-
PDF
- Split View
-
Views
-
Cite
Cite
Huey-Ling Chen, Wan-Hsin Wen, Mei-Hwei Chang, Management of Pregnant Women and Children: Focusing on Preventing Mother-to-Infant Transmission, The Journal of Infectious Diseases, Volume 216, Issue suppl_8, 15 October 2017, Pages S785–S791, https://doi.org/10.1093/infdis/jix429
Close - Share Icon Share
Abstract
Hepatitis B virus (HBV) immunization has been effectively preventing chronic HBV infection with >90% efficacy in countries with universal neonatal immunization. Perinatal mother-to-infant transmission of HBV remains the major cause of chronic HBV infection despite immunization. Maternal hepatitis B e-antigen (HBeAg) and high viral load have been noted to be the most important risk factors for transmission. In recent years, short-term antiviral therapy for pregnant women in the third trimester has been shown to be highly effective in reducing 90% of vaccine failure in children. It is important to monitor maternal aminotransferase elevations postpartum. Long-term outcome of mothers and children is needed and awaits further investigations. Despite the above-mentioned preventive measures, it is also important to monitor high-risk children at 1 year of age with hepatitis B surface antigen and anti-hepatitis B to identify those with chronic HBV infection. Most of the children with chronic HBV infection were in the immune-tolerant phase. The goals for antiviral treatment in children are to reduce severity of liver injury, achieve HBeAg seroconversion, and prevent development of liver fibrosis and cancer. Studies on antiviral therapy are undergoing to elucidate the optimal indication and drug treatment for children. The ideal future goal of treatment is to eradicate chronic HBV infection globally.
Hepatitis B virus (HBV) infection and its related liver disease and mortality are a worldwide health problem [1, 2]. In endemic areas, mother-to-infant transmission (MTIT) is a major cause of chronic HBV infection in children in large populations [3–5]. Therefore, effective prevention and management of HBV infection is recommended for mothers and children before preganancy, during pregnancy, and after delivery.
Since the development of HBV vaccines and hepatitis B immunoglobulin (HBIG) in the 1980s, approximately 90% of mother-to-infant HBV transmission can be blocked. In countries with universal neonatal immunization, the HBV epidemiology has been largely changed in the past 30 years [6, 7]. However, neonatal immunization has not completely eradicated mother-to-infant HBV transmission [8]. There is still a large population of women and children who are infected with HBV and are at risk of subsequent disease-related complications.
MOTHER-TO-INFANT TRANSMISSION: RISKS AND CLINICAL OUTCOMES
Mother-to-infant transmission is associated with more severe morbidity and mortality than horizontal transmission. Chronic infections and subsequent complications, ie, cirrhosis and hepatocellular carcinoma (HCC), are more likely to occur in persons infected during infancy or early childhood [3, 5]. Immuno-prophylaxis with HBIG and hepatitis B vaccines reduce but do not completely eradicate MTIT. Mother-to-infant transmission remains a major source of chronic HBV infection in the era of universal hepatitis B vaccination [7–9].
The terms “mother-to-child-transmission” or “vertical transmission of HBV” commonly used in recent or previous studies are similar to the more precise term “mother-to-infant transmission”, which we prefer to use here. Most MTIT occurs at delivery when the infant encounters infected maternal secretions and blood [5, 10]. To a lesser extent, maternal transmission takes place intrauterine or postnatally [11–14]. Hepatitis B virus infection has an incubation period of 1 to 6 months [15]. If an infant is positive for hepatitis B surface antigen (HBsAg) at birth or within 1 month after birth, the baby is considered to be infected during the intrauterine period rather than during delivery [11, 12]. In HBV-infected children, the causes of immunoprophylactic failure include the following: (1) intrauterine infection, which cannot be prevented by prophylaxis administered at birth; (2) peripartum infection, breakthrough infection occurring at delivery; and, rarely, (3) postnatal infection, occurring in a small number of children who have inadequate immune response to neonatal immunoprophylaxis [16, 17] (Table 1).
| Mode of Transmissions . | Time of Viral Exposure . | Time of HBsAg Detection . | Children Infection Rate (Born to HBsAg/HBeAg Mothers) . | Rate of Infected Children to Become Chronically Infected . |
|---|---|---|---|---|
| Mother-to-Infant (Child) Transmission | ||||
| Intrauterine infection | From 1st to 3rd trimester of gestation | At birth or within 1 month after birth | ~2.4% of mother-to-infant transmission with immunoprophylaxis [12] | ~100% |
| Peripartum infection | Shortly before or during partum | After incubation period, 1–6 months of age | 90% without immunoprophylaxis [10, 26] 10% with immunoprophylaxis [8] | 90% |
| Postnatal infection | After birth | Variable, after incubation period | 57% at 1–3 years without immunoprophyalaxis [14] Infrequent with immunoprophylaxis [17] | 50%–90% (decrease with age of infection) [5] |
| Horizontal Transmission | ||||
| Blood transfusion/needle stick/medical procedures/sexual transmission | At the time of event | After incubation period (1–6 months) | Variable | Depend on age and immunity |
| Mode of Transmissions . | Time of Viral Exposure . | Time of HBsAg Detection . | Children Infection Rate (Born to HBsAg/HBeAg Mothers) . | Rate of Infected Children to Become Chronically Infected . |
|---|---|---|---|---|
| Mother-to-Infant (Child) Transmission | ||||
| Intrauterine infection | From 1st to 3rd trimester of gestation | At birth or within 1 month after birth | ~2.4% of mother-to-infant transmission with immunoprophylaxis [12] | ~100% |
| Peripartum infection | Shortly before or during partum | After incubation period, 1–6 months of age | 90% without immunoprophylaxis [10, 26] 10% with immunoprophylaxis [8] | 90% |
| Postnatal infection | After birth | Variable, after incubation period | 57% at 1–3 years without immunoprophyalaxis [14] Infrequent with immunoprophylaxis [17] | 50%–90% (decrease with age of infection) [5] |
| Horizontal Transmission | ||||
| Blood transfusion/needle stick/medical procedures/sexual transmission | At the time of event | After incubation period (1–6 months) | Variable | Depend on age and immunity |
Abbreviations: HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
| Mode of Transmissions . | Time of Viral Exposure . | Time of HBsAg Detection . | Children Infection Rate (Born to HBsAg/HBeAg Mothers) . | Rate of Infected Children to Become Chronically Infected . |
|---|---|---|---|---|
| Mother-to-Infant (Child) Transmission | ||||
| Intrauterine infection | From 1st to 3rd trimester of gestation | At birth or within 1 month after birth | ~2.4% of mother-to-infant transmission with immunoprophylaxis [12] | ~100% |
| Peripartum infection | Shortly before or during partum | After incubation period, 1–6 months of age | 90% without immunoprophylaxis [10, 26] 10% with immunoprophylaxis [8] | 90% |
| Postnatal infection | After birth | Variable, after incubation period | 57% at 1–3 years without immunoprophyalaxis [14] Infrequent with immunoprophylaxis [17] | 50%–90% (decrease with age of infection) [5] |
| Horizontal Transmission | ||||
| Blood transfusion/needle stick/medical procedures/sexual transmission | At the time of event | After incubation period (1–6 months) | Variable | Depend on age and immunity |
| Mode of Transmissions . | Time of Viral Exposure . | Time of HBsAg Detection . | Children Infection Rate (Born to HBsAg/HBeAg Mothers) . | Rate of Infected Children to Become Chronically Infected . |
|---|---|---|---|---|
| Mother-to-Infant (Child) Transmission | ||||
| Intrauterine infection | From 1st to 3rd trimester of gestation | At birth or within 1 month after birth | ~2.4% of mother-to-infant transmission with immunoprophylaxis [12] | ~100% |
| Peripartum infection | Shortly before or during partum | After incubation period, 1–6 months of age | 90% without immunoprophylaxis [10, 26] 10% with immunoprophylaxis [8] | 90% |
| Postnatal infection | After birth | Variable, after incubation period | 57% at 1–3 years without immunoprophyalaxis [14] Infrequent with immunoprophylaxis [17] | 50%–90% (decrease with age of infection) [5] |
| Horizontal Transmission | ||||
| Blood transfusion/needle stick/medical procedures/sexual transmission | At the time of event | After incubation period (1–6 months) | Variable | Depend on age and immunity |
Abbreviations: HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
Maternal viral load is the most important risk factor for MTIT. Recent studies using sensitive commercial HBV deoxyribonucleic acid (DNA) assays showed that a maternal viral load <6 log10 copies/mL (equivalent to 5.3 log10 IU/mL) seldom causes HBV transmission under adequate neonatal immunoprophylaxis. In highly viremic mothers with a viral load level ranging from 6 to 9 log10 copies/mL, the rate of MTIT increased stepwise [9, 16, 18, 19]. Estimated rates of chronic infection in infants at maternal HBV DNA levels of 6, 7, 8, and 9 log10 IU/mL were 2.5% (95% confidence interval [CI], 0.1–4.9; P = .041), 5.7% (95% CI, 2.3–9.1; P = .001), 12.4% (95% CI, 7.1–17.7; P < .001), and 24.7% (95% CI, 12.0–37.3; P < .001) [16]. These findings form the basis in support for the use of antivirals in highly viremic HBV-infected pregnant women, who are still at high risk of transmission under current HBV immunization strategy.
Positive maternal hepatitis B e-antigen (HBeAg) is a well known risk marker for MTIT of HBV. The association of maternal HBeAg positivity and HBV transmission is not only because positive maternal HBeAg indicates high levels of maternal HBV DNA [16], but also because transplacental maternal HBeAg may induce immune tolerance, which contributes to establish chronic infection in infants [20].
The question at issue has been whether delivery mode affects MTIT of HBV. Some data showed that mothers in the elective cesarean delivery group had a lower rate of transmission than mothers in the vaginal delivery group, especially when the maternal viral load was above 6 log10 copies/mL, equivalent to 5.3 log10 IU/mL [21, 22]. However, the quality of evidence and benefit over risks is not high enough to alter regular obstetric practices. Similarly, studies have documented no difference in rates of infection between breastfed and formula-fed vaccinated infants born to HBV-infected mothers [23]. Current guidelines recommend that HBV-infected women be encouraged to breastfeed as long as the infant receives appropriate immunoprophylaxis after birth [22, 24].
PRENATAL SCREENING AND HEPATITIS B VIRUS IMMUNIZATION PROGRAMS
To prevent mother-to-infant HBV transmission that mainly occurs during delivery, immunoprophylaxis should be administered to neonates as soon as possible after birth. The World Health Organization strongly recommends that all infants receive a birth dose of hepatitis B vaccine within 24 hours of birth, followed by 2 or 3 doses to complete the primary series [15]. By 2015, 185 countries had introduced the hepatitis B vaccine into their national infant immunization schedules, and 97 countries had introduced a birth dose. The global coverage with 3 doses of hepatitis B containing vaccine and the global coverage of a birth dose was estimated at 84% and 39%, respectively. These findings underscore the need to improve the implementation of hepatitis B vaccination, especially the birth dose [15, 25]. The combination of HBIG with HBV vaccines have improved the preventive efficacy to 94% compared with 75% with vaccines alone in infants born to HBV infected mothers [26, 27]. In many countries, HBIG have been added to neonates born to HBV-infected mothers in the immunization programs, in addition to HBV vaccines.
During the past 3 decades, universal pregnant women HBV screening has gradually replaced selective approaches targeting only high-risk groups due to less effective outcomes with selective approaches [28]. There are 2 main prenatal maternal screening strategies. Some countries screen pregnant women for HBV infection by testing HBsAg and administering HBIG to newborns of HBsAg-positive mothers [9, 18]. Some countries perform prenatal screening with both maternal HBsAg and HBeAg and add on HBIG to newborns of HBeAg-positive mothers [7, 8]. For infants born to HBsAg-positive and HBeAg-positive mothers, the benefit of HBIG in addition to vaccine is nonquestionable. However, for infants born to HBsAg-positive and HBeAg-negative mothers, the benefit of HBIG is still an issue of discussion [8, 29].
The original purpose for pregnant women HBV screening is for stratified HBV immunization programs. In future perspectives, the strategy of pregnant women screening is not only for immunization protocols, but it is also closely related to pregnant women antiviral treatment and postimmunization surveillance of children at 1 year of age. In concordance with the prenatal screening strategy, some countries screen infants born to HBsAg-positive mothers, and some countries screen infants born to both HBsAg- and HBeAg-positive mothers. Thus, the selection of strategies for screening pregnant women should be carefully evaluated based on local epidemiology, risk group identification, availability of medical resources, and budgets (Figure 1).
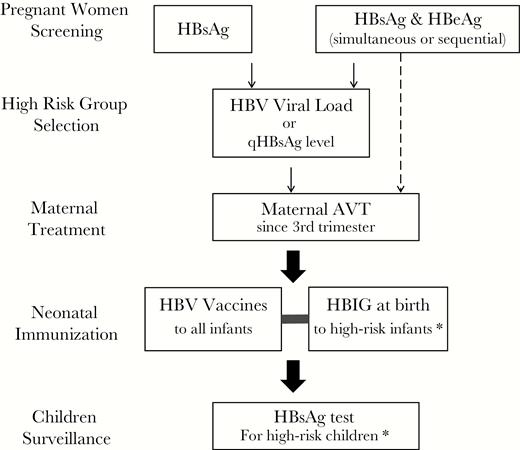
Strategy of pregnant women screening that is tightly linked with high-risk group selection, maternal antiviral treatment (AVT), neonatal immunization, and postimmunization surveillance for hepatitis B surface antigen (HBsAg) positivity in children. *In concordance with antenatal screening strategy, some countries target infants born to HBsAg-positive mothers for hepatitis B immunoglobulin (HBIG) administration and postimmunization HBsAg surveillance; some countries target infants born to both HBsAg and hepatitis B e-antigen (HBeAg)-positive mothers for HBIG administration and postimmunization HBsAg surveillance.
IMPROVING MATERNAL LIVER HEALTH AND PREVENTING MOTHER-TO-INFANT TRANSMISSION STARTING BEFORE PREGNANCY
Management of Hepatitis B Virus-Infected Women in Childbearing Age Before Pregnancy
Chronic HBV-infected women in the childbearing age should be notified regarding pregnancy-related health issues before the women become pregnant. The impact of pregnancy in relation to mother and children’s health should be fully discussed with physician and patient, regarding choices of antiviral therapy for maternal disease and for preventing MTIT.
For women who are in the immune-active phase indicated for antiviral therapy and have near-future plan for pregnancy, interferon (IFN) treatment maybe preferred because of definite and shorter duration of treatment. During IFN treatment, contraception is suggested because of its antiproliferative effect. Nucleos(t)ide analog (NA) can also be used, with the patients’ acknowledgment that treatment may be continued for 2–4 or more years. Alternatively, in women without cirrhosis and with near-future planned pregnancy, treatment may be deferred after delivery.
If women are pregnant during the course of NA treatment, antiviral treatment (AVT) with telbivudine or tenofovir can be continued from first to third trimester. For women under entecavir or other NA treatment, a switch to tenofovir is suggested [30]. Although rare, pregnant women with cirrhosis should start or continue on AVT because they carry a higher risk of hepatic decompensation and increased maternal and fetal complications [31].
Hepatitis B Virus Disease Course During Pregnancy and Peripartum Period
In general, chronically HBV-infected women have smooth pregnancy and delivery course. Alanine aminotransferase (ALT) levels may increase late in pregnancy or in the postpartum period, usually asymptomatic and usually well tolerated. However, severe flare and life-threatening fulminant hepatic failure may occur [32]. In the postpartum period, due to hormonal/immunological changes in pregnancy and rapidly decreased corticosteroid levels postpartum, a significant proportion of women may experience ALT elevations [33]. The incidence and risk factors of ALT flare are not well understood. Variations of frequency and extent of ALT elevations have been reported, based on different study populations and the definition of ALT flare [28, 32, 34–36]. In a control group of pregnant women with high HBV viral load (above 7.5 log10 IU/mL) without AVT and observed from 30 weeks of gestation to 6 months postpartum, the elevation of ALT levels were noted in postpartum 1 to 4 months: 27% of women had ALT elevations >2× upper limit of normal (ULN), and 12.5% of women had ALT elevations >5× ULN. Alanine aminotransferase elevations were usually resolved spontaneously by 6 months after delivery [17].
It has been reported that 12.5% of women experienced HBeAg seroconversion 1 year after delivery. Lower HBV DNA (3.75 × 105 vs 3 × 108 copies/mL) and lower HBeAg levels were observed in the e-seroconverted group compared with nonconverted group [37]. Some studies have reported chronic HBV infection associated risks of obstetric complications, including gestational diabetes mellitus, antepartum hemorrhage, and threatened preterm labor <37 weeks [38].
Management of Pregnant Women With Hepatitis B Virus Infection
Pregnant women should receive HBV screening in the first trimester of pregnancy [30]. If women are detected to have HBV infection for the first time, careful physical examination, family history, HBeAg/anti-HBe, ALT level, and abdominal ultrasonography should be checked to evaluate the liver disease status. Alpha-fetoprotein (AFP) should not be tested in pregnant women for the purpose of HCC surveillance because AFP can be high due to fetal production. If women have ALT elevations during pregnancy or with cirrhosis, NA treatment can be started any time and continue through and after delivery, preferentially using tenofovir.
For pregnant women with positive HBeAg and HBsAg, and normal or mildly elevated ALT without cirrhosis, HBV DNA level can be checked to consider short-term maternal AVT if resources are available. In mothers with high viral load, amniocentesis has been reported to be associated with increased risk of HBV transmission. A practical measure is to notify and to discuss the risk of mother-to-infant HBV transmission with high viral load mothers (HBV DNA above >7 log10 IU/mL) when amniocentesis or choriocentesis is indicated and if noninvasive measures are available [22, 28]. Elective cesarean section was reported to have a lower rate on MTIT than vaginal delivery or emergent cesarean section in high viral load mothers. However, current guidelines do not recommend cesarean section to be performed for the sole indication to reduce mother-to-infant HBV transmission [22, 24, 28].
There have been limited data regarding the optimal monitoring schedule for pregnant women with HBV infection. In addition to initial evaluation (including ALT, HBeAg, and HBV DNA), follow-up for ALT in the third trimester and 1 to 3 monthly follow-ups postpartum for 6 months are suggested in HBeAg-positive or high viral load mothers because pregnant women tend to have elevated ALT late in pregnancy and after delivery [17, 35, 36]. For HBeAg-positive mothers, we suggest to check HBV DNA levels by 28 weeks of gestation for considering AVT to prevent MTIT.
Antiviral Therapy for Pregnant Women
With increasing evidence of efficacy and safety, the latest practice guidelines recommend short-term antiviral therapy in HBV-infected highly viremic pregnant women with stable liver disease to prevent MTIT [24, 30, 39] (Table 2). Lamivudine, telbivudine, and tenofovir disoproxil fumarate (prodrug of tenofovir) are the only antivirals that have been studied in pregnant women and have been analyzed in meta-analysis to effectively reduce the risk of MTIT of HBV [17, 35, 36, 40–45]. Tenofovir is considered a preferred choice because of its antiviral potency, more safety data in pregnant women, and lower rates of resistance. In an Australian observational study recruiting pregnant women with high viral load (>7 log IU/mL), maternal AVT have reduced perinatal transmission to 2% and 0% in tenofovir and lamivudine cohorts, compared with 20% in untreated [41]. A prospective well controlled trial in Taiwan recruiting pregnant women with HBV DNA ≥7.5 log10 IU/mL has shown a reduction of HBsAg positivity of infants from 10.71% to 1.54% (P = .0481), with an odds ratioof 0.10 (P = .0434) in the tenofovir treated group [17]. An open-label, randomized, controlled trial of tenofovir in pregnant women with HBV DNA level >200 000 IU/mL in China has shown a reduction of infant HBsAg positivity from 18% to 5% (P = .007) in the intention-to-treat analysis, and a reduction from 7% to 0% (P = .01) in the per-protocol analysis [44]. Prophylaxis failure may still occur after maternal AVT and may be associated with intrauterine infection or inadequate host responses to HBV vaccines [17].
Current Recommendations by International Expert Societies for Pregnant Women Antiviral Therapy to Prevent Mother-to-Infant Hepatits B Virus Transmissions
| Expert Societies . | Maternal Viral Load . | Time to Start AVT (Gestational Age) . | Time to Stop AVT . | Drug Choice . | Breastfeeding During AVT* . | Other Recommendations . |
|---|---|---|---|---|---|---|
| AASLD (2016) [23] | >2 × 105 IU/mL | 28–32 weeks of gestation | At delivery or up to 3 months postpartum | Lamivudine, telbivudine, tenofovir | Not contraindicated. The unknown risk of exposure should be discussed with mothers | Maternal ALT monitoring every 3 months up to 6 months after discontinuation of treatment |
| EASL (2017) [30] | >2 × 105 IU/mL; or HBsAg levels >104 IU/mL | 24–28 weeks of gestation | 12 weeks after delivery | Tenofovir | Not contraindicated | - |
| APASL (2014) [39] | >106–7 IU/mL | 28–32 weeks of gestation | Birth | Tenofovir or telbivudine | Discouraged | For maternal ALT flare, continuation of AVT may be indicated |
| Australian, UK, and NZ leaders (2016) [28] | >107 IU/mL | 28–32 weeks of gestation | At delivery or up to 12 weeks postpartum | Tenofovir, (telbivudine or lamivudine are alternatives) | Not contraindicated | Long-term follow-up of babies needed |
| Society for Maternal- Fetal Medicine (SMFM) (2016) [22] | >106–8 copies/mL | 3rd trimester | - | Tenofovir | - | - |
| ESPGHAN (2013) [46] | >106 IU/mL | 3rd trimester | - | Tenofovir | - | Pregnant teens may apply |
| Expert Societies . | Maternal Viral Load . | Time to Start AVT (Gestational Age) . | Time to Stop AVT . | Drug Choice . | Breastfeeding During AVT* . | Other Recommendations . |
|---|---|---|---|---|---|---|
| AASLD (2016) [23] | >2 × 105 IU/mL | 28–32 weeks of gestation | At delivery or up to 3 months postpartum | Lamivudine, telbivudine, tenofovir | Not contraindicated. The unknown risk of exposure should be discussed with mothers | Maternal ALT monitoring every 3 months up to 6 months after discontinuation of treatment |
| EASL (2017) [30] | >2 × 105 IU/mL; or HBsAg levels >104 IU/mL | 24–28 weeks of gestation | 12 weeks after delivery | Tenofovir | Not contraindicated | - |
| APASL (2014) [39] | >106–7 IU/mL | 28–32 weeks of gestation | Birth | Tenofovir or telbivudine | Discouraged | For maternal ALT flare, continuation of AVT may be indicated |
| Australian, UK, and NZ leaders (2016) [28] | >107 IU/mL | 28–32 weeks of gestation | At delivery or up to 12 weeks postpartum | Tenofovir, (telbivudine or lamivudine are alternatives) | Not contraindicated | Long-term follow-up of babies needed |
| Society for Maternal- Fetal Medicine (SMFM) (2016) [22] | >106–8 copies/mL | 3rd trimester | - | Tenofovir | - | - |
| ESPGHAN (2013) [46] | >106 IU/mL | 3rd trimester | - | Tenofovir | - | Pregnant teens may apply |
Abbreviations: ALT, alanine aminotransferase; AVT, antiviral therapy; HBIG, hepatitis B immunoglobulin; HBsAg, hepatitis B surface antigen; HBV, hepatits B virus.
*(1) Breastfeeding of HBV-infected mothers (not on antiviral therapy) is recommended as long as the infant receives appropriate immunoprophylaxis after birth (HBIG and HB vaccine). (2) Breastfeeding of HBV-infected mothers who are on antiviral therapy is still controversial.
Current Recommendations by International Expert Societies for Pregnant Women Antiviral Therapy to Prevent Mother-to-Infant Hepatits B Virus Transmissions
| Expert Societies . | Maternal Viral Load . | Time to Start AVT (Gestational Age) . | Time to Stop AVT . | Drug Choice . | Breastfeeding During AVT* . | Other Recommendations . |
|---|---|---|---|---|---|---|
| AASLD (2016) [23] | >2 × 105 IU/mL | 28–32 weeks of gestation | At delivery or up to 3 months postpartum | Lamivudine, telbivudine, tenofovir | Not contraindicated. The unknown risk of exposure should be discussed with mothers | Maternal ALT monitoring every 3 months up to 6 months after discontinuation of treatment |
| EASL (2017) [30] | >2 × 105 IU/mL; or HBsAg levels >104 IU/mL | 24–28 weeks of gestation | 12 weeks after delivery | Tenofovir | Not contraindicated | - |
| APASL (2014) [39] | >106–7 IU/mL | 28–32 weeks of gestation | Birth | Tenofovir or telbivudine | Discouraged | For maternal ALT flare, continuation of AVT may be indicated |
| Australian, UK, and NZ leaders (2016) [28] | >107 IU/mL | 28–32 weeks of gestation | At delivery or up to 12 weeks postpartum | Tenofovir, (telbivudine or lamivudine are alternatives) | Not contraindicated | Long-term follow-up of babies needed |
| Society for Maternal- Fetal Medicine (SMFM) (2016) [22] | >106–8 copies/mL | 3rd trimester | - | Tenofovir | - | - |
| ESPGHAN (2013) [46] | >106 IU/mL | 3rd trimester | - | Tenofovir | - | Pregnant teens may apply |
| Expert Societies . | Maternal Viral Load . | Time to Start AVT (Gestational Age) . | Time to Stop AVT . | Drug Choice . | Breastfeeding During AVT* . | Other Recommendations . |
|---|---|---|---|---|---|---|
| AASLD (2016) [23] | >2 × 105 IU/mL | 28–32 weeks of gestation | At delivery or up to 3 months postpartum | Lamivudine, telbivudine, tenofovir | Not contraindicated. The unknown risk of exposure should be discussed with mothers | Maternal ALT monitoring every 3 months up to 6 months after discontinuation of treatment |
| EASL (2017) [30] | >2 × 105 IU/mL; or HBsAg levels >104 IU/mL | 24–28 weeks of gestation | 12 weeks after delivery | Tenofovir | Not contraindicated | - |
| APASL (2014) [39] | >106–7 IU/mL | 28–32 weeks of gestation | Birth | Tenofovir or telbivudine | Discouraged | For maternal ALT flare, continuation of AVT may be indicated |
| Australian, UK, and NZ leaders (2016) [28] | >107 IU/mL | 28–32 weeks of gestation | At delivery or up to 12 weeks postpartum | Tenofovir, (telbivudine or lamivudine are alternatives) | Not contraindicated | Long-term follow-up of babies needed |
| Society for Maternal- Fetal Medicine (SMFM) (2016) [22] | >106–8 copies/mL | 3rd trimester | - | Tenofovir | - | - |
| ESPGHAN (2013) [46] | >106 IU/mL | 3rd trimester | - | Tenofovir | - | Pregnant teens may apply |
Abbreviations: ALT, alanine aminotransferase; AVT, antiviral therapy; HBIG, hepatitis B immunoglobulin; HBsAg, hepatitis B surface antigen; HBV, hepatits B virus.
*(1) Breastfeeding of HBV-infected mothers (not on antiviral therapy) is recommended as long as the infant receives appropriate immunoprophylaxis after birth (HBIG and HB vaccine). (2) Breastfeeding of HBV-infected mothers who are on antiviral therapy is still controversial.
The criteria for antiviral therapy are slightly different among studies. The threshold of viral load at which intervention is recommended ranges from 6.0–7.7 log10 copies/mL, equivalent to 5.3–7.0 log10 IU/mL in different treatment guidelines [22, 24, 28, 30, 39, 46]. In most studies, antiviral therapy was started at 28–32 weeks of gestation. Alternatively, antiviral therapy may also be considered in pregnant women with an HBsAg level above 4 log10 IU/mL to interrupt MTIT [47].
Breastfeeding is not suggested during antiviral therapy for unknown risk of low-level exposure to infants, and drug labels recommended avoidance of breastfeeding during maternal AVT. However, studies have shown that the levels of tenofovir are 12 500-fold lower than therapeutic dose in infants plasma, and 94% of infants’ plasma had undetectable levels of tenofovir. Lamivudine level was only 2% of the recommended treatment dose for infants. Some recent guidelines suggested that breastfeeding is not contraindicated during maternal AVT [24, 30, 48, 49].
The safety of mother and children is an important issue. Close monitoring of maternal ALT elevation at 1 to 3 monthly follow-ups for at least 6 months after delivery is suggested [24]. Most studies stopped maternal AVT 1–3 months after delivery to prevent ALT flares associated with postpartum withdraw of hormones and viral load rebound after discontinuing AVT. Continuation or restart of AVT should be considered in mothers with elevated ALT. More comprehensive data on the long-term follow-up of growth and development in children with intrauterine exposure to maternal antivirals are required.
Surveillance of Children With Hepatitis B Virus Infection
Infants born to HBV-infected mothers, especially those with high viral load, are still at risk of infection despite immunoprophylaxis. These high-risk infants should be screened to identify those with HBV infection. In concordance with prenatal screening strategy, some countries screen infants born to HBsAg-positive mothers, and some countries screen infants born to both HBsAg- and HBeAg-positive mothers [8, 50]. Because it may take several months to develop antibody after hepatitis B vaccination or to become HBsAg-positive after perinatal infection, it is suggested that high-risk infants be tested for HBV serological markers at age 9–18 months [50]. It is also suggested that high-risk children who are negative for anti-HBs receive booster HB vaccines.
Natural Course of Children Infected With Hepatitis B Virus
Most children contract HBV infection and become chronically infected during the perinatal period or early childhood. During childhood, most HBsAg-positive children are in the immune-tolerant phase, remain HBeAg and HBV DNA positive, but with normal ALT levels. Inflammatory phase (or immune-active phase) usually start from the second to fourth decade in many patients, but it may occur at any age, including childhood. The annual HBeAg clearance rate has been reported to be <2% in children younger than 3 years of age and approximately 5% in those above 3 years [51]. Patients with genotype C are older at time of HBeAg clearance (50th percentile at 47.8 years) than those with genotype A, B, D, and F (50th percentile at 16–19 years) [52]. Host factors including the onset of adrenarche and puberty, phenotype of cytokines, innate immunity, and human leukocyte antigens are associated with the onset of immune clearance and severity of hepatic inflammation [53]. Many patients with ALT elevations and liver inflammations on liver histology during the immune-active phase may be asymptomatic and remained unnoticed [54, 55]. Spontaneous HBsAg seroclearance occurs in few cases, with an average annual clearance rate of 0.58% [56]. Except for cases with jaundice, or HBV markers check-up by screening because of maternal HBV positivity, patients are usually not aware of their HBV-related liver diseases.
Hepatitis B e-antigen seroconversion has been found to occur before 3 years of age in small number of children. Despite that HBeAg seroconversion is generally considered as a favorable event and is considered to be an important endpoint in antiviral therapies, these early HBeAg-seroconverted children have been noted to have at higher risk of developing liver cirrhosis and HCC [57, 58].
On the other hand, fulminant hepatic failure occurs mainly in infants born to HBeAg-negative mothers at 3–9 months of age. Transplacental HBeAg exposure to fetus has been proposed to induce immune tolerance [59, 60]. It is speculated that children born to HBeAg-negative mothers have not been exposed to maternal HBeAg and associated immune modulation. A more severe hepatic inflammation may occur at the stage of acute HBV infection.
Hepatocellular carcinoma has been found to develop in children with chronic HBV infection. Hepatitis B virus integration has been found to occur in 60% of children with HCC. Risk factors included maternal HBsAg positivity, early HBeAg seroconversion, male sex, persistent high viral replication, HBV genotype C, D, or F1, pre-S2, or core promoter mutants, and with family history of HCC [61, 62]. It is proved that universal immunization has decreased the incidence of HCC of childhood and young adult (6–26 years old) from 0.92 per 100 000 person-years in the unvaccinated cohort to 0.23 in the vaccinated birth cohorts [63]. However, the proportion of reduction of HCC after universal immunization is not as effective as the >90% reduction in the chronic HBV-infected population [64]. This fact highlighted the importance of surveillance of children born to HBV-infected mothers with HBsAg positivity. Six to twelve monthly follow-ups for surveillance and management of hepatic inflammation and HCC should be started as soon as HBsAg is detected.
Management of Children With Hepatitis B Virus Infection
Currently, the goals of AVT for HBV-infected children are to decrease hepatic inflammation, decrease viral replication, and accelerate HBeAg seroconversion [65]. Elimination of HBV infection or HBsAg loss is a rare event. It is of note that many children may achieve spontaneous HBeAg seroconversion after a variable period of ALT elevations. Careful considerations before AVT to children should include the natural course of HBV infection, extent of liver injury, risk of liver-disease related complications, adverse reactions of the antivirals, treatment duration, and treatment tolerability for children and family.
Interferon treatment has been reported to be effective in children, with successful suppression of viral replication in 20%–50% of children compared with 8%–17% in the control group [59, 66]. In children with ALT elevations >2× ULN, 35% became negative for HBeAg and HBV DNA at end of treatment. Hepatitis B surface antigen seroconversion occurred in 10% of treated children compared with 1% in the control group [67]. It has the advantages of a shorter and definite duration (6–12 months) of treatment than oral AVTs, and it is not associated with viral resistance. Treatment response may continue to occur 6–12 months after stopping treatment. Significant side effects are associated with IFN treatment including fever, flu-like symptoms, fatigue, depression, thyroid dysfunction, and bone marrow toxicity. Currently, conventional IFN-α is approved for use in children above 1 year of age. Pegylated IFN-α has not been approved in HBV-infected children. Clinical trials using pegylated IFN-α for 24 weeks are now underway with promising results. In the treatment group, 25.7% vs 6.0% (0 = 0.0043) achieved HBeAg seroconversion at 24 weeks of follow-up [68].
Nucleos(t)ide analogs have been shown to be safe and well tolerated in children with HBV infection. The ages approved to be used in children are ≥2 years for lamivudine, ≥12 years for adefovir, ≥2 years for entecavir, and ≥12 years for tenofovir. Clinical trials of tenofovir for children below age 12 are undergoing. In children in the immune-tolerant phase with normal ALT, treatment is not indicated unless there is moderate liver histology change or cirrhosis. In children in the immune-active phase, ALT elevations above 1.3× ULN (usually >60 U/L) on at least 2 occasions for 6 months or more may be considered to receive treatment. The treatment duration may last for 2–4 or more years. Consolidation treatment for 12 months after HBeAg seroconversion and close monitoring for at least 12 months after discontinuing treatment are recommended. For HBeAg-negative hepatitis, ALT elevations above 1.5× ULN for at least 3 occasions over 12 months and HBV DNA >2 × 104 IU/mL may consider treatment [24, 69]. The treatment duration for an adult is indefinite for HBeAg-negative hepatitis. It is debatable whether to treat children life-long with antiviral drugs.
Children receiving liver transplantation from HBsAg(−)/anti-HBc(+) donors should receive antivirals to prevent reactivation of virus after transplantation. Children with HBsAg positivity before liver transplantation, or de novo HBV infection after transplantation, should receive long-term AVT.
The future perspective will be aimed at effective HBV covalently closed circular DNA eradiation to achieve long-term HBsAg seroconversion. When the new treatment is available, children, adolescents, or young adults can be timely treated before late complications occur.
CONCLUSIONS
Perinatal mother-to-infant transmission of HBV remains the major cause of chronic HBV infection despite immunization. Short-term antiviral therapy for highly viremic pregnancy women has been shown to be highly effective in preventing children from vaccine failure. Long-term outcome of mothers and children awaits further investigations. Surveillance of high risk children at one year of age is important to identify children with chronic HBV infection despite the above-mentioned preventive measures. Goals of antiviral treatment for HBV-infected children are to reduce severity of liver injury, achieve HBeAg seroconversion, and hopefully to prevent development of liver fibrosis and cancer. Ideal future goal of treatment is to eradicate chronic HBV infection globally.
Notes
Supplement sponsorship. This work is part of a supplement sponsored by the Hepatitis Research Center at the National Taiwan University Hospital.
Potential conflicts of interest. Professor M.-H. C. has conducted research grant from Gilead (IN-US-174-0171). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




