-
PDF
- Split View
-
Views
-
Cite
Cite
David X Liu, Donna L Perry, Lisa Evans DeWald, Yingyun Cai, Katie R Hagen, Timothy K Cooper, Louis M Huzella, Randy Hart, Amanda Bonilla, John G Bernbaum, Krisztina B Janosko, Ricky Adams, Reed F Johnson, Jens H Kuhn, Matthias J Schnell, Ian Crozier, Peter B Jahrling, Juan C de la Torre, Persistence of Lassa Virus Associated With Severe Systemic Arteritis in Convalescing Guinea Pigs (Cavia porcellus), The Journal of Infectious Diseases, Volume 219, Issue 11, 1 June 2019, Pages 1818–1822, https://doi.org/10.1093/infdis/jiy641
Close - Share Icon Share
Abstract
Lassa fever (LF) survivors develop various clinical manifestations including polyserositis, myalgia, epididymitis, and hearing loss weeks to months after recovery from acute infection. We demonstrate a systemic lymphoplasmacytic and histiocytic arteritis and periarteritis in guinea pigs more than 2 months after recovery from acute Lassa virus (LASV) infection. LASV was detected in the arterial tunica media smooth muscle cells by immunohistochemistry, in situ hybridization, and transmission electron microscopy. Our results suggest that the sequelae of LASV infection may be due to virus persistence resulting in systemic vascular damage. These findings shed light on the pathogenesis of LASV sequelae in convalescent human survivors.
Lassa fever (LF) is an acute, severe, and sometimes fatal hemorrhagic fever disease caused by Lassa virus (LASV), a mammarenavirus of the Arenaviridae family that causes perennial outbreaks in endemic areas in Western Africa, mainly in the Republic of Guinea, Sierra Leone, Liberia, and Nigeria [1]. Although the annual incidence of LASV infection is estimated to be more than 300000 cases, only approximately 20% of infections result in moderate to severe hemorrhagic disease [2]. Lassa virus first targets dendritic cells, macrophages, endothelial cells, adrenal cortical cells, and hepatocytes. End-stage lethal disease is characterized by an uncontrolled cytokine response that results in multiorgan failure and hemorrhagic shock [1, 2].
Approximately 66.67% of hospitalized patients develop post-LF syndrome [3]. The symptoms include polyserositis, dysmorphopsias (visual distortion), vertigo, epididymitis, back pain, and partial or permanent hearing loss. These symptoms can persist for weeks to months after recovery from LASV infection [1, 4]. However, little is known about the pathogenic processes underlying the clinical symptoms observed in these convalescing human patients that recovered from acute LF. Studies of the pathogenesis of chronic sequelae in convalescent LF patients have been hampered by a lack of autopsy samples and animal models to study the sequelae of acute LF [5].
Inbred (strain 13) and outbred (Hartley) guinea pigs ([GPs] Cavia porcellus) are commonly used to model acute LASV infection [6, 7]. Disease course in GPs experimentally infected with LASV includes fever, weight loss, anorexia, and viremia. Guinea pigs typically succumb to LASV infection 1–3 weeks postinoculation. Interstitial pneumonia, myocarditis, and hepatic degeneration and necrosis are the consistent histopathological findings in GPs that succumb to LF.
The perennial nature and common occurrence of sequelae in survivors of acute LF underscores the importance of having appropriate animal models to understand the pathogenesis and consequences of complications in LF survivors [8]. In the current study, we demonstrate that LASV persists in the smooth muscle cells of the tunica media of arteries in GPs that survived acute LASV infection. Lassa virus persistence is associated with severe, systemic vascular inflammation, indicating that GPs could serve as a model to investigate the consequences of chronic LASV infection in humans.
MATERIALS AND METHODS
Animals, Viruses, and Clinical Observations
Twelve GP survivors in this study were from 4 separate and independent experiments. Two, 6- to 12-week-old, male inbred strain 13 GPs (GPS13 1 and 2) survived a confirmation of virulence study after intraperitoneal (IP) inoculation with 1000 plaque-forming units (pfu) of wild-type LASV Josiah strain (wtLASV-J) (GenBank nos. KY425638.1 [S segment] and KY425632.1 [L segment]). These GPS13s were euthanized at the end of the study at day postinfection (dpi) 41. Six, 6- to 12-week-old GPS13s survived subcutaneous exposure with 1000 pfu of recombinant LASV-Josiah (rLASV-J). Three (GPS13 3 through 5, 1 male and 2 females) were euthanized at dpi 42, and the other 3 (GPS13 6 through 8, 2 males and 1 female) were euthanized at dpi 92. The rLASV-J was generated using reverse genetics based wtLASV-J. Two (1 male and 1 female), 6- to 8-week-old outbred Hartley GPs (GPH 1 and 2) recovered from a confirmation of virulence study after IP exposure with 100 pfu of GP-adapted LASV (gpa-LASV) (GenBank nos. KY425643.1 [S segment] and KY425651.1 [L segment]). They were euthanized at the end of the study, dpi 35. Two additional 6- to 8-week-old, female GPHs (GPH 3 and 4) survived intramuscular exposure with 10000 pfu of gpa-LASV. These 2 GPHs served as unvaccinated controls and were euthanized at the end of study, dpi 47.
All animals were weighed, body temperature measured, and observed daily for clinical signs of disease. Samples of peripheral blood and fresh tissues from liver, spleen, and lung were collected at necropsy for viral plaque assay or real-time quantitative RT-PCR (see the detail protocols in the Supplemental Methods and Materials). All studies were performed under a National Institute of Allergy and Infectious Diseases Division of Clinical Research Animal Care and Use Committee-approved protocol in compliance with the Animal Welfare Act, Public Health Service Policy, the Guide for the Care and Use of Laboratory Animals, National Research Council (2011), and federal statutes and regulations relating to experiments involving animals. The research was conducted at the Integrated Research Facility, an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility.
Gross and Histological Pathology
Guinea pigs were humanely euthanized at the end of each study and complete necropsies were performed. All major organs were collected and fixed in 10% neutral-buffered formalin for at least 72 hours in biosafety level 4 containment before being processed routinely in a Tissue-Tek VIP-6 tissue processor (Sakura Finetek USA, Torrance, CA) and paraffin-embedded. The inner ear bones were decalcified in 12.5% neutral ethylenediaminetetraacetic acid (catalog no. DCVERGAL; American MasterTech, Lodi, CA) before routine tissue processing. Paraffin-embedded tissues were routinely processed for histopathology, immunohistochemistry (IHC), and RNAscope in situ hybridization (ISH).
Immunohistochemistry for Lassa Virus
Lassa virus IHC was performed with rabbit-anti-LASV primary antibody at a dilution of 1:200 (for the decalcified inner ears) and 1:500 (other tissues) (Thermo Scientific Pierce Protein Biology, Waltham, MA) followed by an alkaline phosphatase-conjugated anti-rabbit secondary polymer (catalog no. MRAP536L; Biocare Medical, Concord, CA). Positive staining was visualized with Biocare Warp Red chromogen (catalog no. WR806S; Biocare Medical) and counterstained with hematoxylin.
RNAscope In Situ Hybridization for Lassa Virus
RNAscope ISH was performed to detect LASV genomic ribonucleic acid (RNA) in formalin-fixed, paraffin-embedded tissues using the RNAscope 2.5 HD RED kit (catalog no. 322360; Advanced Cell Diagnostics, Newark, CA) according to the manufacturer’s instructions. The probe pairs targeting the LASV genomic Z protein (Z) and polymerase (L) genes (catalog no. 463761) were used (Advanced Cell Diagnostics).
Electron Microscopy
Samples from paraffin-embedded blocks were processed according to Lighezan et al [9] and sectioned using a Leica UC7 ultramicrotome. Sections 70 to 80 nm in thickness were collected on 200-mesh copper grids and poststained with Reynold’s lead citrate. Samples were examined using a FEI Tecnai Spirit Twin transmission electron microscope (Thermo Fisher Scientific, Waltham, MA) operating at 80 kV.
RESULTS
Lassa Virus Induces Mild to Moderate Clinical Signs in Surviving Guinea Pigs
The 2 surviving GPS13s exposed to wtLASV-J IP developed moderate clinical signs consisting of fever, anorexia, and reduced body weight at dpi 10 that resolved gradually from 15 to 35 dpi. These 2 GPs remained normal until the end of study at 41 dpi. By comparison, minimal to mild changes in clinical status, including appetite and body weight, were seen in the in 4 GPH survivors exposed to gpa-LASV and the 6 GPS13 survivors exposed to rLASV-J, respectively. Guinea pigs that succumbed to acute infection in these experiments showed typical clinical signs consistent with acute, lethal LASV infection [6, 7] and will only be discussed as comparators for histopathology and viral detection in surviving GPs.
Some Guinea Pig Survivors Aere Lassa Virus-Negative by Quantitative Reverse-Transcription Polymerase Chain Reaction or Plaque Assay
One GPS13 at dpi 41 (exposed to wtLASV-J), 2 GPS13s at dpi 92 (exposed to rLASV-J), and 1 GPH at dpi 47 (exposed to gpa-LASV) tested negative for LASV in the blood and selected tissues by plaque assay or quantitative reverse-transcription polymerase chain reaction (see Supplementary Table S1).
Lassa Virus Surviving Guinea Pigs Develop Systemic Lymphoplasmacytic and Histiocytic Arteritis and Periarteritis
There were no significant gross pathology findings at necropsy in any surviving GP. However, histopathologically surviving GPs demonstrated mild (GPH) to severe (GPS13) systemic lymphoplasmacytic and histiocytic arteritis (Supplementary Table S2). Multifocally, the affected arteries were lined by hypertrophic and/or degenerate endothelial cells, and the tunica intima was mildly to moderately expanded and infiltrated by lymphocytes, plasma cells, and monocytes. Multifocally to segmentally, the tunica media was mildly to moderately expanded by hyperplastic smooth muscle cells admixed with rare apoptotic cellular debris and inflammatory cells. Eccentrically, the tunica adventitia was mildly to severely expanded by edema, fibrin, fibrosis, numerous lymphocytes, plasma cells, and macrophages. (Figure 1A and Supplementary Figure S1) The tricuspid valves of GPS13 were also multifocally expanded by edema and mononuclear infiltrates. The parenchyma of these affected organs was generally within normal limits. Additional histopathological findings in surviving GPs are presented in Supplementary Table S2 and Supplementary Figure S2.
Lassa Virus Persistence in the Tunica Media Smooth Muscle Cells Is Associated With Systemic Arteritis
Lassa virus was detected in the smooth muscle cells in the tunicae mediae of the medium and large arteries in the hearts, aortas, kidneys, pancreases, and mesenteries by both IHC and ISH (Table 1). The intensity of IHC and ISH positivity generally trended with the severity of perivasculitis and was stronger in the GPS13 than in the GPH. Immunohistochemistry demonstrated that LASV antigen expression centered around nuclei and extended along the sarcoplasm of smooth muscle cells (Figure 1B and Supplementary Figure S3A and B), which correlated with detection of LASV RNA by ISH (Figure 1C and Supplementary Figure S3C and D). The tunicae mediae of positive IHC- or ISH-stained arteries had no to rare inflammatory cells. By comparison, in GPs that succumbed to wtLASV-J infection acutely, only arterial endothelial cells were LASV positive (Supplementary Figure S3E and F). Lassa virus antigen or RNA was not detected in brain, lung, liver, spleen, lymph nodes, gonads, eyes, and ears by IHC or ISH.
| Guinea Pig Strain | GPs13 | GPH | ||||
| LASV isolate | wtLASV-J | rLASV-J | gpa-LASV | |||
| Dosage (pfu)/route | 1000/IP | 1000/SC | 100/IP | 10000/IM | ||
| dpi (animal no.) | 41 (GPs13 1 and 2) | 42 (GPs13 3 to 5) | 92 (GPs13 6 to 8) | 35 (GPH 1 and 2) | 47 (GPH 3 and 4) | |
| Heart and Aorta | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | ++ (1/2) | + (2/2) |
| IHC | ++ (2/2) | + (1/3) | + (1/3) | − (2/2) | + (1/2) | |
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | − (2/2) | |
| Kidney | H&E | +++ (2/2) | ++ (3/3) | + (3/3) | ++ (2/2) | ++ (2/2) |
| IHC | +++ (2/2) | + (2/3); ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| ISH | ++ (2/2) | ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| Pancreas and Mesentery | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | na | +++ (2/2) |
| IHC | +++ (2/2) | + (2/3) | + (1/3) | ++ (1/2) | ||
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | ||
| Guinea Pig Strain | GPs13 | GPH | ||||
| LASV isolate | wtLASV-J | rLASV-J | gpa-LASV | |||
| Dosage (pfu)/route | 1000/IP | 1000/SC | 100/IP | 10000/IM | ||
| dpi (animal no.) | 41 (GPs13 1 and 2) | 42 (GPs13 3 to 5) | 92 (GPs13 6 to 8) | 35 (GPH 1 and 2) | 47 (GPH 3 and 4) | |
| Heart and Aorta | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | ++ (1/2) | + (2/2) |
| IHC | ++ (2/2) | + (1/3) | + (1/3) | − (2/2) | + (1/2) | |
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | − (2/2) | |
| Kidney | H&E | +++ (2/2) | ++ (3/3) | + (3/3) | ++ (2/2) | ++ (2/2) |
| IHC | +++ (2/2) | + (2/3); ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| ISH | ++ (2/2) | ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| Pancreas and Mesentery | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | na | +++ (2/2) |
| IHC | +++ (2/2) | + (2/3) | + (1/3) | ++ (1/2) | ||
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | ||
Abbreviations: dpi, day postinoculation; gpa-LASV, guinea pig-adapted LASV; GPH, outbred Hartley guinea pig; GPs13, inbred guinea pig strain 13; H&E, the scores of periarteritis and arteritis on hematoxylin and eosin stained slides; IHC, immunohistochemistry; IM, intramuscular; IP, intraperitoneal; ISH, in situ hybridization; LASV, Lassa virus; na, not available; pfu, plaque-forming unit; rLASV-J, recombinant LASV-Josiah isolate; SC, subcutaneous; wtLASV-J, wild-type LASV Josiah isolate; −, negative staining; +, weak staining; ++, moderate staining; +++, strong staining.
| Guinea Pig Strain | GPs13 | GPH | ||||
| LASV isolate | wtLASV-J | rLASV-J | gpa-LASV | |||
| Dosage (pfu)/route | 1000/IP | 1000/SC | 100/IP | 10000/IM | ||
| dpi (animal no.) | 41 (GPs13 1 and 2) | 42 (GPs13 3 to 5) | 92 (GPs13 6 to 8) | 35 (GPH 1 and 2) | 47 (GPH 3 and 4) | |
| Heart and Aorta | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | ++ (1/2) | + (2/2) |
| IHC | ++ (2/2) | + (1/3) | + (1/3) | − (2/2) | + (1/2) | |
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | − (2/2) | |
| Kidney | H&E | +++ (2/2) | ++ (3/3) | + (3/3) | ++ (2/2) | ++ (2/2) |
| IHC | +++ (2/2) | + (2/3); ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| ISH | ++ (2/2) | ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| Pancreas and Mesentery | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | na | +++ (2/2) |
| IHC | +++ (2/2) | + (2/3) | + (1/3) | ++ (1/2) | ||
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | ||
| Guinea Pig Strain | GPs13 | GPH | ||||
| LASV isolate | wtLASV-J | rLASV-J | gpa-LASV | |||
| Dosage (pfu)/route | 1000/IP | 1000/SC | 100/IP | 10000/IM | ||
| dpi (animal no.) | 41 (GPs13 1 and 2) | 42 (GPs13 3 to 5) | 92 (GPs13 6 to 8) | 35 (GPH 1 and 2) | 47 (GPH 3 and 4) | |
| Heart and Aorta | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | ++ (1/2) | + (2/2) |
| IHC | ++ (2/2) | + (1/3) | + (1/3) | − (2/2) | + (1/2) | |
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | − (2/2) | |
| Kidney | H&E | +++ (2/2) | ++ (3/3) | + (3/3) | ++ (2/2) | ++ (2/2) |
| IHC | +++ (2/2) | + (2/3); ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| ISH | ++ (2/2) | ++ (1/3) | − (3/3) | + (1/2) | − (2/2) | |
| Pancreas and Mesentery | H&E | +++ (2/2) | ++ (3/3) | ++ (3/3) | na | +++ (2/2) |
| IHC | +++ (2/2) | + (2/3) | + (1/3) | ++ (1/2) | ||
| ISH | ++ (2/2) | ++ (1/3) | + (1/3) | − (2/2) | ||
Abbreviations: dpi, day postinoculation; gpa-LASV, guinea pig-adapted LASV; GPH, outbred Hartley guinea pig; GPs13, inbred guinea pig strain 13; H&E, the scores of periarteritis and arteritis on hematoxylin and eosin stained slides; IHC, immunohistochemistry; IM, intramuscular; IP, intraperitoneal; ISH, in situ hybridization; LASV, Lassa virus; na, not available; pfu, plaque-forming unit; rLASV-J, recombinant LASV-Josiah isolate; SC, subcutaneous; wtLASV-J, wild-type LASV Josiah isolate; −, negative staining; +, weak staining; ++, moderate staining; +++, strong staining.
Transmission electron microscopy revealed the presence of LASV particles and budding events in the smooth muscle cells of the tunica media. Virions were 100–120 nm in diameter with a granular core (Figure 1D).
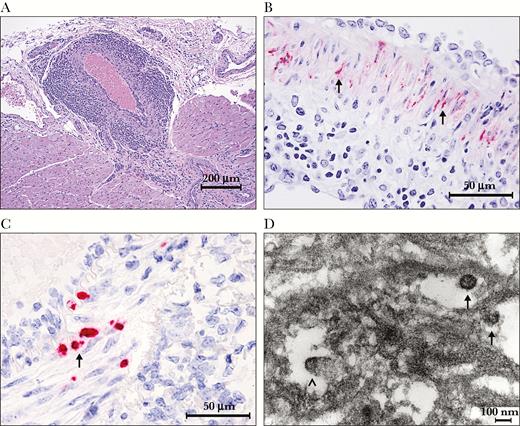
Representative images of histopathology, immunohistochemistry (IHC), in situ hybridization (ISH), and electron microscopy. (A) Histopathology of severe lymphoplasmacytic and histiocytic arteritis and periarteritis of a coronary artery in the heart from strain 13 guinea pigs (GPS13) 2 inoculated with wild-type Lassa virus Josiah strain (wtLASV-J), days postinfection (dpi) 41. Hematoxylin and eosin stain: (B) IHC demonstrates that the smooth muscle cells in the tunica media of the mesenteric artery are strong positive for LASV antigen (black arrows, red chromogen), from GPS13 2 inoculated with wtLASV-J, dpi 41; (C) the smooth muscle cells in the tunica media of the mesenteric artery from GPS13 2 inoculated with wtLASV-J, dpi 41, are positive (black arrow, red chromogen) for LASV genomic ribonucleic acid by ISH; (D) electron micrograph from the tunica media of an artery in the pancreas from GPS13 2 exposed to wtLASV-J, dpi 41. Lassa virions (black arrows) were approximately 100 nm in diameter and had granular cores. Budding virus particles were seen (black arrowhead).
Discussion
This study demonstrates that GPs surviving acute LASV infection can develop long-term systemic vasculitis and periarteritis associated with LASV persistence in the tunicae mediae of affected vessels. The results of histopathology, IHC, ISH, and electron microscopy confirm that LASV can persist in the smooth muscle cells of the arterial tunica media for up to 92 days after LASV exposure, in the absence of viremia. Additional sequelae observed in these GPs include lymphoplasmacytic adrenal medullitis, ventricular encephalitis, cataracts, and lymphoid hyperplasia. These histopathological findings are distinct from those observed in fatal LF in GPs or human cases of acute LASV infection wherein hepatocellular necrosis, lymphoid depletion, adrenocortical necrosis, and necrotizing pneumonia are the predominant lesions [6, 7].
Despite GPs from 2 different genetic backgrounds, 3 different LASV isolates, 3 inoculation routes, and 3 viral dosages, all GP survivors developed similar histopathologic lesions associated with LASV persistence in the tunicae mediae of arteries. It is notable that a rhesus and 2 cynomolgus macaques that survived acute LF developed systemic arteritis, meningoencephalitis, and myelitis months after LASV exposure [10, 11]. These data suggest a common pathogenesis of LF sequelae in animal models of LASV infection. In addition, chronic sequelae in convalescing LF human patients suggest chronic, systemic inflammation in multiple organs directly or secondary to LASV persistent infection [12, 13]. Lassa virus persistence may be associated with a continual increase in neutralizing antibody titers for several months during convalescence [14]. Although the pathogenesis underlying these symptoms is unknown because a lack of well documented pathology in convalescent human patients [5], our data suggest that persistence of LASV in these patients may be the inciting factor underlying the observed increases in antibody LASV titer during the convalescent period.
Our IHC and ISH results also demonstrated that only the endothelial cells in the cardiovascular system are LASV positive in the GPs that succumbed to acute LASV infection. These results are consistent with previous studies that LASV first targets the endothelial cells of blood vessels in the acute stage [15]. Lassa virus may then infect, replicate, and persist in the smooth muscle cells of the tunicae mediae of arteries, which may contribute to chronic sequelae in convalescing LASV human patients.
Conclusions
The systemic vascular damage resulting from LASV persistence in the tunicae mediae of arteries throughout the body may be the cause of sequelae in human convalescent patients. Our findings indicate that LASV infection of the guinea pig may serve as a model to investigate how LASV persistence can contribute to the pathogenesis of the sequela of LASV infection in convalescing human patients that have survived acute LF. This, in turn, may facilitate novel therapeutic strategies to combat the significant and very prevalent sequelae of LASV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D.X.L., D.L.P., T.K.C., and L.M.H. performed necropsy, tissue collection, histopathology evaluation, and data interpretation; L.M.E.D., Y.C., K.R.H., R.F.J., J.H.K., I.C., M.J.S., P.B.J., and J.C.d.T. implemented the study and designed experiments; R.H. and A.B. performed immunohistochemistry and RNAscope in situ hybridization; J.G.B., D.L.P., D.X.L., and T.K.C. performed electron microscopy; K.B.J. and R.A. performed plaque assay and reverse-transcription polymerase chain reaction; D.X.L., D.L.P., R.F.J., and T.K.C. wrote the manuscript.
Acknowledgments. We thank Drs. Lisa Hensley and Michael Holbrook and Linda Coe and Daniela Pusl for supporting this study. We also thank Laura Bollinger for technical writing services and Jiro Wada for figure preparation.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), Division of Intramural Research, Division of Clinical Research, and through Battelle Memorial Institute’s prime contract with the NIAID (contract HHSN272200700016I).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services or the institutions and companies affiliated with the authors.




