-
PDF
- Split View
-
Views
-
Cite
Cite
Nicolas A. Margot, Pamela Wong, Rima Kulkarni, Kirsten White, Danielle Porter, Michael E. Abram, Christian Callebaut, Michael D. Miller, Commonly Transmitted HIV-1 Drug Resistance Mutations in Reverse-Transcriptase and Protease in Antiretroviral Treatment–Naive Patients and Response to Regimens Containing Tenofovir Disoproxil Fumarate or Tenofovir Alafenamide, The Journal of Infectious Diseases, Volume 215, Issue 6, 15 March 2017, Pages 920–927, https://doi.org/10.1093/infdis/jix015
Close - Share Icon Share
Abstract
The presence of transmitted drug resistance mutations (TDRMs) in antiretroviral treatment (ART)–naive patients can adversely affect the outcome of ART.
Resistance testing was conducted in 6704 ART-naive subjects predominantly from the United States and Europe in 9 clinical studies conducted by Gilead Sciences from 2000 to 2013.
The presence of TDRMs increased during this period (from 5.2% to 11.4%), primarily driven by an increase in nonnucleoside reverse-transcriptase (RT) inhibitor (NNRTI) resistance mutations (from 0.3% to 7.1%), particularly K103N/S (increase from 0.3% to 5.3%). Nucleoside/nucleotide RT inhibitor mutations were found in 3.1% of patients. Only 1 patient had K65R (0.01%) and 7 had M184V/I (0.1%), despite high use of tenofovir disoproxil fumarate (TDF), emtricitabine, and lamivudine and potential transmission of resistance to these drugs. At least 1 thymidine-analogue mutations was present in 2.7% of patients with 0.07% harboring T215Y/F and 2.7% harboring T215 revertant mutations (T215rev). Patients with the combination of M41L + L210W + T215rev showed full human immunodeficiency virus RNA suppression while receiving a TDF- or tenofovir alafenamide–containing regimen.
There was an overall increase of TDRMs among patients enrolling in clinical trials from 2000 through 2013, driven primarily by an increase in NNRTI resistance. However, the presence of common TDRMs, including thymidine-analogue mutations/T215rev, showed no impact on response to TDF- or tenofovir alafenamide–containing regimens.
The research and discovery of chemical entities targeting human immunodeficiency virus (HIV) replication have provided the medical community with an arsenal of drugs to combat the advance of the AIDS epidemic [1]. However, concurrent to the use of antiretroviral (ARV) therapies with suboptimal viral suppression and owing to the error-prone replication of HIV reverse-transcriptase (RT), drug-resistant strains of HIV have emerged [2–4]. A direct consequence of the emergence of drug resistance is that transmission of drug-resistant viruses has the potential to fuel the epidemic with viruses harboring preexisting drug resistance. Indeed, the risk of transmission of drug-resistant viruses in ART-naive patients has been documented as far back as 1992 [5], and more recent reports have estimated that close to 10% of ART-naive patients harbor HIV with transmitted drug resistance mutations (TDRMs) [6–9].
The extent to which TDRMs persist as a major species in the absence of selective pressure from drug treatment after transmission depends mostly on the fitness costs associated with the mutations and the time that has elapsed since the transmission event. For example, the M184V/I RT substitution conferring resistance to emtricitabine (FTC) and lamivudine, which is known to impart a replication defect to the virus [10, 11], was found to revert to wild type more readily than mutations with little or no fitness defects such as the M41L and K103N RT substitutions, or the protease substitution L90M [10, 12–14]. Similarly, the T215Y/F RT thymidine-analogue mutation (TAM) was found to evolve away from the mutant residues at a comparable rate as M184V/I [14], but because the mutations that confer T215Y or T215F are the product of 2-nucleotide changes from wild type, resistant mutants do not typically revert to wild type but rather to nonresistant mutants with a 1-nucleotide change from the resistant mutants, such as T215C/D/S/E, termed T215 revertants (T215rev)[15].
It was recognized in the late 1990s that testing patients for the presence of ARV resistance mutations before initiation of ARV treatment (ART) would be key to selecting the most appropriate treatment regimen and thus ensuring the best treatment outcomes for HIV-infected patients [16]. As a consequence, resistance testing by population-based viral sequencing has become routine practice in many regions [17–19], using either local methods or commercial assays [20, 21]. The interpretation of the sequencing data is fairly straightforward in the presence of signature ARV resistance mutations—for example, the presence of K103N indicates resistance to the nonnucleoside RT inhibitor (NNRTI) efavirenz, and the presence of K65R, resistance to the nucleotide RT inhibitor (NRTI) tenofovir disoproxil fumarate (TDF). However, complex mixtures of mutations may be harder to interpret. Thus, genotypic algorithms for the interpretation of the mutation data have been developed, including proprietary methods (linked to commercial assays) and open-source methods (Stanford HIV database, Rega Institute, Agence Nationale de Recherche sur le SIDA). For TDF, a key aspect of these resistance algorithms involves the presence of ≥3 TAMs, notably M41L + L210W + T215Y/F. However, T215rev mutations are often included along with T215Y/F, even though these substitutions have not been shown to confer resistance to TDF in vitro.
In the present study, we performed a retrospective analysis of viral genotypes obtained after resistance testing of baseline plasma samples from a large cohort of ART-naive patients before enrollment in clinical studies conducted at Gilead Sciences from 2000 to 2013. We looked at the overall prevalence of TDRMs at baseline, with a special focus on the prevalence of TAMs and T215rev mutations, and we studied the response to TDF- or tenofovir alafenamide (TAF) –containing regimens in the presence of TDRMs.
MATERIALS AND METHODS
Study Subjects
The analysis included ART-treatment-naive patients with pretreatment genotypic data from 9 TDF- and/or TAF-based phase 3 studies initiated at Gilead Sciences between 2000 and 2013 (Table 1) [22–29]. All 9 studies contained an active control treatment group, which was provided as either open-label treatment or double-blind, double-dummy treatment. Studies were conducted at multiple sites across multiple countries (except studies 236-0102 and 216-0130, which were in the United States only). Patients’ informed consent for the use of biological samples had been obtained at initiation of the studies. In total, the data set comprised pretreatment genotypic data from 6704 patients, of whom 5990 were enrolled in the studies.
| Study . | Program . | Enrollment Year . | HIV-1 RNA, Log10 Copies/mLa . | CD4 Cell Count, Cells/ mm3a . | Geographic Origin . | Patients, No.b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Screened . | Enrolled . | TDF Regimen . | TAF Regimen . | ||||||
| 99-903 | TDF | 2000 | 4.91 | 279 | US, Europe, SA | 598 | 598 | 299 | … |
| 01-934 | TDF + FTC + EFV | 2003 | 5.01 | 237 | US, Europe | 501 | 479 | 240 | … |
| 236-0102 | E/COBI/FTC/TDF | 2010 | 4.76 | 380 | US | 799 | 700 | 700 | … |
| 236-0103 | E/COBI/FTC/TDF | 2010 | 4.87 | 357 | NA, Europe, Australia, Mexico,Thailand | 853 | 708 | 708 | … |
| 216-0114 | COBI | 2010 | 4.81 | 343 | NA, Europe, Australia, Brazil, Mexico, DR, Thailand | 772 | 692 | 692 | … |
| 264-0110 | RPV/FTC/TDF | 2011 | 4.80 | 374 | NA, Europe, Australia | 905 | 786 | 786 | … |
| 216-0130 | DRV + COBI | 2011 | 4.80 | 370 | US | 336 | 295 | 294 | … |
| 292-0104 | E/COBI/FTC/TAF | 2013 | 4.61 | 404 | NA, Europe, Australia, Japan, Thailand | 972 | 866 | 432 | 434 |
| 292-0111 | E/COBI/FTC/TAF | 2013 | 4.55 | 406 | NA, Europe, Mexico, DR | 968 | 866 | 435 | 431 |
| Total | 6704 | 5990 | 4586 | 865 | |||||
| Study . | Program . | Enrollment Year . | HIV-1 RNA, Log10 Copies/mLa . | CD4 Cell Count, Cells/ mm3a . | Geographic Origin . | Patients, No.b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Screened . | Enrolled . | TDF Regimen . | TAF Regimen . | ||||||
| 99-903 | TDF | 2000 | 4.91 | 279 | US, Europe, SA | 598 | 598 | 299 | … |
| 01-934 | TDF + FTC + EFV | 2003 | 5.01 | 237 | US, Europe | 501 | 479 | 240 | … |
| 236-0102 | E/COBI/FTC/TDF | 2010 | 4.76 | 380 | US | 799 | 700 | 700 | … |
| 236-0103 | E/COBI/FTC/TDF | 2010 | 4.87 | 357 | NA, Europe, Australia, Mexico,Thailand | 853 | 708 | 708 | … |
| 216-0114 | COBI | 2010 | 4.81 | 343 | NA, Europe, Australia, Brazil, Mexico, DR, Thailand | 772 | 692 | 692 | … |
| 264-0110 | RPV/FTC/TDF | 2011 | 4.80 | 374 | NA, Europe, Australia | 905 | 786 | 786 | … |
| 216-0130 | DRV + COBI | 2011 | 4.80 | 370 | US | 336 | 295 | 294 | … |
| 292-0104 | E/COBI/FTC/TAF | 2013 | 4.61 | 404 | NA, Europe, Australia, Japan, Thailand | 972 | 866 | 432 | 434 |
| 292-0111 | E/COBI/FTC/TAF | 2013 | 4.55 | 406 | NA, Europe, Mexico, DR | 968 | 866 | 435 | 431 |
| Total | 6704 | 5990 | 4586 | 865 | |||||
Abbreviations: COBI, cobicistat; DR, Dominican Republic; DRV, darunavir; E, elvitegravir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; NA, North America (United States and Canada); RPV, rilpivirine; SA, South America; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; US, United States (may include sites in Puerto Rico).
aMedian pretreatment (baseline) values.
bPatients with available genotypic data.
| Study . | Program . | Enrollment Year . | HIV-1 RNA, Log10 Copies/mLa . | CD4 Cell Count, Cells/ mm3a . | Geographic Origin . | Patients, No.b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Screened . | Enrolled . | TDF Regimen . | TAF Regimen . | ||||||
| 99-903 | TDF | 2000 | 4.91 | 279 | US, Europe, SA | 598 | 598 | 299 | … |
| 01-934 | TDF + FTC + EFV | 2003 | 5.01 | 237 | US, Europe | 501 | 479 | 240 | … |
| 236-0102 | E/COBI/FTC/TDF | 2010 | 4.76 | 380 | US | 799 | 700 | 700 | … |
| 236-0103 | E/COBI/FTC/TDF | 2010 | 4.87 | 357 | NA, Europe, Australia, Mexico,Thailand | 853 | 708 | 708 | … |
| 216-0114 | COBI | 2010 | 4.81 | 343 | NA, Europe, Australia, Brazil, Mexico, DR, Thailand | 772 | 692 | 692 | … |
| 264-0110 | RPV/FTC/TDF | 2011 | 4.80 | 374 | NA, Europe, Australia | 905 | 786 | 786 | … |
| 216-0130 | DRV + COBI | 2011 | 4.80 | 370 | US | 336 | 295 | 294 | … |
| 292-0104 | E/COBI/FTC/TAF | 2013 | 4.61 | 404 | NA, Europe, Australia, Japan, Thailand | 972 | 866 | 432 | 434 |
| 292-0111 | E/COBI/FTC/TAF | 2013 | 4.55 | 406 | NA, Europe, Mexico, DR | 968 | 866 | 435 | 431 |
| Total | 6704 | 5990 | 4586 | 865 | |||||
| Study . | Program . | Enrollment Year . | HIV-1 RNA, Log10 Copies/mLa . | CD4 Cell Count, Cells/ mm3a . | Geographic Origin . | Patients, No.b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Screened . | Enrolled . | TDF Regimen . | TAF Regimen . | ||||||
| 99-903 | TDF | 2000 | 4.91 | 279 | US, Europe, SA | 598 | 598 | 299 | … |
| 01-934 | TDF + FTC + EFV | 2003 | 5.01 | 237 | US, Europe | 501 | 479 | 240 | … |
| 236-0102 | E/COBI/FTC/TDF | 2010 | 4.76 | 380 | US | 799 | 700 | 700 | … |
| 236-0103 | E/COBI/FTC/TDF | 2010 | 4.87 | 357 | NA, Europe, Australia, Mexico,Thailand | 853 | 708 | 708 | … |
| 216-0114 | COBI | 2010 | 4.81 | 343 | NA, Europe, Australia, Brazil, Mexico, DR, Thailand | 772 | 692 | 692 | … |
| 264-0110 | RPV/FTC/TDF | 2011 | 4.80 | 374 | NA, Europe, Australia | 905 | 786 | 786 | … |
| 216-0130 | DRV + COBI | 2011 | 4.80 | 370 | US | 336 | 295 | 294 | … |
| 292-0104 | E/COBI/FTC/TAF | 2013 | 4.61 | 404 | NA, Europe, Australia, Japan, Thailand | 972 | 866 | 432 | 434 |
| 292-0111 | E/COBI/FTC/TAF | 2013 | 4.55 | 406 | NA, Europe, Mexico, DR | 968 | 866 | 435 | 431 |
| Total | 6704 | 5990 | 4586 | 865 | |||||
Abbreviations: COBI, cobicistat; DR, Dominican Republic; DRV, darunavir; E, elvitegravir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; NA, North America (United States and Canada); RPV, rilpivirine; SA, South America; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; US, United States (may include sites in Puerto Rico).
aMedian pretreatment (baseline) values.
bPatients with available genotypic data.
Genotypic Data
Patients’ HIV-1 RNA was extracted from plasma samples and amplified by RT polymerase chain reaction, as described elsewhere [20, 21, 30]. Population sequencing of the RT and protease segments of HIV-1 pol was conducted at either Virco Laboratories, Monogram Biosciences, or Gilead Sciences. Sequences were compared with the HIV-1NL4-3 reference sequence and amino acid changes from reference at resistance residues were tabulated. Amino acid mixtures of mutant and wild type were counted as mutant.
Definition of Resistance
The list of resistance-associated mutations used in these analyses was identical to the 2009 update of the list published by the World Health Organization [31], except for position RT T215, where T215Y/F and T215rev (any substitution other than Y or F at position RT T215) were tabulated separately. NRTI resistance (NRTI-R)–associated mutations were M41L, K65R, D67N/G/E, T69 insertions, T69D, K70R/E, L74V/I, V75M/T/A/S, F77L, Y115F, F116Y, Q151M, M184V/I, L210W, T215Y/F, and K219Q/E/N/R in RT. NNRTI resistance (NNRTI-R)–associated mutations were L100I, K101E/P, K103N/S, V106A/M, V179F, Y181C/I/V, Y188L/C/H, G190S/A/E, P225H, and M230L in RT. Protease inhibitor (PI) primary resistance- associated mutations were L23I, L24I, D30N, V32I, M46I/L, I47V/A, G48V/M, I50L/V, I54M/L/V/A/T/S, G73S/T/C/A, L76V, V82A/F/T/S/L/C/M, N83D, I84V/A/C, I85V, N88D/S, and L90M in protease.
Treatment Response and Statistical Analyses
Response to TDF- or TAF-based treatment at week 48 was calculated using Food and Drug Administration (FDA) algorithms, as originally defined in the clinical study protocols [32]. Earlier studies (studies 99-903 and 01-934) used the FDA time to loss of virologic response (TLOVR) algorithm, and later studies (all others) used the FDA snapshot analysis algorithm. The resistance trend analysis over time was conducted using the Cochran-Armitage trend test. The statistical analysis of treatment response at week 48 per baseline virologic category and all other comparisons were conducted using the Fisher exact test.
RESULTS
Data Set Characteristics
We investigated the prevalence of transmitted RT and protease resistance mutations in HIV-infected ART-naive patients who were enrolled or screened in 9 Gilead clinical studies between 2000 and 2013 (Table 1). The majority of patients who were screened in the course of the various studies (screened data set; n = 6704) fit the study criteria and were eventually enrolled in the studies (enrolled data set; n = 5990). Within the enrolled data set, 4586 patients received a TDF-containing regimen and 865 received a TAF-containing regimen. HIV-1 subtype B was the most prevalent subtype (89.9% of screened patients; 6026 of 6704), followed by subtypes AE (3.8%; 255 of 6704), C (1.4%; 91 of 6704), and AG (1.3%; 87 of 6704). The predominance of HIV-1 subtype B was consistent with the geographic origin of the patients, who came mostly from North America or Western Europe. The median age of enrolled patients ranged from 34 to 38 years across all 9 studies.
Prevalence of Pretreatment Genotypic Resistance
The overall prevalence of any genotypic resistance across all studies was 11.2% in the screened data set (Table 2). Single–drug class resistance was found in 9.9% of patients, dual–drug class resistance in 1.1%, and triple–drug class resistance in 0.3%. Resistance to the NNRTI class was the most prevalent (6.0%), with 4.5% of screened patients harboring K103N/S substitutions in RT. The prevalence of NNRTI-R was significantly higher among US than among European patients (8.0% vs 2.1%, respectively; P < .001), with K103N/S found in 6.1% of US patients compared with 1.4% of European patients (P < .001).
| Drug Resistance Mutation . | Patients, No. (%) . | |||
|---|---|---|---|---|
| Screened (Total) (n = 6704) . | Screened (US Only) (n = 4414) . | Screened (Europe Only) (n = 1191) . | Enrolled (n = 5990) . | |
| Any resistance | 752 (11.2) | 607 (13.8) | 76 (6.4) | 604 (10.1) |
| NNRTI associateda | 401 (6.0) | 352 (8.0) | 25 (2.1) | 301 (5.0) |
| K103N/S | 302 (4.5) | 270 (6.1) | 17 (1.4) | 230 (3.8) |
| NRTI associatedb | 205 (3.1) | 151 (3.4) | 27 (2.3) | 165 (2.8) |
| K65R | 1 (0.01) | 1 (0.02) | 0 | 0 |
| K70E | 0 | 0 | 0 | 0 |
| M184I/V | 7 (0.1) | 4 (0.1) | 2 (0.2) | 3 (0.1) |
| TAMs c | 182 (2.7) | 134 (3.0) | 25 (2.1) | 145 (2.4) |
| M41L | 99 (1.5) | 78 (1.8) | 9 (0.8) | 77 (1.3) |
| D67N | 39 (0.6) | 24 (0.5) | 9 (0.8) | 28 (0.5) |
| K70R | 10 (0.1) | 7 (0.2) | 1 (0.1) | 8 (0.1) |
| L210W | 32 (0.5) | 25 (0.6) | 4 (0.3) | 21 (0.4) |
| T215Y/F | 5 (0.07) | 2 (0.05) | 1 (0.08) | 0 |
| K219E/N/Q/R | 59 (0.9) | 42 (1.0) | 10 (0.8) | 44 (0.7) |
| T215revd | 183 (2.7) | 138 (3.1) | 25 (2.1) | 151 (2.5) |
| PI associatede | 159 (2.4) | 127 (2.9) | 14 (1.2) | 124 (2.1) |
| M46I/L | 62 (0.9) | 49 (1.1) | 5 (0.4) | 46 (0.8) |
| L90M | 56 (0.8) | 48 (1.1) | 4 (0.3) | 43 (0.7) |
| Drug Resistance Mutation . | Patients, No. (%) . | |||
|---|---|---|---|---|
| Screened (Total) (n = 6704) . | Screened (US Only) (n = 4414) . | Screened (Europe Only) (n = 1191) . | Enrolled (n = 5990) . | |
| Any resistance | 752 (11.2) | 607 (13.8) | 76 (6.4) | 604 (10.1) |
| NNRTI associateda | 401 (6.0) | 352 (8.0) | 25 (2.1) | 301 (5.0) |
| K103N/S | 302 (4.5) | 270 (6.1) | 17 (1.4) | 230 (3.8) |
| NRTI associatedb | 205 (3.1) | 151 (3.4) | 27 (2.3) | 165 (2.8) |
| K65R | 1 (0.01) | 1 (0.02) | 0 | 0 |
| K70E | 0 | 0 | 0 | 0 |
| M184I/V | 7 (0.1) | 4 (0.1) | 2 (0.2) | 3 (0.1) |
| TAMs c | 182 (2.7) | 134 (3.0) | 25 (2.1) | 145 (2.4) |
| M41L | 99 (1.5) | 78 (1.8) | 9 (0.8) | 77 (1.3) |
| D67N | 39 (0.6) | 24 (0.5) | 9 (0.8) | 28 (0.5) |
| K70R | 10 (0.1) | 7 (0.2) | 1 (0.1) | 8 (0.1) |
| L210W | 32 (0.5) | 25 (0.6) | 4 (0.3) | 21 (0.4) |
| T215Y/F | 5 (0.07) | 2 (0.05) | 1 (0.08) | 0 |
| K219E/N/Q/R | 59 (0.9) | 42 (1.0) | 10 (0.8) | 44 (0.7) |
| T215revd | 183 (2.7) | 138 (3.1) | 25 (2.1) | 151 (2.5) |
| PI associatede | 159 (2.4) | 127 (2.9) | 14 (1.2) | 124 (2.1) |
| M46I/L | 62 (0.9) | 49 (1.1) | 5 (0.4) | 46 (0.8) |
| L90M | 56 (0.8) | 48 (1.1) | 4 (0.3) | 43 (0.7) |
Abbreviations: NNRTI, nonnucleoside reverse-transcriptase (RT) inhibitor; NRTI, nucleoside/nucleotide RT inhibitor; PI, protease inhibitor; T215rev, T215 revertants; TAMs, thymidine-analogue mutations.
aNNRTI resistance mutations include L100I, K101E/P, K103N/S, V106M/A, V179F, Y181C/I/V, Y188C/H/L, G190A/E/S, P225H, and M230L in RT (World Health Organization 2009).
bNRTI resistance mutations include M41L, K65R, D67N/G/E, T69 insertion, T69D, K70E/R, L74V/I, V75M/T/A/S, F77L, Y115F, F116Y, Q151M, M184V/I, L210W, T215Y/F, and K219E/Q/N/R in RT (WHO 2009).
cTAMs include M41L, D67N, K70R, L210W, T215Y/F, and K219E/Q/N/R in RT.
dRT T215rev mutations observed were T215A/C/D/E/G/H/I/L/N/P/Q/R/S/V.
ePrimary PI resistance mutations include L23I, L24I, D30N, V32I, M46I/L, I47V/A, G48V/M, I50V/L, F53L/Y, I54A/V/T/S/M/L, G73S/T/C/A, L76V, V82A/F/L/S/T/C/M, N83D, I84V/A/C, I85V, N88S/D, and L90M in protease (World Health Organization 2009).
| Drug Resistance Mutation . | Patients, No. (%) . | |||
|---|---|---|---|---|
| Screened (Total) (n = 6704) . | Screened (US Only) (n = 4414) . | Screened (Europe Only) (n = 1191) . | Enrolled (n = 5990) . | |
| Any resistance | 752 (11.2) | 607 (13.8) | 76 (6.4) | 604 (10.1) |
| NNRTI associateda | 401 (6.0) | 352 (8.0) | 25 (2.1) | 301 (5.0) |
| K103N/S | 302 (4.5) | 270 (6.1) | 17 (1.4) | 230 (3.8) |
| NRTI associatedb | 205 (3.1) | 151 (3.4) | 27 (2.3) | 165 (2.8) |
| K65R | 1 (0.01) | 1 (0.02) | 0 | 0 |
| K70E | 0 | 0 | 0 | 0 |
| M184I/V | 7 (0.1) | 4 (0.1) | 2 (0.2) | 3 (0.1) |
| TAMs c | 182 (2.7) | 134 (3.0) | 25 (2.1) | 145 (2.4) |
| M41L | 99 (1.5) | 78 (1.8) | 9 (0.8) | 77 (1.3) |
| D67N | 39 (0.6) | 24 (0.5) | 9 (0.8) | 28 (0.5) |
| K70R | 10 (0.1) | 7 (0.2) | 1 (0.1) | 8 (0.1) |
| L210W | 32 (0.5) | 25 (0.6) | 4 (0.3) | 21 (0.4) |
| T215Y/F | 5 (0.07) | 2 (0.05) | 1 (0.08) | 0 |
| K219E/N/Q/R | 59 (0.9) | 42 (1.0) | 10 (0.8) | 44 (0.7) |
| T215revd | 183 (2.7) | 138 (3.1) | 25 (2.1) | 151 (2.5) |
| PI associatede | 159 (2.4) | 127 (2.9) | 14 (1.2) | 124 (2.1) |
| M46I/L | 62 (0.9) | 49 (1.1) | 5 (0.4) | 46 (0.8) |
| L90M | 56 (0.8) | 48 (1.1) | 4 (0.3) | 43 (0.7) |
| Drug Resistance Mutation . | Patients, No. (%) . | |||
|---|---|---|---|---|
| Screened (Total) (n = 6704) . | Screened (US Only) (n = 4414) . | Screened (Europe Only) (n = 1191) . | Enrolled (n = 5990) . | |
| Any resistance | 752 (11.2) | 607 (13.8) | 76 (6.4) | 604 (10.1) |
| NNRTI associateda | 401 (6.0) | 352 (8.0) | 25 (2.1) | 301 (5.0) |
| K103N/S | 302 (4.5) | 270 (6.1) | 17 (1.4) | 230 (3.8) |
| NRTI associatedb | 205 (3.1) | 151 (3.4) | 27 (2.3) | 165 (2.8) |
| K65R | 1 (0.01) | 1 (0.02) | 0 | 0 |
| K70E | 0 | 0 | 0 | 0 |
| M184I/V | 7 (0.1) | 4 (0.1) | 2 (0.2) | 3 (0.1) |
| TAMs c | 182 (2.7) | 134 (3.0) | 25 (2.1) | 145 (2.4) |
| M41L | 99 (1.5) | 78 (1.8) | 9 (0.8) | 77 (1.3) |
| D67N | 39 (0.6) | 24 (0.5) | 9 (0.8) | 28 (0.5) |
| K70R | 10 (0.1) | 7 (0.2) | 1 (0.1) | 8 (0.1) |
| L210W | 32 (0.5) | 25 (0.6) | 4 (0.3) | 21 (0.4) |
| T215Y/F | 5 (0.07) | 2 (0.05) | 1 (0.08) | 0 |
| K219E/N/Q/R | 59 (0.9) | 42 (1.0) | 10 (0.8) | 44 (0.7) |
| T215revd | 183 (2.7) | 138 (3.1) | 25 (2.1) | 151 (2.5) |
| PI associatede | 159 (2.4) | 127 (2.9) | 14 (1.2) | 124 (2.1) |
| M46I/L | 62 (0.9) | 49 (1.1) | 5 (0.4) | 46 (0.8) |
| L90M | 56 (0.8) | 48 (1.1) | 4 (0.3) | 43 (0.7) |
Abbreviations: NNRTI, nonnucleoside reverse-transcriptase (RT) inhibitor; NRTI, nucleoside/nucleotide RT inhibitor; PI, protease inhibitor; T215rev, T215 revertants; TAMs, thymidine-analogue mutations.
aNNRTI resistance mutations include L100I, K101E/P, K103N/S, V106M/A, V179F, Y181C/I/V, Y188C/H/L, G190A/E/S, P225H, and M230L in RT (World Health Organization 2009).
bNRTI resistance mutations include M41L, K65R, D67N/G/E, T69 insertion, T69D, K70E/R, L74V/I, V75M/T/A/S, F77L, Y115F, F116Y, Q151M, M184V/I, L210W, T215Y/F, and K219E/Q/N/R in RT (WHO 2009).
cTAMs include M41L, D67N, K70R, L210W, T215Y/F, and K219E/Q/N/R in RT.
dRT T215rev mutations observed were T215A/C/D/E/G/H/I/L/N/P/Q/R/S/V.
ePrimary PI resistance mutations include L23I, L24I, D30N, V32I, M46I/L, I47V/A, G48V/M, I50V/L, F53L/Y, I54A/V/T/S/M/L, G73S/T/C/A, L76V, V82A/F/L/S/T/C/M, N83D, I84V/A/C, I85V, N88S/D, and L90M in protease (World Health Organization 2009).
NRTI-R was detected in 3.1% of screened patients, with 182 patients (2.7% of screened patients) harboring ≥1 TAM, and 183 (2.7% of total) harboring T215rev mutations. T215rev mutations were enriched in patients with HIV-1 subtype B; 97.8% of subjects (179 of 183) with T215rev had HIV-1 subtype B, compared with 89.9% of subjects overall (6026 of 6704; P < .001). The most frequent T215rev mutations were T215S (n = 67), T215D (n = 48), T215E (n = 28), T215L (n = 21), and T215C (n = 20); others included T215I/A/V/H/N/P/G/Q/R. Five patients had ≥3 TAMs, including 3 with M41L + L210W + T215Y. Only 1 patient (0.1%) carried the K65R substitution (1 of 6704), and 7 (0.1%) carried the M184V/I substitutions. Primary PI resistance (PI-R) was detected in 3% of patients, with M46I/L (0.9%) and L90M (0.8%) the most prevalent. The proportions of patients harboring NRTI-R or PI-R were similar in US and European patients.
There was a statistically significant increase in the presence of any resistance mutations (from 5.2% to 11.4%), NNRTI-R (from 0.3% to 7.1%), and/or K103N/S (from 0.3% to 5.3%) over time from 2000 to 2013 (Figure 1A; P < .001). The presence of PI-R also showed an increase (from 0.7% to 2.4%) from 2000 to 2013 (P = .02). The presence of NRTI-R, TAMs, and T215rev remained essentially unchanged during the period (from 4.2% to 3.6%, 2.8% to 2.3%, and 2.5% to 1.8%, respectively, from 2000 to 2013). The trend analysis for US patients (Figure 1B; n = 4414) showed an increase over time in the any resistance category (from 8.2% to 14.6%;), the NNRTI-R category (from 0.7% to 9.8%), and the K103N/S category (from 0.7% to 7.5% (all P < .001). For European patients (Figure 1C; n = 1191), the trend analysis showed no statistically significant changes over time across any of the resistance categories.
![Proportion of patients with preexisting resistance mutations over time. Prospective subjects were sequenced at screening (A, all patients [n = 6704]; B, US patients [n = 4414]; C, European patients [n = 1191]) before study entry (Virco, Monogram, or Gilead) from the start of human immunodeficiency virus type 1 (HIV-1) protease through HIV-1 reverse-transcriptase (RT) up to position 305. The mutations (World Health Organization 2009) included in each category are listed in Materials and Methods, unless specified. *Statistically significant increase for specific mutation categories over time (P ≤ .01, Cochran-Armitage trend test). A, B, P <. 001 for any resistance, nonnucleoside RT inhibitor resistance (NNRTI-R), and K103N/S. Abbreviations: NRTI-R, nucleoside/nucleotide RT inhibitor resistance; PI-R protease inhibitor resistance; TAMs, thymidine-analogue mutations. The total number of sequences is indicated at the bottom of each graph.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/215/6/10.1093_infdis_jix015/3/m_jix01501.jpeg?Expires=1748038352&Signature=Y4TFXEjMD0FKc9sWSj3SI2yABIgu2ovzP06S0wAkLU6ZtJryZXPp2lu6VLca4hnPPb~6FymZ1TFo2UiXnM-6Htg7nuoxAiRxYYiICH8ITiALz~hxom99anBFO-JvCqLk3kGITZQQtdBo84FokNtgrQbQV3BDyRr7f2npcdrVUCRRxsIGBd~WWTIG~UouHghoFaUEQZhkkQgXXj~SmSBNGjJvjddIztqxgdssIV~RmUPcIhhHnXVZ8ruy7M7bql76omkN3H~vMFV-v9Zo~r0Y5Ypj-Gn2v5GaK0g5MCz6c1gmPu9Dbz7OSp8-htY~wD9J5ylq91eeYzADoCKiY1mwfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Proportion of patients with preexisting resistance mutations over time. Prospective subjects were sequenced at screening (A, all patients [n = 6704]; B, US patients [n = 4414]; C, European patients [n = 1191]) before study entry (Virco, Monogram, or Gilead) from the start of human immunodeficiency virus type 1 (HIV-1) protease through HIV-1 reverse-transcriptase (RT) up to position 305. The mutations (World Health Organization 2009) included in each category are listed in Materials and Methods, unless specified. *Statistically significant increase for specific mutation categories over time (P ≤ .01, Cochran-Armitage trend test). A, B, P <. 001 for any resistance, nonnucleoside RT inhibitor resistance (NNRTI-R), and K103N/S. Abbreviations: NRTI-R, nucleoside/nucleotide RT inhibitor resistance; PI-R protease inhibitor resistance; TAMs, thymidine-analogue mutations. The total number of sequences is indicated at the bottom of each graph.
Among the 6704 ART-naive patients who were screened to potentially receive a regimen containing FTC + TDF or FTC + TAF, only 8 (0.1%) were excluded from enrollment per protocol owing to the presence of TDRMs in their screening genotype and potential TDF, TAF, or FTC resistance (2 with M184V/I, 2 with M184V/I + ≥3 TAMs, 3 with ≥3 TAMs, and 1 with K65R). In contrast, of the 2803 of 6704 patients who were screened to potentially receive an NNRTI (studies 903, 934, 236-0102, and 264-0110), 78 patients (2.8%) were excluded from enrollment owing to the presence of NNRTI-R in the screening genotype. Nonenrollment for the remainder of patients was due to other protocol-based reasons.
Distribution of TAMs at Baseline
Because the presence of ≥3 TAMs has been documented to affect treatment response to TDF in ART-experienced patients with incomplete viral suppression [33], we studied the distribution of TAMs in the ART-naive patients enrolled in the studies described herein (Figure 2). Using the strict definition of TAMs (M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E/N/R in RT), 112 patients had 1 TAM and 33 had 2 TAMs at enrollment (the presence of ≥3 TAMs was an exclusion criterion) (Figure 2). Among patients with 2 TAMs, most carried the D67N + K219Q combination (20 of 33; 61%) (Supplementary Figure 1A), followed by the M41L + L210W combination (10 of 33. 30%). Among patients with 1 TAM, M41L was the most frequent (65 of 112; 58%), followed by K219Q/E/N/R (24 of 112; 21%).
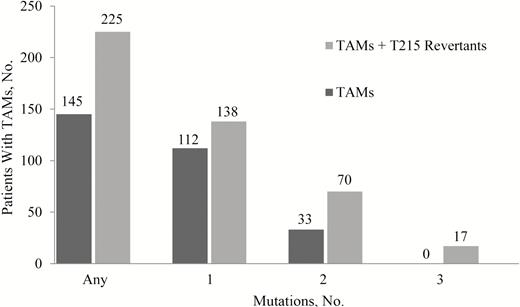
Pretreatment distribution of thymidine-analogue mutations (TAMs) in enrolled patients (n = 5990). The distributions of TAMs (dark gray bars) or TAMs including T215 revertants (eg, T215S/D/E/C/L/I/A; light gray bars) in the enrollment sequence data set are shown. TAMs are M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E/N/R in reverse-transcriptase.
When T215rev mutations were included in the TAM count (TAMs/rev), the number of patients with 2 TAMs/rev increased to 70, and 17 patients were found to carry 3 TAMs/rev. Most of the patients with 2 TAMs/rev carried an M41L + T215rev combination (43 of 70; 61%) (Supplementary Figure 1B), followed by patients carrying a D67N + K219Q combination (16 of 70; 23%). The majority of patients with 3 TAMs/rev carried a combination of M41L + L210W + T215rev (T215D or S or C or D/E) (10 of 17; 59%), followed by a combination of D67N + K219Q + T215 rev (T215S or L or A/V) (4 of 17; 24%).
Treatment Response at Week 48
Most of the 5990 patients who met the enrollment criteria in any of the 9 studies received a treatment regimen that contained either TDF (n = 4586) or TAF (n = 865). We investigated whether the presence of TDRMs affected treatment response at week 48 (Table 3). The overall proportions of patients with treatment success at week 48 was 85.7% and 92.5% for patients receiving a TDF- or TAF-containing regimen, respectively. Only 1.7% and 0.8% of patients receiving a TDF- or TAF-containing regimen, respectively, had virologic failure with emerging resistance to their treatment regimen. Nonsuccess in the remaining 12.6% and 6.7% of patients receiving TDF or TAF, respectively, was due to other events, such as noncompliance, adverse events, or withdrawal of consent [25, 29, 34–39]. Similarly as for the overall population, the presence of preexisting NNRTI-R, K103N/S, NRTI-R, TAMs (excluding or including T215rev mutations), or PI-R had no significant impact on the observed response to treatment.
TDF- and TAF-Based Treatment Response at Week 48 by Baseline Virologic Category
| Category . | Proportion of Patients with Treatment Success at Week 48, % (No./Total)a . | |
|---|---|---|
| TDF . | TAF . | |
| All patients | 85.7 (3932/4586) | 92.5 (800/865) |
| B | 85.5 (3557/4158) | 92.7 (689/743) |
| Non-B | 87.7 (372/424)b | 91.0 (111/122) |
| NNRTI-Rc | 89.0 (210/236) | 90.6 (58/64) |
| No NNRTI-R | 85.6 (3722/4350) | 92.6 (742/801) |
| K103N/Sc | 87.8 (159/181) | 91.7 (44/48) |
| No K103N/S | 85.7 (3773/4405) | 92.5 (756/817) |
| NRTI-R | 88.0 (169/192) | 96.7 (29/30) |
| No NRTI-R | 85.6 (3763/4394) | 92.3 (771/835) |
| TAMs | 87.0 (94/108) | 95.2 (20/21) |
| No TAMs | 85.7 (3838/4478) | 92.4 (780/844) |
| TAMs + T215rev | 90.4 (160/177) | 96.4 (27/28) |
| No TAMs + T215rev | 85.6 (3772/4409) | 92.4 (773/837) |
| PI-Rd | 86.6 (84/97) | 84.2 (16/19) |
| No PI-R | 85.7 (3848/4489) | 92.7 (784/846) |
| Category . | Proportion of Patients with Treatment Success at Week 48, % (No./Total)a . | |
|---|---|---|
| TDF . | TAF . | |
| All patients | 85.7 (3932/4586) | 92.5 (800/865) |
| B | 85.5 (3557/4158) | 92.7 (689/743) |
| Non-B | 87.7 (372/424)b | 91.0 (111/122) |
| NNRTI-Rc | 89.0 (210/236) | 90.6 (58/64) |
| No NNRTI-R | 85.6 (3722/4350) | 92.6 (742/801) |
| K103N/Sc | 87.8 (159/181) | 91.7 (44/48) |
| No K103N/S | 85.7 (3773/4405) | 92.5 (756/817) |
| NRTI-R | 88.0 (169/192) | 96.7 (29/30) |
| No NRTI-R | 85.6 (3763/4394) | 92.3 (771/835) |
| TAMs | 87.0 (94/108) | 95.2 (20/21) |
| No TAMs | 85.7 (3838/4478) | 92.4 (780/844) |
| TAMs + T215rev | 90.4 (160/177) | 96.4 (27/28) |
| No TAMs + T215rev | 85.6 (3772/4409) | 92.4 (773/837) |
| PI-Rd | 86.6 (84/97) | 84.2 (16/19) |
| No PI-R | 85.7 (3848/4489) | 92.7 (784/846) |
Abbreviations: NNRTI-R, nonnucleoside reverse-transcriptase (RT) inhibitor resistance; NRTI, nucleoside/nucleotide RT inhibitor resistance; PI-R, protease inhibitor resistance; T215rev, T215 revertants; TAF, tenofovir alafenamide; TAMs, thymidine-analogue mutations; TDF, tenofovir disoproxil fumarate.
aTreatment success was determined based on the Food and Drug Administration algorithms (time to loss of virologic response [TLOVR] 50 algorithm for studies 903 and 934; snapshot analysis algorithm [<50 copies/mL] for all other studies).
bFour patients with missing human immunodeficiency virus type 1 subtype data are not represented.
cPatients with transmitted NNRTI-R mutations received either an integrase strand transfer inhibitor-based or a PI-based treatment regimen.
dPatients with transmitted PI resistance mutations may have received a PI-based treatment regimen, depending on the mutations present before treatment.
TDF- and TAF-Based Treatment Response at Week 48 by Baseline Virologic Category
| Category . | Proportion of Patients with Treatment Success at Week 48, % (No./Total)a . | |
|---|---|---|
| TDF . | TAF . | |
| All patients | 85.7 (3932/4586) | 92.5 (800/865) |
| B | 85.5 (3557/4158) | 92.7 (689/743) |
| Non-B | 87.7 (372/424)b | 91.0 (111/122) |
| NNRTI-Rc | 89.0 (210/236) | 90.6 (58/64) |
| No NNRTI-R | 85.6 (3722/4350) | 92.6 (742/801) |
| K103N/Sc | 87.8 (159/181) | 91.7 (44/48) |
| No K103N/S | 85.7 (3773/4405) | 92.5 (756/817) |
| NRTI-R | 88.0 (169/192) | 96.7 (29/30) |
| No NRTI-R | 85.6 (3763/4394) | 92.3 (771/835) |
| TAMs | 87.0 (94/108) | 95.2 (20/21) |
| No TAMs | 85.7 (3838/4478) | 92.4 (780/844) |
| TAMs + T215rev | 90.4 (160/177) | 96.4 (27/28) |
| No TAMs + T215rev | 85.6 (3772/4409) | 92.4 (773/837) |
| PI-Rd | 86.6 (84/97) | 84.2 (16/19) |
| No PI-R | 85.7 (3848/4489) | 92.7 (784/846) |
| Category . | Proportion of Patients with Treatment Success at Week 48, % (No./Total)a . | |
|---|---|---|
| TDF . | TAF . | |
| All patients | 85.7 (3932/4586) | 92.5 (800/865) |
| B | 85.5 (3557/4158) | 92.7 (689/743) |
| Non-B | 87.7 (372/424)b | 91.0 (111/122) |
| NNRTI-Rc | 89.0 (210/236) | 90.6 (58/64) |
| No NNRTI-R | 85.6 (3722/4350) | 92.6 (742/801) |
| K103N/Sc | 87.8 (159/181) | 91.7 (44/48) |
| No K103N/S | 85.7 (3773/4405) | 92.5 (756/817) |
| NRTI-R | 88.0 (169/192) | 96.7 (29/30) |
| No NRTI-R | 85.6 (3763/4394) | 92.3 (771/835) |
| TAMs | 87.0 (94/108) | 95.2 (20/21) |
| No TAMs | 85.7 (3838/4478) | 92.4 (780/844) |
| TAMs + T215rev | 90.4 (160/177) | 96.4 (27/28) |
| No TAMs + T215rev | 85.6 (3772/4409) | 92.4 (773/837) |
| PI-Rd | 86.6 (84/97) | 84.2 (16/19) |
| No PI-R | 85.7 (3848/4489) | 92.7 (784/846) |
Abbreviations: NNRTI-R, nonnucleoside reverse-transcriptase (RT) inhibitor resistance; NRTI, nucleoside/nucleotide RT inhibitor resistance; PI-R, protease inhibitor resistance; T215rev, T215 revertants; TAF, tenofovir alafenamide; TAMs, thymidine-analogue mutations; TDF, tenofovir disoproxil fumarate.
aTreatment success was determined based on the Food and Drug Administration algorithms (time to loss of virologic response [TLOVR] 50 algorithm for studies 903 and 934; snapshot analysis algorithm [<50 copies/mL] for all other studies).
bFour patients with missing human immunodeficiency virus type 1 subtype data are not represented.
cPatients with transmitted NNRTI-R mutations received either an integrase strand transfer inhibitor-based or a PI-based treatment regimen.
dPatients with transmitted PI resistance mutations may have received a PI-based treatment regimen, depending on the mutations present before treatment.
Of note, patients with preexisting NNRTI-R did not receive a treatment regimen that contained an NNRTI, and those with preexisting PI-R may have received a treatment regimen that contained a PI (either atazanavir or darunavir) to which they were considered sensitive based on genotype. Patients with treatment failure in the TAMs + T215rev group (17 of 177 receiving a TDF-based regimen and 1 of 28 receiving a TAF-based regimen) failed mostly for reasons other than virologic failure and did not develop additional resistance, including no additional TAMs or T215Y/F (data not shown). In addition, similar treatment responses to either TDF- or TAF-containing regimens were observed in patients with HIV-1 subtype B or non-B. Notably, all 14 of the 17 patients with 3 TAMs/rev (Table 4) who received a TDF- or TAF-containing regimen were virologically suppressed (HIV RNA, <50 copies/mL) thoughout the duration of the studies, including 8 with M41L + L210W + T215rev who remained suppressed through a maximum follow-up of ≥144 weeks.
TDF- and TAF-based Treatment Response in Patients With 2 TAMs Plus T215 Revertants
| Patient . | Mutation at TAM Position . | Treatment Regimen . | Treatment Response (HIV RNA <50 Copies/mL)a . | |||||
|---|---|---|---|---|---|---|---|---|
| M41 . | D67 . | K70 . | L210 . | T215 . | K219 . | |||
| 1 | M41L | … | … | L210W | T215C | … | EFV/3TC/TDF | Through wk 144 |
| 2 | M41L | … | … | L210W | T215D | … | EFV/3TC/TDF | Through wk 168 |
| 3 | M41L | … | … | L210W | T215C | … | TDF + FTC + EFV | Through wk 168 |
| 4 | M41L | … | … | L210W | T215S | … | ATV/C/F/TDF | Through wk 156 |
| 5 | M41L | … | … | L210W | T215S | … | ATV/r/F/TDF | Through wk 144 |
| 6 | M41L | … | … | L210W | T215S | … | EFV/F/TDF | Through wk 144 |
| 7 | M41L | … | … | L210W | T215D | … | ATV/r/F/TDF | Through wk 144 |
| 8 | M41L | … | … | L210W | T215D | … | E/C/F/TDF | Through wk 168 |
| 9 | M41L | … | K70R | … | T215E | … | ATV/r/F/TDF | Through wk 144 |
| 10 | M41L | … | K70K/R | … | T215E | … | ATV/C/F/TDF | Through wk 152 |
| 11 | … | D67N | … | … | T215L | K219Q | E/C/F/TAF | Through wk 168 |
| 12 | … | D67N | … | … | T215S | K219Q | EFV/F/TDF | Through wk 144 |
| 13 | … | D67N | … | … | T215A/V | K219Q | E/C/F/TDF | Through wk 24 (DC) |
| 14 | … | D67N | … | … | T215S | K219Q | RPV/F/TDF | Through wk 48 |
| Patient . | Mutation at TAM Position . | Treatment Regimen . | Treatment Response (HIV RNA <50 Copies/mL)a . | |||||
|---|---|---|---|---|---|---|---|---|
| M41 . | D67 . | K70 . | L210 . | T215 . | K219 . | |||
| 1 | M41L | … | … | L210W | T215C | … | EFV/3TC/TDF | Through wk 144 |
| 2 | M41L | … | … | L210W | T215D | … | EFV/3TC/TDF | Through wk 168 |
| 3 | M41L | … | … | L210W | T215C | … | TDF + FTC + EFV | Through wk 168 |
| 4 | M41L | … | … | L210W | T215S | … | ATV/C/F/TDF | Through wk 156 |
| 5 | M41L | … | … | L210W | T215S | … | ATV/r/F/TDF | Through wk 144 |
| 6 | M41L | … | … | L210W | T215S | … | EFV/F/TDF | Through wk 144 |
| 7 | M41L | … | … | L210W | T215D | … | ATV/r/F/TDF | Through wk 144 |
| 8 | M41L | … | … | L210W | T215D | … | E/C/F/TDF | Through wk 168 |
| 9 | M41L | … | K70R | … | T215E | … | ATV/r/F/TDF | Through wk 144 |
| 10 | M41L | … | K70K/R | … | T215E | … | ATV/C/F/TDF | Through wk 152 |
| 11 | … | D67N | … | … | T215L | K219Q | E/C/F/TAF | Through wk 168 |
| 12 | … | D67N | … | … | T215S | K219Q | EFV/F/TDF | Through wk 144 |
| 13 | … | D67N | … | … | T215A/V | K219Q | E/C/F/TDF | Through wk 24 (DC) |
| 14 | … | D67N | … | … | T215S | K219Q | RPV/F/TDF | Through wk 48 |
Abbreviations: 3TC, lamivudine; ATV, atazanavir; C, cobicistat; DC, discontinuation; E, elvitegravir; EFV, efavirenz; F, emtricitabine; HIV-1, human immunodeficiency virus type; r, ritonavir; RPV, rilpivirine; TAF, tenofovir alafenamide; TAM, thymidine-analogue mutation; TDF, tenofovir disoproxil fumarate;.
aThrough the end of the study at the time indicated.
TDF- and TAF-based Treatment Response in Patients With 2 TAMs Plus T215 Revertants
| Patient . | Mutation at TAM Position . | Treatment Regimen . | Treatment Response (HIV RNA <50 Copies/mL)a . | |||||
|---|---|---|---|---|---|---|---|---|
| M41 . | D67 . | K70 . | L210 . | T215 . | K219 . | |||
| 1 | M41L | … | … | L210W | T215C | … | EFV/3TC/TDF | Through wk 144 |
| 2 | M41L | … | … | L210W | T215D | … | EFV/3TC/TDF | Through wk 168 |
| 3 | M41L | … | … | L210W | T215C | … | TDF + FTC + EFV | Through wk 168 |
| 4 | M41L | … | … | L210W | T215S | … | ATV/C/F/TDF | Through wk 156 |
| 5 | M41L | … | … | L210W | T215S | … | ATV/r/F/TDF | Through wk 144 |
| 6 | M41L | … | … | L210W | T215S | … | EFV/F/TDF | Through wk 144 |
| 7 | M41L | … | … | L210W | T215D | … | ATV/r/F/TDF | Through wk 144 |
| 8 | M41L | … | … | L210W | T215D | … | E/C/F/TDF | Through wk 168 |
| 9 | M41L | … | K70R | … | T215E | … | ATV/r/F/TDF | Through wk 144 |
| 10 | M41L | … | K70K/R | … | T215E | … | ATV/C/F/TDF | Through wk 152 |
| 11 | … | D67N | … | … | T215L | K219Q | E/C/F/TAF | Through wk 168 |
| 12 | … | D67N | … | … | T215S | K219Q | EFV/F/TDF | Through wk 144 |
| 13 | … | D67N | … | … | T215A/V | K219Q | E/C/F/TDF | Through wk 24 (DC) |
| 14 | … | D67N | … | … | T215S | K219Q | RPV/F/TDF | Through wk 48 |
| Patient . | Mutation at TAM Position . | Treatment Regimen . | Treatment Response (HIV RNA <50 Copies/mL)a . | |||||
|---|---|---|---|---|---|---|---|---|
| M41 . | D67 . | K70 . | L210 . | T215 . | K219 . | |||
| 1 | M41L | … | … | L210W | T215C | … | EFV/3TC/TDF | Through wk 144 |
| 2 | M41L | … | … | L210W | T215D | … | EFV/3TC/TDF | Through wk 168 |
| 3 | M41L | … | … | L210W | T215C | … | TDF + FTC + EFV | Through wk 168 |
| 4 | M41L | … | … | L210W | T215S | … | ATV/C/F/TDF | Through wk 156 |
| 5 | M41L | … | … | L210W | T215S | … | ATV/r/F/TDF | Through wk 144 |
| 6 | M41L | … | … | L210W | T215S | … | EFV/F/TDF | Through wk 144 |
| 7 | M41L | … | … | L210W | T215D | … | ATV/r/F/TDF | Through wk 144 |
| 8 | M41L | … | … | L210W | T215D | … | E/C/F/TDF | Through wk 168 |
| 9 | M41L | … | K70R | … | T215E | … | ATV/r/F/TDF | Through wk 144 |
| 10 | M41L | … | K70K/R | … | T215E | … | ATV/C/F/TDF | Through wk 152 |
| 11 | … | D67N | … | … | T215L | K219Q | E/C/F/TAF | Through wk 168 |
| 12 | … | D67N | … | … | T215S | K219Q | EFV/F/TDF | Through wk 144 |
| 13 | … | D67N | … | … | T215A/V | K219Q | E/C/F/TDF | Through wk 24 (DC) |
| 14 | … | D67N | … | … | T215S | K219Q | RPV/F/TDF | Through wk 48 |
Abbreviations: 3TC, lamivudine; ATV, atazanavir; C, cobicistat; DC, discontinuation; E, elvitegravir; EFV, efavirenz; F, emtricitabine; HIV-1, human immunodeficiency virus type; r, ritonavir; RPV, rilpivirine; TAF, tenofovir alafenamide; TAM, thymidine-analogue mutation; TDF, tenofovir disoproxil fumarate;.
aThrough the end of the study at the time indicated.
DISCUSSION
We conducted a retrospective analysis of the genotypic resistance observed in 6704 patients before enrollment in clinical studies conducted at Gilead Sciences between 2000 and 2013. We observed an increase in the overall presence of TDRMs throughout the period, from 5.2% of screened patients in 2000 to 11.4% in 2013. The increase in pretreatment resistance during the period was primarily driven by an increase in the number of patients with NNRTI resistance (6.0% overall), notably with the K103N/S substitutions in RT (4.5% overall).
The prevalence of NNRTI-R and K103N/S was significantly higher in US patients (6.1% of patients with K103N/S) than in European patients (1.4% of patients with K103N/S). In the United States, the prevalence of K103N/S increased from <1% in 2000 to 7.4% in 2010 and remained mostly stable through 2013. In contrast, the prevalence of K103N/S in European patients remained stable near or below 2% from 2003 through 2013. Longitudinal studies from the SPREAD study conducted in Europe from 1996 to 2010 have shown a similar trend for the presence of K103N/S over time, which has remained stable at about 1.5% in the European ART-naive population [6, 8, 40]. Similar to our observations, studies conducted in the United States found a consistently higher prevalence of K103N/S than those conducted in Europe, with a prevalence of 5.2% [9], or 8.6%–10.1% [41] at time periods similar to those used in our analysis. These differences are probably multifactorial, including differential management of HIV treatment and differential ARV drug usage between the United States and Europe.
In contrast to the prevalence of NNRTI-R, the prevalence of NRTI-R was very similar between US and European patients. Signature NRTI-R mutations, such as K65R or M184V/I, were very rarely observed in our data set (0.01% and 0.1%, respectively), despite the prevalent use of drugs potentially selecting for these mutations during the period studied, such as TDF (K65R or K70E) and FTC, lamivudine, or abacavir (M184V/I). Other studies have reported consistently low frequency or absence of K65R (<0.5%), whereas the frequency of M184V/I has shown more variability across studies (ranging from 0.3% to 4.3%) [6, 8, 9, 40, 41]. The higher frequency of M184V/I compared with K65R correlates with reported rates of emergence of these mutations in clinical studies [26, 27, 34].
In addition, the overall rare observation of these mutations in ART-naive patients is probably a consequence of the reduced fitness of viruses harboring these mutations [10, 11, 42], resulting in reduced transmission rates and/or a higher rates of reversion to wild type in the absence of drug pressure. Indeed, M184V/I was found at higher frequencies in acutely/recently infected ART-naive patients than in chronically infected ART-naive patients (8.2% and 2.5%, respectively) [43], and M184V/I was reported to have a particularly high reversion rate in the absence of drug pressure [14] compared with more stable mutations, such as PI-R L90M or the NNRTI-R K103N [13]. Overall, it seems that the impairment conferred by the M184V/I or K65R substitutions on viral fitness reduces their prevalence in ART-naive patients.
The TAMs (M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E/N/R in RT) represent another set of NRTI-R signature mutations. In addition to conferring resistance to zidovudine, with the T215Y/F substitutions playing a major role [2], TAMs have been associated with cross-resistance to most NRTIs [44, 45]. In particular, the combination of ≥3 TAMs including M41L, L210W, and T215Y was associated with significantly reduced response to TDF treatment in heavily ART-experienced patients [33]. TAMs were altogether found in 2%–3% of ART-naive patients throughout the period we studied, with only minor variation between the United States and Europe, indicating relative ease of transmissibility and persistence in the ART-naive population compared with M184V/I or K65R. The M41L substitution was the most persistently observed TAM in our data set (1.5%), in agreement with published data (1.5%) [40]. On the other hand, T215Y/F was very rarely observed (<0.05%), probably because of the impaired fitness associated with these substitutions in the absence of drug selective pressure [10], and consequently, T215rev mutations (T215A/C/D/E/G/H/I/L/N/P/Q/R/S or V) were readily detected (2.7% of patients), similar to prior data (2.5%) [40].
Some current resistance algorithms include all changes from wild type at position T215 (Y/F as well as revertants) as potential resistant mutants. However, the observed extended virologic response to TDF- or TAF-containing regimens through ≥144 weeks in patients with 2 TAMs + T215rev mutations in our data set suggests that the T215rev mutations did not act as resistant mutants against TDF or TAF. In addition, the overall response to TDF- or TAF-containing regimens at week 48 was slightly higher when T215rev mutations were included in the TAM total (Table 3), and back-reversion of T215rev to T215Y/F was never observed in patients with virologic failure. Taken together, these data suggest that T215rev mutants are not associated with resistance to TDF or TAF, and therefore resistance algorithms may need to be revised to reflect this lack of correlation. These findings are supported by a prior study [46] that also showed the absence of a resistance phenotype linked to the presence of T215rev mutants.
Finally, we have shown that the overall presence of commonly transmitted drug resistance mutations, most notably NRTI-R mutations, had no impact on treatment response to TDF- or TAF-containing regimens at week 48. Of note, patients with specific drug resistance mutations to any of the components of the study regimens, such as K65R or ≥3 TAMs, were excluded from the trials, and the impact of these mutations on treatment response to TDF- or TAF-containing regimen could not be assessed. However, this resulted in only minimal exclusions (8 patients) based on TDF, TAF, or FTC resistance, given the very low prevalence of K65R, M184V, and ≥3 TAMs. In contrast, many more patients with NNRTI-R were excluded from enrollment in NNRTI-containing clinical studies owing to transmitted NNRTI-R (78 patients). Importantly, patients with transmitted NNRTI-R were treated in our studies with integrase strand transfer inhibitor-based or PI-based regimens and obtained high virologic success rates, indicating the absence of cross-class resistance.
In summary, we have shown that the presence of common TDRMs in ART-naive patients, particularly the T215rev mutations, had no measurable impact on treatment-response to TDF- or TAF-based regimens. This further suggests that the presence of T215rev mutations should not be a factor in the estimation of genotypic resistance to TDF or TAF.
Notes
Acknowledgments. We thank the patients who participated in the clinical studies, as well as the personnel at the clinical sites. Additional thanks to Richard Haubrich for his thorough review of the manuscript and to Ya-Pei Liu for helping with the statistical data analyses.
Financial support. This work was supported by Gilead Sciences.
Potential conflicts of interest. The authors are employees and stockholders of Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: HIV Drug Resistance Workshop, Boston, Massachusetts, 20–21 February 2016.
Correspondence: N. A. Margot, Gilead Sciences, 333 Lakeside Dr, Foster City, CA 94404 ([email protected]).




