-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy F Murphy, Charmaine Kirkham, Mary C Gallo, Yang Yang, Gregory E Wilding, Melinda M Pettigrew, Immunoglobulin A Protease Variants Facilitate Intracellular Survival in Epithelial Cells By Nontypeable Haemophilus influenzae That Persist in the Human Respiratory Tract in Chronic Obstructive Pulmonary Disease, The Journal of Infectious Diseases, Volume 216, Issue 10, 15 November 2017, Pages 1295–1302, https://doi.org/10.1093/infdis/jix471
Close - Share Icon Share
Abstract
Nontypeable Haemophilus influenzae (NTHi) persists in the airways in chronic obstructive pulmonary disease (COPD). NTHi expresses 4 immunoglobulin (Ig)A protease variants (A1, A2, B1, B2) with distinct cleavage specificities for human IgA1. Little is known about the different roles of IgA protease variants in NTHi infection.
Twenty-six NTHi isolates from a 20-year longitudinal study of COPD were analyzed for IgA protease expression, survival in human respiratory epithelial cells, and cleavage of lysosomal-associated membrane protein 1 (LAMP1).
IgA protease B1 and B2-expressing strains showed greater intracellular survival in host epithelial cells than strains expressing no IgA protease (P < .001) or IgA protease A1 or A2 (P < .001). Strains that lost IgA protease expression showed reduced survival in host cells compared with the same strain that expressed IgA protease B1 (P = .006) or B2 (P = .015). IgA proteases B1 and B2 cleave LAMP1. Passage of strains through host cells selected for expression of IgA proteases B1 and B2 but not A1.
IgA proteases B1 and B2 cleave LAMP1 and mediate intracellular survival in respiratory epithelial cells. Intracellular persistence of NTHi selects for expression of IgA proteases B1 and B2. The variants of NTHi IgA proteases play distinct roles in pathogenesis of infection.
The natural ecological niche of nontypeable Haemophilus influenzae (NTHi) is the human upper respiratory tract. Nontypeable Haemophilus influenzae also inhabits the lower respiratory tract of adults with chronic obstructive pulmonary disease (COPD) and is the most common bacterial cause of exacerbations that characterize the course of COPD [1, 2]. As an exclusively human pathogen that causes infection in a uniquely human disease, NTHi has evolved virulence factors that mediate survival of the organism in the human respiratory tract [2]. One such virulence factor, immunoglobulin (Ig)A1 protease, cleaves the hinge region of human IgA1, a peptide region that is present in the IgA1 of humans and higher primates [3–6]. Colonization of the human respiratory tract places strong selective pressure on NTHi to express IgA protease, consistent with a key role for IgA protease in colonization and infection of the human respiratory tract [7].
Although IgA proteases were named because of their specificity for human IgA1, these enzymes contribute to bacterial infection through several other mechanisms [8–10]. Furthermore, strains of NTHi express 4 distinct IgA protease variants, encoded by 2 separate loci (IgA proteases A1, A2, B1, and B2), each with its own specificity for the human IgA1 hinge region [11]. Recent studies in one strain showed that IgA protease B1 cleaves human lysosomal-associated membrane protein 1 (LAMP1) and mediates intracellular survival in human respiratory epithelial cells [10]. The evolution of NTHi IgA proteases with different proteolytic specificities leads to the intriguing hypothesis that the proteases play different roles in the pathogenesis of human respiratory tract infection.
Some strains of NTHi persist for months to years in the respiratory tract in COPD [12]. Although NTHi has long been viewed as an extracellular pathogen, several research groups have shown that NTHi invades and survives in a variety of human respiratory epithelial cells, subepithelial cells, and macrophages in vitro and ex vivo, including the bronchi of adults with chronic lung disease [13–21]. Intracellular survival provides protection from immune pressures and, thus, may provide temporary niches or even long-term reservoirs for chronic NTHi infection in COPD [13].
Little is known about the relative contributions of the IgA protease variants to the pathogenesis of NTHi infection. The goals of the present study are to (1) assess the role of the 4 variants of IgA protease in intracellular survival by studying clinical isolates from adults with COPD, (2) analyze selected strains of NTHi that alter expression during persistence in human airways, (3) determine the specificity of the 4 variants of IgA protease for cleavage of human LAMP1, and (4) test the hypothesis that intracellular persistence of NTHi selects for expression of IgA proteases. This work advances our understanding of mechanisms by which NTHi persists in the human respiratory tract, leading to new approaches to interventions to eradicate NTHi in COPD airways.
METHODS
Bacterial Strains and Growth Conditions
Haemophilus influenzae strains were identified using standard techniques and were further distinguished from Haemophilus haemolyticus using monoclonal antibody 7F3, which recognizes an epitope on the P6 protein [22]. All but 1 strain was isolated from the sputum of adults with COPD as part of a 20-year prospective study in Buffalo, NY, described previously (Table 1) [23]. Strains were passaged twice from the original isolation. Immunoglobulin A protease knockout mutants were described previously [11, 24]. Strains were grown on chocolate agar or in brain heart infusion broth supplemented with hemin and nicotinamide adenine dinucleotide, both at 10 µg/mL.
| Straina . | Duration of Carriageb . | IgA Protease Expression . | ||||
|---|---|---|---|---|---|---|
| None . | A1 . | A2 . | B1 . | B2 . | ||
| 12P56H2 | Cleared | X | ||||
| 26P1H1 | 35 | X | ||||
| 27P38H1 | 35 | X | ||||
| 29P30H1 | Cleared | X | ||||
| 46P8H1 | Cleared | X | ||||
| 5P1H1 | 1036 | X | ||||
| 14P41H1 | 150 | X | ||||
| 19P49H1 | 1422 | X | ||||
| 32P8H3 | Cleared | X | ||||
| 102P16H1 | Cleared | X | ||||
| 72P12H1 | 70 | X | ||||
| 84P12H1 | 117 | X | ||||
| 94P40H1 | Cleared | X | ||||
| 124P3H1 | Cleared | X | ||||
| 126P11H1 | 344 | X | ||||
| 3P14H1 | 339 | X | ||||
| 6P24H2 | 252 | X | ||||
| 11P6H | Cleared | X | ||||
| 33P46H1 | Cleared | X | ||||
| 56P54H1 | Cleared | X | ||||
| 118P3H1 | Cleared | X | ||||
| 56P34H4 | 217 | X | ||||
| 91P109H1 | 70 | X | ||||
| 128P1H1 | 932 | X | ||||
| 133P17H1 | 572 | X | ||||
| 2019c | NA | X | ||||
| 138P2H1 | 721 | X | ||||
| 138P39H1 | 721 | X | ||||
| 93P10H1 | 543 | X | ||||
| 93P28H1 | 543 | X | ||||
| 91P114H1 | 70 | X | ||||
| 103P3H1 | 565 | X | ||||
| 103P20H1 | 565 | X | ||||
| Straina . | Duration of Carriageb . | IgA Protease Expression . | ||||
|---|---|---|---|---|---|---|
| None . | A1 . | A2 . | B1 . | B2 . | ||
| 12P56H2 | Cleared | X | ||||
| 26P1H1 | 35 | X | ||||
| 27P38H1 | 35 | X | ||||
| 29P30H1 | Cleared | X | ||||
| 46P8H1 | Cleared | X | ||||
| 5P1H1 | 1036 | X | ||||
| 14P41H1 | 150 | X | ||||
| 19P49H1 | 1422 | X | ||||
| 32P8H3 | Cleared | X | ||||
| 102P16H1 | Cleared | X | ||||
| 72P12H1 | 70 | X | ||||
| 84P12H1 | 117 | X | ||||
| 94P40H1 | Cleared | X | ||||
| 124P3H1 | Cleared | X | ||||
| 126P11H1 | 344 | X | ||||
| 3P14H1 | 339 | X | ||||
| 6P24H2 | 252 | X | ||||
| 11P6H | Cleared | X | ||||
| 33P46H1 | Cleared | X | ||||
| 56P54H1 | Cleared | X | ||||
| 118P3H1 | Cleared | X | ||||
| 56P34H4 | 217 | X | ||||
| 91P109H1 | 70 | X | ||||
| 128P1H1 | 932 | X | ||||
| 133P17H1 | 572 | X | ||||
| 2019c | NA | X | ||||
| 138P2H1 | 721 | X | ||||
| 138P39H1 | 721 | X | ||||
| 93P10H1 | 543 | X | ||||
| 93P28H1 | 543 | X | ||||
| 91P114H1 | 70 | X | ||||
| 103P3H1 | 565 | X | ||||
| 103P20H1 | 565 | X | ||||
Abbreviations: COPD, chronic obstructive pulmonary disease; IgA, immunoglobulin A; NA, not available.
aAll strains were isolated as part of a prospective study unless otherwise noted [23].
b“Cleared” indicates the strain was isolated at a single monthly clinic visit as part of a prospective study. A number indicates that the strain persisted for 2 or more monthly clinical visits and notes the number of days between the first and last positive cultures [23].
cStrain 2019 is a sputum isolate from an adult with COPD in Buffalo as part a separate study [32].
| Straina . | Duration of Carriageb . | IgA Protease Expression . | ||||
|---|---|---|---|---|---|---|
| None . | A1 . | A2 . | B1 . | B2 . | ||
| 12P56H2 | Cleared | X | ||||
| 26P1H1 | 35 | X | ||||
| 27P38H1 | 35 | X | ||||
| 29P30H1 | Cleared | X | ||||
| 46P8H1 | Cleared | X | ||||
| 5P1H1 | 1036 | X | ||||
| 14P41H1 | 150 | X | ||||
| 19P49H1 | 1422 | X | ||||
| 32P8H3 | Cleared | X | ||||
| 102P16H1 | Cleared | X | ||||
| 72P12H1 | 70 | X | ||||
| 84P12H1 | 117 | X | ||||
| 94P40H1 | Cleared | X | ||||
| 124P3H1 | Cleared | X | ||||
| 126P11H1 | 344 | X | ||||
| 3P14H1 | 339 | X | ||||
| 6P24H2 | 252 | X | ||||
| 11P6H | Cleared | X | ||||
| 33P46H1 | Cleared | X | ||||
| 56P54H1 | Cleared | X | ||||
| 118P3H1 | Cleared | X | ||||
| 56P34H4 | 217 | X | ||||
| 91P109H1 | 70 | X | ||||
| 128P1H1 | 932 | X | ||||
| 133P17H1 | 572 | X | ||||
| 2019c | NA | X | ||||
| 138P2H1 | 721 | X | ||||
| 138P39H1 | 721 | X | ||||
| 93P10H1 | 543 | X | ||||
| 93P28H1 | 543 | X | ||||
| 91P114H1 | 70 | X | ||||
| 103P3H1 | 565 | X | ||||
| 103P20H1 | 565 | X | ||||
| Straina . | Duration of Carriageb . | IgA Protease Expression . | ||||
|---|---|---|---|---|---|---|
| None . | A1 . | A2 . | B1 . | B2 . | ||
| 12P56H2 | Cleared | X | ||||
| 26P1H1 | 35 | X | ||||
| 27P38H1 | 35 | X | ||||
| 29P30H1 | Cleared | X | ||||
| 46P8H1 | Cleared | X | ||||
| 5P1H1 | 1036 | X | ||||
| 14P41H1 | 150 | X | ||||
| 19P49H1 | 1422 | X | ||||
| 32P8H3 | Cleared | X | ||||
| 102P16H1 | Cleared | X | ||||
| 72P12H1 | 70 | X | ||||
| 84P12H1 | 117 | X | ||||
| 94P40H1 | Cleared | X | ||||
| 124P3H1 | Cleared | X | ||||
| 126P11H1 | 344 | X | ||||
| 3P14H1 | 339 | X | ||||
| 6P24H2 | 252 | X | ||||
| 11P6H | Cleared | X | ||||
| 33P46H1 | Cleared | X | ||||
| 56P54H1 | Cleared | X | ||||
| 118P3H1 | Cleared | X | ||||
| 56P34H4 | 217 | X | ||||
| 91P109H1 | 70 | X | ||||
| 128P1H1 | 932 | X | ||||
| 133P17H1 | 572 | X | ||||
| 2019c | NA | X | ||||
| 138P2H1 | 721 | X | ||||
| 138P39H1 | 721 | X | ||||
| 93P10H1 | 543 | X | ||||
| 93P28H1 | 543 | X | ||||
| 91P114H1 | 70 | X | ||||
| 103P3H1 | 565 | X | ||||
| 103P20H1 | 565 | X | ||||
Abbreviations: COPD, chronic obstructive pulmonary disease; IgA, immunoglobulin A; NA, not available.
aAll strains were isolated as part of a prospective study unless otherwise noted [23].
b“Cleared” indicates the strain was isolated at a single monthly clinic visit as part of a prospective study. A number indicates that the strain persisted for 2 or more monthly clinical visits and notes the number of days between the first and last positive cultures [23].
cStrain 2019 is a sputum isolate from an adult with COPD in Buffalo as part a separate study [32].
Immunoglobulin A Protease Assays
The expression of IgA proteases and the cleavage specificity of the IgA proteases were determined using previously described methods [11, 24, 25].
H292 Respiratory Epithelial Cell Invasion and Intracellular Survival Assays
Twenty-four-well plates were seeded with suspensions of the H292 human respiratory epithelial cell line (2 × 105 cells per well) and grown using previously described methods [10]. Assays were performed in duplicate wells. Broth cultures were inoculated with NTHi grown on chocolate agar overnight to an optical density (OD)600 of 0.08 and grown to mid-log phase (OD600 of ~0.4) with shaking. H292 cells were washed twice with fresh sRPMI medium, inoculated with NTHi at a multiplicity of infection (MOI) of 1 bacterium per H292 cell, and the plates were centrifuged at 170 × g for 5 minutes at room temperature to facilitate contact with bacteria. Cells and bacteria were incubated for 3 hours at 37°C with 5% CO2. To assess intracellular survival of NTHi in the epithelial cells, infected monolayers were washed 3 times with phosphate-buffered saline and sRPMI containing 50 μg/mL gentamicin and incubated for 1 hour at 37°C. After washing, 200 μL trypsin (0.25%) was added to each well, and plates were incubated at 37°C for 10 minutes to remove adherent cells. A 300-μL volume of 1% saponin was applied to each well to lyse cells, pipetted into microcentrifuge tubes, and after vigorous vortexing were plated in duplicate to perform bacterial cell counts. Intracellular survival was expressed as colony-forming units recovered from the lysed cells. Trypan blue exclusion assays were conducted to control for cytotoxicity.
Statistical Analysis of Intracellular Survival Assays
To analyze the results of intracellular survival in respiratory epithelial cells by 26 clinical isolates, the number of intracellular bacteria was based on a standard linear modeling approach with number of intracellular bacteria specified as a function of group (based on IgA protease type expressed) and strain, nested with group. The log transformation was applied to the dependent variable to meet statistical assumptions. Once the model was fit, specific linear contrasts based on the estimated model parameters were constructed to test for differences among the groups in a pairwise fashion. All statistical tests were 2-sided, tested in conjunction with a 0.05 nominal significance level, and standard diagnostic plots were used to assess model fit. Analyses were carried out using SAS version 9.4 statistical software (Cary, NC). A paired, 2-tailed Student t test was used to compare the intracellular survival in respiratory epithelial cells by the same strain at acquisition and after persistence in the airways (ie, paired strains).
Lysosomal-Associated Membrane Protein 1 Cleavage Assay
Assays were performed as previously described except that the image was acquired using an Alpha Innotech Imager using the ECL filter [10].
RESULTS
Immunoglobulin A Protease Expression of Nontypeable Haemophilus influenzae Clinical Isolates in Chronic Obstructive Pulmonary Disease
To characterize the role of the 4 variants of IgA protease in invasion of human respiratory epithelial cells, we studied 26 sputum isolates that were each previously characterized with regard to IgA protease expression [11]. Figure 1 shows the expression pattern of 1 strain of each of the IgA protease variants and the cleavage specificity of each variant.
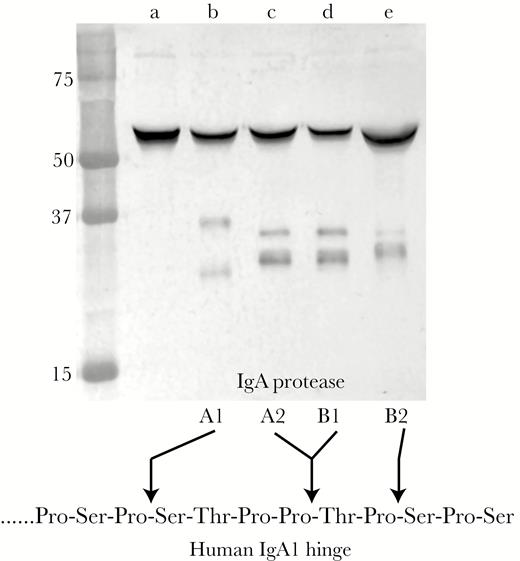
Immunoblot assay showing cleavage pattern of purified human immunoglobulin (Ig)A incubated with epithelial cell culture supernatant collected 3 hours after inoculation of cell cultures with bacterial strains. Lanes (a) strain 29P30H1, which expresses no IgA protease; (b) strain 19P49H1, which expresses IgA protease A1; (c) strain 84P12H1, which expresses IgA protease A2; (d) strain 33P46H1, which expresses IgA protease B1; (e) 56P34H4, which expresses IgA protease B2. The cleavage specificity of the human IgA1 hinge region is shown at the bottom. Molecular mass markers are noted in kilodaltons on the left.
Intracellular Survival in Human Respiratory Epithelial Cells by Clinical Isolates
Results of assays with the H292 human respiratory epithelial cell line with 26 sputum isolates of NTHi are shown in Figure 2. We chose an MOI of 1 to reflect the concentration of bacteria in vivo at the time of inoculation and early infection. This concentration increases over the course of the infection.
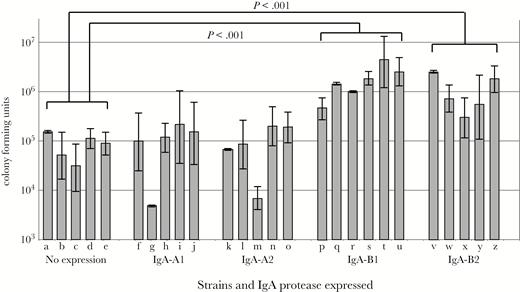
Results of invasion and intracellular survival assays of human respiratory epithelial cells with 26 clinical isolates of nontypeable Haemophilus influenzae recovered from sputum samples of adults with chronic obstructive pulmonary disease. Y-axis shows colony-forming units of bacteria that invaded cells. X-axis shows strains with immunoglobulin (Ig)A protease expression as noted. Lanes contain strains: (a) 12P56H2, (b) 26P1H1, (c) 27P38H1, (d) 29P30H1, (e) 46P8H1, (f) 5P1H1, (g) 19P49H1, (h) 14P41H1, (i) 32P8H3, (j) 102p16H1, (k) 72P12H1, (l) 84P12H1, (m) 94P40H1, (n) 124P3H1, (o) 40P92H1, (p) 3P14H1, (q) 6P24H2, (r) 33P46H1, (s) 56P54H1, (t) 118P3H1, (u) 11P6H, (v) 56P34H4, (w) 91P109H1, (x) 128P1H1, (y) 133P17H1, (z) 2019. Individual bars show 3 independent replicates for each strain, and error bars show standard errors. Invasion by strains that express IgA-B1 protease (P < .001) and IgA-B2 protease (P < .001) are significantly greater than invasion by strains that do not express IgA protease as noted. Strains that express IgA-A1 protease and IgA-A2 protease are no different in invasion compared with nonexpressing strains.
Survival in host cells after 3 hours of incubation with strains that express IgA protease B1 and B2 was greater than for strains that expressed no IgA protease (P < .001). By contrast, intracellular survival by strains that expressed IgA protease A1 and A2 was no different from strains that expressed no IgA protease (P = .68 for IgA-A1 and P = .76 for IgA-A2). Complete results of statistical analysis are shown in Table 2.
Statistical Comparison of Invasion of Human Respiratory Epithelial Cells by Strains of NTHi With Different IgA Protease Expression
| IgA Protease Expression Group . | IgA Protease Expression Groupa . | P Values . |
|---|---|---|
| No expression | A1 | .6836 |
| No expression | A2 | .7553 |
| No expression | B1 | <.0001 |
| No expression | B2 | <.0001 |
| A1 | A2 | .4729 |
| A1 | B1 | <.0001 |
| A1 | B2 | <.0001 |
| A2 | B1 | <.0001 |
| A2 | B2 | <.0001 |
| B1 | B2 | .0696 |
| IgA Protease Expression Group . | IgA Protease Expression Groupa . | P Values . |
|---|---|---|
| No expression | A1 | .6836 |
| No expression | A2 | .7553 |
| No expression | B1 | <.0001 |
| No expression | B2 | <.0001 |
| A1 | A2 | .4729 |
| A1 | B1 | <.0001 |
| A1 | B2 | <.0001 |
| A2 | B1 | <.0001 |
| A2 | B2 | <.0001 |
| B1 | B2 | .0696 |
Abbreviations: IgA, immunoglobulin A; NTHi, nontypeable Haemophilus influenzae.
aIn instances in which statistically significant differences were observed, invasion by strains in this column was significantly greater than invasion by the comparator strains.
Statistical Comparison of Invasion of Human Respiratory Epithelial Cells by Strains of NTHi With Different IgA Protease Expression
| IgA Protease Expression Group . | IgA Protease Expression Groupa . | P Values . |
|---|---|---|
| No expression | A1 | .6836 |
| No expression | A2 | .7553 |
| No expression | B1 | <.0001 |
| No expression | B2 | <.0001 |
| A1 | A2 | .4729 |
| A1 | B1 | <.0001 |
| A1 | B2 | <.0001 |
| A2 | B1 | <.0001 |
| A2 | B2 | <.0001 |
| B1 | B2 | .0696 |
| IgA Protease Expression Group . | IgA Protease Expression Groupa . | P Values . |
|---|---|---|
| No expression | A1 | .6836 |
| No expression | A2 | .7553 |
| No expression | B1 | <.0001 |
| No expression | B2 | <.0001 |
| A1 | A2 | .4729 |
| A1 | B1 | <.0001 |
| A1 | B2 | <.0001 |
| A2 | B1 | <.0001 |
| A2 | B2 | <.0001 |
| B1 | B2 | .0696 |
Abbreviations: IgA, immunoglobulin A; NTHi, nontypeable Haemophilus influenzae.
aIn instances in which statistically significant differences were observed, invasion by strains in this column was significantly greater than invasion by the comparator strains.
Intracellular survival was also assessed at 24 hours. Each of the 26 clinical isolates showed the same relationship as observed at 3 hours among the strains expressing different IgA protease variants. In particular, strains that express IgA protease B1 and B2 showed greater survival after 24 hours than strains that expressed no IgA protease and strains that expressed IgA protease A1 or A2 (P < .001 for each comparison) (data not shown). The bacterial counts at 24 hours were ~2 logs lower than those observed after 3 hours.
To confirm that strains continued to express IgA proteases during the course of the assay with epithelial cells, NTHi were isolated from cell culture media at the end of the 3-hour assay and tested for IgA protease expression. For each strain, expression of IgA protease (or no expression in the case of the no expression group) was present at the end of the assay, matching expression that was determined in vitro before the assay. Figure 1 shows example results with 5 strains at the end of the 3-hour incubation period of the assays; IgA protease expression patterns matched those at the start of the assay for each strain (Table 1). We conclude that strains of NTHi that express IgA protease B1 and B2 show greater intracellular survival than all other groups, providing evidence that IgA protease B1 and B2 both play a role in mediating survival in human respiratory epithelial cells by NTHi.
Immunoglobulin A Protease Expression and Intracellular Survival
To more precisely assess the role of specific IgA proteases in survival inside host cells, we performed assays on 2 pairs of isolates of the same strain when acquired by the patient and at the time it was cleared after persistence in the respiratory tract. Both strain pairs showed changes in expression of IgA protease during persistence. Strain 103P3H1 expressed IgA protease B1 upon acquisition by the patient. After persistence in the patient’s respiratory tract for 565 days, the strain no longer expressed IgA protease B1 due to a 49-base pair (bp) deletion that caused the igaB1 gene to be out of frame (M.C.G., C.K., M.M.P, T.F.M manuscript in preparation). The latter isolate (103P20H1), which does not express IgA protease B1, showed significantly reduced survival inside respiratory epithelial cells compared with the same strain that expresses IgA protease B1 (P = .006) (Figure 3). Immunoglobulin A protease assays confirmed that expression by each strain was unchanged at the end of the assay compared with the start of the assay, excluding the confounding variable that NTHi alters expression in the conditions of the assay. This observation supports the conclusion that IgA protease B1 mediated intracellular survival of NTHi inside human respiratory epithelial cells.
Strain 91P109H1 expressed IgA protease B2 upon acquisition by the patient. After persistence in the patient’s respiratory tract for 70 days, the strain no longer expressed IgA protease B2 due to a change in the number of upstream repeats that caused the igaB2 gene to be out of frame. The latter isolate (91P114H1), which does not express IgA protease B2, showed significantly reduced survival inside respiratory epithelial cells compared with the same strain that expresses IgA protease B2 (P = .015) (Figure 3). Immunoglobulin A protease assays confirmed that expression by each strain was unchanged at the end of the assay compared with the start of the assay. This observation supports the conclusion that IgA protease B2 mediates survival of NTHi inside human respiratory epithelial cells.
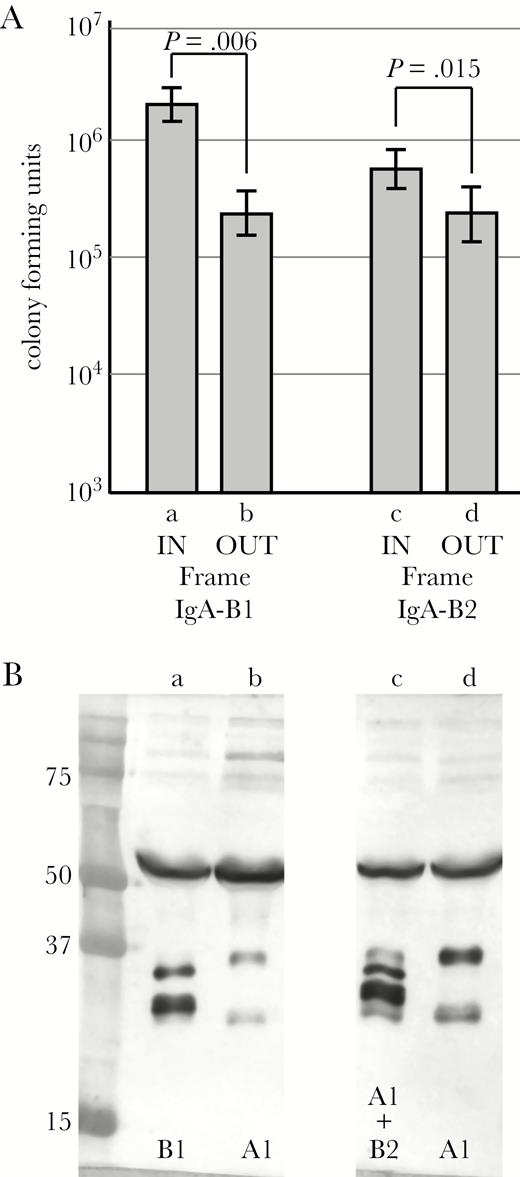
(A) Results of invasion and intracellular survival assays of human respiratory epithelial cells comparing strains at acquisition and after persistence in the airways. Lane a, strain 103P3H1 upon acquisition by the patient (igaB1 gene in frame and expressed); lane b, strain 103P20H1 (igaB1 gene out of frame and not expressed), which is the same strain as in lane a after persistence for 565 days. Lane c, strain 91P109H1 upon acquisition by the patient (igaB2 gene in frame and expressed); lane d, strain 91P114H1 (igaB2 gene out of frame and not expressed), which is the same strain as in lane c after persistence for 70 days. Y-axis is colony-forming units of intracellular bacteria after 3 hour assay. P values are noted based on paired 2-tailed Student t test. (B) Immunoblot assay showing immunoglobulin (Ig)A protease expression at the end of the 3-hour assays confirming that expression did not change during the assay. Lanes are the same as in A. IgA protease expression is noted at the bottom. Molecular mass standards are noted on the left in kilodaltons.
Lysosomal-Associated Membrane Protein 1 Cleavage Specificity of Immunoglobulin A Protease Variants
Previous work with a single strain demonstrated that IgA protease B1 cleaved LAMP 1 and that cleavage was correlated with intracellular survival [10]. We assessed the activity of the 4 IgA protease variants in cleaving LAMP1 (Figure 4). The large top band is intact LAMP1, and the smaller bands, noted by arrows, are LAMP1 cleavage fragments. The figure shows that IgA proteases B1 and B2 cleave LAMP 1 (Figure 4, lanes d and f), whereas IgA proteases A1 and A2 do not (Figure 4, lanes a and b). These conclusions are further supported by the absence of cleavage by the isogenic igaB1 and igaB2 knockout mutants (Figure 4, lanes e and g).

Lysosomal-associated membrane protein 1 (LAMP1) cleavage assay. Aliquots of cell culture medium from H292 cells infected for 24 hours with wild-type and mutant strains as indicated were filter sterilized and incubated with recombinant human LAMP1 at 37°C overnight. Digestions were separated on protein gels, transferred to polyvinylidene difluoride membrane, probed with antihuman LAMP1 antibody, and visualized by chemiluminescence. The large top band is intact LAMP1, and the smaller bands at the arrows are LAMP1 cleavage fragments. The IgA protease expressed by each strain is noted at the bottom; knockout mutants are noted by Δ. Lanes contain nontypeable Haemophilus influenzae strains: (a) 105P29H1, (b) 124P34H1, (c) igaA2 knockout mutant of 124P34H1, (d) 11P6H, (e) igaB1 knockout mutant of 11P6H, (f) 2019, (g) igaB2 knockout mutant of 2019.
Selection of Immunoglobulin A Protease Expression
Expression of IgA protease B2 is regulated by slipped strand mispairing of a 7-bp repeat that is upstream of the igaB2 gene [7, 11]. The expression of IgA-B2 changes during persistence in the human respiratory tract, suggesting that conditions in the human respiratory tract regulate expression of IgA protease B2 [7] (M.C.G., et al. manuscript in preparation). To test the hypothesis that intracellular persistence in human respiratory epithelial cells selects for expression of IgA protease B2, we performed serial passage in epithelial cells of a strain of NTHi whose igaB2 gene was out of frame. After 8 passages through cells, alternating with 10 passages on plates to collect cells for the next passage through cells (18 total passages), strain 56P34H2 converted from no expression to expression of IgA protease B2 during passage. The same strain passaged an equivalent number of times (18 passages) on agar plates as a control showed no IgA protease B2 expression (Figure 5A). Analysis of sequences indicated that the nonexpressing original strain had 12 upstream TCAAAAT repeats, placing the gene out of frame, whereas the epithelial cell-passaged strain that expressed IgA protease B2 had 14 upstream repeats, placing the gene in frame. We conclude that intracellular persistence of NTHi selected for expression of IgA protease B2.
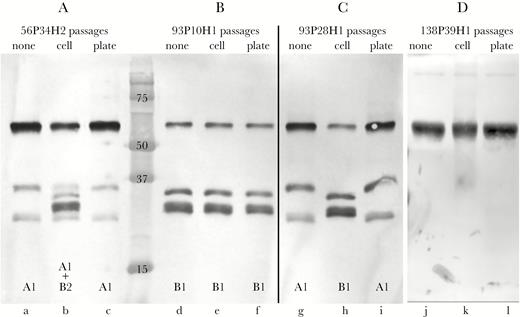
Immunoblot assays showing effect of passage of strains through human bronchial respiratory epithelial cells. (A) Immunoglobulin (Ig)A protease expression of strain 56P34H2 upon original isolation from sputum (lane a), after passaging through epithelial cells (lane b), and after passage on agar plates (lane c, control). (B) The IgA protease expression of strain 93P10H1 upon original isolation from sputum (lane d), after passage through epithelial cells (lane e), and after passage on agar plates (lane f, control). (C) The IgA protease expression of strain 93P28H1, which is the same strain as 93P10H1 that persisted in the respiratory tract for 543 days, upon original isolation from sputum (lane g), after passage through epithelial cells (lane h), and after passage on agar plates (lane I, control). (D) IgA protease expression of strain 138P39H1 upon original isolation from sputum (lane j), after passage through epithelial cells (lane k), and after passage on agar plates (lane l, control). A, B, and C are the same immunoblot. IgA protease expression is noted at the bottom of each lane. Molecular mass markers are noted in kilodaltons.
The upstream region of the igaB1 gene lacks repeats or other obvious regulatory sequences. However, selected strains alter expression of IgA protease B1 during persistence in the human respiratory tract. Strain 93P10H1 expressed IgA protease B1 upon acquisition by patient 93, but stopped expressing when isolated after 543 days of persistence. Analysis of sequences revealed that the persisting strain (93P28H1) underwent deletion of a single A in a run of 7 A’s within the open reading frame that placed the igaB1 gene out of frame, accounting for loss of expression. To test the hypothesis that passage in human respiratory epithelial cells selects for expression of IgA protease B1, we passaged the nonexpressing strain 93P28H1 11 times through cells, alternating with 14 passages on plates (25 total passages). Figure 5C shows that passage through cells resulted in conversion from absent expression (Figure 5C, lane g) to expression of IgA protease B1 (Figure 5C, lane h). Sequence analysis confirmed that the igaB1 gene in the strain that is expressing IgA-B1 after passage in cells is now in frame due to the acquisition of an A in the repeat of 7 A’s. As a negative control, the same strain was passaged an equivalent number of times on agar plates (25 passages) and showed no induction of expression of IgA protease B1 (Figure 5C, lane i). As an additional control, we passaged the IgA protease B1-expressing strain in cells, and this strain continues to express IgA protease B1, as expected (Figure 5B). We conclude that intracellular persistence of NTHi selected for expression of IgA protease B1.
The upstream region of igaA1 lacks repeats or other obvious regulatory sequences. Strain 138P2H1 expressed IgA protease A1 upon acquisition at visit 2 by patient 138, but it stopped expressing when isolated at visit 39 after 721 days of persistence. Analysis of sequences revealed that the persisting strain (138P39H1) acquired a G in a run of 12 Gs within the open reading frame that placed the igaA1 gene out of frame, accounting for loss of expression. To test the hypothesis that passage in human respiratory epithelial cells selects for expression of IgA protease A1, the nonexpressing strain 138P39H1 underwent 11 passages through cells, alternating with 14 passages on plates (25 total passages). The strain continued to show absence of expression after passaging through cells (Figure 5D). We conclude that, in contrast to IgA proteases B1 and B2, intracellular persistence did not select for expression of IgA protease A1.
DISCUSSION
This work advances our understanding of the role of IgA proteases in how NTHi interfaces with the human respiratory tract during infection by establishing that variants of IgA proteases expressed by strains of NTHi show different functions. We show here that IgA proteases B1 and B2 mediate intracellular survival in human respiratory epithelial cells and cleave LAMP1. By contrast, IgA proteases A1 or A2 do not enhance intracellular survival over strains that lack IgA protease expression, and they do not cleave LAMP1. These conclusions are based on experiments performed with 26 minimally passaged, well characterized clinical isolates collected as part of a longitudinal study in adults with COPD. The conclusions are further supported by assays in pairs of isolates of strains that underwent alteration of expression of IgA proteases during persistence in the human respiratory tract. These observations have important implications in understanding the role of IgA proteases in persistence of NTHi in the human respiratory tract.
An additional novel observation is that persistence of NTHi in human respiratory epithelial cells induces expression of IgA proteases B1 and B2 but not IgA protease A1. Expression of IgA protease B2 is regulated by slipped strand mispairing of repeats upstream of the gene, and IgA protease B1 expression in some strains appears to be regulated by a similar mechanism through a segment of single nucleotide repeats within the open reading frame. The observation that, in each case, the equivalent number of passages of the same strains in parallel on agar plates did not result in transition to expression, further supports the conclusion that passage in cells places selective pressure to express IgA proteases B1 and B2. The observation that clinical isolates that altered their IgA protease expression in vivo, in combination with the results of in vitro experiments on selected strains, provides a proof of principle that NTHi possesses the capacity to alter its expression of IgA proteases in response to conditions in the human respiratory tract. Based on this observation, we speculate that intracellular conditions select for expression specifically of the IgA protease variants (B1 and B2) that promote intracellular survival.
Analysis of 1 additional pair each of isolates that change expression of IgA protease B1 and B2 during persistence showed a trend toward greater expression of the isolate that expressed IgA protease, but these did not reach statistical significance. The most likely explanation for this observation, which is in contrast to the observations with the 2 strain pairs described above and in Figure 3, is that the overall effect of IgA proteases on invasion of host cells varies from strain to strain, with different virulence factors playing relatively different roles in host cell invasion among strains of NTHi [26].
Cell culture experiments demonstrated that intracellular persistence selects “for” expression of IgA protease B1 and B2. This in vitro observation is consistent with the results of a human challenge model, which demonstrated strong selective pressure for expression of IgA protease B2 after challenge of human volunteers with NTHi [7]. Of interest, the results in Figure 3 show 2 examples of strains that expressed IgA protease B1 and B2 upon acquisition by the patient and subsequently stopped expression, suggesting selective pressure “against” expression of IgA proteases. During colonization and infection of the human respiratory tract, NTHi resides in the lumen, bound to mucus, adherent to epithelial cells, inside multiple cell types (epithelial cells, subepithelial cells, macrophages) and between cells [14–21, 27–31]. The present study shows that clinical isolates of NTHi have the capacity to alter expression of IgA protease to survive in the multiple microenvironments of the human airways.
Comparison of intracellular survival of clinical isolates that express IgA proteases B1 and B2 reveals a trend toward higher expression in IgA protease B1 isolates compared with IgA B2-expressing isolates (Table 2). The LAMP1 cleavage assay shows apparent greater cleavage by the IgA protease B1-expressing strain compared with the B2 strain (Figure 4). Although this is not a quantitative assay, it is interesting that the results of the invasion assays and LAMP1 cleavage assays parallel one another, consistent with the concept that LAMP1 cleavage mediates survival in lysosomes, which facilitates intracellular persistence [10].
It is curious that IgA protease A2 and B1 have identical cleavage specificities of the IgA1 hinge region, yet have different effects in mediating survival in epithelial cells. Immunoglobulin A protease B1-expressing clinical isolates show significantly greater intracellular survival compared with IgA protease A2-expressing isolates. In addition, IgA protease B1 cleaves LAMP1, whereas IgA protease A2 does not. The cleavage sequence of LAMP1 may differ from that of the human IgA1 hinge region, accounting for the different functional effects. An alternative explanation may be that the IgA protease B1-expressing strains have other virulence mechanisms of invasion that are not present in IgA protease A2-expressing strains.
CONCLUSIONS
The functional activity of IgA proteases of NTHi has been studied previously in a limited number of selected strains. In this study, we report the results of functional activity of IgA proteases and cell invasion in multiple longitudinally collected, minimally passaged clinical isolates, including strain pairs that underwent altered expression of their IgA proteases during persistence in human airways. This work advances understanding of the functional role of IgA proteases in facilitating survival of NTHi in human epithelial cells, an important mechanism by which NTHi persists in COPD airways. The 4 IgA protease variants display different functional activities and regulation of expression during infection in COPD. Immunoglobulin A proteases B1 and B2 cleave LAMP1 and mediate intracellular survival of human respiratory epithelial cells. Intracellular persistence of NTHi selects for expression of IgA proteases B1 and B2. In addition to the implications in understanding mechanisms of pathogenesis, these observations suggest that IgA proteases represent a potential therapeutic target to shorten or eradicate persistence of NTHi in COPD, which would be of enormous benefit in this clinical setting.
Acknowledgments
Financial support. This work was supported by National Institutes of Health grant R01 AI19641 (to T. F. M. and M. M. P.) and by National Center for Advancing Translational Sciences award UL1 TR001412 (to the University at Buffalo).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




