-
PDF
- Split View
-
Views
-
Cite
Cite
Deepa Gamage, Ondrej Mach, Samitha Ginige, William C Weldon, M Steven Oberste, Visalakshi Jeyaseelan, Roland W Sutter, Poliovirus Type 2 Seroprevalence Following Full- or Fractional-Dose Inactivated Poliovirus Vaccine in the Period After Sabin Type 2 Withdrawal in Sri Lanka, The Journal of Infectious Diseases, Volume 219, Issue 12, 15 June 2019, Pages 1887–1892, https://doi.org/10.1093/infdis/jiz026
Close - Share Icon Share
Abstract
In July 2016, Sri Lanka replaced 1 intramuscular dose of inactivated poliovirus vaccine (IPV) with 2 doses of intradermal fractional-dose IPV (fIPV) in its routine immunization schedule. We carried out a survey of seroprevalence of antipolio antibodies in children who received 2 fIPV doses and compared it with those who received 1 full IPV dose.
Children born between March and December 2016 were randomly selected from 3 Sri Lankan districts (Colombo, Badulla, and Anuradhapura). Serum samples were collected and tested for presence of neutralizing antibodies to poliovirus types 1, 2, and 3.
Seroprevalence of antipolio antibodies was 100% in all districts for poliovirus type 1 and poliovirus type 3; it ranged between 90% and 93% for poliovirus type 2 (PV2) in children who received 1 full IPV dose and between 78% and 100% in those receiving 2 fIPV doses (P = .22). The median reciprocal titers of anti-PV2 antibodies were similar in children who received full-dose IPV and those who received fIPV (1:64 vs 1:45, respectively; P = .11).
Our study demonstrated not only that Sri Lanka succeeded in maintaining very high primary immunization coverage also but that it is feasible for a national immunization program to implement fIPV immunization and achieve high coverage with intradermal application. The seroprevalence of anti-PV2 antibodies did not decrease after the introduction of fIPV.
Wild poliovirus has been eliminated from all but a few endemic areas of Nigeria, Pakistan, and Afghanistan [1]. To complete eradication, the Global Polio Eradication Initiative (GPEI) must also eliminate polioviruses emanating from the use of live oral poliovirus vaccine (OPV) because in rare circumstances the Sabin virus from OPV may revert, regain neurovirulence, and start to circulate, causing outbreaks of circulating vaccine-derived poliovirus (cVDPV) [2]. For this reason, GPEI has developed and implemented its “Polio Eradication and Endgame Strategic Plan 2013–2018” [3]. This plan includes a phased withdrawal of OPV starting with poliovirus type 2 (PV2); the global switch from trivalent OPV (tOPV) to bivalent OPV (bOPV), which contains poliovirus types 1 and 3 (PV1 and PV3), was carried out in a synchronized manner globally in April 2016. Since then, no country in the world has used vaccine containing live PV2 in their routine immunization program. Monovalent oral PV2 (mOPV2) is being used for outbreak control of cVDPV type 2 but only when explicitly authorized by the director general of the World Health Organization.
In order to mitigate risk of a type 2 cVDPV emergence or a threatening outbreak scenario, the Strategic Advisory Group of Experts (SAGE) recommended the universal introduction of 1 dose of inactivated poliovirus vaccine (IPV) to routine immunization schedules in countries that had been using only OPV [4]. Data suggest that 1 dose of IPV induces an immunity base (ie, seroconversion and priming) that can be rapidly boosted by a second dose of IPV, demonstrated by high antibody titers that would be expected to mitigate the consequences of a type 2 event by reducing the number of paralytic cases [5–8].
Following SAGE’s recommendation, Sri Lanka added 1 dose of IPV (administered at 4 months of age) into their routine immunization schedule in July 2015 and, together with all other countries in the world, switched from tOPV to bOPV on 30 April 2016. However, starting in 2016, GPEI has been experiencing an acute IPV supply shortage that affected initially almost 50 countries and caused either delays in IPV introduction or stockouts in countries that had already introduced IPV in their routine immunization programs [9]. To extend the available IPV supplies, intradermal administration of one-fifth of a full IPV dose (0.1 mL instead of 0.5 mL, referred to as fractional-dose IPV [fIPV]) has been evaluated, with a conclusion that a schedule of 2 doses of fIPV provides superior immunogenicity compared with 1 full dose of IPV and stretches the existing supply of IPV [10–12]. Based on the results of this evaluation, the use of a 2-dose fIPV schedule for routine immunization was recommended by SAGE [13] for all OPV-using countries.
To guarantee adequate IPV supply for its children, Sri Lanka decided to replace 1 full IPV dose with 2 doses of fIPV administered intradermally at 2 and 4 months of age, effective July 2016. Apart from Sri Lanka, other countries in the region, notably India, Bangladesh, and Nepal, have also recently introduced intradermal fIPV in their routine immunization schedules.
A polio antibody seroprevalence survey conducted in Sri Lanka in 2014 demonstrated >90% seroprevalence against all 3 poliovirus serotypes [14]. This corresponds with consistently high vaccination coverage reported from Sri Lanka; coverage with the third dose of poliovirus vaccine has been >95% for the past 5 years [15].
In the current article, we present results from a polio seroprevalence survey conducted in Sri Lanka between December 2017 and March 2018 with the objective to quantify levels of serological protection against PV1, PV2, and PV3 in children who completed the primary immunization schedule after the switch from tOPV to bOPV. In addition, we compared the seroprevalence for PV2 in children who received 1 full IPV dose with those who received 2 fIPV doses.
METHODS
We performed a cross-sectional community-based survey in 3 districts of Sri Lanka: Colombo, Badulla, and Anuradhapura. The study area represented lower and medium socio-economic strata as well as a mix of rural, periurban, and urban families.
Children from 3 age groups were selected to participate in the study. Group 1a included children born just before the tOPV-to-bOPV switch (born between 1 March and 30 April 2016). Although children in group 1a were born before the switch, they had not yet received tOPV because they reached 2 months of age after the switch and therefore received 3 doses of bOPV (at 2, 3, and 4 months of age) and 1 full IPV dose at 4 months of age. Group 1b included children born right after the switch (between 1 and 15 May 2016); they also received 3 doses of bOPV and a full IPV dose at 4 months of age. Group 2 included children born after the switch (between 1 June and 31 December 2016); they received 2 fIPV doses at 2 and 4 months of age in addition to 3 bOPV doses. We intentionally did not sample from children born between 15 May and 1 June 2016 because these children could have received either 1 full IPV dose or 2 fIPV doses.
The children were randomly selected from field-level health registers kept with the Medical Officers of Health. Parents of eligible children were approached during regular visits of public health midwives, provided consent, and were enrolled. On the same day, the children and parents were transported to the nearest health center, where a 2-mL blood sample was collected and a short questionnaire was administered to the parents. The study was approved by the Ethics Review Committee of the Ministry of Health, Sri Lanka, and by the World Health Organizations’s Ethics Review Committee in Geneva, Switzerland.
The blood samples were allowed to clot. Serum samples were separated and transported to Colombo, where they were stored at −20°C until shipment to the Centers for Disease Control and Prevention in Atlanta, Georgia, where the serum samples were tested for the presence of poliovirus neutralizing antibodies using standard neutralization assays [16]. Seropositivity was defined as a reciprocal titer of poliovirus neutralizing antibodies ≥1:8. The maximum reciprocal titer reported was 1:1448; therefore, the value of 1:1448 indicated a reciprocal titer ≥1:1448 [16].
The vaccination histories of the enrolled children were recorded from vaccination cards when available; otherwise, histories were obtained through parental recall. A sample size of 300 children was calculated to be sufficient to detect, at the 95% confidence level, a seroprevalence point estimate, with a precision of approximately ±5% assuming ≥80% seroprevalence for PV2. It was decided to have 150 children each in groups 1 and 2 (75 each in groups 1a and 1b).
Seroprevalence was expressed in percentages, and exact 95% confidence intervals (CIs) were calculated. The comparison of seroprevalence for the 3 serotypes was made using the χ2 test, and the median titers were compared using the Kruskal-Wallis test. The median titers were also presented with percentile 95% bootstrap CIs [17]. Differences were considered statistically significant at P < . 05.
RESULTS
The community health workers approached 332 children in the 3 targeted districts; 330 of 332 children (99%) were enrolled (the other 2 were ill during the health worker’s visit). All enrolled children provided sufficient blood samples; we included 327 of 330 children in the final analysis. Three children were excluded because they had a history of receiving hexavalent vaccine containing IPV, which had been administered outside the routine immunization schedule. There were 80, 72, and 175 children included in the analysis from age groups 1a, 1b, and 2 respectively.
The baseline characteristics for the per-protocol population are presented in Table 1. There were no significant differences in baseline characteristics between the districts. The rate of vaccination card retention was >98% in our sample, and vaccination history based on information from vaccination cards indicated >95% immunization coverage with all antigens as per the routine immunization schedule.
| . | Children by District, No. (%)a . | . | . | . |
|---|---|---|---|---|
| Characteristic . | Anuradhapura (n = 107) . | Badulla (n = 114) . | Colombo (n = 106) . | Total . |
| Age, median (range), mo | 18 (10–21) | 20 (13–23) | 19 (12–22) | 18 (10–23) |
| Female sex | 55 (51.4) | 47 (41.2) | 46 (43.4) | 148 (45.3) |
| Mother’s educational level | ||||
| Up to grade 5 | 12 (11.2) | 3 (2.6) | 3 (2.8) | 18 (5.4) |
| Up to ordinary levelb | 43 (40.2) | 58 (50.9) | 47 (44.3) | 148 (45.3) |
| Up to advanced levelc | 36 (33.6) | 40 (35.1) | 41 (38.7) | 117 (35.8) |
| Degree/diploma (university) | 16 (15.0) | 13 (11.4) | 15 (14.2) | 44 (13.5) |
| Father’s educational level | ||||
| Up to grade 5 | 11 (10.3) | 8 (7.0) | 7 (6.6) | 26 (8.0) |
| Up to ordinary levelb | 54 (50.5) | 59 (51.8) | 55 (51.9) | 168 (51.4) |
| Up to advanced levelc | 35 (32.7) | 39 (34.2) | 27 (25.5) | 101 (30.9) |
| Degree/diploma (university) | 7 (6.5) | 8 (7.0) | 17 (16.0) | 32 (9.8) |
| Family monthly income | ||||
| <25 000 SLRs (~$150) | 25 (23.4) | 27 (23.7) | 16 (15.1) | 68 (20.8) |
| 25 001–50 000 SLRs (~$150–$300) | 62 (57.9) | 59 (51.8) | 74 (69.8) | 195 (59.6) |
| >50 000 SLRs (~$300) | 20 (18.7) | 27 (23.7) | 16 (15.1) | 63 (19.3) |
| Stunting | ||||
| Moderate | 7 (6.5) | 6 (5.3) | 6 (5.7) | 19 (5.8) |
| Severe | 0 | 3 (2.6) | 3 (2.8) | 6 (1.8) |
| Wasting | ||||
| Moderate | 28 (26.2) | 24 (21.1) | 16 (15.1) | 68 (20.8) |
| Severe | 2 (1.9) | 4 (3.5) | 2 (1.9) | 8 (2.4) |
| . | Children by District, No. (%)a . | . | . | . |
|---|---|---|---|---|
| Characteristic . | Anuradhapura (n = 107) . | Badulla (n = 114) . | Colombo (n = 106) . | Total . |
| Age, median (range), mo | 18 (10–21) | 20 (13–23) | 19 (12–22) | 18 (10–23) |
| Female sex | 55 (51.4) | 47 (41.2) | 46 (43.4) | 148 (45.3) |
| Mother’s educational level | ||||
| Up to grade 5 | 12 (11.2) | 3 (2.6) | 3 (2.8) | 18 (5.4) |
| Up to ordinary levelb | 43 (40.2) | 58 (50.9) | 47 (44.3) | 148 (45.3) |
| Up to advanced levelc | 36 (33.6) | 40 (35.1) | 41 (38.7) | 117 (35.8) |
| Degree/diploma (university) | 16 (15.0) | 13 (11.4) | 15 (14.2) | 44 (13.5) |
| Father’s educational level | ||||
| Up to grade 5 | 11 (10.3) | 8 (7.0) | 7 (6.6) | 26 (8.0) |
| Up to ordinary levelb | 54 (50.5) | 59 (51.8) | 55 (51.9) | 168 (51.4) |
| Up to advanced levelc | 35 (32.7) | 39 (34.2) | 27 (25.5) | 101 (30.9) |
| Degree/diploma (university) | 7 (6.5) | 8 (7.0) | 17 (16.0) | 32 (9.8) |
| Family monthly income | ||||
| <25 000 SLRs (~$150) | 25 (23.4) | 27 (23.7) | 16 (15.1) | 68 (20.8) |
| 25 001–50 000 SLRs (~$150–$300) | 62 (57.9) | 59 (51.8) | 74 (69.8) | 195 (59.6) |
| >50 000 SLRs (~$300) | 20 (18.7) | 27 (23.7) | 16 (15.1) | 63 (19.3) |
| Stunting | ||||
| Moderate | 7 (6.5) | 6 (5.3) | 6 (5.7) | 19 (5.8) |
| Severe | 0 | 3 (2.6) | 3 (2.8) | 6 (1.8) |
| Wasting | ||||
| Moderate | 28 (26.2) | 24 (21.1) | 16 (15.1) | 68 (20.8) |
| Severe | 2 (1.9) | 4 (3.5) | 2 (1.9) | 8 (2.4) |
Abbreviation: SLR, Sri Lankan rupees.
aData represent No. (%) of children unless otherwise specified.
bGrade 10 or 11.
cGrade 12 or 13.
| . | Children by District, No. (%)a . | . | . | . |
|---|---|---|---|---|
| Characteristic . | Anuradhapura (n = 107) . | Badulla (n = 114) . | Colombo (n = 106) . | Total . |
| Age, median (range), mo | 18 (10–21) | 20 (13–23) | 19 (12–22) | 18 (10–23) |
| Female sex | 55 (51.4) | 47 (41.2) | 46 (43.4) | 148 (45.3) |
| Mother’s educational level | ||||
| Up to grade 5 | 12 (11.2) | 3 (2.6) | 3 (2.8) | 18 (5.4) |
| Up to ordinary levelb | 43 (40.2) | 58 (50.9) | 47 (44.3) | 148 (45.3) |
| Up to advanced levelc | 36 (33.6) | 40 (35.1) | 41 (38.7) | 117 (35.8) |
| Degree/diploma (university) | 16 (15.0) | 13 (11.4) | 15 (14.2) | 44 (13.5) |
| Father’s educational level | ||||
| Up to grade 5 | 11 (10.3) | 8 (7.0) | 7 (6.6) | 26 (8.0) |
| Up to ordinary levelb | 54 (50.5) | 59 (51.8) | 55 (51.9) | 168 (51.4) |
| Up to advanced levelc | 35 (32.7) | 39 (34.2) | 27 (25.5) | 101 (30.9) |
| Degree/diploma (university) | 7 (6.5) | 8 (7.0) | 17 (16.0) | 32 (9.8) |
| Family monthly income | ||||
| <25 000 SLRs (~$150) | 25 (23.4) | 27 (23.7) | 16 (15.1) | 68 (20.8) |
| 25 001–50 000 SLRs (~$150–$300) | 62 (57.9) | 59 (51.8) | 74 (69.8) | 195 (59.6) |
| >50 000 SLRs (~$300) | 20 (18.7) | 27 (23.7) | 16 (15.1) | 63 (19.3) |
| Stunting | ||||
| Moderate | 7 (6.5) | 6 (5.3) | 6 (5.7) | 19 (5.8) |
| Severe | 0 | 3 (2.6) | 3 (2.8) | 6 (1.8) |
| Wasting | ||||
| Moderate | 28 (26.2) | 24 (21.1) | 16 (15.1) | 68 (20.8) |
| Severe | 2 (1.9) | 4 (3.5) | 2 (1.9) | 8 (2.4) |
| . | Children by District, No. (%)a . | . | . | . |
|---|---|---|---|---|
| Characteristic . | Anuradhapura (n = 107) . | Badulla (n = 114) . | Colombo (n = 106) . | Total . |
| Age, median (range), mo | 18 (10–21) | 20 (13–23) | 19 (12–22) | 18 (10–23) |
| Female sex | 55 (51.4) | 47 (41.2) | 46 (43.4) | 148 (45.3) |
| Mother’s educational level | ||||
| Up to grade 5 | 12 (11.2) | 3 (2.6) | 3 (2.8) | 18 (5.4) |
| Up to ordinary levelb | 43 (40.2) | 58 (50.9) | 47 (44.3) | 148 (45.3) |
| Up to advanced levelc | 36 (33.6) | 40 (35.1) | 41 (38.7) | 117 (35.8) |
| Degree/diploma (university) | 16 (15.0) | 13 (11.4) | 15 (14.2) | 44 (13.5) |
| Father’s educational level | ||||
| Up to grade 5 | 11 (10.3) | 8 (7.0) | 7 (6.6) | 26 (8.0) |
| Up to ordinary levelb | 54 (50.5) | 59 (51.8) | 55 (51.9) | 168 (51.4) |
| Up to advanced levelc | 35 (32.7) | 39 (34.2) | 27 (25.5) | 101 (30.9) |
| Degree/diploma (university) | 7 (6.5) | 8 (7.0) | 17 (16.0) | 32 (9.8) |
| Family monthly income | ||||
| <25 000 SLRs (~$150) | 25 (23.4) | 27 (23.7) | 16 (15.1) | 68 (20.8) |
| 25 001–50 000 SLRs (~$150–$300) | 62 (57.9) | 59 (51.8) | 74 (69.8) | 195 (59.6) |
| >50 000 SLRs (~$300) | 20 (18.7) | 27 (23.7) | 16 (15.1) | 63 (19.3) |
| Stunting | ||||
| Moderate | 7 (6.5) | 6 (5.3) | 6 (5.7) | 19 (5.8) |
| Severe | 0 | 3 (2.6) | 3 (2.8) | 6 (1.8) |
| Wasting | ||||
| Moderate | 28 (26.2) | 24 (21.1) | 16 (15.1) | 68 (20.8) |
| Severe | 2 (1.9) | 4 (3.5) | 2 (1.9) | 8 (2.4) |
Abbreviation: SLR, Sri Lankan rupees.
aData represent No. (%) of children unless otherwise specified.
bGrade 10 or 11.
cGrade 12 or 13.
Seroprevalence of antipolio antibodies was 100% in all age groups and in all districts for PV1 and PV3 and ranged between 78% and 96% for PV2; however, the difference in seroprevalence between age groups or between districts did not reach statistical significance (P = .35 for age groups; P = .42 for districts) (Figure 1). In addition, neither place of residence nor any other demographic factor was significantly associated with PV2 seroprevalence (P > .10).
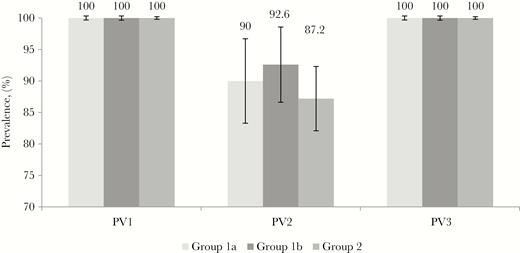
Seroprevalence of antipolio antibodies against poliovirus types 1, 2, and 3 (PV1, PV2, and PV3, respectively), by age group. Group 1a, children born between 1 March and 30 April 2016, received full-dose inactivated poliovirus vaccine (IPV) at age 4 months; group 1b, children born between 1 and 15 May 2016, received full-dose IPV at 4 months; and group 2, children born between 1 June and 31 December 2016, received 2 doses of fractional-dose IPV, at 2 and 4 months.
The median reciprocal titers of antipolio antibodies among seropositive children were ≥1:1448 for PV1 in all 3 age groups (P = .99); they were between 1:45 and 1:72 for PV2 (P = .10) and between 1:910 and 1:1152 for PV3 (P = .22). The reverse cumulative curves of antibody titers demonstrated similar distributions of reciprocal titers among the age groups (Figure 2). The median reciprocal titers of anti-PV2 antibodies did not decline significantly with time since fIPV administration; the median titer was 5.83 (95% CI, 5.17–6.17) in children who had received fIPV 12–15 months earlier and 5.5 (4.83–6.17) in those who had received fIPV 16–18 months earlier (P = .52). We did not observe significant differences in seroprevalence of PV2 antibodies in children of different ages at the time of the survey (Figure 3) (P > .10).
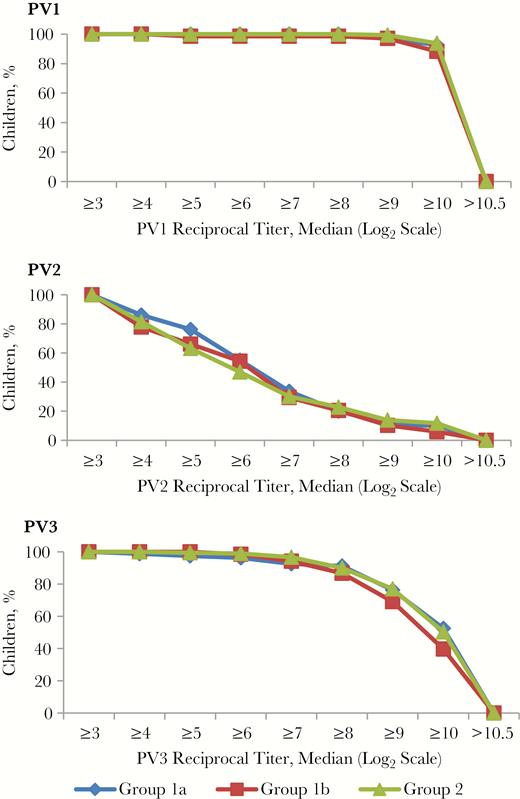
Reverse cumulative curves of antibody titer distribution among seropositive children . Group 1a, children born between 1 March and 30 April 2016, received full-dose inactivated poliovirus vaccine (IPV) at age 4 months; group 1b, children born between 1 and 15 May 2016 received full-dose IPV at 4 months; and group 2, children born between 1 June and 31 December 2016 received 2 doses of fractional-dose IPV at 2 and 4 months. Abbreviations: PV1, PV2, and PV3, poliovirus types 1, 2, and 3, respectively.
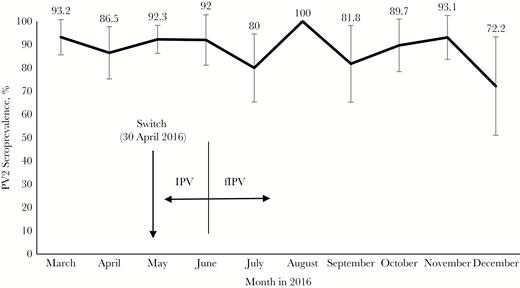
Seroprevalence of antibodies against poliovirus type 2 (PV2) among children born in different months of 2016. Children born before May 15 received 1 full inactivated poliovirus vaccine (IPV) dose, and those born on or after June 1 received 2 fractional IPV (fIPV) doses.
DISCUSSION
To our knowledge this is the first study to evaluate the seroprevalence of anti-PV2 antibodies achieved in a national routine immunization program with intradermal fIPV. We demonstrated that the seroprevalence of anti-PV2 antibodies did not differ significantly between children who received 1 full IPV dose and those who received 2 fIPV doses. It is, however, important to note that some children in our study may have been exposed to PV2 outside of vaccination; previous studies indicated that polioviruses persist in the environment for up to 3 months after OPV use [18, 19]. This may explain the higher-than-expected seroprevalence for PV2, especially in the age groups born around the date of the switch. Data obtained from clinical trials estimated PV2 seroconversion after 1 full IPV dose administered at 4 months to be 63% (compared with >90% in our study), and seroconversion after 2 fIPV doses administered at 2 and 4 months to be 72% (compared with 87% in our study) [10].
Our study demonstrated that Sri Lanka has maintained very high primary immunization coverage; the seroprevalence for PV1 and PV3 was 100% in all age groups, providing further evidence that a successfully executed routine immunization program is sufficient in providing complete protection against PV1 and PV3.
Our study had a limitation: we were unable to quantify the level of exposure to PV2 in children born around the date of the switch, and therefore seroprevalence achieved by IPV in those children is probably overestimated because of secondary transmission.
Before fIPV introduction, intradermal administration of BCG vaccine in Sri Lanka was conducted at the delivery sites (usually a maternal hospital). As part of the preparation for fIPV introduction, the vaccinators at the Expanded Program on Immunization (EPI) health centers were trained by the midwives to administer fractional intradermal doses of IPV during a 1-day course of theory and practice sessions. Our findings attest to the effectiveness of the training, which perhaps can serve as a blueprint for other EPI programs considering introduction of fIPV
The Sri Lankan immunization program carried out an evaluation of the introduction of fIPV and concluded that the introduction of this intradermal vaccine was successful and that health authorities at all levels reported public acceptance of the additional injections of the new schedule to be high [20].
Sri Lankan immunization program demonstrated that it is feasible for a national EPI program to train vaccinators in successful delivery of intradermal fIPV administrated with BCG needle and syringe and to achieve high coverage and seroprevalence with the intradermal application. In Sri Lanka, we demonstrated that the change from IPV to fIPV led to no failure to vaccinate as well as no failure to induce immune response. Other countries deciding whether to adopt fIPV in their routine immunization schedules may be reassured by both the feasibility of implementation and immunogenicity of such a schedule.
Notes
Acknowledgments. We thank the staff of the Epidemiology Unit for coordinating and conducting the study and the staff of the Division of Viral Diseases at the Centers for Disease Control and Prevention in Atlanta—namely, Deborah Moore, Yiting Zhang, Sharla McDonald, William Hendley, Mario Nicolas, and Kathryn Manly—for timely analysis of the serum samples. We also acknowledge the staff of the health facilities in the selected district for their extraordinary efforts to complete the enrollments and study procedures within a tight timeline, and the enrolled children and their parents for agreeing to participate in the study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention and other contributing agencies.
Financial support. This work was supported by Rotary International and the World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Global Polio Eradication Initiative.
Global Polio Eradication Initiative.
Strategic Advisory Group of Experts.
World Health Organization.
World Health Organization.
Wikipedia.




