-
PDF
- Split View
-
Views
-
Cite
Cite
Robine Donken, Tessa M. Schurink-van‘t Klooster, Rutger M. Schepp, Fiona R. M. van der Klis, Mirjam J. Knol, Chris J. L. M. Meijer, Hester E. de Melker, Immune Responses After 2 Versus 3 Doses of HPV Vaccination up to 4½ Years After Vaccination: An Observational Study Among Dutch Routinely Vaccinated Girls, The Journal of Infectious Diseases, Volume 215, Issue 3, 1 February 2017, Pages 359–367, https://doi.org/10.1093/infdis/jiw588
Close - Share Icon Share
Abstract
In 2014 the Netherlands switched from 3 to 2 doses for routine vaccination with the prophylactic bivalent human papillomavirus (HPV) vaccine. The current study explored whether antibody responses are noninferior after 2 versus 3 doses in girls.
Girls vaccinated at 12 years of age with 2 (at 0 and 6 months) or 3 doses (at 0, 1, and 6 months) of the bivalent HPV vaccine were identified in the vaccination registration system. Type-specific antibody concentrations and avidity against HPV-16/18/31/33/45/52/58 were assessed. Analyses were stratified for time since the first dose (0–2, 2–3, 3–4, or 4–4½ years). Noninferiority (margin, 0.5) of the 2- versus the 3-dose schedule in girls was examined.
Geometric mean concentrations (GMCs) for vaccine types were only noninferior for 2 versus 3 doses for HPV-18 (at 2–3 years after the first dose; GMC ratio, 0.89; 95% confidence interval, .57–1.38) For vaccine types and cross-protective types (HPV-16/18/31/33/45), the avidity index was noninferior for the 2-dose compared with the 3-dose schedule, except for HPV-31 at 4–4½ years after the first dose and HPV-33 at 3–4 and 4–4½ years.
GMCs for HPV-16/18 were not noninferior for 2 versus 3 doses, except for HPV-18 (at 2–3 years after first dose). However, antibody avidity for these types showed noninferiority, independent of concentrations.
Among women worldwide, cervical cancer is the fourth most common cancer [1]. A persistent infection with a high-risk type of the human papillomavirus (HPV) is the causative agent in the development of cervical cancer [2]. Among sexually active women, approximately 80% will contract an HPV infection during their lifetime. Most of these infections are transient, but in approximately 20% of women the high-risk HPV infection does not clear, and a productive infection develops. Ultimately, a transforming infection that leads to (epi)genetic changes develops in only 3%–5% of women with a persistent infection [3–5]. Since 2006, primary prevention against HPV-related cancers has been enabled by prophylactic vaccination. Three vaccines are currently available: the bivalent vaccine (Cervarix; GlaxoSmithKline), protecting against HPV-16/18; the quadrivalent vaccine (Gardasil; Merck), which protects against HPV-6/11/16/18; and a relatively new nonavalent vaccine (Gardasil9; Merck), which includes the same types as the quadrivalent vaccine and also protects against HPV-31/33/45/52/58 [6, 7].
All vaccines were initially licensed in a 3-dose schedule. Based on available evidence, the European Medicines Agency licensed a 2-dose schedule for all 3 vaccines [8–10]. Licensure was based on so-called immune-bridging studies [11, 12]. These studies assumed, given the shown efficacy in young adults, that with comparable immunogenicity also efficacy would be comparable. Therefore, antibody concentrations after 2 doses in preadolescents were compared with 3 doses among young adults. However, the antibody concentrations after HPV vaccination are generally higher among younger recipients [11, 13]. On the other hand, longer protection might be needed for this age group. In addition to antibody concentrations, other aspects of the immune response may contribute to the success of HPV vaccination in preventing HPV-associated cancers, such as the induction of immunological memory and the avidity of antibodies [14, 15]. The aim of the current study was to compare the antibody concentrations and avidity up to 4½ years after the first dose within the same age group—namely, young girls who had received 2 or 3 doses of the prophylactic HPV-16/18 L1 vaccine.
MATERIAL AND METHODS
Study Design
This observational study (HPV2D) was designed to evaluate the immune responses of 2 doses (at 0 and 6 months) compared with 3 doses (at 0, 1, and 6 months) of the bivalent HPV vaccine (HPV-16/18) in a population-based setting. We examined noninferiority of antibody concentrations and avidity after a 2- versus a 3-dose schedule up to 4½ years after vaccination.
Ethical Approval
The study protocol was approved by the medical ethics committee of VU University Medical Center (NL48754.029.14; protocol 2014.230), Amsterdam, the Netherlands, and was conducted in adherence to the Declaration of Helsinki. The study is registered in the Dutch Trial Registry (NTR 4719).
Study Population and Procedures
We used the Dutch vaccination registry Praeventis [16] to obtain a sample of girls who were routinely vaccinated between 2010 and 2013 (birth cohorts 1997–2000). Analyses were stratified by cohorts, defined by time since first dose of HPV vaccine: <2, 2–3, 3–4, or 4–4½ years. Stratification was done because previous research showed a time trend in geometric mean concentration (GMC) ratio for HPV-18 [17]. Girls were eligible if they received 3 doses of the bivalent HPV vaccine, the recommended schedule at that time, or 2 doses with ≥5 months between doses, at age 12 years. Because the 3-dose schedule was the standard at the time the girls were vaccinated, 2-dose recipients were noncompliant.
To show noninferiority of 2 versus 3 doses for antibody concentrations and avidity, we used a noninferiority margin of 0.5. This margin was based on and equal to those used in previous studies on this topic [17–24]. Sample size calculations showed that ≥50 girls should be included for each dosing group and birth cohort to show noninferiority of antibody concentrations. Taking into account a response rate of 15%, we invited 2870 girls, divided equally among birth cohorts and schedules, to participate in the study. Girls and their parents received an invitation letter supplemented by an informed consent form in September 2014. If both the girl and her parent(s) or guardian(s) provided written consent, the girl received a research package at home, including a link to an online questionnaire and materials for capillary blood drawing. Capillary blood samples were obtained by the use of protein saver cards (Whatman 903 Protein Saver Card; GE Healthcare). After participants had completed the questionnaire and the blood sampling, they received an incentive of €25.
Serological Evaluation
All serological analysis was performed in a blinded manner. The protein saver cards containing the dried blood spot (DBS) samples were stored at −20°C until analysis. A previous validation pilot (unpublished) showed that for all 7 examined serotypes the correlation between DBS and conventional serum measurements was high (R2, 0.94–0.99; correlation coefficients, 0.91–1.07). A single small (3-mm) DBS punch from every sample was collected in 250 µL of assay buffer (phosphate-buffered saline [PBS], 1% bovine serum albumin, and 0.2% Tween 20) and incubated overnight at 4°C on a shaking platform, resulting in an approximately 200-fold serum sample dilution. All samples, diluted 200- and 5000-fold in assay buffer, were determined simultaneously within a single assay run using an HPV multiplex assay, as described elsewhere by Scherpenisse et al [25]. Viruslike particles (VLPs), kindly donated by GlaxoSmithKline, were coupled to distinct fluorescent microspheres (Luminex), and HPV-specific immunoglobulin G (IgG) antibodies were analyzed using a Bioplex system 200 with Bioplex software (Bio-Rad Laboratories). For each analyte, the median fluorescent intensity was converted to Luminex units (LU) per milliliter using a 2-fold serial dilution of a reference standard (intravenous immunoglobulin; lot LE12H227AF; Baxter) with interpolation of the median fluorescent intensity data through a 5-parameter curve-fitting algorithm. Cutoffs for seropositivity had been previously determined at 9, 13, 27, 11, 19, 14, and 31 LU/mL for HPV types 16, 18, 31, 33, 45, 52, and 58, respectively [25].
Evaluation of Antibody Avidity
The avidity of IgG-specific antibodies was determined by a modification of the VLP multiplex immunoassay, as described elsewhere by Scherpenisse et al [26]. In short, serum samples were diluted to a HPV antibody concentration of 0.05–0.75 LU/mL and incubated at room temperature for 1 hour with VLP-conjugated beads. After 3 washes, 50 µL of a 2.5 mol/L ammonium thiocyanate solution (Sigma-Aldrich) in PBS was added and incubated for 10 minutes at room temperature, followed by 3 washes with PBS. Residual bound antibodies were measured. The avidity index (AI) was calculated as the percentage of IgG antibodies still binding after being eluted with the thiocyanate solution, in comparison with concentrations after addition of (only) PBS (set at 100%). The AI for 2 and 3 doses was assessed in a subset of approximately 230 randomly selected samples per serotype (range, 208–236 samples per serotype). To minimize assay variability, assays were performed in a single run and by a single laboratory technician (R. M. S.).
Statistical Analysis
Differences between participants receiving 2 or 3 doses in sociodemographic characteristics were compared using Fisher exact test, and a 2-sample median test was used for differences in ages and time since vaccination. IgG GMCs for HPV type-specific antibodies were calculated. We calculated the GMC ratio with corresponding 95% confidence intervals (CIs) for 2 versus 3 doses, and noninferiority was concluded if the 95% CI did not include the margin of 0.5. To assess noninferiority for the geometric mean AI, the AI ratio (the AI for the 2-dose schedule divided by that for the 3 dose schedule) and the corresponding 95% CI were calculated, and a margin of 0.5 was also used here. Data analysis was performed using SAS software package 9.3 (SAS Institute).
Sensitivity Analysis
We cross-checked the data on vaccination status obtained from the vaccination registry with that self-reported in the questionnaire. We selected those participants with consistent information regarding vaccination status from these 2 sources. Next, we calculated the GMC ratios comparing the 2- with 3–dose schedule, for HPV-16 and HPV-18, respectively. We did the same for the antibody AI.
RESULTS
Sociodemographic Characteristics
In total 466 girls (response rate, 16.2%) participated in the study, of whom 37 did not complete the questionnaire (19 had received 3 and 18 had received 2 doses). For participants without a questionnaire, only age and number of doses was known. Of participants who filled out the questionnaire, 298 participants had received 3 and 131 had received 2 doses. The required sample size was not reached for all cohorts; in these birth cohorts, unfortunately, no more 2-dose recipients were available to be invited from the vaccination registry.
Time since the first dose was comparable between different dosing schedules for the cohorts who had received the first dose 2–3 years or 4–4½ years earlier (Table 1). For the cohorts <2 years after the first dose and the cohort who had received the first dose 3–4 years earlier, the median time span since the first dose was smaller in the 2-dose group.
For 2 doses, the median time between doses was 189 days (range, 151–752 days). For 3 doses, the median time between the first and second doses was 34 days (range, 23–218 days), and the median time between the second and third doses was 161 days (range, 119–845 days). We did not observe a correlation between time between doses and antibody concentrations (data not shown).
Age and Time Since The First Dose in Participants Stratified by Dosing Schedulea
| . | 0–2 y Since 1st Dose (n = 145) . | 2–3 y Since 1st Dose (n = 118) . | 3–4 y Since 1st Dose (n = 106) . | 4–4½ y Since 1st Dose (n = 97) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 Doses (n = 89) . | 2 Doses (n = 56) . | P Value . | 3 Doses (n = 81) . | 2 Doses (n = 37) . | P Value . | 3 Doses (n = 73) . | 2 Doses (n = 33) . | P Value . | 3 Doses (n = 74) . | 2 Doses (n = 23) . | P Value . |
| Age, median (range), y | 14 (13–15) | 14 (13–15) | .73 | 15 (14–16) | 15 (14–16) | .10 | 16 (15–17) | 16 (15–17) | .75 | 17 (16–17) | 17 (16–17) | .83 |
| Duration since 1st dose, median (range), d | 562 (525–728) | 549.5 (337–728) | .02 | 924 (895–940) | 915 (742–1092) | .10 | 1290 (1237–1449) | 1265 (1120–1456) | .03 | 1625.5 (1568–1647) | 1624 (1478–1644) | .18 |
| . | 0–2 y Since 1st Dose (n = 145) . | 2–3 y Since 1st Dose (n = 118) . | 3–4 y Since 1st Dose (n = 106) . | 4–4½ y Since 1st Dose (n = 97) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 Doses (n = 89) . | 2 Doses (n = 56) . | P Value . | 3 Doses (n = 81) . | 2 Doses (n = 37) . | P Value . | 3 Doses (n = 73) . | 2 Doses (n = 33) . | P Value . | 3 Doses (n = 74) . | 2 Doses (n = 23) . | P Value . |
| Age, median (range), y | 14 (13–15) | 14 (13–15) | .73 | 15 (14–16) | 15 (14–16) | .10 | 16 (15–17) | 16 (15–17) | .75 | 17 (16–17) | 17 (16–17) | .83 |
| Duration since 1st dose, median (range), d | 562 (525–728) | 549.5 (337–728) | .02 | 924 (895–940) | 915 (742–1092) | .10 | 1290 (1237–1449) | 1265 (1120–1456) | .03 | 1625.5 (1568–1647) | 1624 (1478–1644) | .18 |
aData in Table 1 represent all participants.
Age and Time Since The First Dose in Participants Stratified by Dosing Schedulea
| . | 0–2 y Since 1st Dose (n = 145) . | 2–3 y Since 1st Dose (n = 118) . | 3–4 y Since 1st Dose (n = 106) . | 4–4½ y Since 1st Dose (n = 97) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 Doses (n = 89) . | 2 Doses (n = 56) . | P Value . | 3 Doses (n = 81) . | 2 Doses (n = 37) . | P Value . | 3 Doses (n = 73) . | 2 Doses (n = 33) . | P Value . | 3 Doses (n = 74) . | 2 Doses (n = 23) . | P Value . |
| Age, median (range), y | 14 (13–15) | 14 (13–15) | .73 | 15 (14–16) | 15 (14–16) | .10 | 16 (15–17) | 16 (15–17) | .75 | 17 (16–17) | 17 (16–17) | .83 |
| Duration since 1st dose, median (range), d | 562 (525–728) | 549.5 (337–728) | .02 | 924 (895–940) | 915 (742–1092) | .10 | 1290 (1237–1449) | 1265 (1120–1456) | .03 | 1625.5 (1568–1647) | 1624 (1478–1644) | .18 |
| . | 0–2 y Since 1st Dose (n = 145) . | 2–3 y Since 1st Dose (n = 118) . | 3–4 y Since 1st Dose (n = 106) . | 4–4½ y Since 1st Dose (n = 97) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 Doses (n = 89) . | 2 Doses (n = 56) . | P Value . | 3 Doses (n = 81) . | 2 Doses (n = 37) . | P Value . | 3 Doses (n = 73) . | 2 Doses (n = 33) . | P Value . | 3 Doses (n = 74) . | 2 Doses (n = 23) . | P Value . |
| Age, median (range), y | 14 (13–15) | 14 (13–15) | .73 | 15 (14–16) | 15 (14–16) | .10 | 16 (15–17) | 16 (15–17) | .75 | 17 (16–17) | 17 (16–17) | .83 |
| Duration since 1st dose, median (range), d | 562 (525–728) | 549.5 (337–728) | .02 | 924 (895–940) | 915 (742–1092) | .10 | 1290 (1237–1449) | 1265 (1120–1456) | .03 | 1625.5 (1568–1647) | 1624 (1478–1644) | .18 |
aData in Table 1 represent all participants.
Participants who had received 2 doses did not differ significantly from those who had received 3 doses in age, current educational level, oral contraceptive use, whether they had had sex, age at sexual debut, whether they were immunocompromised or used immunosuppressive medication, or whether they had reached menarche. Most participants were born in the Netherlands (Table 2).
Sociodemographic Characteristics of Participants Stratified by Dosing Schedulea
| . | 0–2 y Since 1st Dose (n = 136) . | 2–3 y Since 1st Dose (n = 108) . | 3–4 y Since 1st Dose (n = 97) . | 4–4½ y Since 1st Dose (n = 90) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | 3 Doses (n = 83) . | 2 Doses (n = 53) . | P Value . | 3 Doses (n = 77) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 68) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 70) . | 2 Doses (n = 20) . | P Value . |
| Current educational level, No. (%) | ||||||||||||
| Low | 14 (17) | 12 (23) | .49 | 11 (14) | 9 (31) | .23 | 9 (13) | 3 (10) | .82 | 4 (6) | 0 (0) | .45 |
| Middle | 21 (25) | 13 (25) | 22 (29) | 6 (21) | 24 (35) | 12 (41) | 32 (46) | 7 (35) | ||||
| High | 48 (58) | 27 (51) | 43 (56) | 14 (48) | 35 (51) | 14 (48) | 34 (49) | 13 (65) | ||||
| Unknown | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Country of birth, No. (%) | ||||||||||||
| Netherlands | 79 (100) | 49 (98) | .39 | 72 (97) | 26 (96) | .32 | 68 (100) | 28 (97) | .30 | 68 (97) | 17 (85) | .03 |
| Europe | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (3) | 2 (3) | 1 (5) | ||||
| Other | 0 (0) | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | ||||
| Country of birth mother, No. (%) | ||||||||||||
| Netherlands | 80 (96) | 45 (85) | .05 | 73 (95) | 22 (76) | <.01 | 65 (96) | 22 (76) | .01 | 63 (90) | 17 (85) | .70 |
| Europe | 1 (1) | 2 (4) | 3 (4) | 2 (7) | 1 (1) | 2 (7) | 3 (4) | 1 (5) | ||||
| Other | 2 (2) | 6 (11) | 1 (1) | 5 (17) | 2 (3) | 4 (14) | 4 (6) | 2 (10) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Country of birth father, No. (%) | ||||||||||||
| Netherlands | 79 (95) | 45 (87) | .17 | 75 (97) | 18 (62) | <.01 | 66 (97) | 22 (76) | <.01 | 65 (93) | 15 (75) | .03 |
| Europe | 1 (1) | 1 (2) | 2 (3) | 2 (7) | 1 (1) | 1 (3) | 2 (3) | 0 (0) | ||||
| Other | 3 (4) | 6 (12) | 0 (0) | 8 (28) | 1 (1) | 5 (17) | 3 (4) | 5 (25) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Oral contraceptive use, No. (%) | ||||||||||||
| Current user | 4 (5) | 4 (8) | .87 | 10 (13) | 9 (31) | .08 | 27 (40) | 14 (48) | .20 | 51 (73) | 13 (65) | .69 |
| Past user | 2 (2) | 1 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (7) | 5 (7) | 1 (5) | ||||
| No | 77 (93) | 48 (91) | 65 (84) | 20 (69) | 40 (59) | 13 (45) | 14 (20) | 6 (30) | ||||
| Ever had sex, No. (%) | ||||||||||||
| Never | 82 (99) | 51 (96) | .56 | 71 (92) | 26 (90) | .43 | 57 (84) | 19 (66) | .06 | 37 (53) | 12 (60) | .62 |
| Yes | 1 (1) | 2 (4) | 6 (8) | 2 (7) | 11 (16) | 10 (34) | 33 (47) | 8 (40) | ||||
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Age at sexual debut, median (range), y | 15 (15-15) | 14 (14-14) | .16 | 14 (13–15) | 14.5 (14–15) | .82 | 15 (13–16) | 15 (15-15) | .90 | 15 (12–16) | 16 (13–17) | .37 |
| Immuuncompromised, No. (%) | ||||||||||||
| No | 82 (99) | 53 (100) | >.99 | 77 (100) | 29 (100) | >.99 | 68 (100) | 29 (100) | >.99 | 70 (100) | 20 (100) | >.99 |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t Know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Immuunsupressive medication, No. (%) | ||||||||||||
| No | 79 (95) | 53 (100) | .41 | 74 (96) | 29 (100) | .56 | 61 (90) | 29 (100) | .27 | 67 (96) | 19 (95) | >.99 |
| Yes | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t know | 2 (2) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 1 (5) | ||||
| Had menarche, No. (%) | ||||||||||||
| Yes | 72 (87) | 46 (87) | >.99 | 73 (95) | 27 (93) | .66 | 67 (99) | 28 (97) | .51 | 70 (100) | 20 (100) | >.99 |
| No | 11 (13) | 7 (13) | 4 (5) | 2 (7) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | ||||
| . | 0–2 y Since 1st Dose (n = 136) . | 2–3 y Since 1st Dose (n = 108) . | 3–4 y Since 1st Dose (n = 97) . | 4–4½ y Since 1st Dose (n = 90) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | 3 Doses (n = 83) . | 2 Doses (n = 53) . | P Value . | 3 Doses (n = 77) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 68) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 70) . | 2 Doses (n = 20) . | P Value . |
| Current educational level, No. (%) | ||||||||||||
| Low | 14 (17) | 12 (23) | .49 | 11 (14) | 9 (31) | .23 | 9 (13) | 3 (10) | .82 | 4 (6) | 0 (0) | .45 |
| Middle | 21 (25) | 13 (25) | 22 (29) | 6 (21) | 24 (35) | 12 (41) | 32 (46) | 7 (35) | ||||
| High | 48 (58) | 27 (51) | 43 (56) | 14 (48) | 35 (51) | 14 (48) | 34 (49) | 13 (65) | ||||
| Unknown | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Country of birth, No. (%) | ||||||||||||
| Netherlands | 79 (100) | 49 (98) | .39 | 72 (97) | 26 (96) | .32 | 68 (100) | 28 (97) | .30 | 68 (97) | 17 (85) | .03 |
| Europe | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (3) | 2 (3) | 1 (5) | ||||
| Other | 0 (0) | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | ||||
| Country of birth mother, No. (%) | ||||||||||||
| Netherlands | 80 (96) | 45 (85) | .05 | 73 (95) | 22 (76) | <.01 | 65 (96) | 22 (76) | .01 | 63 (90) | 17 (85) | .70 |
| Europe | 1 (1) | 2 (4) | 3 (4) | 2 (7) | 1 (1) | 2 (7) | 3 (4) | 1 (5) | ||||
| Other | 2 (2) | 6 (11) | 1 (1) | 5 (17) | 2 (3) | 4 (14) | 4 (6) | 2 (10) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Country of birth father, No. (%) | ||||||||||||
| Netherlands | 79 (95) | 45 (87) | .17 | 75 (97) | 18 (62) | <.01 | 66 (97) | 22 (76) | <.01 | 65 (93) | 15 (75) | .03 |
| Europe | 1 (1) | 1 (2) | 2 (3) | 2 (7) | 1 (1) | 1 (3) | 2 (3) | 0 (0) | ||||
| Other | 3 (4) | 6 (12) | 0 (0) | 8 (28) | 1 (1) | 5 (17) | 3 (4) | 5 (25) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Oral contraceptive use, No. (%) | ||||||||||||
| Current user | 4 (5) | 4 (8) | .87 | 10 (13) | 9 (31) | .08 | 27 (40) | 14 (48) | .20 | 51 (73) | 13 (65) | .69 |
| Past user | 2 (2) | 1 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (7) | 5 (7) | 1 (5) | ||||
| No | 77 (93) | 48 (91) | 65 (84) | 20 (69) | 40 (59) | 13 (45) | 14 (20) | 6 (30) | ||||
| Ever had sex, No. (%) | ||||||||||||
| Never | 82 (99) | 51 (96) | .56 | 71 (92) | 26 (90) | .43 | 57 (84) | 19 (66) | .06 | 37 (53) | 12 (60) | .62 |
| Yes | 1 (1) | 2 (4) | 6 (8) | 2 (7) | 11 (16) | 10 (34) | 33 (47) | 8 (40) | ||||
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Age at sexual debut, median (range), y | 15 (15-15) | 14 (14-14) | .16 | 14 (13–15) | 14.5 (14–15) | .82 | 15 (13–16) | 15 (15-15) | .90 | 15 (12–16) | 16 (13–17) | .37 |
| Immuuncompromised, No. (%) | ||||||||||||
| No | 82 (99) | 53 (100) | >.99 | 77 (100) | 29 (100) | >.99 | 68 (100) | 29 (100) | >.99 | 70 (100) | 20 (100) | >.99 |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t Know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Immuunsupressive medication, No. (%) | ||||||||||||
| No | 79 (95) | 53 (100) | .41 | 74 (96) | 29 (100) | .56 | 61 (90) | 29 (100) | .27 | 67 (96) | 19 (95) | >.99 |
| Yes | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t know | 2 (2) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 1 (5) | ||||
| Had menarche, No. (%) | ||||||||||||
| Yes | 72 (87) | 46 (87) | >.99 | 73 (95) | 27 (93) | .66 | 67 (99) | 28 (97) | .51 | 70 (100) | 20 (100) | >.99 |
| No | 11 (13) | 7 (13) | 4 (5) | 2 (7) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | ||||
aData in Table 2 represent only participants who filled out the questionnaire.
Sociodemographic Characteristics of Participants Stratified by Dosing Schedulea
| . | 0–2 y Since 1st Dose (n = 136) . | 2–3 y Since 1st Dose (n = 108) . | 3–4 y Since 1st Dose (n = 97) . | 4–4½ y Since 1st Dose (n = 90) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | 3 Doses (n = 83) . | 2 Doses (n = 53) . | P Value . | 3 Doses (n = 77) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 68) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 70) . | 2 Doses (n = 20) . | P Value . |
| Current educational level, No. (%) | ||||||||||||
| Low | 14 (17) | 12 (23) | .49 | 11 (14) | 9 (31) | .23 | 9 (13) | 3 (10) | .82 | 4 (6) | 0 (0) | .45 |
| Middle | 21 (25) | 13 (25) | 22 (29) | 6 (21) | 24 (35) | 12 (41) | 32 (46) | 7 (35) | ||||
| High | 48 (58) | 27 (51) | 43 (56) | 14 (48) | 35 (51) | 14 (48) | 34 (49) | 13 (65) | ||||
| Unknown | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Country of birth, No. (%) | ||||||||||||
| Netherlands | 79 (100) | 49 (98) | .39 | 72 (97) | 26 (96) | .32 | 68 (100) | 28 (97) | .30 | 68 (97) | 17 (85) | .03 |
| Europe | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (3) | 2 (3) | 1 (5) | ||||
| Other | 0 (0) | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | ||||
| Country of birth mother, No. (%) | ||||||||||||
| Netherlands | 80 (96) | 45 (85) | .05 | 73 (95) | 22 (76) | <.01 | 65 (96) | 22 (76) | .01 | 63 (90) | 17 (85) | .70 |
| Europe | 1 (1) | 2 (4) | 3 (4) | 2 (7) | 1 (1) | 2 (7) | 3 (4) | 1 (5) | ||||
| Other | 2 (2) | 6 (11) | 1 (1) | 5 (17) | 2 (3) | 4 (14) | 4 (6) | 2 (10) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Country of birth father, No. (%) | ||||||||||||
| Netherlands | 79 (95) | 45 (87) | .17 | 75 (97) | 18 (62) | <.01 | 66 (97) | 22 (76) | <.01 | 65 (93) | 15 (75) | .03 |
| Europe | 1 (1) | 1 (2) | 2 (3) | 2 (7) | 1 (1) | 1 (3) | 2 (3) | 0 (0) | ||||
| Other | 3 (4) | 6 (12) | 0 (0) | 8 (28) | 1 (1) | 5 (17) | 3 (4) | 5 (25) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Oral contraceptive use, No. (%) | ||||||||||||
| Current user | 4 (5) | 4 (8) | .87 | 10 (13) | 9 (31) | .08 | 27 (40) | 14 (48) | .20 | 51 (73) | 13 (65) | .69 |
| Past user | 2 (2) | 1 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (7) | 5 (7) | 1 (5) | ||||
| No | 77 (93) | 48 (91) | 65 (84) | 20 (69) | 40 (59) | 13 (45) | 14 (20) | 6 (30) | ||||
| Ever had sex, No. (%) | ||||||||||||
| Never | 82 (99) | 51 (96) | .56 | 71 (92) | 26 (90) | .43 | 57 (84) | 19 (66) | .06 | 37 (53) | 12 (60) | .62 |
| Yes | 1 (1) | 2 (4) | 6 (8) | 2 (7) | 11 (16) | 10 (34) | 33 (47) | 8 (40) | ||||
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Age at sexual debut, median (range), y | 15 (15-15) | 14 (14-14) | .16 | 14 (13–15) | 14.5 (14–15) | .82 | 15 (13–16) | 15 (15-15) | .90 | 15 (12–16) | 16 (13–17) | .37 |
| Immuuncompromised, No. (%) | ||||||||||||
| No | 82 (99) | 53 (100) | >.99 | 77 (100) | 29 (100) | >.99 | 68 (100) | 29 (100) | >.99 | 70 (100) | 20 (100) | >.99 |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t Know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Immuunsupressive medication, No. (%) | ||||||||||||
| No | 79 (95) | 53 (100) | .41 | 74 (96) | 29 (100) | .56 | 61 (90) | 29 (100) | .27 | 67 (96) | 19 (95) | >.99 |
| Yes | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t know | 2 (2) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 1 (5) | ||||
| Had menarche, No. (%) | ||||||||||||
| Yes | 72 (87) | 46 (87) | >.99 | 73 (95) | 27 (93) | .66 | 67 (99) | 28 (97) | .51 | 70 (100) | 20 (100) | >.99 |
| No | 11 (13) | 7 (13) | 4 (5) | 2 (7) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | ||||
| . | 0–2 y Since 1st Dose (n = 136) . | 2–3 y Since 1st Dose (n = 108) . | 3–4 y Since 1st Dose (n = 97) . | 4–4½ y Since 1st Dose (n = 90) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | 3 Doses (n = 83) . | 2 Doses (n = 53) . | P Value . | 3 Doses (n = 77) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 68) . | 2 Doses (n = 29) . | P Value . | 3 Doses (n = 70) . | 2 Doses (n = 20) . | P Value . |
| Current educational level, No. (%) | ||||||||||||
| Low | 14 (17) | 12 (23) | .49 | 11 (14) | 9 (31) | .23 | 9 (13) | 3 (10) | .82 | 4 (6) | 0 (0) | .45 |
| Middle | 21 (25) | 13 (25) | 22 (29) | 6 (21) | 24 (35) | 12 (41) | 32 (46) | 7 (35) | ||||
| High | 48 (58) | 27 (51) | 43 (56) | 14 (48) | 35 (51) | 14 (48) | 34 (49) | 13 (65) | ||||
| Unknown | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Country of birth, No. (%) | ||||||||||||
| Netherlands | 79 (100) | 49 (98) | .39 | 72 (97) | 26 (96) | .32 | 68 (100) | 28 (97) | .30 | 68 (97) | 17 (85) | .03 |
| Europe | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (3) | 2 (3) | 1 (5) | ||||
| Other | 0 (0) | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | ||||
| Country of birth mother, No. (%) | ||||||||||||
| Netherlands | 80 (96) | 45 (85) | .05 | 73 (95) | 22 (76) | <.01 | 65 (96) | 22 (76) | .01 | 63 (90) | 17 (85) | .70 |
| Europe | 1 (1) | 2 (4) | 3 (4) | 2 (7) | 1 (1) | 2 (7) | 3 (4) | 1 (5) | ||||
| Other | 2 (2) | 6 (11) | 1 (1) | 5 (17) | 2 (3) | 4 (14) | 4 (6) | 2 (10) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Country of birth father, No. (%) | ||||||||||||
| Netherlands | 79 (95) | 45 (87) | .17 | 75 (97) | 18 (62) | <.01 | 66 (97) | 22 (76) | <.01 | 65 (93) | 15 (75) | .03 |
| Europe | 1 (1) | 1 (2) | 2 (3) | 2 (7) | 1 (1) | 1 (3) | 2 (3) | 0 (0) | ||||
| Other | 3 (4) | 6 (12) | 0 (0) | 8 (28) | 1 (1) | 5 (17) | 3 (4) | 5 (25) | ||||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||||
| Oral contraceptive use, No. (%) | ||||||||||||
| Current user | 4 (5) | 4 (8) | .87 | 10 (13) | 9 (31) | .08 | 27 (40) | 14 (48) | .20 | 51 (73) | 13 (65) | .69 |
| Past user | 2 (2) | 1 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (7) | 5 (7) | 1 (5) | ||||
| No | 77 (93) | 48 (91) | 65 (84) | 20 (69) | 40 (59) | 13 (45) | 14 (20) | 6 (30) | ||||
| Ever had sex, No. (%) | ||||||||||||
| Never | 82 (99) | 51 (96) | .56 | 71 (92) | 26 (90) | .43 | 57 (84) | 19 (66) | .06 | 37 (53) | 12 (60) | .62 |
| Yes | 1 (1) | 2 (4) | 6 (8) | 2 (7) | 11 (16) | 10 (34) | 33 (47) | 8 (40) | ||||
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Age at sexual debut, median (range), y | 15 (15-15) | 14 (14-14) | .16 | 14 (13–15) | 14.5 (14–15) | .82 | 15 (13–16) | 15 (15-15) | .90 | 15 (12–16) | 16 (13–17) | .37 |
| Immuuncompromised, No. (%) | ||||||||||||
| No | 82 (99) | 53 (100) | >.99 | 77 (100) | 29 (100) | >.99 | 68 (100) | 29 (100) | >.99 | 70 (100) | 20 (100) | >.99 |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t Know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Immuunsupressive medication, No. (%) | ||||||||||||
| No | 79 (95) | 53 (100) | .41 | 74 (96) | 29 (100) | .56 | 61 (90) | 29 (100) | .27 | 67 (96) | 19 (95) | >.99 |
| Yes | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 0 (0) | ||||
| Don’t know | 2 (2) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 0 (0) | 3 (4) | 1 (5) | ||||
| Had menarche, No. (%) | ||||||||||||
| Yes | 72 (87) | 46 (87) | >.99 | 73 (95) | 27 (93) | .66 | 67 (99) | 28 (97) | .51 | 70 (100) | 20 (100) | >.99 |
| No | 11 (13) | 7 (13) | 4 (5) | 2 (7) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | ||||
aData in Table 2 represent only participants who filled out the questionnaire.
GMC Data
For the vaccine types HPV-16/18, GMCs were lower after 2 than after 3 doses (Figure 1). Noninferiority was concluded only at 2–3 years after the first dose for HPV-18. For nonvaccine types, GMCs were also lower for the 2-dose than for the 3-dose schedule, except for HPV-33/52/58 at 2–3 years and HPV-52 at 3–4 years after the first dose (Table 3). For the examined HPV types, noninferiority was concluded only at 2–3 years after the first dose for HPV-31 and at 3–4 years for HPV-52 (Table 3). The difference between 2 and 3 doses was smallest at 2–3 years (GMC ratio closest to 1) after the first dose for all examined HPV types. For vaccine types HPV-16/18 it seems that the differences are larger up to <2 years after vaccination, become smaller at 2–3 years, and increase again thereafter.
GMCs for IgG Antibodies Against 7 HPV Types Among Girls Who Received a 2- or 3-Dose Schedule of HPV Vaccinationa
| . | GMC (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | GMC Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 1619.7 (1312.9–1998.2) | 4582.5 (3827.6–5431.7) | 0.35 (.27–.46) |
| HPV-18 | 1043.1 (804.3–1352.9) | 1900.7 (1571.8–2275.6) | 0.55 (.40–.76) |
| HPV-31 | 18.5 (13.9–24.5) | 46.1 (37.3–56.8) | 0.40 (.28–.57) |
| HPV-33 | 12.2 (8.8–16.6) | 22.2 (18.4–26.8) | 0.55 (.38–.79) |
| HPV-45 | 49.4 (34.8–69.4) | 85.6 (68.7–105.6) | 0.58 (.38–.87) |
| HPV-52 | 13.9 (10.4–18.5) | 22.9 (18.9–27.7) | 0.61 (.43–.86) |
| HPV-58 | 21.8 (15.8–30.0) | 46.5 (38.1–57.4) | 0.47 (.32–.68) |
| 2–3 y | |||
| HPV-16 | 1808.4 (1312.9–2514.9) | 2591.5 (2121.8–3197.1) | 0.73 (.49–1.02) |
| HPV-18 | 871.3 (584.1–1299.8) | 982.4 (812.4–1199.9) | 0.89 (.57–1.38)b |
| HPV-31 | 24.3 (16.6–35.5) | 29.7 (22.9–38.1) | 0.82 (.52–1.29)b |
| HPV-33 | 17.1 (12.1–24.3) | 15.0 (11.6–18.5) | 1.14 (.76–1.71)b |
| HPV-45 | 45.6 (41.7–72.2) | 47.5 (37.7–60.3) | 0.96 (.67–1.38)b |
| HPV-52 | 18.5 (13.5–25.8) | 16.8 (13.6–20.7) | 1.11 (.75–1.63)b |
| HPV-58 | 34.8 (24.0–50.9) | 31.8 (25.3–40.0) | 1.09 (.70–1.70)b |
| 3–4 y | |||
| HPV-16 | 1737.1 (1248.9–2440.6) | 2751.8 (2164.6–3463.4) | 0.63 (.42–.95) |
| HPV-18 | 699.2 (473.4–1032.8) | 888.9 (685.4–1164.4) | 0.79 (.49–1.26) |
| HPV-31 | 20.1 (14.4–27.7) | 33.8 (25.8–44.7) | 0.59 (.39–.91) |
| HPV-33 | 10.5 (7.8–14.3) | 16.4 (13.1–20.7) | 0.64 (.44–.93) |
| HPV-45 | 28.5 (18.7–43.8) | 40.9 (31.8–53.0) | 0.70 (.43–1.15) |
| HPV-52 | 15.3 (11.3–21.1) | 19.5 (15.2–25.3) | 0.79 (.52–1.18)b |
| HPV-58 | 23.6 (16.6–33.4) | 37.3 (28.5–48.9) | 0.63 (.41–.98) |
| 4–4½ y | |||
| HPV-16 | 1176.1 (862.6–1587.6) | 2321.6 (1900.7–2835.6) | 0.51 (.35–.73) |
| HPV-18 | 483.0 (337.0–692.3) | 972.6 (982.4–1261.4) | 0.49 (.32–.76) |
| HPV-31 | 15.6 (9.7–25.3) | 30.6 (24.0–39.3) | 0.51 (.30–.88) |
| HPV-33 | 8.8 (6.0–13.2) | 15.5 (12.4–19.3) | 0.57 (.36–.90) |
| HPV-45 | 21.8 (13.9–34.1) | 42.9 (33.1–56.3) | 0.51 (.30–.85) |
| HPV-52 | 9.9 (5.9–16.6) | 18.4 (14.6–22.9) | 0.54 (.31–.94) |
| HPV-58 | 16.9 (11.1–25.8) | 31.2 (24.5–39.6) | 0.54 (.33–.88) |
| . | GMC (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | GMC Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 1619.7 (1312.9–1998.2) | 4582.5 (3827.6–5431.7) | 0.35 (.27–.46) |
| HPV-18 | 1043.1 (804.3–1352.9) | 1900.7 (1571.8–2275.6) | 0.55 (.40–.76) |
| HPV-31 | 18.5 (13.9–24.5) | 46.1 (37.3–56.8) | 0.40 (.28–.57) |
| HPV-33 | 12.2 (8.8–16.6) | 22.2 (18.4–26.8) | 0.55 (.38–.79) |
| HPV-45 | 49.4 (34.8–69.4) | 85.6 (68.7–105.6) | 0.58 (.38–.87) |
| HPV-52 | 13.9 (10.4–18.5) | 22.9 (18.9–27.7) | 0.61 (.43–.86) |
| HPV-58 | 21.8 (15.8–30.0) | 46.5 (38.1–57.4) | 0.47 (.32–.68) |
| 2–3 y | |||
| HPV-16 | 1808.4 (1312.9–2514.9) | 2591.5 (2121.8–3197.1) | 0.73 (.49–1.02) |
| HPV-18 | 871.3 (584.1–1299.8) | 982.4 (812.4–1199.9) | 0.89 (.57–1.38)b |
| HPV-31 | 24.3 (16.6–35.5) | 29.7 (22.9–38.1) | 0.82 (.52–1.29)b |
| HPV-33 | 17.1 (12.1–24.3) | 15.0 (11.6–18.5) | 1.14 (.76–1.71)b |
| HPV-45 | 45.6 (41.7–72.2) | 47.5 (37.7–60.3) | 0.96 (.67–1.38)b |
| HPV-52 | 18.5 (13.5–25.8) | 16.8 (13.6–20.7) | 1.11 (.75–1.63)b |
| HPV-58 | 34.8 (24.0–50.9) | 31.8 (25.3–40.0) | 1.09 (.70–1.70)b |
| 3–4 y | |||
| HPV-16 | 1737.1 (1248.9–2440.6) | 2751.8 (2164.6–3463.4) | 0.63 (.42–.95) |
| HPV-18 | 699.2 (473.4–1032.8) | 888.9 (685.4–1164.4) | 0.79 (.49–1.26) |
| HPV-31 | 20.1 (14.4–27.7) | 33.8 (25.8–44.7) | 0.59 (.39–.91) |
| HPV-33 | 10.5 (7.8–14.3) | 16.4 (13.1–20.7) | 0.64 (.44–.93) |
| HPV-45 | 28.5 (18.7–43.8) | 40.9 (31.8–53.0) | 0.70 (.43–1.15) |
| HPV-52 | 15.3 (11.3–21.1) | 19.5 (15.2–25.3) | 0.79 (.52–1.18)b |
| HPV-58 | 23.6 (16.6–33.4) | 37.3 (28.5–48.9) | 0.63 (.41–.98) |
| 4–4½ y | |||
| HPV-16 | 1176.1 (862.6–1587.6) | 2321.6 (1900.7–2835.6) | 0.51 (.35–.73) |
| HPV-18 | 483.0 (337.0–692.3) | 972.6 (982.4–1261.4) | 0.49 (.32–.76) |
| HPV-31 | 15.6 (9.7–25.3) | 30.6 (24.0–39.3) | 0.51 (.30–.88) |
| HPV-33 | 8.8 (6.0–13.2) | 15.5 (12.4–19.3) | 0.57 (.36–.90) |
| HPV-45 | 21.8 (13.9–34.1) | 42.9 (33.1–56.3) | 0.51 (.30–.85) |
| HPV-52 | 9.9 (5.9–16.6) | 18.4 (14.6–22.9) | 0.54 (.31–.94) |
| HPV-58 | 16.9 (11.1–25.8) | 31.2 (24.5–39.6) | 0.54 (.33–.88) |
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; HPV, human papillomavirus; IgG, immunoglobulin G.
aGMC ratios represent GMC for 2-dose schedule divided by GMC for 3-dose schedule. If the 95% CI of the GMC ratio is >.5, noninferiority can be concluded.
bNoninferior (lower boundary 95% CI, >.5).
GMCs for IgG Antibodies Against 7 HPV Types Among Girls Who Received a 2- or 3-Dose Schedule of HPV Vaccinationa
| . | GMC (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | GMC Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 1619.7 (1312.9–1998.2) | 4582.5 (3827.6–5431.7) | 0.35 (.27–.46) |
| HPV-18 | 1043.1 (804.3–1352.9) | 1900.7 (1571.8–2275.6) | 0.55 (.40–.76) |
| HPV-31 | 18.5 (13.9–24.5) | 46.1 (37.3–56.8) | 0.40 (.28–.57) |
| HPV-33 | 12.2 (8.8–16.6) | 22.2 (18.4–26.8) | 0.55 (.38–.79) |
| HPV-45 | 49.4 (34.8–69.4) | 85.6 (68.7–105.6) | 0.58 (.38–.87) |
| HPV-52 | 13.9 (10.4–18.5) | 22.9 (18.9–27.7) | 0.61 (.43–.86) |
| HPV-58 | 21.8 (15.8–30.0) | 46.5 (38.1–57.4) | 0.47 (.32–.68) |
| 2–3 y | |||
| HPV-16 | 1808.4 (1312.9–2514.9) | 2591.5 (2121.8–3197.1) | 0.73 (.49–1.02) |
| HPV-18 | 871.3 (584.1–1299.8) | 982.4 (812.4–1199.9) | 0.89 (.57–1.38)b |
| HPV-31 | 24.3 (16.6–35.5) | 29.7 (22.9–38.1) | 0.82 (.52–1.29)b |
| HPV-33 | 17.1 (12.1–24.3) | 15.0 (11.6–18.5) | 1.14 (.76–1.71)b |
| HPV-45 | 45.6 (41.7–72.2) | 47.5 (37.7–60.3) | 0.96 (.67–1.38)b |
| HPV-52 | 18.5 (13.5–25.8) | 16.8 (13.6–20.7) | 1.11 (.75–1.63)b |
| HPV-58 | 34.8 (24.0–50.9) | 31.8 (25.3–40.0) | 1.09 (.70–1.70)b |
| 3–4 y | |||
| HPV-16 | 1737.1 (1248.9–2440.6) | 2751.8 (2164.6–3463.4) | 0.63 (.42–.95) |
| HPV-18 | 699.2 (473.4–1032.8) | 888.9 (685.4–1164.4) | 0.79 (.49–1.26) |
| HPV-31 | 20.1 (14.4–27.7) | 33.8 (25.8–44.7) | 0.59 (.39–.91) |
| HPV-33 | 10.5 (7.8–14.3) | 16.4 (13.1–20.7) | 0.64 (.44–.93) |
| HPV-45 | 28.5 (18.7–43.8) | 40.9 (31.8–53.0) | 0.70 (.43–1.15) |
| HPV-52 | 15.3 (11.3–21.1) | 19.5 (15.2–25.3) | 0.79 (.52–1.18)b |
| HPV-58 | 23.6 (16.6–33.4) | 37.3 (28.5–48.9) | 0.63 (.41–.98) |
| 4–4½ y | |||
| HPV-16 | 1176.1 (862.6–1587.6) | 2321.6 (1900.7–2835.6) | 0.51 (.35–.73) |
| HPV-18 | 483.0 (337.0–692.3) | 972.6 (982.4–1261.4) | 0.49 (.32–.76) |
| HPV-31 | 15.6 (9.7–25.3) | 30.6 (24.0–39.3) | 0.51 (.30–.88) |
| HPV-33 | 8.8 (6.0–13.2) | 15.5 (12.4–19.3) | 0.57 (.36–.90) |
| HPV-45 | 21.8 (13.9–34.1) | 42.9 (33.1–56.3) | 0.51 (.30–.85) |
| HPV-52 | 9.9 (5.9–16.6) | 18.4 (14.6–22.9) | 0.54 (.31–.94) |
| HPV-58 | 16.9 (11.1–25.8) | 31.2 (24.5–39.6) | 0.54 (.33–.88) |
| . | GMC (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | GMC Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 1619.7 (1312.9–1998.2) | 4582.5 (3827.6–5431.7) | 0.35 (.27–.46) |
| HPV-18 | 1043.1 (804.3–1352.9) | 1900.7 (1571.8–2275.6) | 0.55 (.40–.76) |
| HPV-31 | 18.5 (13.9–24.5) | 46.1 (37.3–56.8) | 0.40 (.28–.57) |
| HPV-33 | 12.2 (8.8–16.6) | 22.2 (18.4–26.8) | 0.55 (.38–.79) |
| HPV-45 | 49.4 (34.8–69.4) | 85.6 (68.7–105.6) | 0.58 (.38–.87) |
| HPV-52 | 13.9 (10.4–18.5) | 22.9 (18.9–27.7) | 0.61 (.43–.86) |
| HPV-58 | 21.8 (15.8–30.0) | 46.5 (38.1–57.4) | 0.47 (.32–.68) |
| 2–3 y | |||
| HPV-16 | 1808.4 (1312.9–2514.9) | 2591.5 (2121.8–3197.1) | 0.73 (.49–1.02) |
| HPV-18 | 871.3 (584.1–1299.8) | 982.4 (812.4–1199.9) | 0.89 (.57–1.38)b |
| HPV-31 | 24.3 (16.6–35.5) | 29.7 (22.9–38.1) | 0.82 (.52–1.29)b |
| HPV-33 | 17.1 (12.1–24.3) | 15.0 (11.6–18.5) | 1.14 (.76–1.71)b |
| HPV-45 | 45.6 (41.7–72.2) | 47.5 (37.7–60.3) | 0.96 (.67–1.38)b |
| HPV-52 | 18.5 (13.5–25.8) | 16.8 (13.6–20.7) | 1.11 (.75–1.63)b |
| HPV-58 | 34.8 (24.0–50.9) | 31.8 (25.3–40.0) | 1.09 (.70–1.70)b |
| 3–4 y | |||
| HPV-16 | 1737.1 (1248.9–2440.6) | 2751.8 (2164.6–3463.4) | 0.63 (.42–.95) |
| HPV-18 | 699.2 (473.4–1032.8) | 888.9 (685.4–1164.4) | 0.79 (.49–1.26) |
| HPV-31 | 20.1 (14.4–27.7) | 33.8 (25.8–44.7) | 0.59 (.39–.91) |
| HPV-33 | 10.5 (7.8–14.3) | 16.4 (13.1–20.7) | 0.64 (.44–.93) |
| HPV-45 | 28.5 (18.7–43.8) | 40.9 (31.8–53.0) | 0.70 (.43–1.15) |
| HPV-52 | 15.3 (11.3–21.1) | 19.5 (15.2–25.3) | 0.79 (.52–1.18)b |
| HPV-58 | 23.6 (16.6–33.4) | 37.3 (28.5–48.9) | 0.63 (.41–.98) |
| 4–4½ y | |||
| HPV-16 | 1176.1 (862.6–1587.6) | 2321.6 (1900.7–2835.6) | 0.51 (.35–.73) |
| HPV-18 | 483.0 (337.0–692.3) | 972.6 (982.4–1261.4) | 0.49 (.32–.76) |
| HPV-31 | 15.6 (9.7–25.3) | 30.6 (24.0–39.3) | 0.51 (.30–.88) |
| HPV-33 | 8.8 (6.0–13.2) | 15.5 (12.4–19.3) | 0.57 (.36–.90) |
| HPV-45 | 21.8 (13.9–34.1) | 42.9 (33.1–56.3) | 0.51 (.30–.85) |
| HPV-52 | 9.9 (5.9–16.6) | 18.4 (14.6–22.9) | 0.54 (.31–.94) |
| HPV-58 | 16.9 (11.1–25.8) | 31.2 (24.5–39.6) | 0.54 (.33–.88) |
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; HPV, human papillomavirus; IgG, immunoglobulin G.
aGMC ratios represent GMC for 2-dose schedule divided by GMC for 3-dose schedule. If the 95% CI of the GMC ratio is >.5, noninferiority can be concluded.
bNoninferior (lower boundary 95% CI, >.5).
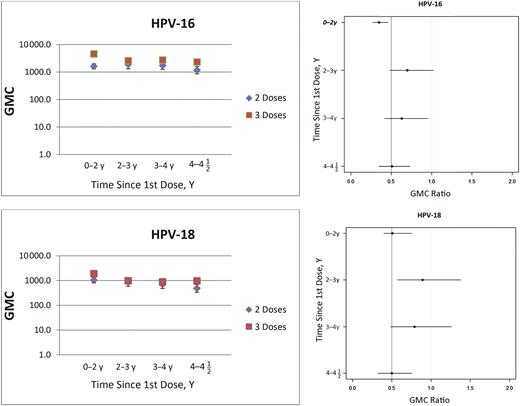
Geometric mean concentrations (GMCs) (left) and GMC ratios (right) for vaccine human papillomavirus (HPV) types; ratios represent GMC for 2-dose schedule divided by GMC for 3-dose schedule. Dashed lines in the right panel represent noninferiority margin of .5.
Avidity
There were no significant differences in sociodemographic characteristics between participants in whom avidity was examined and those in whom avidity was not determined, except for country of birth of the mother (data not shown). In participants in whom avidity was examined, the country of birth of the mother was more often outside Europe. In general, geometric mean AIs were higher for vaccine types than for the other HPV types measured (Figure 2). For HPV-16, the geometric mean AI varied between 74% and 77% for 3 and between 67% and 80% for 2 doses. For HPV-18 this was 63%–67% and 54%–79%, respectively. For vaccine types HPV-16/18, the geometric mean AI was noninferior up to 4½ years after vaccination for 2 compared with 3 doses. Moreover, at most time points noninferiority for cross-protective types HPV-31/33/45 could be concluded, except for HPV-31 at 4–4½ years and HPV-33 at 3–4 and 4–4½ years after vaccination (Table 4).
| . | Geometric Mean AI (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | Geometric Mean AI Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 69 (64–74) | 76 (73–79) | 0.91 (.84–1.00)b |
| HPV-18 | 60 (51–71) | 63 (56–71) | 0.95 (.78–1.16)b |
| HPV-31 | 20 (15–27) | 22 (18–27) | 0.93 (.66–1.32)b |
| HPV-33 | 31 (24–40) | 32 (28–38) | 0.96 (.71–1.29)b |
| HPV-45 | 21 (17–26) | 27 (23–32) | 0.76 (.58–1.01)b |
| HPV-52 | 17 (14–22) | 18 (14–22) | 0.95 (.70–1.30)b |
| HPV-58 | 17 (13–23) | 20 (13–23) | 0.87 (.60–1.25)b |
| 2–3 y | |||
| HPV-16 | 69 (64–76) | 74 (72–77) | 0.93 (.85–1.02)b |
| HPV-18 | 65 (58–73) | 67 (61–74) | 0.97 (.82–1.13)b |
| HPV-31 | 26 (20–33) | 24 (19–29) | 1.09 (.79–1.51)b |
| HPV-33 | 36 (23–55) | 40 (35–46) | 0.90 (.57–1.40)b |
| HPV-45 | 27 (22–36) | 27 (23–32) | 0.99 (.74–1.33)b |
| HPV-52 | 15 (12–19) | 18 (15–22) | 0.85 (.63–1.15)b |
| HPV-58 | 15 (12–19) | 19 (15–24) | 0.79 (.42–1.47) |
| 3–4 y | |||
| HPV-16 | 80 (73–86) | 77 (74–80) | 1.04 (.95–1.14)b |
| HPV-18 | 79 (59–74) | 63 (56–72) | 1.25 (1.05–1.48)b |
| HPV-31 | 65 (59–73) | 22 (18–26) | 3.00 (2.40–3.76)b |
| HPV-33 | 15 (11–20) | 34 (29–40) | 0.45 (.32–.63) |
| HPV-45 | 32 (22–45) | 29 (25–34) | 1.07 (.73–1.58)b |
| HPV-52 | 26 (21–31) | 15 (13–18) | 1.67 (1.29–2.16)b |
| HPV-58 | 11 (8–16) | 20 (16–25) | 0.57 (.39–.83) |
| 4–4½ y | |||
| HPV-16 | 67 (62–73) | 74 (70–77) | 0.91 (.83–1.00)b |
| HPV-18 | 54 (39–74) | 66 (59–76) | 0.81 (.57–1.14)b |
| HPV-31 | 16 (8–31) | 23 (18–28) | 0.69 (.34–1.40) |
| HPV-33 | 30 (13–75) | 29 (23–38) | 1.03 (.41–2.60) |
| HPV-45 | 33 (18–60) | 30 (26–36) | 1.09 (.59–2.04)b |
| HPV-52 | 16 (10–24) | 17 (14–20) | 0.92 (.58–1.48)b |
| HPV-58 | 16 (6–45) | 18 (15–23) | 0.88 (.31–2.49) |
| . | Geometric Mean AI (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | Geometric Mean AI Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 69 (64–74) | 76 (73–79) | 0.91 (.84–1.00)b |
| HPV-18 | 60 (51–71) | 63 (56–71) | 0.95 (.78–1.16)b |
| HPV-31 | 20 (15–27) | 22 (18–27) | 0.93 (.66–1.32)b |
| HPV-33 | 31 (24–40) | 32 (28–38) | 0.96 (.71–1.29)b |
| HPV-45 | 21 (17–26) | 27 (23–32) | 0.76 (.58–1.01)b |
| HPV-52 | 17 (14–22) | 18 (14–22) | 0.95 (.70–1.30)b |
| HPV-58 | 17 (13–23) | 20 (13–23) | 0.87 (.60–1.25)b |
| 2–3 y | |||
| HPV-16 | 69 (64–76) | 74 (72–77) | 0.93 (.85–1.02)b |
| HPV-18 | 65 (58–73) | 67 (61–74) | 0.97 (.82–1.13)b |
| HPV-31 | 26 (20–33) | 24 (19–29) | 1.09 (.79–1.51)b |
| HPV-33 | 36 (23–55) | 40 (35–46) | 0.90 (.57–1.40)b |
| HPV-45 | 27 (22–36) | 27 (23–32) | 0.99 (.74–1.33)b |
| HPV-52 | 15 (12–19) | 18 (15–22) | 0.85 (.63–1.15)b |
| HPV-58 | 15 (12–19) | 19 (15–24) | 0.79 (.42–1.47) |
| 3–4 y | |||
| HPV-16 | 80 (73–86) | 77 (74–80) | 1.04 (.95–1.14)b |
| HPV-18 | 79 (59–74) | 63 (56–72) | 1.25 (1.05–1.48)b |
| HPV-31 | 65 (59–73) | 22 (18–26) | 3.00 (2.40–3.76)b |
| HPV-33 | 15 (11–20) | 34 (29–40) | 0.45 (.32–.63) |
| HPV-45 | 32 (22–45) | 29 (25–34) | 1.07 (.73–1.58)b |
| HPV-52 | 26 (21–31) | 15 (13–18) | 1.67 (1.29–2.16)b |
| HPV-58 | 11 (8–16) | 20 (16–25) | 0.57 (.39–.83) |
| 4–4½ y | |||
| HPV-16 | 67 (62–73) | 74 (70–77) | 0.91 (.83–1.00)b |
| HPV-18 | 54 (39–74) | 66 (59–76) | 0.81 (.57–1.14)b |
| HPV-31 | 16 (8–31) | 23 (18–28) | 0.69 (.34–1.40) |
| HPV-33 | 30 (13–75) | 29 (23–38) | 1.03 (.41–2.60) |
| HPV-45 | 33 (18–60) | 30 (26–36) | 1.09 (.59–2.04)b |
| HPV-52 | 16 (10–24) | 17 (14–20) | 0.92 (.58–1.48)b |
| HPV-58 | 16 (6–45) | 18 (15–23) | 0.88 (.31–2.49) |
Abbreviations: AI, avidity index; CI, confidence interval; HPV, human papillomavirus.
aGeometric mean AI ratio represents the geometric mean AI after the 2-dose schedule divided by that after the 3-dose schedule. Noninferiority can be concluded if the 95% CI is >.5.
bNoninferior (lower boundary 95% CI, >.5).
| . | Geometric Mean AI (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | Geometric Mean AI Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 69 (64–74) | 76 (73–79) | 0.91 (.84–1.00)b |
| HPV-18 | 60 (51–71) | 63 (56–71) | 0.95 (.78–1.16)b |
| HPV-31 | 20 (15–27) | 22 (18–27) | 0.93 (.66–1.32)b |
| HPV-33 | 31 (24–40) | 32 (28–38) | 0.96 (.71–1.29)b |
| HPV-45 | 21 (17–26) | 27 (23–32) | 0.76 (.58–1.01)b |
| HPV-52 | 17 (14–22) | 18 (14–22) | 0.95 (.70–1.30)b |
| HPV-58 | 17 (13–23) | 20 (13–23) | 0.87 (.60–1.25)b |
| 2–3 y | |||
| HPV-16 | 69 (64–76) | 74 (72–77) | 0.93 (.85–1.02)b |
| HPV-18 | 65 (58–73) | 67 (61–74) | 0.97 (.82–1.13)b |
| HPV-31 | 26 (20–33) | 24 (19–29) | 1.09 (.79–1.51)b |
| HPV-33 | 36 (23–55) | 40 (35–46) | 0.90 (.57–1.40)b |
| HPV-45 | 27 (22–36) | 27 (23–32) | 0.99 (.74–1.33)b |
| HPV-52 | 15 (12–19) | 18 (15–22) | 0.85 (.63–1.15)b |
| HPV-58 | 15 (12–19) | 19 (15–24) | 0.79 (.42–1.47) |
| 3–4 y | |||
| HPV-16 | 80 (73–86) | 77 (74–80) | 1.04 (.95–1.14)b |
| HPV-18 | 79 (59–74) | 63 (56–72) | 1.25 (1.05–1.48)b |
| HPV-31 | 65 (59–73) | 22 (18–26) | 3.00 (2.40–3.76)b |
| HPV-33 | 15 (11–20) | 34 (29–40) | 0.45 (.32–.63) |
| HPV-45 | 32 (22–45) | 29 (25–34) | 1.07 (.73–1.58)b |
| HPV-52 | 26 (21–31) | 15 (13–18) | 1.67 (1.29–2.16)b |
| HPV-58 | 11 (8–16) | 20 (16–25) | 0.57 (.39–.83) |
| 4–4½ y | |||
| HPV-16 | 67 (62–73) | 74 (70–77) | 0.91 (.83–1.00)b |
| HPV-18 | 54 (39–74) | 66 (59–76) | 0.81 (.57–1.14)b |
| HPV-31 | 16 (8–31) | 23 (18–28) | 0.69 (.34–1.40) |
| HPV-33 | 30 (13–75) | 29 (23–38) | 1.03 (.41–2.60) |
| HPV-45 | 33 (18–60) | 30 (26–36) | 1.09 (.59–2.04)b |
| HPV-52 | 16 (10–24) | 17 (14–20) | 0.92 (.58–1.48)b |
| HPV-58 | 16 (6–45) | 18 (15–23) | 0.88 (.31–2.49) |
| . | Geometric Mean AI (95% CI) . | . | |
|---|---|---|---|
| HPV Type by Time Since 1st Dose . | Geometric Mean AI Ratio (95% CI) . | 2-Dose Schedule . | 3-Dose Schedule . |
| 0–2 y | |||
| HPV-16 | 69 (64–74) | 76 (73–79) | 0.91 (.84–1.00)b |
| HPV-18 | 60 (51–71) | 63 (56–71) | 0.95 (.78–1.16)b |
| HPV-31 | 20 (15–27) | 22 (18–27) | 0.93 (.66–1.32)b |
| HPV-33 | 31 (24–40) | 32 (28–38) | 0.96 (.71–1.29)b |
| HPV-45 | 21 (17–26) | 27 (23–32) | 0.76 (.58–1.01)b |
| HPV-52 | 17 (14–22) | 18 (14–22) | 0.95 (.70–1.30)b |
| HPV-58 | 17 (13–23) | 20 (13–23) | 0.87 (.60–1.25)b |
| 2–3 y | |||
| HPV-16 | 69 (64–76) | 74 (72–77) | 0.93 (.85–1.02)b |
| HPV-18 | 65 (58–73) | 67 (61–74) | 0.97 (.82–1.13)b |
| HPV-31 | 26 (20–33) | 24 (19–29) | 1.09 (.79–1.51)b |
| HPV-33 | 36 (23–55) | 40 (35–46) | 0.90 (.57–1.40)b |
| HPV-45 | 27 (22–36) | 27 (23–32) | 0.99 (.74–1.33)b |
| HPV-52 | 15 (12–19) | 18 (15–22) | 0.85 (.63–1.15)b |
| HPV-58 | 15 (12–19) | 19 (15–24) | 0.79 (.42–1.47) |
| 3–4 y | |||
| HPV-16 | 80 (73–86) | 77 (74–80) | 1.04 (.95–1.14)b |
| HPV-18 | 79 (59–74) | 63 (56–72) | 1.25 (1.05–1.48)b |
| HPV-31 | 65 (59–73) | 22 (18–26) | 3.00 (2.40–3.76)b |
| HPV-33 | 15 (11–20) | 34 (29–40) | 0.45 (.32–.63) |
| HPV-45 | 32 (22–45) | 29 (25–34) | 1.07 (.73–1.58)b |
| HPV-52 | 26 (21–31) | 15 (13–18) | 1.67 (1.29–2.16)b |
| HPV-58 | 11 (8–16) | 20 (16–25) | 0.57 (.39–.83) |
| 4–4½ y | |||
| HPV-16 | 67 (62–73) | 74 (70–77) | 0.91 (.83–1.00)b |
| HPV-18 | 54 (39–74) | 66 (59–76) | 0.81 (.57–1.14)b |
| HPV-31 | 16 (8–31) | 23 (18–28) | 0.69 (.34–1.40) |
| HPV-33 | 30 (13–75) | 29 (23–38) | 1.03 (.41–2.60) |
| HPV-45 | 33 (18–60) | 30 (26–36) | 1.09 (.59–2.04)b |
| HPV-52 | 16 (10–24) | 17 (14–20) | 0.92 (.58–1.48)b |
| HPV-58 | 16 (6–45) | 18 (15–23) | 0.88 (.31–2.49) |
Abbreviations: AI, avidity index; CI, confidence interval; HPV, human papillomavirus.
aGeometric mean AI ratio represents the geometric mean AI after the 2-dose schedule divided by that after the 3-dose schedule. Noninferiority can be concluded if the 95% CI is >.5.
bNoninferior (lower boundary 95% CI, >.5).
Sensitivity Analysis
Among recipients with 2 or 3 doses documented in the vaccination registry, the self-reported vaccination status was consistent with the vaccination registry in 70.4% and 80.4%, respectively. Restricting the analysis to participants who reported the same number of doses in the questionnaire as documented in the vaccination registry showed that the differences in GMCs for HPV-16 and HPV-18 between dosing schedules were larger than found in the original analyses. Noninferiority could be concluded for none of the time points (Supplementary Table 1). We also analyzed the antibody avidity using this reclassification. The number of participants who had received 2 doses based on the vaccination registry and self-reported vaccination status between 4–4½ years after the first dose was very small (n = 2) and resulted in very broad CIs around the geometric mean AI and corresponding ratio. All other time points showed noninferior antibody avidity for HPV-16 and HPV-18, which was in line with findings based on the vaccination registry only (Supplementary Table 2).
DISCUSSION
This study aimed to compare the quantity and quality of antibody responses up to 4½ years after the first dose in routinely vaccinated young girls who had received 2 or 3 doses of HPV vaccination, by measuring antibody concentration and avidity. Most studies on this topic so far have been clinical trials and/or have compared antibody concentrations of 2 doses in girls to 3 doses in young adults. We found that GMCs for vaccine and cross-protective types after 2 doses were only noninferior to 3 doses at 2–3 years after vaccination for vaccine type HPV-18 and cross-protective types HPV-31/33/45. However, for HPV-16/18 at all time points and for cross-protecting types, AIs showed noninferiority at almost all time points.
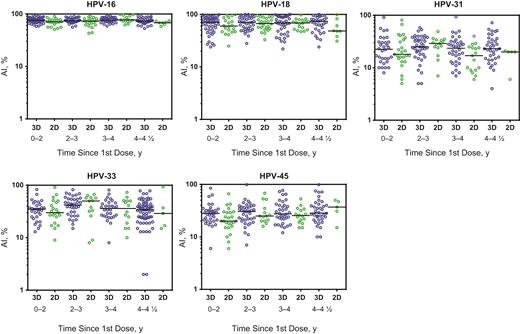
Avidity index (AI) for vaccine and cross-protective types up until 4 1/2 years since the first dose with the 2-dose (2D) and 3-dose (3D) schedules. Black lines indicate the median antibody AI for each group. Abbreviation: HPV, human papillomavirus.
The observed GMCs after both 2 and 3 doses are high. In accordance with findings of previous studies comparing 2 with 3 doses within the same age group, the antibody concentrations after 3 doses were generally higher [19, 22]. In a population-based study, Lazcano-Ponce et al [19] showed noninferior estimates for HPV-16/18 up to 21 months after the first dose (GMT ratio for 3 vs 2 doses received at age 9–10 years, 1.7 and 1.7, respectively; 95% CI, 1.5–1.8 and 1.5–1.9). In contrast, for girls aged 9–14 years, Romanowski et al [22] did not show noninferiority of 2 doses at 7 and 24 months after the first dose for HPV-16 and at 24 months for HPV-18. A study comparing immunogenicity and effectiveness after different dosing schedules of the quadrivalent vaccine also showed noninferior antibody levels up to 48 months within the same age group [23]. In comparing antibody concentrations over time in the current study it should be taken into account that measurements at different time points were performed in different participants, given the study design.
Although we found some differences in the level of antibody concentrations between 2 and 3 doses, implication of these differences is unknown. Despite that registration of the 2-dose schedule was mainly based on findings on antibody concentrations, a threshold of protection for HPV is unknown [8, 9]. Furthermore, besides antibody concentrations, other immune factors might also influence the protection and impact of HPV vaccination [14].
The geometric mean AI ratios show a more or less stable pattern over time. Compared with GMC ratios, the AI ratios are closer to 1, meaning less difference between the schedules. Hence, a 2-dose schedule generates geometric mean AIs that are noninferior to those generated by the 3-dose schedule for both vaccine and cross-protective types. This is in line with data from Boxus et al [27], who observed no differences in avidity between 2 or 3 doses of the quadrivalent vaccine (in girls aged 9–14 years) for HPV-16/18 up to month 48 [27]. Furthermore, for the quadrivalent HPV vaccine, the AI was shown to be noninferior in girls aged 10–18 years [23]. Collectively, these data indicate that although the levels of antibodies may differ between dosing schedules, the AIs remain similar whether 2 or 3 doses of HPV vaccine are given [27–30]. In this context, the suggestion from Scherpenisse et al [26] is interesting—that is, that in addition to antibody concentrations AI levels could be used to differentiate HPV-protective from nonprotective antibodies and could therefore serve as a possible immune surrogate. Avidity is an indicator of antibody quality. Low concentrations of high-avidity antibodies might be able to provide sufficient protection. On the other hand, with higher antibody concentrations, the total number of highly avid antibodies becomes higher. In the absence of a correlate of protection, the exact mechanism is difficult to predict. However, Safaeian et al [31] showed that persons who developed an incident HPV-31 infection were more likely to have lower HPV-16 antibody avidity.
The current study focused on immunological aspects after different doses of HPV vaccine. Effectiveness was not taken into account. At this time, several studies have looked into efficacy after 2-dose schedules; most of them were post hoc analyses that were not powered for this comparison, in groups who had accidentally received alternate dosage schedules and in whom the second dose was mostly not given within the recommended time window. No significant differences were observed between different dosing schedules for vaccine types; however, only 3 and 2 doses given 6 months apart showed significant efficacy against cross-protective types HPV-31/33/45 [32]. A Scottish study examining HPV prevalence found no significant differences between different schedules for vaccine types, but a significant reduction for cross-protective types HPV-31/33/45 was shown only after 3 doses [33].
To our knowledge, ours is the first study to determine antibody concentrations and antibody avidity for different dosing schedules in young girls against cross-protective HPV vaccination types up to 4½ years after the first dose for the bivalent vaccine. To minimize assay variability for the AI measurement, all assays were performed in a single run by the same technician. We found some differences between the vaccination status as reported in the vaccination registry and the self-reported vaccination status. Although previous studies have shown that the vaccination status from a registry might be more reliable than the self-reported vaccination status [34, 35], we explored the influence of possible registration errors in sensitivity analyses. These analyses strengthen our previous findings. Limitations of our study include the lack of efficacy data and possibly suboptimal power, because the ideal sample size for both schedules could not always be realized for all cohorts. In addition, girls receiving 2 doses were originally eligible for 3 doses, although they had received the doses ≥5 months apart.
In conclusion, our data show that while there are differences in antibody concentrations between 2 and 3 doses among young girls, the antibody avidity does not differ. However, until a correlate of protection has been detected, long-term evaluation of the protective effect of reduced dosing schedules against cervical intraepithelial neoplasia lesions (and intermediate end points) in studies designed and powered for this issue is important.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participating girls and their parents for their contributions to this study. We also thank Marjan van Maurik (Center for Infectious Disease Control, National Institute for Public Health and the Environment) for her help with the serological evaluation of the samples and Petra Oomen (Department of Vaccine Supply and Prevention Programs, National Institute for Public Health and the Environment) for selecting eligible girls from the vaccination registry.
Financial support. This work was supported by and performed at the Dutch National Institute for Public Health and the Environment.
Potential conflicts of interest. C. J. L. M. M. reports personal fees from Qiagen, Merck/Sanofi Pasteur MSD, GlaxoSmithKline, Roche Diagnostics, and Genticel and lecture fees from Seegene and Menarini, outside the submitted work. He also has a patented high-risk HPV test and patented methylation markers for cervical screening licensed to Self-Screen and is minority shareholder in Self-screen, a spin-off company of VU University Medical Center. Until 1 April 2016, he was minority stockholder in Diassay, a spin-off company of VU University Medical Center, and until 2014 he held a small number of certificates of shares in Delphi Biosciences, which went into receivership in 2014. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 30th International Papillomavirus Conference; Lisbon, Portugal; 17–21 September 2015. Abstract HPV15-0523.
Correspondence: R. Donken, MSc, National Institute for Public Health and the Environment, PO Box 1, 3720 BA, the Netherlands ([email protected]).




