-
PDF
- Split View
-
Views
-
Cite
Cite
Zhisheng Her, Yiu-Wing Kam, Victor C. Gan, Bernett Lee, Tun-Linn Thein, Jeslin J. L. Tan, SIgN Immunomonitoring Platform, Linda K. Lee, Katja Fink, David C. Lye, Laurent Rénia, Yee-Sin Leo, Lisa F. P. Ng, Severity of Plasma Leakage Is Associated With High Levels of Interferon γ–Inducible Protein 10, Hepatocyte Growth Factor, Matrix Metalloproteinase 2 (MMP-2), and MMP-9 During Dengue Virus Infection, The Journal of Infectious Diseases, Volume 215, Issue 1, 1 January 2017, Pages 42–51, https://doi.org/10.1093/infdis/jiw494
Close - Share Icon Share
Dengue virus infection typically causes mild dengue fever, but, in severe cases, life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) occur. The pathophysiological hallmark of DHF and DSS is plasma leakage that leads to enhanced vascular permeability, likely due to a cytokine storm.
Ninety patients with dengue during 2010–2012 in Singapore were prospectively recruited and stratified according to their disease phase, primary and secondary infection status, and disease severity, measured by plasma leakage. Clinical parameters were recorded throughout the disease progression. The levels of various immune mediators were quantified using comprehensive multiplex microbead-based immunoassays for 46 immune mediators.
Associations between clinical parameters and immune mediators were analyzed using various statistical methods. Potential immune markers, including interleukin 1 receptor antagonist, interferon γ–inducible protein 10, hepatocyte growth factor, soluble p75 tumor necrosis factor α receptor, vascular cell adhesion molecule 1, and matrix metalloproteinase 2, were significantly associated with significant plasma leakage. Secondary dengue virus infections were also shown to influence disease outcome in terms of disease severity.
This study identified several key markers for exacerbated dengue pathogenesis, notably plasma leakage. This will allow a better understanding of the molecular mechanisms of DHF and DSS in patients with dengue.
Dengue is an important human arboviral disease that is caused by infection with 4 antigenically related strains of dengue virus (DENV-1–4), which belong to the Flaviviridae family [1]. Despite extensive worldwide efforts, it remains a major public health concern: 55% of the world's population is estimated to be at risk for DENV infection, and there are approximately 390 million DENV infections annually, of which 96 million are symptomatic [2]. Infection by any of the 4 DENV serotypes can cause a wide spectrum of disease manifestations that ranges from mild, self-limiting dengue hemorrhagic fever (DHF) to severe, life-threatening dengue shock syndrome (DSS). The major pathophysiological hallmark that determines disease severity and differentiates DHF and DSS from dengue fever is the degree of plasma leakage, characterized by rising hematocrit, hypoalbuminemia, ascites, or pleural effusion [1, 3]. Plasma leakage is a consequence of increased vascular permeability, in which a dysfunction of endothelial cells and abnormal hemostasis occurs during the critical phase of the disease (3–7 days of illness onset) [1, 4]. In cases where plasma leakage becomes extensive, patients can develop profound hypovolemic DSS with fatal outcome [5, 6].
Although the pathogenesis of DHF remains elusive, evidence from clinical studies has indicated extensive immune activation with the overproduction of cytokines [5]. A cytokine storm was associated with enhancement of vascular permeability, leading to plasma leakage in dengue [7, 8]. Furthermore, DENV infection in immature dendritic cells and macrovascular endothelial cells increased endothelial permeability through the overproduction of matrix metalloproteinase 9 (MMP-9) and MMP-2, respectively [9, 10]. Collectively, these studies highlight the need to examine the role and regulation of host factors in dengue-associated vascular pathophysiology. While the underlying mechanism of the cytokine storm in DENV infections is still unknown, various factors and activities, including immune complexes, enhancing antibodies, complement, cross-reactive memory T-cell activation, virus virulence, and host genetic polymorphisms, have been postulated [11].
In this study, we sought to identify immune mediators of plasma leakage in 90 patients with dengue prospectively recruited during 2010–2012 as part of the Prospective Adult Dengue Study at Tan Tock Seng Hospital, Singapore [12]. Patients were classified according to phase of DENV infection, primary versus secondary infection, and severity of plasma leakage. Comprehensive multiplex microbead arrays, clinical parameters, differential patterns of immune mediators, and MMPs were analyzed to temporally associate with the severity of plasma leakage and the important influence of secondary DENV infections. These findings will provide useful predictors of significant plasma leakage and have important implications in the development of novel and effective therapeutic strategies to manage severe DENV infections.
MATERIALS AND METHODS
Ethical Approval
Written informed consent was obtained from all participants in accordance with the principles of the Declaration of Helsinki. All procedures adopted in this study were approved by the National Healthcare Group Domain-Specific Review Board (DSRB/E/09/00432).
Patients and Plasma Collection
We investigated 90 patients with dengue who were selected from a group of 400 patients recruited at the Communicable Disease Centre, Tan Tock Seng Hospital, between January 2010 and September 2012 [11]. Inclusion criteria included fever ≤5 days after onset of fever and the availability of paired sera. Diagnostic testing was performed by the Environmental Health Institute, a World Health Organization Collaborating Center for Reference and Research on Arbovirus and Its Associated Vectors, in Singapore. Patients received a diagnosis of DENV infection on the basis of detection of DENV NS1 antigen and DENV RNA [12]. Crossing-point values were used to derive viral loads from a standard curve generated by 10-fold serial dilutions of a stock containing 108 plaque-forming units/mL. Patients with influenza virus infection confirmed by findings of polymerase chain reaction analysis at the Department of Laboratory Medicine, Tan Tock Seng Hospital, were included as controls. Data on demographic characteristics and comorbidities were collected on admission. Daily clinical data and findings of hematological and biochemical laboratory testing were collected prospectively from patients until they were discharged from the hospital or reached the recovery phase of illness. Patients were classified according to their phase of disease (febrile, critical, and convalescent), type of infection (primary and secondary), and severity of plasma leakage (none, mild, and significant) [1, 13]. Significant plasma leakage was defined by a hematocrit change of >20% in the full blood count or by clinical detection of free fluid in lungs or abdomen by the treating physician. Patients with a low serum total protein level (<63 g/L; normal range, 64–83 g/L) without apparent fluid accumulation were classified as having mild plasma leakage. Patients who did not fulfill these criteria are classified as having no plasma leakage. Plasma specimens from patients with dengue and 4 patients with influenza (as controls) were collected, aliquoted, and stored at −80°C until use.
Cytokine Quantification in Plasma, Using a Microbead-Based Immunoassay
Cytokine levels in human plasma were measured simultaneously, using a premixed 36-plex Procarta multiplex microbead-based immunoassay (Affymetrix). The 36 cytokines and chemokines assayed included epidermal growth factor (EGF), granulocyte colony-stimulating factor, hepatocyte growth factor (HGF), interferon (IFN) α2, IFN-γ, interleukin 1β, interleukin 10 (IL-10), interleukin 13 (IL-13), interleukin 17A (IL-17A), interleukin 17F (IL-17F), interleukin 1 receptor antagonist (IL-1RA), serum-soluble interleukin 2 receptor antagonist (sIL-2RA), interleukin 4 (IL-4), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 8 (IL-8), interleukin 9 (IL-9), IFN-γ–inducible protein 10 (IP-10), IFN-inducible T-cell α chemoattractant, monocyte chemoattractant protein 1 (MCP-1), MCP-3, macrophage migration inhibitory factor, monokine induced by IFN-γ, macrophage inflammatory protein (MIP) 1α, MIP-1β, plasminogen activator inhibitor type 1, platelet-derived growth factor BB (PDGF-BB), regulated on activation, normal T-cell expressed and secreted (RANTES), stem cell factor, transforming growth factor β1 (TGF-β1), tumor necrosis factor α (TNF-α), soluble p75 TNF-α receptor (sTNFRp750, thrombopoietin, serum-soluble TNF-related apoptosis-inducing ligand (sTRAIL), vascular cell adhesion molecule 1 (VCAM-1), and vascular endothelial growth factor α (VEGF-α). Plasma levels were also screened for 10 human MMPs, which included EMMPRIN, MMP-1–3, MMP-7–10, MMP-12, and MMP-13, using a custom-designed multiplex microbead-based immunoassay (R&D System). Samples were prepared, reagents used, and immunoassays performed according to manufacturers' instructions. Data were acquired using Luminex FlexMap 3D instrument (Millipore) and analyzed using Bio-plex Manager 6.0 software (Bio-Rad), based on standard curves plotted through a 5-parameter logistic curve setting. Levels of IL-17A, IL-17F, and MCP-3 were below detection limits and therefore excluded from further analysis.
Data Analysis
Associations between continuous variables and categorical variables with 2 unique values were tested statistically, using the Mann–Whitney U test. If the categorical variable had >2 unique values, Kruskal–Wallis tests were performed, followed by Dunn post hoc tests, to determine the significant comparison pairs. Associations between categorical variables were tested using Fisher exact tests or χ2 tests. P values of <.05 were considered statistically significant. The relationship between clinical parameters and cytokines was determined using Spearman rank correlation analysis. Correlations characterized by a Spearman correlation coefficient (r) of >0.5 were considered strong and are presented. All data analysis was performed using Prism 6.0 (GraphPad software). Two-way hierarchical clustering was performed as described elsewhere [14].
RESULTS
Secondary DENV Infection Increased the Risk of Plasma Leakage
The 90 adult patients with laboratory-confirmed DENV infection during the study period were classified according to their severity of plasma leakage; 30 had no plasma leakage, 30 had mild plasma leakage, and 30 had significant plasma leakage [12]. Four patients with influenza were analyzed as controls. The majority of the patients were male (70 of 90 [77.8%]) and Chinese (79 of 90 [87.8%]; Table 1). The median age of this cohort was 38 years (interquartile range, 30.00–43.25 years), and the age of patients with significant plasma leakage was significantly higher than that of patients without plasma leakage (Table 1 and Supplementary Figure 1A). Patients with secondary DENV infection had an increased risk of mild (56.7%) and significant (70%) plasma leakage as compared to those without prior infection (Table 1). The odds of having significant plasma leakage, given a secondary infection, were 3.231 times the odds of having no plasma leakage (P = .0329, by the χ2 test). The odds of having mild plasma leakage, given secondary infection, were 1.902 times the odds of having no plasma leakage. This association with severity of plasma leakage was affirmed, as patients without plasma leakage composed 43.3% of the group with secondary DENV infection.
| Characteristic . | Influenza B Virus–Infected Patients (n = 4) . | Severity of Plasma Leakage . | ||
|---|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | ||
| Sex | ||||
| Male | 2 | 26 (86.6) | 20 (66.7) | 24 (80) |
| Female | 2 | 4 (13.4) | 10 (33.3) | 6 (20) |
| Ethnicity | ||||
| Chinese | 3 | 25 (83.3) | 29 (96.7) | 25 (83.3) |
| Indian | 1 | 4 (13.4) | 0 | 2 (6.7) |
| Malay | 0 | 0 | 1 (3.3) | 2 (6.7) |
| Others | 0 | 1 (3.3) | 0 | 1 (3.3) |
| Age (years)a | 31.50 (28.50–34.50) | 33.0 (28.00–39.50) | 36.5 (26.75–44.00) | 40.50 (36.75–47.75) |
| DENV infection | ||||
| Primary | … | 16 (53.4) | 11 (36.7) | 8 (26.7) |
| Secondary | … | 13 (43.3) | 17 (56.7) | 21 (70) |
| Unknown | … | 1 (3.3) | 2 (6.6) | 1 (3.3) |
| DENV serotype | ||||
| 1 | … | 3 (10) | 7 (23.3) | 4 (13.4) |
| 2 | … | 26 (86.7) | 20 (66.7) | 22 (73.3) |
| 3 | … | 0 | 1 (3.3) | 2 (6.7) |
| 4 | … | 0 | 1 (3.3) | 2 (6.7) |
| Unknown | … | 1 (3.3) | 1 (3.3) | 0 |
| Characteristic . | Influenza B Virus–Infected Patients (n = 4) . | Severity of Plasma Leakage . | ||
|---|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | ||
| Sex | ||||
| Male | 2 | 26 (86.6) | 20 (66.7) | 24 (80) |
| Female | 2 | 4 (13.4) | 10 (33.3) | 6 (20) |
| Ethnicity | ||||
| Chinese | 3 | 25 (83.3) | 29 (96.7) | 25 (83.3) |
| Indian | 1 | 4 (13.4) | 0 | 2 (6.7) |
| Malay | 0 | 0 | 1 (3.3) | 2 (6.7) |
| Others | 0 | 1 (3.3) | 0 | 1 (3.3) |
| Age (years)a | 31.50 (28.50–34.50) | 33.0 (28.00–39.50) | 36.5 (26.75–44.00) | 40.50 (36.75–47.75) |
| DENV infection | ||||
| Primary | … | 16 (53.4) | 11 (36.7) | 8 (26.7) |
| Secondary | … | 13 (43.3) | 17 (56.7) | 21 (70) |
| Unknown | … | 1 (3.3) | 2 (6.6) | 1 (3.3) |
| DENV serotype | ||||
| 1 | … | 3 (10) | 7 (23.3) | 4 (13.4) |
| 2 | … | 26 (86.7) | 20 (66.7) | 22 (73.3) |
| 3 | … | 0 | 1 (3.3) | 2 (6.7) |
| 4 | … | 0 | 1 (3.3) | 2 (6.7) |
| Unknown | … | 1 (3.3) | 1 (3.3) | 0 |
Data are no. or no. (%) of patients, unless otherwise indicated.
Abbreviation: DENV, dengue virus.
a Data are median values (interquartile ranges).
| Characteristic . | Influenza B Virus–Infected Patients (n = 4) . | Severity of Plasma Leakage . | ||
|---|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | ||
| Sex | ||||
| Male | 2 | 26 (86.6) | 20 (66.7) | 24 (80) |
| Female | 2 | 4 (13.4) | 10 (33.3) | 6 (20) |
| Ethnicity | ||||
| Chinese | 3 | 25 (83.3) | 29 (96.7) | 25 (83.3) |
| Indian | 1 | 4 (13.4) | 0 | 2 (6.7) |
| Malay | 0 | 0 | 1 (3.3) | 2 (6.7) |
| Others | 0 | 1 (3.3) | 0 | 1 (3.3) |
| Age (years)a | 31.50 (28.50–34.50) | 33.0 (28.00–39.50) | 36.5 (26.75–44.00) | 40.50 (36.75–47.75) |
| DENV infection | ||||
| Primary | … | 16 (53.4) | 11 (36.7) | 8 (26.7) |
| Secondary | … | 13 (43.3) | 17 (56.7) | 21 (70) |
| Unknown | … | 1 (3.3) | 2 (6.6) | 1 (3.3) |
| DENV serotype | ||||
| 1 | … | 3 (10) | 7 (23.3) | 4 (13.4) |
| 2 | … | 26 (86.7) | 20 (66.7) | 22 (73.3) |
| 3 | … | 0 | 1 (3.3) | 2 (6.7) |
| 4 | … | 0 | 1 (3.3) | 2 (6.7) |
| Unknown | … | 1 (3.3) | 1 (3.3) | 0 |
| Characteristic . | Influenza B Virus–Infected Patients (n = 4) . | Severity of Plasma Leakage . | ||
|---|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | ||
| Sex | ||||
| Male | 2 | 26 (86.6) | 20 (66.7) | 24 (80) |
| Female | 2 | 4 (13.4) | 10 (33.3) | 6 (20) |
| Ethnicity | ||||
| Chinese | 3 | 25 (83.3) | 29 (96.7) | 25 (83.3) |
| Indian | 1 | 4 (13.4) | 0 | 2 (6.7) |
| Malay | 0 | 0 | 1 (3.3) | 2 (6.7) |
| Others | 0 | 1 (3.3) | 0 | 1 (3.3) |
| Age (years)a | 31.50 (28.50–34.50) | 33.0 (28.00–39.50) | 36.5 (26.75–44.00) | 40.50 (36.75–47.75) |
| DENV infection | ||||
| Primary | … | 16 (53.4) | 11 (36.7) | 8 (26.7) |
| Secondary | … | 13 (43.3) | 17 (56.7) | 21 (70) |
| Unknown | … | 1 (3.3) | 2 (6.6) | 1 (3.3) |
| DENV serotype | ||||
| 1 | … | 3 (10) | 7 (23.3) | 4 (13.4) |
| 2 | … | 26 (86.7) | 20 (66.7) | 22 (73.3) |
| 3 | … | 0 | 1 (3.3) | 2 (6.7) |
| 4 | … | 0 | 1 (3.3) | 2 (6.7) |
| Unknown | … | 1 (3.3) | 1 (3.3) | 0 |
Data are no. or no. (%) of patients, unless otherwise indicated.
Abbreviation: DENV, dengue virus.
a Data are median values (interquartile ranges).
All 4 DENV serotypes were detected in the study cohort, with DENV-2 (in 75.6% of patients) being the dominant serotype, followed by DENV-1 (in 15.6%), DENV-3 (in 3.3%), and DENV-4 (in 3.3%; Table 1). The clinical symptoms presented by the patients are listed in Table 2, with nausea (74.4%) being the most common clinical symptom. However, no significant statistical association between clinical symptoms and severity of plasma leakage was observed (Table 2).
| Symptom . | Severity of Plasma Leakage, Patients, No. . | ||
|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | |
| Nausea | 23 | 22 | 22 |
| Vomiting | 9 | 5 | 9 |
| Diarrhea | 17 | 7 | 13 |
| Abdominal pain | 6 | 4 | 11 |
| Rash | 11 | 16 | 10 |
| Petechiae | 4 | 11 | 6 |
| Lymphadenopathy | 2 | 0 | 0 |
| Conjunctivitis | 1 | 1 | 0 |
| Postural hypotension | 0 | 1 | 1 |
| Medical history | |||
| Bleeding (including petechiae) | 12 | 16 | 10 |
| Mucosal bleeding | 10 | 10 | 5 |
| Symptom . | Severity of Plasma Leakage, Patients, No. . | ||
|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | |
| Nausea | 23 | 22 | 22 |
| Vomiting | 9 | 5 | 9 |
| Diarrhea | 17 | 7 | 13 |
| Abdominal pain | 6 | 4 | 11 |
| Rash | 11 | 16 | 10 |
| Petechiae | 4 | 11 | 6 |
| Lymphadenopathy | 2 | 0 | 0 |
| Conjunctivitis | 1 | 1 | 0 |
| Postural hypotension | 0 | 1 | 1 |
| Medical history | |||
| Bleeding (including petechiae) | 12 | 16 | 10 |
| Mucosal bleeding | 10 | 10 | 5 |
Associations between plasma leakage severity with the clinical symptoms were tested using the Fisher exact test. None of the tests were statistically significant.
| Symptom . | Severity of Plasma Leakage, Patients, No. . | ||
|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | |
| Nausea | 23 | 22 | 22 |
| Vomiting | 9 | 5 | 9 |
| Diarrhea | 17 | 7 | 13 |
| Abdominal pain | 6 | 4 | 11 |
| Rash | 11 | 16 | 10 |
| Petechiae | 4 | 11 | 6 |
| Lymphadenopathy | 2 | 0 | 0 |
| Conjunctivitis | 1 | 1 | 0 |
| Postural hypotension | 0 | 1 | 1 |
| Medical history | |||
| Bleeding (including petechiae) | 12 | 16 | 10 |
| Mucosal bleeding | 10 | 10 | 5 |
| Symptom . | Severity of Plasma Leakage, Patients, No. . | ||
|---|---|---|---|
| None (n = 30) . | Mild (n = 30) . | Significant (n = 30) . | |
| Nausea | 23 | 22 | 22 |
| Vomiting | 9 | 5 | 9 |
| Diarrhea | 17 | 7 | 13 |
| Abdominal pain | 6 | 4 | 11 |
| Rash | 11 | 16 | 10 |
| Petechiae | 4 | 11 | 6 |
| Lymphadenopathy | 2 | 0 | 0 |
| Conjunctivitis | 1 | 1 | 0 |
| Postural hypotension | 0 | 1 | 1 |
| Medical history | |||
| Bleeding (including petechiae) | 12 | 16 | 10 |
| Mucosal bleeding | 10 | 10 | 5 |
Associations between plasma leakage severity with the clinical symptoms were tested using the Fisher exact test. None of the tests were statistically significant.
In line with previous reports [2, 15], hematological assessment of this study cohort indicated that patients with mild and significant plasma leakage exhibited hypoproteinemia, hypoalbuminemia, and thrombocytopenia (Supplementary Table 1 and Supplementary Figure 1B–D). Plasma leakage was associated with a significant elevation in the hematocrit change in patients with significant plasma leakage (Supplementary Figure 1E). No statistically significant association was observed between severity of plasma leakage and leukocyte count, differential leukocyte counts, and mean platelet volume at the point of admission (Supplementary Table 1 and Supplementary Figure 1F).
Laboratory diagnostic tests confirmed that all patients were DENV positive, based on the detection of either DENV RNA, by polymerase chain reaction, or NS1 antigen, by enzyme-linked immunosorbent assay (ELISA; Supplementary Table 2). Serologically, 65.5% of the patients were found to be immunoglobulin M positive, while 53.3% and 30% of patients were found to be immunoglobulin G (IgG) positive when assayed using indirect and capture IgG ELISA, respectively (Supplementary Table 2).
Temporal Association of Clinical Parameters With Severity of Plasma Leakage
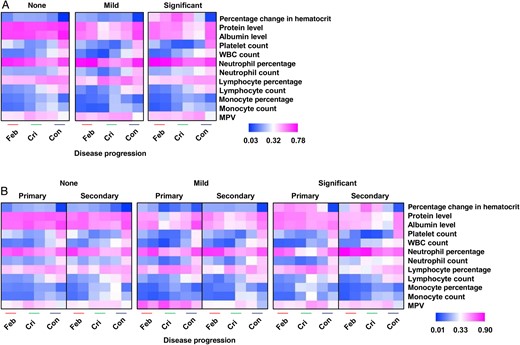
Differential patterns of clinical parameters among dengue virus (DENV)–infected patients, by plasma leakage severity (none, mild, and significant; A) and DENV infection status (primary and secondary; B), at febrile (Feb), critical (Cri), and convalescent (Con) disease phases. Data are for 7–30 individuals per group per time point (A) and 2–21 individuals per group per infection per time point. Data are compared and presented as heat maps of normalized scores. In these presentations, the immune mediator concentrations were scaled between 0 and 1 for each measured immune mediator, and then the average scaled value was computed for each group. Blue colors represent the lowest average scaled value, while pink colors represent the highest average scaled value. Mann–Whitney U tests were used to determine whether there were any significant associations between primary and secondary infection at various disease phases for the group with significant plasma leakage. Significant parameters include total protein levels, albumin levels, platelet counts, white blood cell (WBC) counts, percentages of neutrophils, and neutrophil counts. Abbreviation: MPV, mean platelet volume.
To determine whether secondary DENV infection influenced disease severity, hematological and biochemical laboratory parameters for patients experiencing primary or secondary DENV infections at different phases of illness were evaluated (Figure 1B). Patients with no and those with mild plasma leakage were included for comparison. Patients experiencing secondary DENV infections presented with more-severe clinical features, including significantly lower protein and albumin levels and platelet counts during the critical and convalescent phases (Figure 1B). Significantly higher white blood cell and neutrophil counts during the febrile and critical phases were more prominent in patients with secondary DENV infections (Figure 1B).
Temporal Association of Cytokines Levels With Secondary DENV Infections in Patients With Significant Plasma Leakage
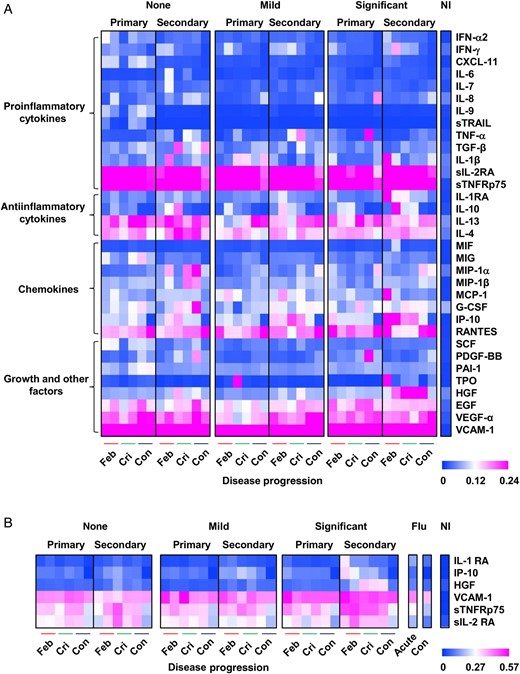
Differential-regulated immune mediator profiles among dengue virus (DENV)–infected patients. A, Patients were first grouped on the basis of plasma leakage severity (none, mild, and significant). Patients were further divided on the basis of DENV infection status (primary and secondary) at febrile (Feb), critical (Cri), and convalescent (Con) disease phases (n = 2–21 individuals per group per infection per time point) during the hospitalization period. B, Plasma samples from influenza B virus–infected patients (Flu) were collected at acute and convalescent disease phases (n = 3–4 individuals per time point) and are included for comparison. Data are compared and presented as heat maps of normalized scores. Immune mediator levels of healthy, noninfected adults (NI; n = 15) are included as a reference point. In the heat map presentation, the immune mediator concentrations were scaled between 0 and 1 for each measured immune mediator, and then the average scaled value was computed for each group. Blue colors represent the lowest average scaled value, whereas pink colors represent the highest average scaled value. Mann–Whitney U tests were used to determine whether there were any significant associations between primary and secondary infection at various disease phases for the group with significant plasma leakage. Significant immune mediators include interleukin 1 receptor antagonist (IL-1RA), hepatocyte growth factor (HGF), interferon γ–inducible protein 10 (IP-10), and soluble p75 tumor necrosis factor α receptor (sTNFRp75). Abbreviations: EGF, epidermal growth factor; G-CSF, granulocyte colony-stimulating factor; IFN-α2, interferon α2; IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-4, interleukin 4; IL-6, interleukin 6; IL-7, interleukin 7; IL-8, interleukin 8; IL-9, interleukin 9; IL-10, interleukin 10; IL-13, interleukin 13; MCP-1, monocyte chemoattractant protein 1; MIF, macrophage migration inhibitory factor; MIG, monokine induced by interferon γ; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; PAI-1, plasminogen activator inhibitor type 1; PDGF-BB, platelet-derived growth factor BB; RANTES, regulated on activation, normal T-cell expressed and secreted; SCF, stem cell factor; sIL-2RA, soluble interleukin 2 receptor antagonist; sTRAIL, serum-soluble tumor necrosis factor–related apoptosis-inducing ligand; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; TPO, thrombopoietin; VCAM-1, vascular cell adhesion molecule 1; VEGF-α, vascular endothelial growth factor α.
Association of MMP-2 and MMP-9 With Severity of Plasma Leakage
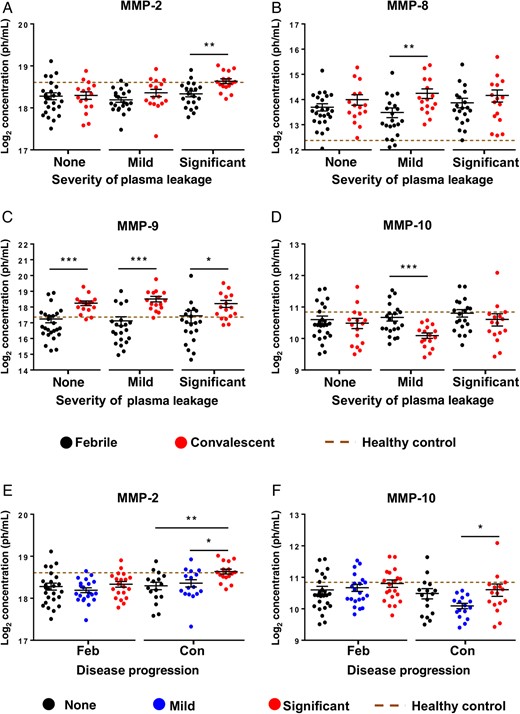
Temporal association of matrix metalloproteinases (MMPs) with severity of plasma leakage. Plasma MMPs levels were determined using a microbead-based immunoassay. A–D, Significant immune mediators between febrile and convalescent disease phases in patients with no, mild, and significant plasma leakage (n = 17–25 individuals/group/time point) include MMP-2 (A), MMP-8 (B), MMP-9 (C), and MMP-10 (D). Data are presented as means ± standard errors of the mean (SEMs). *P < .05, **P < .01, and ***P < .001, by the 2-tailed Mann–Whitney U test (10 MMPs were tested against the disease phase in each of the 3 plasma leakage severity groups). E and F, Comparison among patients with no, mild, and significant plasma leakage at febrile and convalescent disease phases. Data are presented as means ± SEMs. *P < .05, **P < .01, and ***P < .001, by the Kruskal–Wallis test followed by the Dunn multiple comparisons post hoc test (10 MMPs were tested against the plasma leakage severity groups for each of the 2 disease phases). The brown dotted line indicates plasma MMPs levels in healthy controls.
Temporal Relationships Between Immune Mediators and Clinical Parameters
The relationship between the immune mediators and clinical parameters at different phases of illness was evaluated using Spearman's correlation analysis, and statistically significant correlations, defined as those with a Spearman r of >0.5 or less than −0.5, are described in Table 3 and Supplementary Tables 3–4. In patients with significant plasma leakage, increasing changes in hematocrit from the febrile to the critical phases (Figure 1A) were associated with decreasing levels of IL-1RA and IP-10 (Table 3). In parallel, decreasing levels of albumin and protein observed in these patients (Figure 1A) were associated with increasing levels of MMP-10, IL-13, and TGF-β and a decreasing level of EGF (Tables 3 and Supplementary Table 3). Thrombocytopenia (Figure 1A), commonly observed in these patients, was associated with increasing levels of MMP-10 and sTNFRp75 and decreasing levels of EGF during the febrile phase (Table 3). Thrombocytopenia was associated with decreasing levels of RANTES and PDGF-BB and increasing levels of IL-6, IP-10, and IL-10 during the critical phase (Supplementary Table 3), coinciding with the nadir platelet count in these patients (Figure 1A). Increasing leukocyte count, particularly the neutrophil count, was associated with increasing levels of MMP-8 and MMP-9 during the febrile phase (Table 3) and increasing HGF and IL-1RA levels during the critical phase (Supplementary Table 3). Increasing MMP-9 levels were significantly associated with increasing neutrophil counts in all plasma leakage groups during the febrile phase (Table 3) and with mild and significant plasma leakage during the convalescent phase (Supplementary Table 4).
Correlation Analysis of Immune Mediators With Clinical Parameters During the Febrile Phase, by Severity of Plasma Leakage
| Clinical Manifestation . | None . | Mild . | Significant . | |||
|---|---|---|---|---|---|---|
| Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | |
| Percentage change in hematocrit | IL-4 | −0.52b | EGF | 0.62c | IL-1RA | −0.72c |
| VCAM-1 | −0.50b | … | … | IP-10 | −0.52b | |
| Total protein level | … | … | … | … | MMP-10 | −0.69c |
| … | … | … | … | EGF | 0.52b | |
| Albumin level | IL-13 | −0.52b | MCP-1 | −0.64d | MMP-10 | −0.63d |
| … | … | … | … | IL-13 | −0.51b | |
| Platelet count | MMP-10 | −0.52d | RANTES | 0.64c | MMP-10 | −0.57d |
| … | … | TPO | −0.60b | sTNFRp75 | −0.55b | |
| … | … | MMP-9 | 0.58d | EGF | 0.52b | |
| … | … | MCP-1 | −0.58b | … | … | |
| … | … | MMP-2 | −0.57d | … | … | |
| … | … | MMP-1 | 0.51b | … | … | |
| White blood cell count | … | … | MMP-9 | 0.74c | MMP-9 | 0.65d |
| … | … | IFN-γ | −0.72c | MMP-8 | 0.64d | |
| … | … | MMP-8 | 0.64c | PDGF-BB | 0.57d | |
| … | … | IL-10 | −0.61d | … | … | |
| Mean platelet volume | … | … | MMP-12 | −0.54d | … | … |
| … | … | sTNFRp75 | 0.52b | … | … | |
| Neutrophil count | MMP-9 | 0.67c | MMP-8 | 0.75c | MMP-9 | 0.77c |
| … | … | MMP-9 | 0.74c | MMP-8 | 0.69c | |
| … | … | IFN-γ | −0.56d | … | … | |
| Lymphocyte count | MMP-2 | 0.61d | IL-10 | −0.69c | IL-1RA | −0.67d |
| CXCL11 | −0.50b | IFN-γ | −0.68c | IFN-α2 | −0.61d | |
| IL-1RA | −0.50b | VCAM-1 | 0.60d | IL-13 | −0.59b | |
| … | … | PDGF-BB | 0.54d | IL-1β | −0.56b | |
| … | … | MIP-1α | −0.50b | MCP-1 | −0.52b | |
| Monocyte count | … | … | IFN-γ | −0.55d | CXCL-11 | −0.75c |
| … | … | sTRAIL | −0.51b | MIP-1α | −0.53b | |
| Clinical Manifestation . | None . | Mild . | Significant . | |||
|---|---|---|---|---|---|---|
| Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | |
| Percentage change in hematocrit | IL-4 | −0.52b | EGF | 0.62c | IL-1RA | −0.72c |
| VCAM-1 | −0.50b | … | … | IP-10 | −0.52b | |
| Total protein level | … | … | … | … | MMP-10 | −0.69c |
| … | … | … | … | EGF | 0.52b | |
| Albumin level | IL-13 | −0.52b | MCP-1 | −0.64d | MMP-10 | −0.63d |
| … | … | … | … | IL-13 | −0.51b | |
| Platelet count | MMP-10 | −0.52d | RANTES | 0.64c | MMP-10 | −0.57d |
| … | … | TPO | −0.60b | sTNFRp75 | −0.55b | |
| … | … | MMP-9 | 0.58d | EGF | 0.52b | |
| … | … | MCP-1 | −0.58b | … | … | |
| … | … | MMP-2 | −0.57d | … | … | |
| … | … | MMP-1 | 0.51b | … | … | |
| White blood cell count | … | … | MMP-9 | 0.74c | MMP-9 | 0.65d |
| … | … | IFN-γ | −0.72c | MMP-8 | 0.64d | |
| … | … | MMP-8 | 0.64c | PDGF-BB | 0.57d | |
| … | … | IL-10 | −0.61d | … | … | |
| Mean platelet volume | … | … | MMP-12 | −0.54d | … | … |
| … | … | sTNFRp75 | 0.52b | … | … | |
| Neutrophil count | MMP-9 | 0.67c | MMP-8 | 0.75c | MMP-9 | 0.77c |
| … | … | MMP-9 | 0.74c | MMP-8 | 0.69c | |
| … | … | IFN-γ | −0.56d | … | … | |
| Lymphocyte count | MMP-2 | 0.61d | IL-10 | −0.69c | IL-1RA | −0.67d |
| CXCL11 | −0.50b | IFN-γ | −0.68c | IFN-α2 | −0.61d | |
| IL-1RA | −0.50b | VCAM-1 | 0.60d | IL-13 | −0.59b | |
| … | … | PDGF-BB | 0.54d | IL-1β | −0.56b | |
| … | … | MIP-1α | −0.50b | MCP-1 | −0.52b | |
| Monocyte count | … | … | IFN-γ | −0.55d | CXCL-11 | −0.75c |
| … | … | sTRAIL | −0.51b | MIP-1α | −0.53b | |
Immune mediators were tested against continuous clinical parameters (Supplementary Table 1), using Spearman rank correlations. Significant results with a correlation coefficient >0.5 are shown.
Abbreviations: EGF, epidermal growth factor; HGF, hepatocyte growth factor; IFN-α2, interferon α2; IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-1RA, interleukin 1 receptor antagonist; IL-4, interleukin 4; IL-10, interleukin 10; IL-13, interleukin 13; IP-10, interferon γ–inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α; MMP, matrix metalloproteinase; PDGF-BB, platelet-derived growth factor BB; RANTES, regulated on activation, normal T-cell expressed and secreted; sTNFRp75, soluble p75 tumor necrosis factor α receptor; sTRAIL, serum-soluble tumor necrosis factor–related apoptosis-inducing ligand; TPO, thrombopoietin; VCAM-1, vascular cell adhesion molecule 1.
a A Spearman r of 1 indicates perfect positive correlation, whereas a Spearman r of −1 indicates a perfect negative correlation.
bP < .05.
cP < .001.
dP < .01.
Correlation Analysis of Immune Mediators With Clinical Parameters During the Febrile Phase, by Severity of Plasma Leakage
| Clinical Manifestation . | None . | Mild . | Significant . | |||
|---|---|---|---|---|---|---|
| Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | |
| Percentage change in hematocrit | IL-4 | −0.52b | EGF | 0.62c | IL-1RA | −0.72c |
| VCAM-1 | −0.50b | … | … | IP-10 | −0.52b | |
| Total protein level | … | … | … | … | MMP-10 | −0.69c |
| … | … | … | … | EGF | 0.52b | |
| Albumin level | IL-13 | −0.52b | MCP-1 | −0.64d | MMP-10 | −0.63d |
| … | … | … | … | IL-13 | −0.51b | |
| Platelet count | MMP-10 | −0.52d | RANTES | 0.64c | MMP-10 | −0.57d |
| … | … | TPO | −0.60b | sTNFRp75 | −0.55b | |
| … | … | MMP-9 | 0.58d | EGF | 0.52b | |
| … | … | MCP-1 | −0.58b | … | … | |
| … | … | MMP-2 | −0.57d | … | … | |
| … | … | MMP-1 | 0.51b | … | … | |
| White blood cell count | … | … | MMP-9 | 0.74c | MMP-9 | 0.65d |
| … | … | IFN-γ | −0.72c | MMP-8 | 0.64d | |
| … | … | MMP-8 | 0.64c | PDGF-BB | 0.57d | |
| … | … | IL-10 | −0.61d | … | … | |
| Mean platelet volume | … | … | MMP-12 | −0.54d | … | … |
| … | … | sTNFRp75 | 0.52b | … | … | |
| Neutrophil count | MMP-9 | 0.67c | MMP-8 | 0.75c | MMP-9 | 0.77c |
| … | … | MMP-9 | 0.74c | MMP-8 | 0.69c | |
| … | … | IFN-γ | −0.56d | … | … | |
| Lymphocyte count | MMP-2 | 0.61d | IL-10 | −0.69c | IL-1RA | −0.67d |
| CXCL11 | −0.50b | IFN-γ | −0.68c | IFN-α2 | −0.61d | |
| IL-1RA | −0.50b | VCAM-1 | 0.60d | IL-13 | −0.59b | |
| … | … | PDGF-BB | 0.54d | IL-1β | −0.56b | |
| … | … | MIP-1α | −0.50b | MCP-1 | −0.52b | |
| Monocyte count | … | … | IFN-γ | −0.55d | CXCL-11 | −0.75c |
| … | … | sTRAIL | −0.51b | MIP-1α | −0.53b | |
| Clinical Manifestation . | None . | Mild . | Significant . | |||
|---|---|---|---|---|---|---|
| Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | Immune Mediator . | Spearman ra . | |
| Percentage change in hematocrit | IL-4 | −0.52b | EGF | 0.62c | IL-1RA | −0.72c |
| VCAM-1 | −0.50b | … | … | IP-10 | −0.52b | |
| Total protein level | … | … | … | … | MMP-10 | −0.69c |
| … | … | … | … | EGF | 0.52b | |
| Albumin level | IL-13 | −0.52b | MCP-1 | −0.64d | MMP-10 | −0.63d |
| … | … | … | … | IL-13 | −0.51b | |
| Platelet count | MMP-10 | −0.52d | RANTES | 0.64c | MMP-10 | −0.57d |
| … | … | TPO | −0.60b | sTNFRp75 | −0.55b | |
| … | … | MMP-9 | 0.58d | EGF | 0.52b | |
| … | … | MCP-1 | −0.58b | … | … | |
| … | … | MMP-2 | −0.57d | … | … | |
| … | … | MMP-1 | 0.51b | … | … | |
| White blood cell count | … | … | MMP-9 | 0.74c | MMP-9 | 0.65d |
| … | … | IFN-γ | −0.72c | MMP-8 | 0.64d | |
| … | … | MMP-8 | 0.64c | PDGF-BB | 0.57d | |
| … | … | IL-10 | −0.61d | … | … | |
| Mean platelet volume | … | … | MMP-12 | −0.54d | … | … |
| … | … | sTNFRp75 | 0.52b | … | … | |
| Neutrophil count | MMP-9 | 0.67c | MMP-8 | 0.75c | MMP-9 | 0.77c |
| … | … | MMP-9 | 0.74c | MMP-8 | 0.69c | |
| … | … | IFN-γ | −0.56d | … | … | |
| Lymphocyte count | MMP-2 | 0.61d | IL-10 | −0.69c | IL-1RA | −0.67d |
| CXCL11 | −0.50b | IFN-γ | −0.68c | IFN-α2 | −0.61d | |
| IL-1RA | −0.50b | VCAM-1 | 0.60d | IL-13 | −0.59b | |
| … | … | PDGF-BB | 0.54d | IL-1β | −0.56b | |
| … | … | MIP-1α | −0.50b | MCP-1 | −0.52b | |
| Monocyte count | … | … | IFN-γ | −0.55d | CXCL-11 | −0.75c |
| … | … | sTRAIL | −0.51b | MIP-1α | −0.53b | |
Immune mediators were tested against continuous clinical parameters (Supplementary Table 1), using Spearman rank correlations. Significant results with a correlation coefficient >0.5 are shown.
Abbreviations: EGF, epidermal growth factor; HGF, hepatocyte growth factor; IFN-α2, interferon α2; IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-1RA, interleukin 1 receptor antagonist; IL-4, interleukin 4; IL-10, interleukin 10; IL-13, interleukin 13; IP-10, interferon γ–inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α; MMP, matrix metalloproteinase; PDGF-BB, platelet-derived growth factor BB; RANTES, regulated on activation, normal T-cell expressed and secreted; sTNFRp75, soluble p75 tumor necrosis factor α receptor; sTRAIL, serum-soluble tumor necrosis factor–related apoptosis-inducing ligand; TPO, thrombopoietin; VCAM-1, vascular cell adhesion molecule 1.
a A Spearman r of 1 indicates perfect positive correlation, whereas a Spearman r of −1 indicates a perfect negative correlation.
bP < .05.
cP < .001.
dP < .01.
DISCUSSION
Immune mediators can be detrimental or protective to pathogens [5]. In this prospective cohort study, immune mediator profiles of patients with dengue were extensively studied longitudinally to better understand dengue pathogenesis.
Plasma leakage is a major clinical feature in patients with DHF and DSS [1] and is caused by increased capillary permeability [21]. The risk of DHF development can differ according to the sequence of secondary DENV serotype infections, with patients experiencing secondary DENV-2 infection 5–7 times more likely to develop DHF than those with secondary DENV-1 and DENV3 infections [22, 23]. Vascular perturbations were reflected in this study, in which patients from the significant plasma leakage group exhibited marked hematocrit change, thrombocytopenia, hypoproteinemia, and hypoalbuminemia. Supported by histological assessment, the lack of endothelial cell destruction, coupled with the transient nature of plasma leakage, plasma leakage in dengue is mediated by functional rather than anatomical perturbation of capillary endothelial cells [24, 25]. The altered permeability may be induced by soluble mediators and cross-reactive T-cell activation that alter endothelial cell permeability, leading to plasma leakage [26, 27].
The analysis of multiple immune mediators in serum obtained in this adult dengue cohort study revealed a temporal association between cytokines and the severity of plasma leakage. These immune mediators cover a wide range of functions, including antiinflammatory (IL-1RA), chemotactic (IP-10), growth factor (HGF), soluble receptor (sTNFRp75), adhesion (VCAM-1), and enzymatic (MMP-2) activities. Some are proven prognostic markers for severe DENV infection or reported to be potent permeability enhancing cytokines: TNF-α, IFN-γ, IL-6, IL-8, VEGF-α, IL-2 and RANTES [25, 28–33]. However, apart from these common cytokines, immune mediators such as IL-1RA, MCP-1, TGF-β, HGF, IP-10, and sTNFRp75 were also associated with significant plasma leakage in this cohort.
Since the 1960s, secondary DENV infection was suggested to be a risk factor for severe DENV infection (DHF or DSS) [22, 34]. Earlier studies proposed that antibody-dependent enhancement in secondary DENV infections may lead to DHF or DSS [35]. Here, IL-1RA, HGF, IP-10, and sTNFRp75 were significantly higher in secondary DENV infections. Therefore, IL-1RA, HGF, IP-10, and sTNFRp75 may be used to determine the severity of plasma leakage in adult patients with dengue but also to differentiate between primary and secondary DENV infections. As such, IL-1RA, HGF, IP-10, and sTNFRp75 may be useful markers of adult dengue severity that prompt early medical attention.
It may be an attractive option to include MMP-2 and MMP-9 as predictive markers for plasma leakage in DENV infections because their roles in increasing endothelial permeability have been described [9, 10]. The significant upregulation of MMP-2 during the convalescent phase in this study suggested its role in inducing significant plasma leakage in adult patients with dengue. MMP-9 is a core serum protein responsible for plasma leakage and works with MMP-8 and MMP-10 to exert its functions in dengue pathogenesis. Thus, it is plausible that MMP-9 may induce mild-to-significant plasma leakage and exacerbate disease severity. In contrast, a recent study reported downregulation of MMP-8 and MMP-10 in asymptomatic patients with dengue [36]. Although this raises the complexity of the MMP network in driving various disease outcomes in DENV infections, our study suggests that the severity of plasma leakage is regulated by different levels of multiple MMPs (including MMP-2 and MMP-8–10) between healthy and DENV infection status. Moreover, MMP-2 and MMP-9 were demonstrated to work in concert with or antagonistic to each other [37–40]. It would be an interesting idea to consider administering an MMP-2 and MMP-9 inhibitor (CAS 193807-58-8) to control severe DENV infection.
Owing to the cocirculation of multiple viruses such as influenza virus and other closely related arboviruses within the same geographic locations [41, 42], specific biomarker profiles may be necessary for disease prognosis. HGF and sTNFRp75 levels were shown to remain low during acute influenza virus infection in this study (Figure 2B). Furthermore, although similar markers such as IFN-γ, IL-6, IL-10, MIP-1β, and MCP-1 can be found for acute chikungunya virus infection [43], HGF is unique. Therefore, HGF and perhaps sTNFRp75 may be useful markers to distinguish the 3 different viral infections often coexisting in tropical countries. Currently, although no meta-analysis has summarized the immune mediator profiles of different dengue cohorts, the current study provided a set of immune mediators from adult patients with dengue that may be used to predict dengue disease progression and differentiate dengue from other flaviviral diseases, such as Zika fever, which has similar clinical symptoms during early onset of illness.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and healthy volunteers for their participation in the study; research staff from the Communicable Disease Centre, Tan Tock Seng Hospital, for assistance in blood sample preparation and for patient enrollment, study coordination, and data entry; the clinical staff of Communicable Disease Centre, Tan Tock Seng Hospital, for patient enrollment and care; and the members of the SIgN Immune Monitoring Platform (Alessandra Nardin, Anis Larbi, Michael Poidinger, Brian Abel, Doreen Hasse, Veonice Au, Esther Mok Wing-Hei, Suisheng Tang, and Joni Chong Chou-Yue) and Diane Simarmata from the Singapore Immunology Network, for assistance in the study.
All authors discussed the results and commented on the manuscript. V. C. G., Z. H., L. F. P. N., K. F., L. R., Y.-S. L., and D. C. L. conceived and designed the experiments. Z. H., B. L., Y.-W. K., J. J. L. T., and S. I. P. performed the experiments. Z. H., B. L., Y.-W. K., T.-L. T., and L. F. P. N. analyzed the data. V. C. G., L. K. L., T.-L. T., D. C. L., and Y.-S. L. contributed materials. Z. H., T.-L. T., Y.-W. K., D. C. L., and L. F. P. N. wrote the manuscript.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Biomedical Research Council, A*STAR, and the STOP Dengue Translational Clinical Research Programme, funded by the National Research Foundation through the National Medical Research Council, Singapore (grant NMRC/TCR/005/2008).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Present affiliation: Institute of Molecular and Cell Biology, Agency for Science, Technology, and Research, Singapore.
Z. H., Y.-W. K., V. C. G., and B. L. contributed equally to this work.




