-
PDF
- Split View
-
Views
-
Cite
Cite
Hanna Seppälä, Elina Virtanen, Mika Saarela, Pia Laine, Lars Paulín, Laura Mannonen, Petri Auvinen, Eeva Auvinen, Single-Molecule Sequencing Revealing the Presence of Distinct JC Polyomavirus Populations in Patients With Progressive Multifocal Leukoencephalopathy, The Journal of Infectious Diseases, Volume 215, Issue 6, 15 March 2017, Pages 889–895, https://doi.org/10.1093/infdis/jiw399
Close - Share Icon Share
Progressive multifocal leukoencephalopathy (PML) is a fatal disease caused by reactivation of JC polyomavirus (JCPyV) in immunosuppressed individuals and lytic infection by neurotropic JCPyV in glial cells. The exact content of neurotropic mutations within individual JCPyV strains has not been studied to our knowledge.
We exploited the capacity of single-molecule real-time sequencing technology to determine the sequence of complete JCPyV genomes in single reads. The method was used to precisely characterize individual neurotropic JCPyV strains of 3 patients with PML without the bias caused by assembly of short sequence reads.
In the cerebrospinal fluid sample of a 73-year-old woman with rapid PML onset, 3 distinct JCPyV populations could be identified. All viral populations were characterized by rearrangements within the noncoding regulatory region (NCCR) and 1 point mutation, S267L in the VP1 gene, suggestive of neurotropic strains. One patient with PML had a single neurotropic strain with rearranged NCCR, and 1 patient had a single strain with small NCCR alterations.
We report here, for the first time, full characterization of individual neurotropic JCPyV strains in the cerebrospinal fluid of patients with PML. It remains to be established whether PML pathogenesis is driven by one or several neurotropic strains in an individual.
Progressive multifocal leukoencephalopathy (PML) is a neurodegenerative disorder caused by reactivation of latent JC polyomavirus (JCPyV) leading to lytic infection of permissive myelin-producing oligodendrocytes and astrocytes [1, 2]. Symptoms include cognitive, motor, and visual impairment [3]. Established risk factors for PML are underlying lymphoproliferative disorders, hematopoietic stem cell or organ transplantation, or human immunodeficiency virus infection [4]. Recently a new PML risk group has emerged: patients with autoimmune diseases, such as multiple sclerosis, Crohn's disease, or rheumatoid diseases, who are treated with biological immunomodulatory drugs, such as natalizumab or rituximab [3]. Albeit a rare disease, PML has a high mortality rate, influenced by the underlying immunosuppressive condition and its reversibility. The mortality rate in AIDS-related PML is approximately 50% [5–7], and among natalizumab-treated patients with multiple sclerosis, it is about 30% [8, 9]. The PML mortality rate among solid organ and bone marrow transplant recipients ranges from approximately 50% to as high as 84% [10, 11]. Only restoration of immunocompetence improves patient prognosis [12].
JC polyomavirus was first isolated in 1971 from brain tissue of a patient with PML [13]. JCPyV is usually acquired in childhood as an asymptomatic infection [14], and seroprevalence varies from 30% up to 70% in the general population in many countries, including Finland [15]. Circulating archetype JC polyomaviruses are thought to persist without causing symptoms in the host, most probably in kidney epithelium or in bone marrow [16–18]. PML is always associated with so called neurotropic JCPyV strains, which emerge in the individual and do not circulate in the population. Neurotropic strains may arise already during latency or because of enhanced replication upon reactivation in immunosuppressed individuals [19, 20]. Emergence of neurotropic strains enhances virus spread through the blood-brain barrier, lytic virus replication in glial cells, and ultimately PML.
Active replication may lead to accumulation of deletions and duplications within the noncoding regulatory region (NCCR) and point mutations in the capsid protein gene VP1 of archetype strains, giving rise to neurotropic strains. Neurotropic rearrangements are individual in each patient, but they always occur in the NCCR and often also in VP1. The archetype NCCR has an A-B-C-D-E block structure [21, 22]. The prototype neurotropic strain Mad-1 has a 98–base pair (bp) tandem repeat of promoter/enhancer elements and deletions in B and D blocks compared with the archetype CY strain [20, 21]. Owing to NCCR mutations, neurotropic strains have been shown to replicate more efficiently than archetype strains in neuronal cells [22, 23], and indeed all JCPyV strains found in the cerebrospinal fluid (CSF) of patients with PML seem to harbor mutations within NCCR. VP1 mutations may be located within or near the receptor-binding region and enhance virus tropism and attachment to receptors on the surface of oligodendrocytes and astrocytes in the brain [24]. In a representative study by Reid et al [25], 100% of CSF samples from patients with PML had NCCR rearrangements, and 81% had mutated VP1 sequences. The exact requirements of neurotropic rearrangements within the NCCR and VP1 have not been established.
To precisely characterize the viral populations in PML, we exploited the capacity of single-molecule real-time (SMRT) sequencing technology to reveal kilobases of sequence in a single read. No assembly of short sequence fragments was performed, and thus all sequencing reads represent complete individual viral strains. In 1 patient with PML, 3 distinct viral populations were identified, each representing different sets of neurotropic rearrangements within the NCCR and point mutations within VP1. In 2 other patients with PML, single neurotropic strains were identified. It is noteworthy that no archetype JCPyV sequences were identified in the CSF samples. For the first time, to the best of our knowledge, we present here the identification of distinct viral populations in 3 patients with PML, together with complete sequences representing individual JC virus strains.
PATIENTS AND METHODS
Patients
Of 3 patients with PML, patient 1 was a 74-year-old woman in whom diffuse large B-cell non-Hodgkin lymphoma had been diagnosed in 2009. After prednisolone and x-ray therapy, her lymphoma was in remission in April 2010. Relapse was established in January 2011, followed by 6 courses of bendamustin-rituximab treatment. Rituximab maintenance therapy was continued at 3-month intervals. In January 2013, brain computed tomography (CT) revealed a right-sided occipital large lesion in the white matter, and the diagnosis was PML. The patient died in March 2013.
Patient 2 was an 82-year-old man in whom follicular stage IVA lymphoma had been diagnosed in 2002 and treated initially with chlorambucil. After the first relapse, 6 cycles of bendamustin-rituximab were administered from June to October 2004. The second relapse was treated with 6 cycles of rituximab from October 2007 to February 2008, followed by rituximab maintenance treatment at 3-month intervals until March 2010. At the third relapse in September 2011 the patient presented with pain in the left leg, and MRI showed enlarged lymph nodes in the inguinal region. He refused further examinations and treatment at that time. After the fourth relapse in March 2012, the patient received another 6 cycles of rituximab-bendamustin. In March 2013, PML was diagnosed, and the patient died in April 2013.
Patient 3 was a 59-year-old man with no immunosuppressive medication or condition. In August 2007, he had a partial seizure and left-sided hemiparesis; the brain CT appearance was normal. In September 2007, brain CT showed an infarct in the right frontal lobe. In November 2007, the patient experienced epileptic seizures, cognitive decline, and speech apraxia; brain CT showed several infarcts on both sides. The symptoms progressed, and brain magnetic resonance (MR) imaging in January 2008 showed white matter lesions; the diagnosis was PML without severe immunosuppression. The patient died in February 2008.
Detailed information on the patients and their medications is presented in Supplementary Patient Data. Ethical permission to use patient data in the study was granted by the Coordinating Ethical Committee of the Helsinki and Uusimaa Hospital District.
Quantitative TaqMan Polymerase Chain Reaction
Nucleic acids from the CSF samples (200 µL) were extracted using an automated NucliSENS EasyMAG extraction platform with NucliSENS Nucleic Acid Extraction reagents (bioMérieux), and eluted in 25 µL of elution buffer. The polymerase chain reaction (PCR) reaction mixture consisted of ×1 Taqman universal PCR master mix (Applied Biosystems; Life Technologies), 900 nmol/L concentrations of each primer, 175 nmol/L TAMRA probe (Integrated DNA Technologies), and 10 µL of template in a 50-µL total reaction volume. The primer and probe sequences are listed in Supplementary Table 1. Amplification and detection were performed with an ABI PRISM 7900/7500 cycler (Applied Biosystems) using the following cycling conditions: 50°C for 2 minutes and 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minutes. Quantification was based on an external standard curve, using a series of plasmid dilutions.
Amplification of Full-Length JCPyV Genome
Total nucleic acids from CSF (200 µL) were extracted using an automated Magna Pure LC 1.0 instrument and Magna Pure LC Total Nucleic Acid Isolation kit (Roche Applied Science) and eluted in 50 µL of elution buffer. For primer design, the JCPyV strain Mad-1 complete genome sequence (GenBank database, National Center of Biotechnology; accession No. J02226.1) was used as a query to search for areas of homology with the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The region between nucleotides 3620 and 3663 (Mad-1) within the large T antigen was chosen for primer targeting (Supplementary Table 1). The 25-µL PCR reaction mixture consisted of ×1 Q5 Reaction buffer (New England Biolabs), 200 µmol/L dNTP, 500 nmol/L concentrations of each primer, 0.02 U/µL Q5 Hot Start High-Fidelity DNA polymerase (New England Biolabs), and 1 µL of template.
The PCR reactions were performed with a Tetrad 2 Peltier Thermal cycler (Bio-Rad), as follows: 98°C for 30 seconds, followed by 35 cycles of 98°C for 10 seconds, 64°C for 30 seconds, and 72°C for 3 minutes, and the final extension of 72°C for 2 minutes. The PCR products were visualized in agarose gels. For full-length sequencing using PacBio SMRT technology (Pacific Biosciences), the PCR products were further amplified using the same primers complemented with PacBio barcode sequences (Supplementary Table 1) at their 5′ ends to add individual identifiers to each sample. Robust, correct-sized (about 5.2-kb) amplicons were obtained. Equal molar amounts of all full-length barcoded PCR products were purified for sequencing.
SMRT Sequencing and Sequence Analysis
Amplicon template preparation and sequencing was done according to the PacBio protocol. Briefly, DNA damages and ends were first repaired, and then hairpin adapters were blunt ligated to the purified full-length JCPyV genome amplicons to generate the SMRTbell library. Failed ligation products were removed by exonuclease treatment. The sequencing primer was annealed to the single-stranded hairpin, and DNA polymerase P6 was allowed to bind to the SMRTbell template. Finally, the PacBio (Pacific Biosciences) RS II System with C4 chemistry was used for sequencing. The Long Amplicon Analysis tool, implemented in SMRT Analysis Software (version 2.3.0) with default parameters, was used to find phased circular consensus sequence reads from barcoded amplicon pool. Obtained consensus sequence reads were verified using polymerase reads representing ≥5 full passes of the insert (individual viral genome) with the Reads of Insert mapping tool (SMRT Analysis Software; version 2.3.0) (minimum predicted accuracy, 80%; minimum read length of insert, 4900 bp).
Sequence Alignments
For sequence comparisons, Mad-1 (J02226.1), CY (AB038249.1), and prototype genotype 1A (AF015526.1) and 2B (AF015533.1) sequences were retrieved from GenBank. The numbering of nucleotides and amino acids presented in Results is according to prototype strains of genotypes 1A and 2B. The numbering of nucleotides within NCCR is according to the CY strain.
RESULTS
In this study, we used full-genome PCR and single-molecule sequencing to characterize the JCPyV populations in CSF samples from 3 patients with PML. Full JCPyV genomes were amplified by PCR using primers annealing to conserved regions within the T-antigen sequence (Supplementary Table 1), and barcodes were added for sequencing. The principle of sample preparation and PacBio single-molecule sequencing is described in Figure 1. The crucial difference from other novel sequencing techniques is the capacity of PacBio to cover complete polyomavirus genomes in 1 sequencing read, which enables full characterization of individual virus strains. All sequences are available at https://www.ebi.ac.uk/ under Study accession number PRJEB15261.
![Schematic representation of the principle of JC polyomavirus JCPyV sample preparation and PacBio sequencing workflow. Shown are 2 patient samples, sample 1 containing 3 and sample 2 containing 1 distinct JCPyV sequence type. A, Initial amplification of complete viral genomes using primers targeting large T antigen. B, Second amplification with sample specific barcoded primers (see Supplementary Table 1), with barcode portions of primers marked in red. C, PacBio SMRTbell library preparation. A hairpin adapter is attached to both ends of each amplicon molecule to enable continuous sequencing for several passes of each molecule. D, PacBio sequencing unit (single-molecule real-time [SMRT] cell) containing 150 000-hole zero-mode waveguides. Sequencing of a single amplified viral genome molecule takes place in 1 well of the SMRT cell, shown in the enlargement. E, Single molecule sequencing and definitions of different PacBio reads.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/215/6/10.1093_infdis_jiw399/1/m_jiw39901.jpeg?Expires=1750217957&Signature=JuPXDsoORrVU0IwJI4gtaMJmtLCycv2k21Z4u3Hv0JzJdRrfgc0eskE4UDl6xmf-KIenoUgOg9AhVC3iwL1MW4d1UcbdnZBO9HOQjteHpacLjKIW4Deis~fzjI3iLZ0jRhQh2DHZYquyK3Xm1sxFIwzn95MXvQEIxTgTQ0WgSOhc2rpVKDBOgoAGsiYW7pCKaDyz4~qvnUiKXX9CeUscWAxHWGqElNKVQSyVDAAj5MlzmSH6mQZzfnqjOpoQj03HdgEMlfU~F9uldJI0fBwRrazBdlR4L8srBeG~swT72lNaBk6OUbtEhQXuhkwUvHXzJSM~-V3nBSS3iTpvIy1mkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic representation of the principle of JC polyomavirus JCPyV sample preparation and PacBio sequencing workflow. Shown are 2 patient samples, sample 1 containing 3 and sample 2 containing 1 distinct JCPyV sequence type. A, Initial amplification of complete viral genomes using primers targeting large T antigen. B, Second amplification with sample specific barcoded primers (see Supplementary Table 1), with barcode portions of primers marked in red. C, PacBio SMRTbell library preparation. A hairpin adapter is attached to both ends of each amplicon molecule to enable continuous sequencing for several passes of each molecule. D, PacBio sequencing unit (single-molecule real-time [SMRT] cell) containing 150 000-hole zero-mode waveguides. Sequencing of a single amplified viral genome molecule takes place in 1 well of the SMRT cell, shown in the enlargement. E, Single molecule sequencing and definitions of different PacBio reads.
The main characteristics and findings of the patients with PML are shown in Table 1. All patients fulfilled the criteria for definite PML, including clinical symptoms, typical MR findings, and JCPyV DNA in the CSF [26]; therefore, autopsies were not performed, and postmortem histopathological findings were not available, nor could samples other than CSF be obtained. An MR image of patient 1 is shown in Supplementary Figure 1.
| Patient . | JCPyV Viral Load, Copies/mL . | Strain Length, Nucleotides . | Genotype (Subtype) . | NCCR Arrangement . | NCCR Phenotype . | VP1 Mutations . | Coding Sequence Identity With Archetypea . |
|---|---|---|---|---|---|---|---|
| 1 | 9.38 × 105 | 5051 | 1 (A) | A–B–C–E–F | Neurotropic | S267L | 4732/4738 |
| 5191 | 1 (A) | A–B–C–E–F–(C)–E–F | Neurotropic | S267L | 4732/4737 | ||
| 5243 | 1 (A) | A–B–C–E–A–B–C–E–F | Neurotropic | S267L | 4732/4737 | ||
| 2 | 1.84 × 104 | 5111 | 1 (A) | A–B–C–D–E–Fb | Archetype-like | G8A, L158V, S269F, K345R | 4717/4737 |
| 3 | 1.62 × 104 | 5134 | 2 (B) | A–B–(C)–E–(B)–(C)–E–Fb | Neurotropic | K60N, E69Q, S269F | 4728/4737 |
| Patient . | JCPyV Viral Load, Copies/mL . | Strain Length, Nucleotides . | Genotype (Subtype) . | NCCR Arrangement . | NCCR Phenotype . | VP1 Mutations . | Coding Sequence Identity With Archetypea . |
|---|---|---|---|---|---|---|---|
| 1 | 9.38 × 105 | 5051 | 1 (A) | A–B–C–E–F | Neurotropic | S267L | 4732/4738 |
| 5191 | 1 (A) | A–B–C–E–F–(C)–E–F | Neurotropic | S267L | 4732/4737 | ||
| 5243 | 1 (A) | A–B–C–E–A–B–C–E–F | Neurotropic | S267L | 4732/4737 | ||
| 2 | 1.84 × 104 | 5111 | 1 (A) | A–B–C–D–E–Fb | Archetype-like | G8A, L158V, S269F, K345R | 4717/4737 |
| 3 | 1.62 × 104 | 5134 | 2 (B) | A–B–(C)–E–(B)–(C)–E–Fb | Neurotropic | K60N, E69Q, S269F | 4728/4737 |
Abbreviations: JCPyV, JC polyomavirus; NCCR, noncoding regulatory region; PML, progressive multifocal leukoencephalopathy.
a Coding sequence identity is the proportion of identical nucleotides within protein coding genes compared with the prototype sequence for each genotype.
b A G-to-A point mutation at nucleotide 217 in the F block and a small deletion were also established.
| Patient . | JCPyV Viral Load, Copies/mL . | Strain Length, Nucleotides . | Genotype (Subtype) . | NCCR Arrangement . | NCCR Phenotype . | VP1 Mutations . | Coding Sequence Identity With Archetypea . |
|---|---|---|---|---|---|---|---|
| 1 | 9.38 × 105 | 5051 | 1 (A) | A–B–C–E–F | Neurotropic | S267L | 4732/4738 |
| 5191 | 1 (A) | A–B–C–E–F–(C)–E–F | Neurotropic | S267L | 4732/4737 | ||
| 5243 | 1 (A) | A–B–C–E–A–B–C–E–F | Neurotropic | S267L | 4732/4737 | ||
| 2 | 1.84 × 104 | 5111 | 1 (A) | A–B–C–D–E–Fb | Archetype-like | G8A, L158V, S269F, K345R | 4717/4737 |
| 3 | 1.62 × 104 | 5134 | 2 (B) | A–B–(C)–E–(B)–(C)–E–Fb | Neurotropic | K60N, E69Q, S269F | 4728/4737 |
| Patient . | JCPyV Viral Load, Copies/mL . | Strain Length, Nucleotides . | Genotype (Subtype) . | NCCR Arrangement . | NCCR Phenotype . | VP1 Mutations . | Coding Sequence Identity With Archetypea . |
|---|---|---|---|---|---|---|---|
| 1 | 9.38 × 105 | 5051 | 1 (A) | A–B–C–E–F | Neurotropic | S267L | 4732/4738 |
| 5191 | 1 (A) | A–B–C–E–F–(C)–E–F | Neurotropic | S267L | 4732/4737 | ||
| 5243 | 1 (A) | A–B–C–E–A–B–C–E–F | Neurotropic | S267L | 4732/4737 | ||
| 2 | 1.84 × 104 | 5111 | 1 (A) | A–B–C–D–E–Fb | Archetype-like | G8A, L158V, S269F, K345R | 4717/4737 |
| 3 | 1.62 × 104 | 5134 | 2 (B) | A–B–(C)–E–(B)–(C)–E–Fb | Neurotropic | K60N, E69Q, S269F | 4728/4737 |
Abbreviations: JCPyV, JC polyomavirus; NCCR, noncoding regulatory region; PML, progressive multifocal leukoencephalopathy.
a Coding sequence identity is the proportion of identical nucleotides within protein coding genes compared with the prototype sequence for each genotype.
b A G-to-A point mutation at nucleotide 217 in the F block and a small deletion were also established.
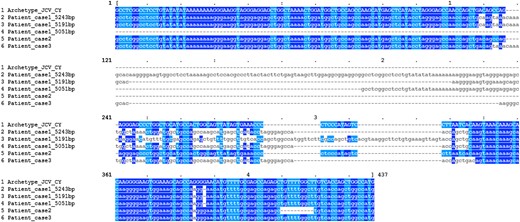
Alignment of the noncoding regulatory regions (NCCR) of patient JC polyomavirus (JCPyV) strains and the CY archetype strain. NCCR sequences of mutant JCPyV strains were compared with the archetype NCCR sequence of the CY prototype strain (in capital letters). Three NCCR sequences were found in the sample from patient 1 (5243, 5191, and 5051 base pairs) but only 1 NCCR sequence in samples from both patients 2 and 3. Sequence identities are highlighted either in dark blue (nucleotides A and G) or light blue (nucleotides C and T).
Patient 2 was an 82-year-old man with follicular stage IVA lymphoma treated with rituximab for a total of 30 cycles. A total of 16 200 subreads were obtained in sequencing, all representing 1 cluster of JCPyV genotype 1A (Table 1, Supplementary Table 2). The NCCR was 246 bp long, and the arrangement was A–B–C–D–E–F (Figure 2). A G-to-A point mutation was found at nucleotide 217 in the F block, and a deletion of nucleotides 243–252. This strain represents an archetype-like rather than an unequivocally neurotropic strain. Point mutations were found within VP1, leading to amino acid changes G8A, L158V, S269F, and K345R (Table 1). Of these, S269F is known to affect the receptor-binding region of VP1.
Patient 3 was a 59-year-old man who developed PML with no preceding immunosuppressive medication or condition. All 17 175 subreads clustered together and represented genotype 2B (Table 1; Supplementary Table 2). The NCCR was 269 nucleotides long and diverged most from archetype NCCR sequences among the PML samples (Figure 2). The arrangement was of type A–B–(C)–E–(B)–(C)–E–F (D deletion; B, C, E duplication). A G-to-A point mutation was found at nucleotide 217 in the F block, and deletion of nucleotides 110–112 affecting the end of the C block. Novel VP1 amino acid changes K60N and E69Q were observed, as well as S269F within the receptor-binding region (Table 1).
DISCUSSION
PML is a devastating disease with a high fatality rate. Its pathogenesis essentially involves the emergence of neurotropic JCPyV strains having better access to glial cells and replicating with higher efficiency than archetype circulating strains. Single-molecule sequencing has for the first time enabled precise characterization of complete neurotropic JCPyV genomes, and this approach has been enforced in the present work. Importantly, along with improved resolution of new sequencing techniques, several viral strains can be differentiated in a population with high precision. In the current study, we characterized the JCPyV populations in CSF samples from 3 patients with PML; in 1 patient, the presence of 3 distinct neurotropic strains was established.
Neurotropic strains associated with PML always carry mutations in the NCCR region, which may alter the composition of transcription factor binding sites [28–30]. All 3 strains from our patient 1 had a deletion of D block with consequent deletion of 1 binding site for specificity protein 1 (Sp-1), deletion or addition of an SpB-binding site, and addition of an nuclear factor-1 (NF-1)–binding site. It is possible that the alterations are partially complemented by each other. Sp-1, Spi-B, and NF-1 are known to enhance JCPyV replication in glial cells [23, 31–35]. Our patient 2 had 1 point mutation and a 10-nucleotide deletion within NCCR, which may be suggestive of a neurotropic strain. In patient 3, considerable rearrangements within the NCCR were observed. Similar to findings in our patients 2 and 3, the G-to-A mutation at nucleotide 217, together with other minor changes, has been reported before in immunocompetent individuals, but more frequently in patients with PML [21].
Many neurotropic strains also have point mutations within the VP1 capsid protein gene, which may alter binding properties of the virus to its receptor or lead to alternative receptor usage [24, 36]. Importantly, neurotropic mutations are always found in CSF and often in plasma samples, whereas archetype sequences are mostly found in urine samples from the same patients. Rare exceptions have been reported [29]. Among our patients with PML, 2 mutations affecting the receptor-binding region of VP1 were identified. An S267L mutation was identified in all 3 JCPyV strains from patient 1, and an S269F mutation in both patients 2 and 3. Both mutations have been reported in PML before, in both CSF and plasma samples from patients with PML, together with another mutation, S267Y [25, 36]. The S267L point mutation has been recognized as a PML-associated site, although another mutation, S267F, seems to occur more frequently, at least in serum samples [25]. It has been speculated that mutation of this site may modify the preference of JCPyV capsids from sialylated glycans outside to nonsialylated glycans inside the central nervous system, which might explain their occurrence and replication in glial cells [24].
In the present study, it is noteworthy that the patient with exceptionally rapid onset of PML had 3 distinct JCPyV strains, which all had different NCCR rearrangements and a mutation putatively affecting receptor binding by VP1. The impact of this finding on the exceptional disease pathogenesis remains to be established. It is indeed possible that NCCR rearrangements alone are insufficient to drive PML pathogenesis, and additional mutations may be needed. Of note, mutations within the JCPyV genome may also generate strains causing abortive infection, where full replication cycle of the virus does not take place—a phenomenon that may lead to additional disease including oncogenic events [37, 38].
Sequencing of complete JCPyV genomes has previously been presented by Reid et al [25]. In that study viral sequences were assembled from Sanger sequencing fragments, which has some drawbacks. First, the resolution of Sanger sequencing is not optimal to identify distinct sequences which may be present in smaller proportions. Second, assembly of sequence fragments conceals their exact origin, and combinations of mutations within a single strain may be missed. No exact information on individual viral strains can be obtained from assembled sequences. However, in that work, cloning of a number of short PCR products revealed the presence of different sequences within an individual. The authors further suggested that NCCR rearrangements in JCPyV strains may exist with or without point mutations within VP1, whereas VP1 mutations never exist without NCCR rearrangements [25]. This is in agreement with our findings, where all JCPyV strains found in CSF had mutations both in NCCR and in the VP1 receptor-binding region.
In our patients with PML, JCPyV genotype 1A, a common European genotype, and an Asian genotype 2B were found [39–42]. The patient carrying genotype 2B was indeed of East Asian origin. Although in this particular patient PML developed without a preceding immunosuppressive condition or medication, which might raise the question of differences between genotypes, PML risk and disease course seem independent of genotype. The other 2 patients with PML in our study both had genotype 1A. It has been suggested that only 1 JCPyV genotype may be found in an individual [25], although a patient on a natalizumab regimen for multiple sclerosis has been reported to harbor 2 genotypes [30].
We found no sequences representing pure archetype JCPyV strains in the CSF samples from patients with PML, although the brain could be the site of selection for neurotropic strains, which would then overgrow archetype strains due to enhanced replication. Theoretically, both archetype and neurotropic strains should be present at some stage of PML pathogenesis in the site of virus strain selection. The possibility cannot be excluded that tiny amounts of archetype strains may have remained undetected despite the great depth of PacBio sequencing. Although neurotropic or altered strains are always found in the brain with PML and their enhanced replication relative to archetype strains has been established, archetype strains have occasionally been detected in the brain as well [20, 43].
To our knowledge this is the first report to characterize individual full-length neurotropic JCPyV strains as well as whole viral populations of patients with PML. Understanding of the biological behavior of different neurotropic JCPyV strains with different combinations of rearrangements is important, because viral load in CSF does not correlate with disease course or survival. The presence of several neurotropic strains within an individual is an intriguing finding. Whether all strains or only one drive PML pathogenesis and whether additive effects take place need further exploration. Information on all neurotropic rearrangements within individual viral strains will contribute to our understanding of JCPyV neurotropism and the pathogenesis of PML.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Harri Kangas for excellent technical assistance.
Financial support. This work was supported by the Finnish Multiple Sclerosis Foundation, Helsinki University Hospital Laboratory (research and development grant), and state funding for university-level health research at Helsinki University Hospital (E. A.).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Correspondence: E. Auvinen, Department of Virology and Immunology, Helsinki University Hospital Laboratory, PO Box 720, 00029 HUS Helsinki, Finland ([email protected]).




