-
PDF
- Split View
-
Views
-
Cite
Cite
Kerry Murphy, Caroline M. Mitchell, The Interplay of Host Immunity, Environment and the Risk of Bacterial Vaginosis and Associated Reproductive Health Outcomes, The Journal of Infectious Diseases, Volume 214, Issue suppl_1, August 2016, Pages S29–S35, https://doi.org/10.1093/infdis/jiw140
Close - Share Icon Share
Abstract
Bacterial vaginosis (BV) is one of the most common causes of vaginal symptoms in US women, but its causal mechanism has not yet been defined. BV is more prevalent in women who are immunosuppressed, and several risk factors for the development of BV are associated with lower quantities of immune mediators in vaginal fluid. In contrast, the poor reproductive health outcomes associated with BV, such as preterm birth and human immunodeficiency virus type 1 acquisition, are associated with increased levels of proinflammatory immune mediators in the genital tract. In this article, we discuss how variations in the host immune profile and environmental effects on host immunity may influence the risk of BV, as well as the risk of complications associated with BV.
Bacterial vaginosis (BV) is present in up to a third of US women, but the root cause of the syndrome is unknown. Epidemiologic and observational studies have identified a number of factors associated with a higher risk for BV. National Health and Nutrition Examination Survey data from 3739 women aged 14–49 years identified independent associations between BV prevalence and race/ethnicity, number of lifetime sex partners, douching frequency, education level, and use of hormonal contraceptive pills [1]. Data from longitudinal studies have also documented associations between absence of H2O2-producing lactobacilli, herpes simplex virus type 2 (HSV-2) seropositivity, and smoking with BV acquisition [2]. Susceptibility to BV is likely driven in part by the host immune response, which may be altered by factors including ethnicity/genetics, environmental and physiologic stressors, and hormonal influences that differ with reproductive status and use of exogenous hormonal contraceptives.
One epidemiologic clue that BV is related to alterations in mucosal immunity is that women infected with human immunodeficiency virus (HIV), who have impaired cellular and innate immunity, have an increased risk of incident and persistent BV [3]. Few studies have assessed the risk of BV in other immunocompromised populations, such as women taking immunosuppressive medications after transplantation. In one small study, 42% of Turkish kidney transplant recipients had BV diagnosed on the basis of the Nugent score, compared with 9% of healthy women [4]. More-mechanistic clues lie in studies showing that polymorphisms in immune-response genes linked to lower production of cytokines and chemokines are associated with a higher risk of BV. An IL1RN polymorphism associated with decreased production of IL-1β by monocytes was associated with higher vaginal pH and higher Nugent scores [5]. Polymorphisms in IL6 associated with less of an immune response to IL-1 and lipopolysaccharide (LPS) were associated with increased BV in black women, and an CXCL8 allele associated with increased IL-8 production is less common in women with BV [5]. Single-nucleotide polymorphisms (SNPs) in Toll-like receptor (TLR) genes, which are key pattern recognition receptors for the innate immune response, have been associated with higher vaginal quantities of the BV-associated species Gardnerella vaginalis [6] and Atopobium vaginae [7], an increased prevalence of BV, and higher rates of systemic infections with gram-negative bacteria [8, 9]. Together, these data suggest that genetic variations that decrease the mucosal innate immune response are associated with an increased risk of BV.
Mucosal homeostasis is maintained by an intricate balance between the host mucosal immune response and the commensal microbiota that colonize mucosal surfaces. Most of the genes found to be associated with the composition of the gut microbial community are related to immunity. People with defects in innate immune sensing pathways, such as nucleotide oligomerization domain receptors, have an altered gut microbiota and increased risk of Crohn disease [10]. Mice with deficiencies in adaptive immune pathways such as Rag 1 have enrichment of the species Akkermansia muciniphila, compared with wild-type mice [11]. However, the microbial community also influences expression of host immunity: germ-free mice develop more-severe colitis when challenged with a hyperosmolar dextran solution as compared to genetically similar mice with normal commensal gut microbiota [12]. The gastrointestinal microbiota can alter the risk of immune-mediated disorders such as asthma and allergies [13], and additional studies have demonstrated that the development of the immune system after birth is partly influenced by the gut microbiome [14, 15]. Which is the chicken and which the egg has not been determined; while the microbial communities of families are more similar than those of genetically unrelated people, studies of monozygotic twins demonstrate significant differences in microbial communities between genetically identical siblings [10]. Understanding how mucosal immunity influences microbial communities and the risk of dysbioses like BV requires a systems biology approach, which calls for a comprehensive evaluation of the factors that may impact the mucosal immune milieu.
In the reproductive tract, most published work focuses on the genital mucosal immune response to BV and how that response alters the risk of reproductive health complications such as HIV acquisition or preterm birth. There is less known about how variations in immune response inform a woman's risk of acquiring BV, response to treatment, or risk of recurrence. Mucosal immune responses that are maladaptive for HIV acquisition risk or term delivery may be beneficial in preventing or clearing vaginal dysbiosis. Alternatively, activation of immune pathways may be profoundly altered by the composition of the microbiome, to either prevent or initiate a disease state. In this article, we discuss the literature on differences in mucosal immunity due to known BV risk factors and how these might impact the vaginal microbiota, the risk of acquisition of BV, and the risk of associated health outcomes, including HIV acquisition and preterm birth. While possible associations with recurrent BV would be of significant interest, there are too few data on differences in mucosal immunity or genetic markers of susceptibility in women with recurrent BV to provide a basis for discussion.
IMPACT OF RISK FACTORS FOR BV ON MUCOSAL IMMUNITY
Race and Ethnicity
Marked racial disparities exist in the prevalence and incidence of BV, as well as sexually transmitted infections (STIs), including HIV infection. Possible explanations for these disparities include differences in socioeconomic status and behavioral factors, but genetic and biologic factors may also be important. In a study of >1100 women in 5 US cities, black race remained an independent predictor of BV, after control for demographic and lifestyle factors [16]. Racial differences in the community structure of the vaginal microbiome have been well documented, with bacterial communities dominated by Lactobacillus species found more commonly in white women (89.7%), compared with black women (61.9%) [17]. Black women were more likely to have a higher vaginal pH, a lower prevalence of Lactobacillus species, and a more diverse bacterial community composed of anaerobes and taxa associated with BV, including Prevotella, Atopobium, Gardnerella, Megasphaera, and Mobiluncus, as compared to white women [17, 18]. Even in women with a normal Nugent score, Lactobacillus species are more common among white women, compared with African American women [18].
Genetic polymorphisms in TLRs and immune mediators may explain some of the racial variability in the vaginal microbiota and risk of BV. In a study of 159 HIV-positive African American adolescents, polymorphisms in TLR4 and TLR9 were significantly associated with BV, after adjustment for gonorrhea, chlamydial infection, CD4+ T-cell count, and douching (Table 1) [9]. The same TLR4 polymorphism, −896A/G, was associated in a different study with an increased quantity of G. vaginalis when the G allele was present but with increased IL1β and IL1ra among individuals with the AA genotype. In other studies, the G allele in TLR4-896A/G has been associated with increased susceptibility to systemic bacterial infections and a decreased responsiveness to LPS. This allele is a minor allele and is reported at similar frequency in populations of European ancestry (allele frequency, 0.0057), compared with African ancestry (0.071), according to 1000 Genomes Project data in dbSNP [23]. However, a closely linked allele, TLR4-1196C/T, for which the T allele is associated with a decreased risk of BV [19], is less prevalent among people of African ancestry (0.005 vs 0.058) [23].
Association of Immune Response Gene Polymorphisms With Bacterial Vaginosis (BV) or Genital Tract Markers of Inflammation
| Gene, Polymorphism(s)a . | Association With BV . | Association With Vaginal Immune Markers . |
|---|---|---|
| TLR1 | ||
| −118G/A (rs5743612) | Increased association among individuals with T allele [9]b | … |
| −743 A/G (rs4833095) | Increased association with BV due to A. vaginae among individuals with TC, compared with homozygotes [7]c | … |
| TLR2 | ||
| −15607A/G (rs1898830) | Increased association among individuals with G allele [9]b | … |
| TLR4 | ||
| −896 A/G (rs4986790) | Increased association among individuals with G allele [9]b; increased association with BV due to G. vaginalis among individuals with G allele [6] | Increased IL1β and IL1ra levels among G. vaginalis–positive or GNR-positive individuals with AA [6] |
| −1196 C/T (rs4986791) | Decreased association among individuals with T allele [19] | … |
| rs1554973 | … | Increased IL1β level among individuals with TT [20]c |
| TLR9 | ||
| rs187084 | Increased association among individuals with T allele [9]b | … |
| rs352140 | Increased association among individuals with T allele [9]b | … |
| CD14 | ||
| −1342 G/T (rs2563298) | Decreased association with BV due to A. vaginae among individuals with TT [7]c | … |
| MD2 | ||
| −1155 G | Increased association with BV due to G. vaginalis among individuals with GG [7]c | … |
| CARD15 | ||
| 14772 A/T (rs2066844) | Decreased association (for BV due to A. vaginae) among individuals with CT, compared with homozygotes [7]c | … |
| IL1β | ||
| −511C/T | Increased association among individuals with CC [5] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| −3954C/T | Decreased association among individuals with CC [5, 19] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| IL1ra | ||
| IL1RN*1 | … | Increased IL1β level among G. vaginalis–positive individuals with *1 allele [5] |
| IL1RN*2 | Increased association among individuals with *2 allele [5]b | Increased IL1ra level and decreased IL-13β level among *2 homozygotes [5] |
| IL6 | ||
| −174 G/C (rs1800795) | Increased association among individuals with C allele [19]b | Increased response to LPS in vitro among individuals with G allele [5]; increased risk of preterm birth among BV-positive individuals with C allele [5] |
| CXCL8 | ||
| −845 T/C (rs2227532) | Decreased association among heterozygotes [19] | … |
| IL10 | ||
| −1082G/A | Decreased association among individuals with G allele [19] | … |
| TNFa | ||
| −308G/A (rs1800629) | No difference [5] | Increased TNF-α level among BV-positive individuals with A allele [5]; increased risk of preterm birth among BV-positive individuals with A allele [21]b |
| CRH | ||
| RI-9-50 | Increased association among individuals with AA [22]c | … |
| BP-15033 | Increased association among individuals with TT [22]c; increased association among smokers with T allele [22]c | … |
| −3362 | Decreased association among individuals with AG, compared with those with AA [22]b | … |
| −1667 | Decreased association among individuals with CT, compared with those with CC [22]b | … |
| −2468 | Increased association among individuals with the T allele [22]b | … |
| Gene, Polymorphism(s)a . | Association With BV . | Association With Vaginal Immune Markers . |
|---|---|---|
| TLR1 | ||
| −118G/A (rs5743612) | Increased association among individuals with T allele [9]b | … |
| −743 A/G (rs4833095) | Increased association with BV due to A. vaginae among individuals with TC, compared with homozygotes [7]c | … |
| TLR2 | ||
| −15607A/G (rs1898830) | Increased association among individuals with G allele [9]b | … |
| TLR4 | ||
| −896 A/G (rs4986790) | Increased association among individuals with G allele [9]b; increased association with BV due to G. vaginalis among individuals with G allele [6] | Increased IL1β and IL1ra levels among G. vaginalis–positive or GNR-positive individuals with AA [6] |
| −1196 C/T (rs4986791) | Decreased association among individuals with T allele [19] | … |
| rs1554973 | … | Increased IL1β level among individuals with TT [20]c |
| TLR9 | ||
| rs187084 | Increased association among individuals with T allele [9]b | … |
| rs352140 | Increased association among individuals with T allele [9]b | … |
| CD14 | ||
| −1342 G/T (rs2563298) | Decreased association with BV due to A. vaginae among individuals with TT [7]c | … |
| MD2 | ||
| −1155 G | Increased association with BV due to G. vaginalis among individuals with GG [7]c | … |
| CARD15 | ||
| 14772 A/T (rs2066844) | Decreased association (for BV due to A. vaginae) among individuals with CT, compared with homozygotes [7]c | … |
| IL1β | ||
| −511C/T | Increased association among individuals with CC [5] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| −3954C/T | Decreased association among individuals with CC [5, 19] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| IL1ra | ||
| IL1RN*1 | … | Increased IL1β level among G. vaginalis–positive individuals with *1 allele [5] |
| IL1RN*2 | Increased association among individuals with *2 allele [5]b | Increased IL1ra level and decreased IL-13β level among *2 homozygotes [5] |
| IL6 | ||
| −174 G/C (rs1800795) | Increased association among individuals with C allele [19]b | Increased response to LPS in vitro among individuals with G allele [5]; increased risk of preterm birth among BV-positive individuals with C allele [5] |
| CXCL8 | ||
| −845 T/C (rs2227532) | Decreased association among heterozygotes [19] | … |
| IL10 | ||
| −1082G/A | Decreased association among individuals with G allele [19] | … |
| TNFa | ||
| −308G/A (rs1800629) | No difference [5] | Increased TNF-α level among BV-positive individuals with A allele [5]; increased risk of preterm birth among BV-positive individuals with A allele [21]b |
| CRH | ||
| RI-9-50 | Increased association among individuals with AA [22]c | … |
| BP-15033 | Increased association among individuals with TT [22]c; increased association among smokers with T allele [22]c | … |
| −3362 | Decreased association among individuals with AG, compared with those with AA [22]b | … |
| −1667 | Decreased association among individuals with CT, compared with those with CC [22]b | … |
| −2468 | Increased association among individuals with the T allele [22]b | … |
Abbreviations: A. vaginae, Atopobium vaginae; CRH, corticotropin-releasing hormone; GNR, gram-negative rods; G. vaginalis, Gardnerella vaginalis; LPS, lipopolysaccharide; rs, reference single-nucleotide polymorphism; TNF-α, tumor necrosis factor α; TLR, Toll-like receptor.
a Identified either by their position and the 2 possible alleles or by their rs number.
b Associations are only studied or only significant in African American populations.
c Associations are only studied or only significant in European American or white populations.
Association of Immune Response Gene Polymorphisms With Bacterial Vaginosis (BV) or Genital Tract Markers of Inflammation
| Gene, Polymorphism(s)a . | Association With BV . | Association With Vaginal Immune Markers . |
|---|---|---|
| TLR1 | ||
| −118G/A (rs5743612) | Increased association among individuals with T allele [9]b | … |
| −743 A/G (rs4833095) | Increased association with BV due to A. vaginae among individuals with TC, compared with homozygotes [7]c | … |
| TLR2 | ||
| −15607A/G (rs1898830) | Increased association among individuals with G allele [9]b | … |
| TLR4 | ||
| −896 A/G (rs4986790) | Increased association among individuals with G allele [9]b; increased association with BV due to G. vaginalis among individuals with G allele [6] | Increased IL1β and IL1ra levels among G. vaginalis–positive or GNR-positive individuals with AA [6] |
| −1196 C/T (rs4986791) | Decreased association among individuals with T allele [19] | … |
| rs1554973 | … | Increased IL1β level among individuals with TT [20]c |
| TLR9 | ||
| rs187084 | Increased association among individuals with T allele [9]b | … |
| rs352140 | Increased association among individuals with T allele [9]b | … |
| CD14 | ||
| −1342 G/T (rs2563298) | Decreased association with BV due to A. vaginae among individuals with TT [7]c | … |
| MD2 | ||
| −1155 G | Increased association with BV due to G. vaginalis among individuals with GG [7]c | … |
| CARD15 | ||
| 14772 A/T (rs2066844) | Decreased association (for BV due to A. vaginae) among individuals with CT, compared with homozygotes [7]c | … |
| IL1β | ||
| −511C/T | Increased association among individuals with CC [5] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| −3954C/T | Decreased association among individuals with CC [5, 19] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| IL1ra | ||
| IL1RN*1 | … | Increased IL1β level among G. vaginalis–positive individuals with *1 allele [5] |
| IL1RN*2 | Increased association among individuals with *2 allele [5]b | Increased IL1ra level and decreased IL-13β level among *2 homozygotes [5] |
| IL6 | ||
| −174 G/C (rs1800795) | Increased association among individuals with C allele [19]b | Increased response to LPS in vitro among individuals with G allele [5]; increased risk of preterm birth among BV-positive individuals with C allele [5] |
| CXCL8 | ||
| −845 T/C (rs2227532) | Decreased association among heterozygotes [19] | … |
| IL10 | ||
| −1082G/A | Decreased association among individuals with G allele [19] | … |
| TNFa | ||
| −308G/A (rs1800629) | No difference [5] | Increased TNF-α level among BV-positive individuals with A allele [5]; increased risk of preterm birth among BV-positive individuals with A allele [21]b |
| CRH | ||
| RI-9-50 | Increased association among individuals with AA [22]c | … |
| BP-15033 | Increased association among individuals with TT [22]c; increased association among smokers with T allele [22]c | … |
| −3362 | Decreased association among individuals with AG, compared with those with AA [22]b | … |
| −1667 | Decreased association among individuals with CT, compared with those with CC [22]b | … |
| −2468 | Increased association among individuals with the T allele [22]b | … |
| Gene, Polymorphism(s)a . | Association With BV . | Association With Vaginal Immune Markers . |
|---|---|---|
| TLR1 | ||
| −118G/A (rs5743612) | Increased association among individuals with T allele [9]b | … |
| −743 A/G (rs4833095) | Increased association with BV due to A. vaginae among individuals with TC, compared with homozygotes [7]c | … |
| TLR2 | ||
| −15607A/G (rs1898830) | Increased association among individuals with G allele [9]b | … |
| TLR4 | ||
| −896 A/G (rs4986790) | Increased association among individuals with G allele [9]b; increased association with BV due to G. vaginalis among individuals with G allele [6] | Increased IL1β and IL1ra levels among G. vaginalis–positive or GNR-positive individuals with AA [6] |
| −1196 C/T (rs4986791) | Decreased association among individuals with T allele [19] | … |
| rs1554973 | … | Increased IL1β level among individuals with TT [20]c |
| TLR9 | ||
| rs187084 | Increased association among individuals with T allele [9]b | … |
| rs352140 | Increased association among individuals with T allele [9]b | … |
| CD14 | ||
| −1342 G/T (rs2563298) | Decreased association with BV due to A. vaginae among individuals with TT [7]c | … |
| MD2 | ||
| −1155 G | Increased association with BV due to G. vaginalis among individuals with GG [7]c | … |
| CARD15 | ||
| 14772 A/T (rs2066844) | Decreased association (for BV due to A. vaginae) among individuals with CT, compared with homozygotes [7]c | … |
| IL1β | ||
| −511C/T | Increased association among individuals with CC [5] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| −3954C/T | Decreased association among individuals with CC [5, 19] | Increased IL1β level among individuals with LPS in vitro and T allele [5] |
| IL1ra | ||
| IL1RN*1 | … | Increased IL1β level among G. vaginalis–positive individuals with *1 allele [5] |
| IL1RN*2 | Increased association among individuals with *2 allele [5]b | Increased IL1ra level and decreased IL-13β level among *2 homozygotes [5] |
| IL6 | ||
| −174 G/C (rs1800795) | Increased association among individuals with C allele [19]b | Increased response to LPS in vitro among individuals with G allele [5]; increased risk of preterm birth among BV-positive individuals with C allele [5] |
| CXCL8 | ||
| −845 T/C (rs2227532) | Decreased association among heterozygotes [19] | … |
| IL10 | ||
| −1082G/A | Decreased association among individuals with G allele [19] | … |
| TNFa | ||
| −308G/A (rs1800629) | No difference [5] | Increased TNF-α level among BV-positive individuals with A allele [5]; increased risk of preterm birth among BV-positive individuals with A allele [21]b |
| CRH | ||
| RI-9-50 | Increased association among individuals with AA [22]c | … |
| BP-15033 | Increased association among individuals with TT [22]c; increased association among smokers with T allele [22]c | … |
| −3362 | Decreased association among individuals with AG, compared with those with AA [22]b | … |
| −1667 | Decreased association among individuals with CT, compared with those with CC [22]b | … |
| −2468 | Increased association among individuals with the T allele [22]b | … |
Abbreviations: A. vaginae, Atopobium vaginae; CRH, corticotropin-releasing hormone; GNR, gram-negative rods; G. vaginalis, Gardnerella vaginalis; LPS, lipopolysaccharide; rs, reference single-nucleotide polymorphism; TNF-α, tumor necrosis factor α; TLR, Toll-like receptor.
a Identified either by their position and the 2 possible alleles or by their rs number.
b Associations are only studied or only significant in African American populations.
c Associations are only studied or only significant in European American or white populations.
However, not all polymorphisms associated with BV are more prevalent in people of African ancestry. A polymorphism in the IL6 gene associated with increased risk of BV (IL6-174, C allele) is more prevalent in people of European ancestry, compared with individuals of African ancestry (0.42 vs 0.018) [23]. An CXCL8 polymorphism that is associated with protection from BV (CXCL8-845, C allele) is less common in people of European ancestry (0.005% vs 0.082%) [23]. These data suggest that genetic differences in immune response may play a role in the known ethnic and racial differences in vaginal bacterial community profile but that these associations are complex.
Chronic Stress
Chronic stress has been linked to alterations in the systemic [24] and vaginal [25] immune responses. Psychological stress is increasingly linked to differences in mucosal immune response and susceptibility to infections in the gut and elsewhere [26], suggesting that a link with BV is biologically plausible. A study in mice showed significant alterations in the vaginal proteome, including decreases in levels of antimicrobial peptides such as lactoferrin, when pregnant dams were subjected to stress. The mice also experienced a decrease in vaginal Lactobacillus species [27]. A separate study showed decreased numbers of natural killer and T cells in the vaginal mucosa of mice subjected to stress [25]. Report of recent stressful life events [28], chronic stress [29], childhood sexual abuse, chronic discrimination [30], and community-level stressors [31] have been associated with higher rates of BV. In a cross-sectional study of pregnant women, African American women had significantly increased odds of BV and were also significantly more likely to report both individual and contextual level stressors as compared to white women. In models controlling for these stressors, the association of black race with BV was attenuated although still significant [31]. Many endocrine hormones and signaling molecules influence the immune system, including corticotropin-releasing hormone (CRH), which can suppress T-helper type 1 responses [32]. In a case-control study of black and white women with and without BV, SNPs in CRH genes were associated with BV [22] (Table 1), and alleles associated with BV were more common in African American women, although this association does not necessarily imply causation. In a separate study, among white women smokers, smokers with the CRH-BP-15033 T allele had an increased risk of BV, but nonsmokers did not. Among African American women, those with the T allele at CRH-2468 had a higher risk of BV [22]. Together, these data suggest that the presence of an environmental stressor may modify risk. Links between neuropsychological stress, immunity, and microbial communities are complex and need further exploration but may contribute to the risk of BV.
Smoking
Cigarette smoking, which has been associated with BV, has a significant effect on innate immunity, which has been studied most extensively in models of the airway epithelium. Smoke exposure both increases inflammatory markers in respiratory secretions and decreases production of inflammatory cytokines by epithelial cells in response to a pathogenic stimulus. Neutrophils and macrophages increase in numbers after exposure to smoke but decrease in functionality [33]. Smokers have a different oral microbiota [34], and smoking cessation profoundly alters the gut microbial community, but whether this is due to direct effects of smoke on microbes, due to systemic nicotine, or occurs through alterations in mucosal immunity has not been explored. Smoking has been shown to have varying effects on markers of cervicovaginal immunity. Two studies found higher IL-10 levels in cervicovaginal secretions in smokers as compared to nonsmokers, one showed lower IL-10 levels, and one showed no difference [35–38]. Only one of these studies examined the concurrent vaginal microbiota, which may account for the variation in levels of cytokines, as a more recent study showed a lower prevalence of lactobacilli in smokers [39].
Viral Coinfections
Viral infections of the genital tract may also influence the immune response and/or susceptibility to BV. Several studies have shown that HSV [40] and HIV [36] are associated with an increased risk of BV, but human papillomavirus (HPV) is not similarly associated [41]. After infection with HSV, cultured uterine epithelial cells secreted lower IL-6 and MCP-1 levels [42], and cervical epithelial cells secreted less SLPI [43]. HIV has well described effects on CD4+ T cells and adaptive immunity, but has also been associated with alterations in innate mucosal immunity. Chronic simian immunodeficiency virus infection causes suppression of pattern recognition receptors on gut mucosa and decreased expression of proinflammatory cytokines [44]. In contrast, keratinocytes infected with HPV showed increased production of inflammatory mediators in response to IL1β and SDF-1α [45].
Hormonal Contraceptives
Hormonal contraceptive use confers decreased risk of incident, prevalent and recurrent BV [46]. Decreased risk of BV has been demonstrated with both combined oral contraceptives (COCs) and progesterone-only contraceptives, despite the fact that, in US women, depot medroxyprogesterone acetate (DMPA) use has been associated with decreased vaginal colonization with H202-producing lactobacilli [47]. In a separate study of Kenyan women, DMPA use was associated with decreased levels of G. vaginalis and increased detection of L. crispatus, which highlights the importance of studying multiple populations when assessing risks for BV [48]. Estradiol has been shown to decrease TLR2 and TLR6 messenger RNA expression and expression of human β defensin 2 (HBD2) and SLPI by vaginal epithelial cells in vitro [49]. Hormonal contraceptives suppress ovulation, resulting in overall decreased serum levels of estrogen and progesterone. In a study of women in Uganda and Zimbabwe, those using COCs who had no STIs and a Nugent score <7 had higher IL-1β, IL-6, IL-8, and IL-1ra expression than women using no hormonal contraception, but this was a cross-sectional study, and detailed analysis of the vaginal microbiota was not reported [50]. In a different analysis from this cohort, COC use was associated with increased IL-1β, IL-6, IL-8, MIP-3α, VEGF, and SLPI expression, while DMPA was associated with elevated RANTES and lower HBD2 expression [51]. In a cross-sectional study of Kenyan and South African women with a normal Nugent score, those using DMPA had higher MIP-1α, MIP1-β, IL-6, IL-8, and RANTES expression as compared to women not using any hormonal contraception [52].
However, not all data support a more robust immune profile in hormonal contraceptive users; in 64 HIV-uninfected South African women using injectable progesterone-containing contraceptives, levels of soluble immune mediators in vaginal fluid were lower than in women not using any hormonal contraceptives, despite no differences in the presence of BV or STI [53]. In reproductive-aged women in Alabama, Langerhans cells were decreased in the mucosa of women using an etonogestrel and ethinyl estradiol vaginal ring and COCs but not in those using DMPA. No concurrent analysis of the vaginal microbial community was performed [54]. Exogenous hormones and the bacterial flora have been shown to influence glycosylation of proteins in cervicovaginal lavage fluid, which in turn affects their ability to function. Women using DMPA, compared with women using other contraceptives, had significantly lower levels of bisecting N-acetyl-D-glucosamine, a monosaccharide that enhances binding of immunoglobulin G Fc to the FcγIIIa receptor to induce antibody-dependent cellular cytotoxicity [55]. This may be one mechanism by which DMPA may enhance the risk of viral infections, but it is unclear whether this influences the vaginal microbial community.
IMMUNITY AND THE RISK OF POOR OUTCOMES WITH BV
Clinically, BV is not an inflammatory condition: the vaginal mucosa is not red or tender, and women do not commonly report pain. However, several of the poor reproductive health outcomes associated with BV seem to be mediated through inflammation. Levels of proinflammatory cytokines like IL1β are often elevated in the vaginal fluid of women with BV, but there is significant heterogeneity in results [20]. Much of this variation is due to differences in the microbial communities between individual women with BV, but some is due to variation in the mucosal immune response, owing to genetic or environmental influences. For example, white women with a TLR4 SNP (rs1554973) had higher levels of IL1β in the presence of BV than women without that allele (but not higher IL1ra or IL8) [56]. Women with the AA genotype of TLR4-896 had elevated IL1ra and IL1 levels in the presence of G. vaginalis or gram-negative rods but not those with the G allele (Table 1) [6].
While genetic polymorphisms associated with lower levels of soluble immune mediators may increase the risk of BV, variants associated with higher levels of inflammation may increase the risk of reproductive health complications (Figure 1). Tumor necrosis factor (TNF) plays a role in the inflammatory response to infection and in a study of pregnant women, carriers of a TNFα polymorphism (TNFA-308G > A) had increased expression of TNFα only in the presence of altered vaginal flora [21]. Women with BV who also carried this TNFα polymorphism had a 6-fold increased risk of preterm birth, an association that was observed mostly in African American women [57]. In a study of 1371 pregnant women, those with a Nugent score of >4 and at least 1 TNF-238 A allele had 2.6 times higher odds of preterm birth as compared to women with the TNF-238 G/G genotype and a Nugent score of <4 [58]. The TT genotype of a SNP in CRH binding protein (CRH-BP) +15033 was shown to be associated with BV in white women, and this SNP has also been associated with preterm birth [59], providing data to support a role for stress and genetics on risk of BV and preterm birth.
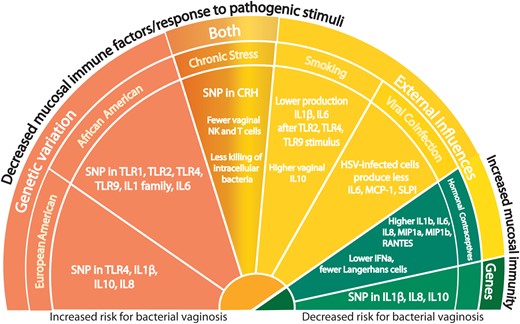
Genetic and environmental factors influence mucosal immunity in the reproductive tract. Factors associated with an increased risk for bacterial vaginosis (BV) are also associated with decreased levels of inflammatory immune markers in cervicovaginal secretions, while factors associated with a decreased risk for BV are associated with increased levels. Abbreviations: CRH, corticotropin-releasing hormone; HSV, herpes simplex virus; IFN, interferon; IL, interleukin; NK, natural killer; SNP, single-nucleotide polymorphism; TLR, Toll-like receptor.
Women with BV have an increased risk of HIV acquisition and transmission [60], likely due in part to increases in proinflammatory mediators and alteration of antimicrobial activity. In a 3-D human vaginal epithelial cell model, colonization with the BV-associated bacterium A. vaginae increased the expression of mucins and proinflammatory mediators, which may in turn disrupt the epithelial barrier to promote HIV acquisition [61]. Low levels of vaginal Lactobacillus colonization have been shown to be an independent risk factor for HIV acquisition [62]. L. crispatus downregulates expression of proinflammatory cytokines produced in response to pathogen stimulation by HeLa cervical cells [63] and vaginal epithelial cells [64]. Women with BV who had H2O2-producing lactobacilli had quantities of BV-associated bacteria similar to those in women without such lactobacilli but had lower levels of IL1β [65]. In a study of healthy HIV-negative South African women, only 37% of had Lactobacillus-dominant vaginal bacterial communities, with the remaining 63% having either Gardnerella-dominant communities or no dominant species but a community composed of at least 10% of Prevotella species. Women in this study with a diverse bacterial community also had higher proinflammatory cytokines in the genital tract as compared to women with Lactobacillus predominance. Women in the highest quartile level of inflammatory cytokines had a significantly higher number of activated CCR5+ CD4+ T cells from cervical cytobrush samples as compared to women in the lowest quartile. Further, in transcriptional analyses using antigen-presenting cells from cervical cytobrush samples, women with diverse vaginal communities had increased upregulation of genes involved in the NF-κB, TNF-α, and TLR pathways when compared to women with Lactobacillus-dominant communities [66]. Several other pathways may be involved in the association between BV and HIV-1 acquisition [67], but most are associated with increased inflammation.
CONCLUSION
The mucosal immune response is modified by multiple factors that are also associated with BV, including ethnicity, hormonal contraception, stress, smoking, and the commensal microbial community. Differences in host genetics appear to be important in a women's predisposition to BV, and although several of the risk factors for BV and adverse outcomes associated with BV differ by race, additional influences on genital tract immune capacity likely act in concert to determine the risk of BV. Factors influencing interactions between the host immune response and the microbiome that decrease the risk of acquiring BV may actually increase the risk of poor reproductive health outcomes once BV is present. Given the complexity of interactions among factors in the genital tract immune milieu, a systems biology approach to elucidating biologic mechanisms mediating the risk of acquisition of BV and subsequent complications is warranted. While this complexity makes research in this area challenging, current data suggest that there may be subgroups of women at higher risk of BV, in whom targeted interventions may have a significant benefit. Longitudinal studies that specifically evaluate the impact of the individual components of the mucosal immune response and their composite interactions on the risk of incident BV or complications associated with BV will move the field forward.
Notes
Supplement sponsorship. This article appears as part of the supplement “Proceedings of the 2015 NIH/NIAID Bacterial Vaginosis Expert Consultation,” sponsored by the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases in partnership with the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Group; contract HHSN272201300012I.
Acknowledgments. We thank Dr Betsy Herold for her support and guidance.
Financial support. This work was supported by Einstein-Montefiore Institutional Mentored Clinical and Translational Research Career Development Award, through the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Award (KL2 award to K. M.); and the Doris Duke Foundation. Clinical Scientist Development Award (to C. M. M.).
Potential conflict of interest. C. M. M. is on an advisory board for Perrigo Pharmaceuticals. K. M. certifies no potential conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: National Institute of Health Bacterial Vaginosis Consultation Meeting.




