-
PDF
- Split View
-
Views
-
Cite
Cite
Richelle C. Charles, Rie Nakajima, Li Liang, Al Jasinskas, Amanda Berger, Daniel T. Leung, Meagan Kelly, Peng Xu, Pavol Kováč, Samantha R. Giffen, James D. Harbison, Fahima Chowdhury, Ashraful I. Khan, Stephen B. Calderwood, Taufiqur Rahman Bhuiyan, Jason B. Harris, Philip L. Felgner, Firdausi Qadri, Edward T. Ryan, Plasma and Mucosal Immunoglobulin M, Immunoglobulin A, and Immunoglobulin G Responses to the Vibrio cholerae O1 Protein Immunome in Adults With Cholera in Bangladesh, The Journal of Infectious Diseases, Volume 216, Issue 1, 1 July 2017, Pages 125–134, https://doi.org/10.1093/infdis/jix253
Close - Share Icon Share
Abstract
Cholera is a severe dehydrating illness of humans caused by toxigenic strains of Vibrio cholerae O1 or O139. Identification of immunogenic V. cholerae antigens could lead to a better understanding of protective immunity in human cholera.
We probed microarrays containing 3652 V. cholerae antigens with plasma and antibody-in-lymphocyte supernatant (ALS, a surrogate marker of mucosal immune responses) from patients with severe cholera caused by V. cholerae O1 in Bangladesh and age-, sex-, and ABO-matched Bangladeshi controls. We validated a subset of identified antigens using enzyme-linked immunosorbent assay.
Overall, we identified 608 immunoreactive V. cholerae antigens in our screening, 59 of which had higher immunoreactivity in convalescent compared with acute-stage or healthy control samples (34 in plasma, 39 in mucosal ALS; 13 in both sample sets). Identified antigens included cholera toxin B and A subunits, V. cholerae O–specific polysaccharide and lipopolysaccharide, toxin coregulated pilus A, sialidase, hemolysin A, flagellins (FlaB, FlaC, and FlaD), phosphoenolpyruvate-protein phosphotransferase, and diaminobutyrate–2-oxoglutarate aminotransferase.
This study is the first antibody profiling of the mucosal and systemic antibody responses to the nearly complete V. cholerae O1 protein immunome; it has identified antigens that may aid in the development of an improved cholera vaccine.
Cholera remains a significant global health problem predominantly affecting impoverished individuals in resource-limited areas of the world. It is estimated that each year, cholera affects 3 million individuals, resulting in >100000 deaths globally [1]. Countries in Asia, sub-Saharan Africa, and the Caribbean bear the highest burden of disease, and outbreaks are increasing in frequency, duration, and associated mortality [2]. The starkest demonstration of this is the large outbreak that has afflicted Haiti over the last 7 years. Since its introduction into Haiti in 2010, cholera has infected 780000 people, killing >9000 [3].
The key to cholera prevention is the provision of safe drinking water and improved sanitation for all; however, it will take decades to implement the infrastructure changes required to achieve this. In the meantime, integrated disease control measures that include use of effective vaccines will be required. There are currently a number of World Health Organization–prequalified cholera vaccines commercially available globally. There are two oral whole cell killed vaccines that contain both the Inaba and Ogawa serotypes of Vibriocholerae O1: a whole cell killed vaccine that contains recombinant B subunit of cholera toxin (CTB), WC/rBS (Dukoral®, Crucell); and the bivalent killed whole cell vaccine, BivWC, (Shanchol®, Shantha Biotechnics, India and Euvichol®, Eubiologics, South Korea) that also includes killed V. cholerae O139 organisms. In older children and adults, these vaccines provide roughly 60% protection for 6–60 months against cholera when used in cholera-endemic areas [4–7]. This level of protection and duration are lower than those afforded by wild-type disease [8]. In addition, these vaccines provide even more limited protection in children aged <5 years even in cholera-endemic areas [4, 5]. A live attenuated oral cholera vaccine containing a single nontoxigenic strain of V. cholerae O1, CVD 103-HgR (Vaxchora, PaxVax, California), was recently approved in the United States. In US volunteers, the vaccine provided high-level protection against challenge for at least 10–90 days after vaccination [9]. Longer-term protection has not been evaluated, nor has the vaccine been evaluated in cholera-endemic areas, nor in individuals <18 years of age.
To improve our current vaccine strategies, we need to have a better understanding of the robust, long-lasting protection provided by natural infection. There is a growing body of evidence that a primary mediator of protection against cholera may be antibodies that target the O-specific polysaccharide (OSP) moiety of V. cholerae lipopolysaccharide (LPS) [10–16]. Other antigens that have been studied and associated with protection include CTB and toxin-coregulated pilus A (TcpA), a major structural subunit of a type IV pilus that is required for intestinal colonization. Elevated serum immunoglobulin A (IgA) antibody levels specific to CTB, TcpA, and LPS/OSP are correlated with protection against cholera in endemic settings [17]. In addition, memory B cell (BMEM) immunoglobulin G (IgG) responses to LPS/OSP have also been associated with protection against cholera [16]. These analyses have all been based on using individual purified antigens, and as such are limited by choice of antigen. To extend our understanding of immune responses during cholera using an unbiased immunoprofiling approach, we used microarrays containing the proteome of V. cholerae O1, as well as LPS and OSP, to identify additional immunogenic V. cholerae O1 antigens. Here, we describe our analysis in plasma and mucosal (antibody-in-lymphocyte supernatant [ALS]) samples from adults with cholera in Dhaka, Bangladesh, a cholera-endemic area.
METHODS
Study Subject Selection and Sample Collection
For our microarray analysis, we enrolled 7 adults aged 18–55 presenting to the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) hospital with acute watery diarrhea and stool culture–confirmed V. cholerae O1 infection. Baseline information and the vibriocidal responses of these patients are summarized in Table 1. Seven healthy Bangladeshis that were age-, sex-, and ABO-matched to the cholera patients and without known recent exposure to cholera were also enrolled. For our confirmatory enzyme-linked immunosorbent assay (ELISA) analysis, we enrolled an additional 35 adult patients with cholera: 13 for plasma-based ELISA analysis, 10 for mucosal ALS ELISA, and 12 for BMEM ALS ELISA. We also included 18 healthy Bangladeshi controls for the plasma (n = 13) and mucosal ALS-based ELISA (n = 5). Venous blood was collected from participants into sodium heparin tubes at the acute phase of infection after clinical stabilization (day 2), and again at convalescent phases of infection (days 7 and 30). This study was approved by the Research and Ethical Review Committees of the icddr,b and the Human Studies Committee of Massachusetts General Hospital. Written informed consent was obtained from all individuals prior to study participation.
| Patient . | Infecting Strain . | Age, y . | Sex . | ABO Blood Group . | Vibriocidal Inaba D2a . | Vibriocidal Ogawa D2a . | Vibriocidal Inaba D7a . | Vibriocidal Ogawa D7a . | Vibriocidal Inaba D30a . | Vibriocidal Ogawa D30a . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O1-Inaba | 46 | F | B+ | 40 | 20 | 640 | 640 | 640 | 320 |
| 2 | O1-Inaba | 36 | M | O+ | 40 | 20 | 640 | 1280 | 640 | 640 |
| 3 | O1-Inaba | 40 | F | O+ | 10 | 5 | 2560 | 2560 | 2560 | 320 |
| 4 | O1-Ogawa | 30 | M | O+ | 10 | 40 | 1280 | 5120 | 640 | 5120 |
| 5 | O1-Ogawa | 44 | F | B+ | 5 | 5 | 5120 | 5120 | 2560 | 1280 |
| 6 | O1-Ogawa | 50 | M | O+ | 20 | 40 | 10240 | 10240 | 1280 | 5120 |
| 7 | O1-Ogawa | 21 | F | B+ | 5 | 40 | 5120 | 10240 | 2560 | 2560 |
| Patient . | Infecting Strain . | Age, y . | Sex . | ABO Blood Group . | Vibriocidal Inaba D2a . | Vibriocidal Ogawa D2a . | Vibriocidal Inaba D7a . | Vibriocidal Ogawa D7a . | Vibriocidal Inaba D30a . | Vibriocidal Ogawa D30a . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O1-Inaba | 46 | F | B+ | 40 | 20 | 640 | 640 | 640 | 320 |
| 2 | O1-Inaba | 36 | M | O+ | 40 | 20 | 640 | 1280 | 640 | 640 |
| 3 | O1-Inaba | 40 | F | O+ | 10 | 5 | 2560 | 2560 | 2560 | 320 |
| 4 | O1-Ogawa | 30 | M | O+ | 10 | 40 | 1280 | 5120 | 640 | 5120 |
| 5 | O1-Ogawa | 44 | F | B+ | 5 | 5 | 5120 | 5120 | 2560 | 1280 |
| 6 | O1-Ogawa | 50 | M | O+ | 20 | 40 | 10240 | 10240 | 1280 | 5120 |
| 7 | O1-Ogawa | 21 | F | B+ | 5 | 40 | 5120 | 10240 | 2560 | 2560 |
aVibriocidal titer to Vibrio cholerae serotypes Ogawa (strain 25049) and Inaba (strain T-19479) as defined as the reciprocal of the highest plasma dilution resulting in >50% reduction in V. cholerae O1 growth (measured by optical density) compared to control wells without plasma.
| Patient . | Infecting Strain . | Age, y . | Sex . | ABO Blood Group . | Vibriocidal Inaba D2a . | Vibriocidal Ogawa D2a . | Vibriocidal Inaba D7a . | Vibriocidal Ogawa D7a . | Vibriocidal Inaba D30a . | Vibriocidal Ogawa D30a . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O1-Inaba | 46 | F | B+ | 40 | 20 | 640 | 640 | 640 | 320 |
| 2 | O1-Inaba | 36 | M | O+ | 40 | 20 | 640 | 1280 | 640 | 640 |
| 3 | O1-Inaba | 40 | F | O+ | 10 | 5 | 2560 | 2560 | 2560 | 320 |
| 4 | O1-Ogawa | 30 | M | O+ | 10 | 40 | 1280 | 5120 | 640 | 5120 |
| 5 | O1-Ogawa | 44 | F | B+ | 5 | 5 | 5120 | 5120 | 2560 | 1280 |
| 6 | O1-Ogawa | 50 | M | O+ | 20 | 40 | 10240 | 10240 | 1280 | 5120 |
| 7 | O1-Ogawa | 21 | F | B+ | 5 | 40 | 5120 | 10240 | 2560 | 2560 |
| Patient . | Infecting Strain . | Age, y . | Sex . | ABO Blood Group . | Vibriocidal Inaba D2a . | Vibriocidal Ogawa D2a . | Vibriocidal Inaba D7a . | Vibriocidal Ogawa D7a . | Vibriocidal Inaba D30a . | Vibriocidal Ogawa D30a . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O1-Inaba | 46 | F | B+ | 40 | 20 | 640 | 640 | 640 | 320 |
| 2 | O1-Inaba | 36 | M | O+ | 40 | 20 | 640 | 1280 | 640 | 640 |
| 3 | O1-Inaba | 40 | F | O+ | 10 | 5 | 2560 | 2560 | 2560 | 320 |
| 4 | O1-Ogawa | 30 | M | O+ | 10 | 40 | 1280 | 5120 | 640 | 5120 |
| 5 | O1-Ogawa | 44 | F | B+ | 5 | 5 | 5120 | 5120 | 2560 | 1280 |
| 6 | O1-Ogawa | 50 | M | O+ | 20 | 40 | 10240 | 10240 | 1280 | 5120 |
| 7 | O1-Ogawa | 21 | F | B+ | 5 | 40 | 5120 | 10240 | 2560 | 2560 |
aVibriocidal titer to Vibrio cholerae serotypes Ogawa (strain 25049) and Inaba (strain T-19479) as defined as the reciprocal of the highest plasma dilution resulting in >50% reduction in V. cholerae O1 growth (measured by optical density) compared to control wells without plasma.
Sample Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation. Plasma was stored at –80°C for subsequent immunological analysis. Fresh PBMCs were used to generate mucosal ALS and BMEM ALS samples, as previously described [10, 18, 19].
Cloning, Microarray Fabrication, and Probing
Proteome microarrays containing 3652 V. cholerae O1 El Tor reference strain N16961 antigens were constructed as previously described [20]. Overall, 3538 open reading frames (ORFs) were represented on the array (95% of the V. cholerae ORFeome). In addition to in vitro transcription/translation (IVTT)–expressed proteins, each array contained “no DNA” control spots consisting of IVTT reaction mixture without the addition of a plasmid, serial dilutions of purified IgG/IgM/IgA spots, and serial dilutions of purified cholera antigens including CTB, TcpA, V. cholerae O1 membrane protein (MP) [21], LPS and OSP (isolated from V. cholerae O1 Inaba strain PIC018 [13] and Ogawa strain PIC158 [10]) conjugated to bovine serum albumin.
The arrays were probed for IgG, IgA, and IgM plasma responses at days 2, 7, and 30 after presentation using the samples of the 7 cholera patients. Because baseline mucosal ALS samples were not available for the cholera patients, mucosal (ALS) responses were measured using day 7 cholera patient samples and compared to responses in 7 healthy matched Bangladeshi controls. For plasma samples, the arrays were probed at 1:100 dilution of sample in protein array blocking buffer (PABB; GVS) supplemented with Escherichia coli lysate (GenScript) at a final concentration of 10% to block anti–E. coli antibodies. After incubation, arrays were washed with Tris-buffered saline/Tween-20 solution, and bound antibody was detected with Alexa Fluor 647–conjugated goat antihuman IgG and Cy3-conjugated goat antihuman IgA secondary antibody mixture (Jackson ImmunoResearch), each diluted at 1:200 in PABB. For detection of IgM signals, arrays were incubated with biotin-conjugated antihuman IgM secondary antibody (Jackson ImmunoResearch), diluted at 1:400 in PABB, followed by incubation with Qdot 800 streptavidin conjugate (Thermo Fisher Scientific) at 1:250 dilution. ALS was diluted 1:5 in PABB, and arrays were processed as described above for plasma samples. The arrays were air-dried by brief centrifugation, and IgG and IgA signals were examined with a ScanArray Express HT confocal laser scanner (PerkinElmer) at a wavelength of 670 nm for Alexa Fluor 647 and 570 nm for Cy3. IgM signals were detected with ArrayCam 400-S Microarray Imaging System (Grace Bio-Labs) for Q800. The array signal intensities were quantified using ProScanArray Express version 3.0.0.0016 (PerkinElmer).
Microarray Analysis
Quantified signals for each antigen corrected for local background signal were considered raw values. “No-DNA” negative controls consisted of IVTT reaction mix without the addition of plasmid template. Escherichia coli background signal from no DNA control spots was subtracted from raw values for each IVTT antigen on the array. Variance stabilizing normalization (VSN) analysis was performed using the R statistical environment (http://www.r-project.org). The VSN method implemented as part of the Bioconductor suite (www.bioconductor.org) was applied to the quantified array intensities. In addition to stabilizing variance, this corrected for nonspecific noise effects by finding maximum likelihood shifting and scaling parameters for each array such that control probe variance was minimized [22, 23]. Antigens were considered “reactive” if the mean reactivity among cholera patients was >2 standard deviations above the mean of the “no DNA” controls. Antigens were further classified as “differentially reactive” if there was differential signal intensity (≥1.5-fold change) in the following comparisons: (1) convalescence (day 7 and/or day 30) compared to acute phase plasma (day 2); or (2) ALS signal intensity of cholera patients at day 7 compared to age-, sex-, and ABO-matched healthy Bangladeshis. A Bayes regularized t test adapted from Cyber-T for protein arrays [24, 25] was applied on normalized values for calculating P values. A P value <.05 was considered significant.
Vibriocidal Antibody Assay
Vibriocidal assays were performed on plasma samples as previously described [10].
Enzyme-Linked Immunosorbent Assay
Vibrio cholerae O1 strain N16961 sialidase, flagellin C (FlaC), and flagellin D (FlaD) were overexpressed from pXT7 cloning vector in E. coli as recombinant polyhistidine proteins. The proteins were purified by affinity chromatography using HisPur Cobalt Spin Columns (Thermo Scientific Pierce) under denaturing conditions and refolded by dialysis into 25 mM Tris-hydrogen chloride pH 8.0 0.15M sodium chloride. Purity was assessed by polyacrylamide gel electrophoresis and Coomassie staining. Hemolysin A (HlyA) was provided by Dr K. Banerjee from the National Institute of Cholera and Enteric Diseases, Kolkata, India [26].
Microplates were coated with FlaC, FlaD, and sialidase at 250 ng/well and HlyA at 100 ng/well. Antigen-specific responses were detected by adding 100 µL of plasma (diluted 1:100, sialidase, FlaC, and FlaD; 1:200 HlyA); ALS (diluted 1:1), or BMEM ALS (undiluted) to the plate. Bound antibodies were detected with antihuman IgG and IgA conjugated with horseradish peroxidase (Jackson ImmunoResearch) at a 1:1000 dilution, and peroxidase activity was measured with the substrate 2,2-azinobis (ethylbenzthiazolinesulfonic acid) for plasma and o-phenylenediamine for ALS and BMEM ALS. To compare across plates, reading samples were divided by readings of an in-house pooled standard and multiplied by 100, and results were expressed as ELISA units. Differences within groups were assessed using the Wilcoxon matched-pairs signed rank sum test and between groups using a Mann-Whitney test. A P value <.05 was considered significant.
RESULTS
Identification of Immunogenic Cholera Antigens
A total of 608 V. cholerae O1 immunoreactive antigens were identified on the antigen array. A subset of these, consisting of 59 antigens, were considered differentially reactive when comparing samples collected in convalescence to acute-stage disease or healthy controls (Supplementary Table 1). In plasma, we identified 60 immunoreactive antigens: 22 for IgG, 41 for IgA, and 11 for IgM. Thirty-four were differentially reactive (13 for IgG, 28 for IgA, and 5 for IgM) with higher immunoreactivity (≥1.5-fold change) at convalescence (day 7 and/or day 30) compared to acute phase plasma (day 2) (Figure 1A and 1B; Supplementary Figure 1). Our top hits included known immunogenic antigens V. cholerae LPS, OSP, MP, CTB, TcpA, and HlyA (also known as V. cholerae cytolysin). In addition, among our top hits were phosphoenolpyruvate-protein phosphotransferase (PtsP), diaminobutyrate–2-oxoglutarate aminotransferase (VBIVibCho83274_1553), sialidase (nanH), outer membrane protein OmpV, flagellin B (FlaB), FlaC, FlaD, and hemolysin-related protein VBIVibCho83274_1804.
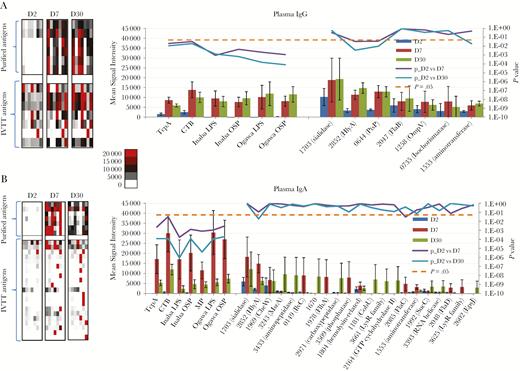
Differentially reactive cholera antigens identified in plasma for cholera patients at acute to convalescent phase of infection. Average immunoglobulin G (IgG) (A) and immunoglobulin A (IgA) (B) plasma signal intensities to differentially reactive antigens (fold change ≥1.5) for cholera patients (n = 7) at the acute (day 2) and convalescent phases (day 7 and day 30) of infection. On the left are heat maps reflecting the overall IgG and IgA reactivity for the purified antigens (top) and in vitro transcription/translation (IVTT) antigens (bottom). A color scale provides the reactivity intensity: White represents no immunoreactivity through to red representing strong immunoreactivity. On the right are representative histograms of the average signal intensities (y-axis) of each differentially reactive antigen (x-axis). The antigens are ordered based on average signal intensity, highest to lowest, with the purified antigens plotted on the left and IVTT antigens plotted on the right. Bayes regularized t test adapted from Cyber-T for protein arrays was applied on normalized values for calculating the P values plotted on the secondary y-axis. P < .05 was considered significant and is symbolized by a dotted line. Error bars indicate the standard error. Proteins are listed according to their PATRIC ID VBIVibCho83274_X.
During the mucosal immunoprofiling using ALS, we identified 578 immunoreactive antigens (16 for IgG, 64 for IgA, and 523 for IgM). Thirty-nine antigens were differentially reactive (14 for IgG, 25 for IgA, 18 for IgM) with higher immunoreactivity (≥1.5-fold change on comparison of cholera patients at day 7 compared to age-, sex-, and ABO-matched healthy Bangladeshis (Figure 2A and 2B; Supplementary Figure 1). One-third (n = 13) of these antigens were also differentially reactive in plasma, and our top hits in ALS included V. cholerae LPS, OSP, MP, CTB, TcpA, sialidase, HlyA, PtsP, diaminobutyrate–2-oxoglutarate aminotransferase, the A subunit of cholera toxin, FlaC, and FlaD.
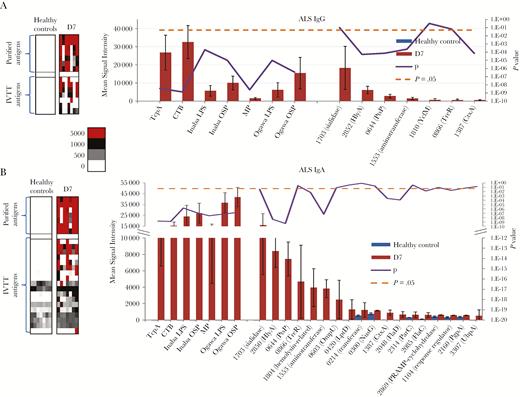
Differentially reactive cholera antigens identified in antibody-in-lymphocyte supernatant (ALS). Average immunoglobulin G (IgG) (A) and immunoglobulin A (IgA) (B) ALS signal intensities to differentially reactive antigens (fold change ≥1.5) for cholera patients (n = 7) at day 7 of infection compared to healthy Bangladeshi controls. On the left are heat maps reflecting the overall IgG and IgA reactivity for the purified antigens (top) and in vitro transcription/translation (IVTT) antigens (bottom). A colorized scale provides the reactivity intensity: White represents no immunoreactivity through to red representing strong immunoreactivity. On the right are representative histograms of the average signal intensities (y-axis) of each differentially reactive antigen (x-axis). The antigens are ordered based on average signal intensity, highest to lowest, with the purified antigens plotted on the left and IVTT antigens plotted on the right. A Bayes regularized t test adapted from Cyber-T for protein arrays was applied on normalized values for calculating the P values plotted on the secondary y-axis. P < .05 was considered significant and symbolized by a dotted line. Error bars indicate standard error. Proteins are listed according to their PATRIC ID VBIVibCho83274_X.
ELISA Validation of Antigens Identified by Microarray
To validate the responses identified using our microarray-based screening, we selected 4 reactive proteins showing a range of immunoreactivity in our microarray analysis to confirm via ELISA: HlyA, sialidase, FlaC, and FlaD. Cholera patients (n = 13) developed a significant plasma IgG and IgA response by day 7 of infection to each of the recombinant antigens, except for IgA reactivity with sialidase (Figure 3). In this cholera-endemic area, measurable plasma antibody responses to FlaC, FlaD, and sialidase were present in healthy individuals.

Anti–Vibrio cholerae plasma immunoglobulin G (IgG) and immunoglobulin A (IgA) responses. Enzyme-linked immunosorbent assay (ELISA) validation of 4 immunogenic proteins identified by the array. Anti-HlyA, sialidase, FlaC, and FlaD IgG and IgA plasma responses in cholera patients at the acute (day 2) and convalescent (day 7) phases of infection and in healthy Bangladeshis (HB). Differences within groups were assessed using the Wilcoxon matched-pairs signed rank test. P < .05 was considered significant.
Cholera patients (n = 10) also had higher IgG and IgA ALS immunoreactivity at day 7 compared to healthy Bangladeshis to HlyA and FlaD (Figure 4A and 4C). Mucosal ALS IgG immunoreactivity was significant for sialidase; however, in this small cohort, mucosal ALS IgA responses to sialidase and IgG and IgA responses to FlaC did not reach statistical significance (Figure 4B and 4D).
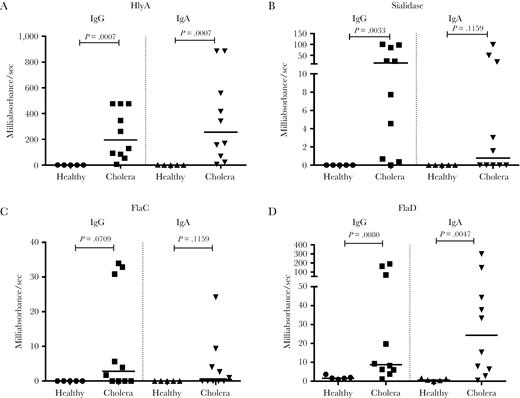
Anti–Vibrio cholerae mucosal antibody-in-lymphocyte supernatant immunoglobulin G (IgG) and immunoglobulin A (IgA) responses. Enzyme-linked immunosorbent assay validation of 4 immunogenic proteins identified by the array. Anti-HlyA, sialidase, FlaC, and FlaD IgG and IgA mucosal responses in cholera patients (n = 10) at day 7 and healthy Bangladeshis (n = 5). Differences between groups were assessed using Mann-Whitney test.
BMEM Responses
To determine whether HlyA, sialidase, FlaC, and FlaD generated long-lived antibody responses, we assessed BMEM ALS responses on day 2 and day 30 via ELISA in 12 cholera patients (Figure 5A–D). Significant BMEM ALS IgG responses were seen at day 30 to HlyA, sialidase, and FlaC. For BMEM ALS IgA responses, only HlyA and FlaC reached statistical significance; however, a trend toward statistical significance was observed for sialidase (P = .0640) and FlaD (P = .1514).
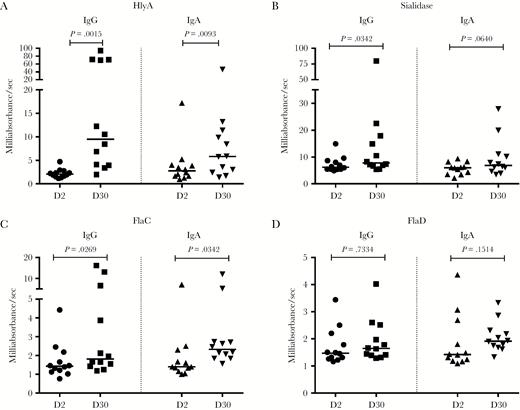
Anti–Vibrio cholerae immunoglobulin G (IgG) and immunoglobulin A (IgA) responses in memory B cell (BMEM) antibody-in-lymphocyte supernatant (ALS). Anti-HlyA, sialidase, FlaC, and FlaD IgG and IgA responses in BMEM ALS from cholera patients at day 2 and day 30. Differences within groups were assessed using the Wilcoxon matched-pairs signed rank test. P < .05 was considered significant.
DISCUSSION
Epidemiological studies have shown that natural infection with cholera results in long-lasting immunity [27, 28]; however, immune correlates of protection against cholera are poorly understood. Administration of currently available oral whole-cell vaccines are able to confer significant vibriocidal, anti-CTB, and anti-LPS/OSP serum responses; however, protective efficacy of vaccination is often short-lived and immune responses in children are often muted [4, 7, 11, 15, 29–31]. A more detailed understanding of immune responses elicited after natural infection could give insights into the mediators and markers of protection from cholera and could lead to improved vaccination strategies.
The goal of our study was to characterize the antigenic repertoire in patients following cholera. We focused this initial analysis on assessing immune responses in adult patients residing in a cholera-endemic area. We probed our arrays not only with plasma, but also with day 7 ALS, a surrogate marker of mucosal immune responses. Activated lymphocytes (ASCs) that are induced by pathogens or vaccines at the mucosal surface transiently migrate in the peripheral blood, peaking within 1 week of infection or vaccination. Many of these circulating activated lymphocytes express gut-homing markers and return to mucosal tissue; thus, these cells provide a glimpse of subsequent immunologic memory at the intestinal surface [32]. These cells can be isolated during their migration in the peripheral blood and evaluated for antigen-specific responses [32]. Alternatively, these cells can be cultured ex vivo without additional antigenic stimulation. During culturing, these already activated lymphocytes secrete antigen-specific antibodies into the culture supernatant (ALS), and this fluid can be used to assess antibody responses from these cells. We have previously demonstrated that ASC/ALS levels correlate well with each other [33].
Using our immunoscreening approach, we identified 59 differentially reactive cholera antigens (13 in both plasma and ALS). The most differentially immunoreactive included V. cholerae OSP, LPS, CTB, and TcpA. Plasma, mucosal, and BMEM responses to these antigens have previously been demonstrated in cholera patients, supporting the validity of our screening approach [10, 15, 34, 35]. We also identified several other antigens in our screening. These included HlyA, sialidase, PtsP, diaminobutyrate–2-oxoglutarate aminotransferase, and flagellins, which were hit multiple times across immunoglobulin isotype and compartment (mucosal and blood samples).
One of the antigens identified in our analysis is sialidase, also known as neuraminidase (nanH). It was recently identified by our group as the third most immunodominant antigen in V. cholerae after cholera toxin (CT) and OSP, based on a protein array immunoscreen of monoclonal antibodies generated from single-cell-sorted plasmablasts of cholera patients [20]. Sialidases, which have highly conserved essential catalytic residues, are widely distributed in nature and are virulence factors required for mucosal colonization in a wide variety of pathogens including Clostridium perfringens, Streptococcus pneumoniae, Salmonella Typhimurium, and influenza virus [36, 37]. The antigenically distinct sialidase of V. cholerae is present in all toxigenic strains and facilitates CT binding to host intestinal epithelial cells by converting cell surface polysialogangliosides to GM1 monogangliosides, the epithelial surface receptor for CT [37]. Our results demonstrate that cholera patients develop not only robust plasma and mucosal reactivity to sialidase, but also long-term BMEM to this antigen.
We also demonstrated robust plasma and mucosal immunoreactivity to HlyA (also known as V. cholerae cytolysin). HlyA is a secreted pore-forming toxin that is both cytotoxic and enterotoxic and has been implicated in V. cholerae virulence in rabbit ileal loop and infant mouse models of cholera infection [38–40]. Present in both toxigenic and nontoxigenic strains of cholera, HlyA has been implicated as the primary virulence factor in non-O1 and non-O139 strains that do not produce CT [38, 41]. We have previously demonstrated that cholera patients develop robust B-cell and T-cell responses to HlyA [21]; these plasma and mucosal findings were confirmed in our analysis. We here report that BMEM develop to HlyA following cholera.
Flagellum-based motility is involved in V. cholerae virulence [42]. The V. cholerae flagellum filament is composed of flagellins A–E [42]. Although FlaA is required for flagellar synthesis, FlaC and FlaD are the most abundant components of the flagella [42]. Purified flagellins are potent inducers of the innate immune response via Toll-like receptor 5; and we found induction of the humoral response with development of plasma, mucosal, and BMEM responses to FlaC and FlaD. Vibrio cholerae minimizes TLR activation and induction of the proinflammatory response by utilizing a stable sheathed flagellum during intestinal colonization [42]. However, this response is not completely abolished and it is the proinflammatory response to flagellins that has been associated with reactogenicity of cholera vaccine strains [43]. Although they may contribute to protective immunity, antiflagellar responses are not required [43] and, due to their reactogenicity, are not candidates for inclusion in a mucosal vaccine.
Two additional novel antigens identified in our screen are PtsP and diaminobutyrate–2-oxoglutarate aminotransferase. The phosphoenolpyruvate phosphotransferase system (PTS) is a multiprotein phosphotransfer cascade that is not only involved in transport of carbohydrates, but also involved in regulatory functions, including those involved in virulence, biofilm formation, and chemotaxis [44–46]. Recently, the carbohydrate PTS (EI, Hpr, and EIIAGlc) of V. cholerae has been found to control the expression of TcpA and CT, through regulation of the expression of tcpPH and aphAB that controls the expression of toxT [44]. PtsP (EINtr) is part of the nitrogen PTS that contains homologues to the sugar PTS: EINtr, NPr, and EIIANtr [45, 46]. This parallel system has no membrane components, so its function may largely pertain to regulation [45, 46]. We detected both significant systemic and mucosal humoral reactivity to PtsP, suggesting that it may be highly expressed in vivo and may play a role in utilizing alternative nutrient sources during infection or have an unidentified regulatory function in humans during V. cholerae infection. In other organisms, the nitrogen PTS system plays a role in phosphate starvation (in E. coli), intramacrophage survival through control of expression of Salmonella pathogenicity island 2 (in S. Typhimurium), and nitrogen metabolism (in Klebsiella pneumoniae), among other regulatory functions [46, 47]. Thus far in V. cholerae, it has been demonstrated that PtsP is not required for intestinal colonization [44].
Diaminobutyrate-2-oxoglutarate aminotransferase participates in glycine, serine, and threonine metabolism and is involved in an alternative polyamine biosynthetic pathway involved in biofilm formation in V. cholerae [48]. There is growing evidence that 2-oxoglutarate functions as a regulatory metabolite that controls the nitrogen PTS and regulates the synthesis of cAMP in E. coli [49]. This metabolite intersects the carbon and nitrogen metabolic pathways, and its intracellular levels fluctuate in response to the availability of carbon and nitrogen [49]. Further studies are required to determine what role this enzyme may play in virulence of V. cholerae.
While the primary goal of this analysis was to characterize the plasma and mucosal humoral immunoprofile of V. cholerae O1–infected adults, the duration of these responses was also of interest. Protection following cholera lasts for years; however, we have previously demonstrated that vibriocidal titers, along with serum acute effector antibody responses to V. cholerae, decline to baseline within 6–12 months of symptomatic cholera, suggesting that these responses are not the primary mediators of protection [19, 35]. We hypothesized that rapid anamnestic responses of BMEM mediate protection against cholera upon reexposure after natural infection, and have demonstrated that CTB and TcpA-specific IgG and IgA BMEM are detectable by day 30 and persist beyond 1 year, and OSP IgG and IgA BMEM are measurable beyond 6 months after cholera infection (the last time point we have thus far evaluated). In this study, we now demonstrate that BMEM responses to HlyA, sialidase, FlaC, and FlaD are induced after cholera infection. Further studies are required to evaluate whether immune responses to these antigens are associated with long-term protection against cholera.
This study has a number of limitations. Although we analyzed across antibody isotype and compartment (plasma and mucosal) for each participant in our immunoscreen, it involved a relatively small cohort of adult patients. Evaluation of responses in children and in vaccinees would also be of interest, in addition to an analysis of responses in adults in non-cholera-endemic areas. Also, our study is one of characterization and antigen identification. To assess potential mechanisms by which these immune responses may mediate protection against cholera will require additional evaluation. Despite these limitations, our study represents the first unbiased antibody-based immunoprofiling of immune responses against the V. cholerae proteome, and has identified immunogenic antigens that induce systemic, mucosal, and memory responses. Further analysis of these antigens and responses, and their potential role in contributing to protection against cholera, is required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We express our gratitude to the patients for participating in this study, and the field workers and research staff at the icddr,b for their support and effort in the sample collection and processing. We also acknowledge the support of Dr Banerjee (National Institute of Cholera and Enteric Diseases, Kolkata, India) for supplying the HlyA used in our confirmatory analysis.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was supported in parts by core grants to the icddr,b. We gratefully acknowledge the following donors: the government of the People’s Republic of Bangladesh; Global Affairs Canada; the Swedish International Development Cooperation Agency; and the UK Department of International Development. This work was also supported by grants from the National Institutes of Health (grant numbers R01 AI106878 to E. T. R. and F.Q.; R01 AI103055 and R01 AI099243 to J. B. H. and F. Q.; K08 AI089721 to R. C. C.; and K08 AI100923 to D. T. L.); the Fogarty International Center Training Grant in Vaccine Development and Public Health (grant number TW005572 to T. R. B.); the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program (award number 72424 to R. C. C.); the Harvard Medical School Office for Diversity Inclusion and Community Partnership Faculty Fellowship Award (to R. C. C.); and the Massachusetts General Hospital Department of Medicine Transformative Scholars Award (to R. C. C.).
Potential conflicts of interest. P. L. F. declares a financial interest in Antigen Discovery, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 65th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, Georgia, October 2016. Abstract 10.
aF. Q. and E. T. R. contributed equally to this work.
Correspondence: R. C. Charles, MD, Division of Infectious Diseases, Massachusetts General Hospital, Jackson 504, 55 Fruit St, Boston, MA 02114 ([email protected]).




