LFUE Cases and Controls Is Most Significant in Younger Children
We next examined the impact of age on the frequency of HHV-6 observed in LFUE cases as compared to controls. When stratifying patients by age, HHV-6 was detected significantly more often in cases compared to controls in patients younger than 6 years (22/38 cases, 8/38 controls, P = .002), but not in children 6 years and older (12/13 cases, 11/13 controls, P = .61). This difference in detection rate was particularly prominent in children younger than 3 years, where HHV-6 was detected in 13/27 cases (48.1%) and 2/27 controls (7.4%) (P = .0009) (Figure 4). However, mean viral load was similar in all age groups. In children younger than 3 years, mean viral load was 2.5 × 105 copies/106 cells (range: 1.5 × 104–1.1 × 106) in cases and 4.2 × 104 copies/106 cells (range: 1.4 × 104–6.9 × 104) in controls (P = .305). In children aged 3 years to younger than 6 years, mean viral load was 1.6 × 105 copies/106 cells in cases (range: 2.8 × 104–4.7 × 105) and 4.2 × 104 copies/106 cells (range: 4.8 × 103–1.2 × 105) in controls (P = .05). In children 6 years and older, mean viral load was 2.1 × 105 copies/106 cells (range: 1.6 × 104–9.9 × 105) in cases and 3.5 × 104 copies/106 cells (range: 1.4 × 103–1.2 × 105) in controls (P = .027).
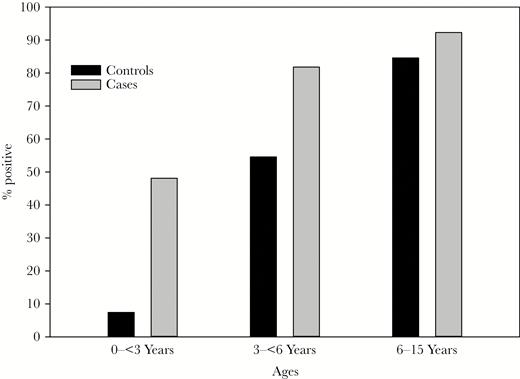
Human herpesvirus 6 (HHV-6) detection rates stratified by age. Frequency of HHV-6 detection in liver failure of unknown etiology cases and controls is shown according to age. HHV-6 was detected in 13/27 (48.15%) cases and 2/27 (7.41%) controls in children younger than 3 years (P = .0009), 9 out of 11 (81.82%) cases and 6 out of 11 (54.55%) controls in children 3 years to younger than 6 years (P = .17), and 12/13 (92.31%) cases and 11/13 (84.62%) controls in children 6 years and older (P = .54).
Identification of Threshold Value for HHV-6 Viral Load
To determine if we could identify a threshold value for HHV-6 viral load suggestive as the etiology for LFUE, ROC curve was plotted, utilizing detected cases as true positives and detected controls as true negatives. This demonstrated an AUC value of 0.78, with optimal cutoff point of 23357 copies/106 cells via Youden index, and sensitivity of 0.853 and specificity of 0.579. We also performed ROC analysis on children younger than 6 years, as well as younger than 3 years, because HHV-6 had been detected significantly more often in cases with LFUE in these age groups. The AUC values in these age groups were similar, at 0.78 in children younger than 6 years and 0.77 in children younger than 3 years (Figure 5). The optimal cutoff point for both children younger than 6 years and younger than 3 years was 73723 (sensitivity 0.591, specificity 0.875 in children younger than 6 years; and sensitivity 0.615, specificity 1.0 in children younger than 3 years) (Table 2). For a maximum sensitivity of 1.0, the optimal cutoff point was 7.3 × 103 copies/106 cells in all age groups [18]. All positive results exceeded the assay threshold of greater than 1000 copies/mL.
Receiver Operator Characteristic Analysis for Patients With Detected HHV-6 Viral Load
| Patients . | Optimal Cutoff (copies/106 cells) . | AUC . | Sensitivity . | Specificity . |
|---|---|---|---|---|
| All patients | 23357 | 0.78 | 0.853 | 0.579 |
| Patients younger than 6 years | 73723 | 0.78 | 0.591 | 0.875 |
| Patients younger than 3 years | 73723 | 0.77 | 0.615 | 1.0 |
| Patients . | Optimal Cutoff (copies/106 cells) . | AUC . | Sensitivity . | Specificity . |
|---|---|---|---|---|
| All patients | 23357 | 0.78 | 0.853 | 0.579 |
| Patients younger than 6 years | 73723 | 0.78 | 0.591 | 0.875 |
| Patients younger than 3 years | 73723 | 0.77 | 0.615 | 1.0 |
Abbreviations: AUC, area under the curve; HHV-6, human herpesvirus 6.
Receiver Operator Characteristic Analysis for Patients With Detected HHV-6 Viral Load
| Patients . | Optimal Cutoff (copies/106 cells) . | AUC . | Sensitivity . | Specificity . |
|---|---|---|---|---|
| All patients | 23357 | 0.78 | 0.853 | 0.579 |
| Patients younger than 6 years | 73723 | 0.78 | 0.591 | 0.875 |
| Patients younger than 3 years | 73723 | 0.77 | 0.615 | 1.0 |
| Patients . | Optimal Cutoff (copies/106 cells) . | AUC . | Sensitivity . | Specificity . |
|---|---|---|---|---|
| All patients | 23357 | 0.78 | 0.853 | 0.579 |
| Patients younger than 6 years | 73723 | 0.78 | 0.591 | 0.875 |
| Patients younger than 3 years | 73723 | 0.77 | 0.615 | 1.0 |
Abbreviations: AUC, area under the curve; HHV-6, human herpesvirus 6.
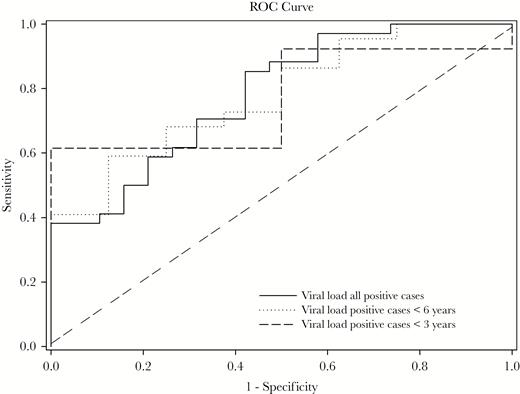
Receiver operator characteristic (ROC) curve of human herpesvirus 6 viral load. ROC curve with area under the curve (AUC) of 0.78 for all positive cases and positive cases younger than 6 years, and AUC of 0.77 for positive cases younger than 3 years. Optimal cutoff point via Youden index is 23357 copies/106 cells for all positive cases, and 73723 copies/106 cells for positive cases younger than 6 years and younger than 3 years.
DISCUSSION
The etiology of hepatic failure in children remains unknown in 40%–50% of cases [19, 20]. Compared to fulminant hepatic failure caused by drugs, hepatitis A, and hepatitis B, LFUE has poorer short-term transplant-free survival [5, 21]. Thus, identifying etiologies for LFUE is critical. This study is the first to evaluate for HHV-6 in FFPE liver explants in pediatric patients transplanted for LFUE, with the largest cohort to date in the literature.
Like other herpesviruses, HHV-6 can establish latency after initial infection, and has been detected in the saliva and peripheral blood mononuclear cells (PBMCs) of 8%–95% of healthy individuals [22–24]. It can reactivate, particularly in immunosuppressed individuals, and higher levels of HHV-6 DNA are found in symptomatic patients [25, 26]. There is evidence suggesting HHV-6 can infect hepatocytes, with in situ hybridization performed on liver tissue localizing HHV-6 DNA more prominently in hepatocytes as opposed to intrahepatic mononuclear cells [10, 17]. In vitro studies have also shown HHV-6 can infect the human hepatoma cell line HepG2 and cause cytokine release, resulting in hepatic cell injury [27, 28]. Thus, while the prevalence of HHV-6–associated liver failure is unknown, it is plausible that one or both species of HHV-6 may be an etiology underlying LFUE. While our assay does not differentiate between HHV-6A and HHV-6B, previous reported cases of HHV-6 detected in patients with liver disease have found HHV-6B as the species present [4, 5, 17].
Several clinical cases of LFUE associated with HHV-6 have been reported, particularly in young children [2, 10, 29–31]. This is consistent with the results of our study, where the rate of HHV-6 detection was particularly evident in children younger than 3 years. Although HHV-6 was overall detected significantly more often in cases compared to controls in our cohort, this significance does not persist in older children when stratified by age. This could be influenced by our cohort having fewer older children (as over half of our patients were younger than 3 years), but may also reflect the natural history of increased HHV-6 prevalence with age. However, detectable viremia (>1000 genome-equivalent copies/mL of blood) is considered the hallmark of active infection [32, 33]. This is different from chromosomally integrated HHV-6 (present in 0.8%–2% of the population), in which there are >1 million viral copies/mL of blood, greatly exceeding the expected values for acute infection, and confirmed if the ratio of viral to human genome is about 1:1 [34]. In our cohort, average detected HHV-6 viral load was nearly 10 times higher in cases compared to controls (213207 copies/106 cells compared to 38115 copies/106 cells), with ROC curve analysis demonstrating an optimal cutoff point of 23357 copies/106 cells. Interestingly, this cutoff was even higher in younger children, at 73723 copies/106 cells in both children younger than 6 years and younger than 3 years, perhaps reflecting absent or less developed anti-HHV6 immunity in younger children. Although our AUC value overall was 0.78, our findings suggest that there is indeed a significant difference in viral load between patients with HHV-6–associated liver failure compared to those without, and that a threshold to identify those with HHV-6–associated liver failure can potentially be established with more evaluation of HHV-6 in patients with LFUE. In addition, despite HHV-6 not being detected significantly more often in cases 6 years and older, there was still a significant difference in detected viral load in this age group. Our overall cohort’s optimal cutoff point for a maximum sensitivity of 1.0 was 7293 copies/106 cells, a threshold that could potentially be used as an initial screen to determine which patients warrant further work-up for HHV-6–associated liver failure. Further studies would be necessary to determine whether the elevations in viral DNA are attributable to active lytic infection, chromosomal integrations of the virus, or increased numbers of latent virions.
Our study found an increased rate of HHV-6 DNA detection in liver explants of children transplanted for LFUE compared to children transplanted for metabolic liver disease, but we acknowledge this does not establish causality between HHV-6 and LFUE. However, the aim of our study was to investigate a potential association between HHV-6 and LFUE using a large biobank of FFPE samples, compared to the more limited availability of fresh frozen samples, to determine whether routine evaluation for HHV-6 may be warranted in children with LFUE. Accordingly, our sample size of 51 cases of children transplanted for LFUE is the largest currently in the literature. Paired sera (before and after the onset of liver failure) demonstrating seroconversion for HHV-6 would provide further support for one or both species of HHV-6 as an etiology for LFUE. However, this is very difficult to obtain, as many children are healthy prior to LFUE and do not have banked sera from prior to their presentation available for testing. The presence of anti-HHV-6 immunoglobulin M, or detection of HHV-6 DNA in the serum, suggests active replication of the virus, although this does not distinguish between primary infection versus reactivation [10]. As HHV-6 can reactivate in times of stress, it is possible HHV-6 can reactivate during liver failure from another etiology, as opposed to being the inciting etiology. However, a study evaluating for HHV-6 in patients with LFUE utilized patients with liver failure secondary to hepatitis B as controls; HHV-6 was detected in 7/11 (63.6%) cases, and 0/4 controls, thus suggesting HHV-6 does not necessarily reactivate during liver failure [10].
We did not evaluate for HHV-6 in PBMCs as banked PBMCs were not available from our cohort. Some previous studies evaluating for HHV-6 utilized PCR analysis of PBMC, as it is known HHV-6 infects and can be latent in PBMCs, and it has not been firmly established that HHV-6 infects hepatocytes. However, several studies evaluating for HHV-6 DNA in both PBMCs and liver biopsies found HHV-6 in liver tissue more frequently than in PBMCs, and there were no cases where HHV-6 was detected in PBMCs and not in liver tissue [4, 17]. Furthermore, previous in situ studies have localized HHV-6 DNA to hepatocytes [10, 17], thus we did not evaluate for PBMC-associated HHV-6 in our study.
Lastly, we acknowledge that while HHV-6 may be an etiology for LFUE, it is most likely not the only etiology. LFUE could have an autoimmune etiology, as evidenced by concurrent development of aplastic anemia in some cases [35]. Among viruses, hepatitis E, adenovirus, echovirus, herpes simplex virus (HSV), and influenza virus, along with rarer causes, such as dengue virus and yellow fever virus, have been implicated in liver failure [36–39]. Metagenomic next-generation sequencing has been used to identify possible viral etiologies in 187 adult patients in the Acute Liver Failure Study Group, and found viruses including HSV-1, parvovirus B19, and human herpesvirus-7. This technique could potentially also be applied to pediatric patients with LFUE [35].
In conclusion, our study detected HHV-6 significantly more often in children transplanted for LFUE compared to controls. These findings support further investigation of one or both species of HHV-6 as a possible etiology for LFUE, including routine evaluation for HHV-6 in livers and sera of patients who present with LFUE, particularly children younger than 3 years. Our institution has now instituted routine evaluation for HHV-6 in all children who present with LFUE. Future studies will evaluate the rate of detection of HHV-6 in sera compared to livers of children transplanted for LFUE, as well as whether intervention with antivirals mitigates the clinical course of these patients. A case report provided evidence that HHV-6–associated liver failure can be successfully treated with valganciclovir [40]; thus early, targeted therapeutic intervention could potentially preempt the need for liver transplant in children with LFUE.
Notes
Presented in part: American Association for the Study of Liver Disease Liver Meeting 2017, Washington DC, 20–24 October 2017, and North American Society for Pediatric Gastroenterology, Hepatology And Nutrition Annual Meeting, Las Vagas, 1–4 November 2017, poster.
Acknowledgment. We thank Peiyi Kan and Amber Trickey for their statistical assistance with this work.
Financial support. This work was supported by the Stanford Child Health Research Institute New Idea Grant.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




