-
PDF
- Split View
-
Views
-
Cite
Cite
Karen M. Gallegos, George L. Drusano, David Z. D′Argenio, Ashley N. Brown, Chikungunya Virus: In Vitro Response to Combination Therapy With Ribavirin and Interferon Alfa 2a, The Journal of Infectious Diseases, Volume 214, Issue 8, 15 October 2016, Pages 1192–1197, https://doi.org/10.1093/infdis/jiw358
Close - Share Icon Share
Abstract
Introduction. We evaluated the antiviral activities of ribavirin (RBV) and interferon (IFN) alfa as monotherapy and combination therapy against chikungunya virus (CHIKV).
Methods. Vero cells were infected with CHIKV in the presence of RBV and/or IFN alfa, and viral production was quantified by plaque assay. A mathematical model was fit to the data to identify drug interactions for effect. We ran simulations using the best-fit model parameters to predict the antiviral activity associated with clinically relevant regimens of RBV and IFN alfa as combination therapy. The model predictions were validated using the hollow fiber infection model (HFIM) system.
Results. RBV and IFN alfa were effective against CHIKV as monotherapy at supraphysiological concentrations. However, RBV and IFN alfa were highly synergistic for antiviral effect when administered as combination therapy. Simulations with our mathematical model predicted that a standard clinical regimen of RBV plus IFN alfa would inhibit CHIKV burden by 2.5 log10 following 24 hours of treatment. In the HFIM system, RBV plus IFN alfa at clinical exposures resulted in a 2.1-log10 decrease in the CHIKV burden following 24 hours of therapy. These findings validate the prediction made by the mathematical model.
Conclusions. These studies illustrate the promise of RBV plus IFN alfa as a potential therapeutic strategy for the treatment of CHIKV infections.
The lack of an antiviral treatment and commercially available vaccine makes chikungunya virus (CHIKV) infection a significant public health concern worldwide. CHIKV infections have been reported throughout the Americas since an outbreak in 2013, affecting >1.2 million people including the United States and US territories, such as Puerto Rico and the Virgin Islands. Local transmission has been observed in these areas [1].
CHIKV is an enveloped alphavirus transmitted through a mosquito vector of the species Aedes aegypti and Aedes albopictus. Symptoms of CHIKV infection include fever (temperature, ≥40°C), polyarthralgias, headache, myalgias, back pain, and polyarthritis [2]. The acute phase of CHIKV infection lasts 3–10 days. In 10% of the cases, various rheumatic symptoms persist for several months to years, deteriorating the patient's quality of life and adversely influencing the economy of affected regions [3, 4].
There are currently no vaccines or antivirals licensed for the treatment of CHIKV infections. Consequently, all therapies for CHIKV fever are strictly supportive. These include analgesic drugs, nonsteroidal antiinflammatory drugs, and corticosteroids [5]. Owing to the rapid spread of the virus to new areas and the increasing number of human infections, there is a great medical need to identify new antiviral agents or treatment strategies against CHIKV.
Ribavirin (RBV; 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) and interferon (IFN) alfa are Food and Drug Administration (FDA)–approved, broad-spectrum antivirals effective against influenza virus, polio virus, and hepatitis C virus (HCV) [6, 7]. Until recently, the combination of RBV and IFN alfa therapy has historically been the standard of care for the treatment of HCV infection. This combination regimen is an attractive therapeutic strategy against CHIKV because the pharmacokinetic and toxicity profiles for both compounds are well defined in humans. Thus, the main objective for this study was to evaluate the antiviral activity of RBV and IFN alfa as both monotherapy and combination therapy against CHIKV to determine their potential as therapeutic options for CHIKV-infected patients.
MATERIALS AND METHODS
Cells, Virus, and Compounds
The CHIKV vaccine strain 181/clone 25 (NR-13222) was obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, Virginia). Virus was propagated in Vero cells (CCL-81) purchased from the American Type Culture Collection (ATCC; Manassas). Vero cells were maintained in Eagle's minimum essential medium (MEM; Corning Cellgro; Manassas) supplemented with 5% fetal bovine serum (FBS; Sigma Aldrich; St. Louis, Missouri) and 1% penicillin-streptomycin solution (HyClone; Logan, Utah) at 37°C, 5% CO2. Human IFN alfa subtype 2a was purchased from PBL assay science (Piscataway, New Jersey), and RBV was obtained from Tokyo Chemical Industry (Portland, Oregon).
Antiviral Drug Activity Assay
Confluent Vero cell monolayers were infected with CHIKV at a multiplicity of infection equivalent to 0.0001 and incubated for 1 hour at 37°C in 5% CO2. Monolayers were then washed with phosphate-buffered saline, and drug-containing medium was added to each well. Drug concentrations ranged from 0 to 1000 µg/mL for RBV monotherapy and from 0 to 10 000 international units (IU)/mL for IFN alfa monotherapy. For combination studies, all concentrations of RBV and IFN alfa were evaluated in combination. Plates were incubated for 3 days at 37°C in 5% CO2. Cell culture supernatants were collected daily and clarified, and infectious virus was quantified by plaque assay.
Plaque Assay
Viral supernatants were serially diluted 10-fold in complete MEM supplemented with 2% FBS. Each dilution (100 µL) was inoculated onto confluent Vero cell monolayers and incubated for 1 hour at 37°C in 5% CO2. After infection, a primary overlay (0.6% agar, MEM, and 5% FBS) was added to every well. A secondary overlay (1% agar containing 1× MEM and 1% FBS, 200 µg/mL diethylaminoethyl-dextran, and 0.008% neutral red) was added 2 days after infection. Plaques were counted with the naked eye 6 hours after the addition of the secondary overlay, and viral burden, reported as plaque forming units (PFU) per milliliter, were calculated.
Statistical Analysis and Mathematical Modeling
System outputs were as follows: Y(1) = RBV or IFN alfa concentrations in the tissue culture vessel, and Y(2) = log10(PFU per milliliter).
For combination studies, the Greco universal response surface approach (URSA) model was used to determine drug interactions for effect between RBV and IFN alfa against CHIKV at day 2 after infection. Day 2 was chosen for the analysis because (1) this time point resulted in 90% cytopathic effect, and (2) monotherapy studies showed that peak viral production occurs at day 2 in the control arm. The results from the combination therapy were analyzed, as indicated by Greco et al [9].
Mathematical Model Prediction of Antiviral Response
The parameter estimates obtained using from the Greco model were used to predict the anti-CHIKV response associated with a standard clinical regimen of RBV (600 mg twice daily orally) and IFN alfa (single injection of 18 million IU). Human PK parameters for RBV [11, 12] and IFN alfa [13, 14] used in this analysis were published elsewhere. All simulations were performed using the SIM module in ADAPT [10].
Experimental Validation
The HFIM system for viral infection has been described previously [15–17]. Briefly, 2 cellulosic hollow-fiber (HF) cartridges (FiberCellSystems, Frederick, Maryland) were inoculated with 108 Vero cells and 100 PFU of CHIKV. One HF cartridge served as a control. RBV (0.3603 µg/mL) and IFN alfa (32 166 IU/mL) were administered into the second HF cartridge as a continuous infusion at exposures equivalent to human plasma 24-hour areas under the concentration time curve (AUCs) during the first 24 hours of therapy with a standard clinical regimen (600 mg twice daily of RBV + 18 million IU of IFN alfa). The exposures simulated were 8.647 mg·h/L for RBV (8.647 mg·h/L/24 hours = 0.36 µg/mL as a continuous infusion) [12] and 771 984 mg·h/L (771 984 mg·h/L/24 hours = 32 166 IU/mL as a continuous infusion) [13] for IFN alfa. Each cartridge was sampled 24 hours after treatment, and the viral burden was quantified by a plaque assay.
RESULTS
RBV Monotherapy Against CHIKV
The antiviral activity of RBV was evaluated as monotherapy against CHIKV. In the absence of drug, CHIKV rapidly replicated reaching a peak of 108.5 PFU/mL at day 2 after infection (Figure 1A). Concentrations of <10 µg/mL failed to inhibit CHIKV, as viral replication kinetics for these treatment arms were nearly identical to those of the control. The 100 µg/mL and 1000 µg/mL concentrations of RBV were effective at suppressing CHIKV at day 1, resulting in a 2-log10 decrease in CHIKV level for the 100 µg/mL concentration and a 4-log10 decrease for the 1000 µg/mL concentration. The EC50 at day 1 for RBV was 102.3 µg/mL, and by day 2 the dose-response effect was largely lost (EC50, 835 µg/mL; Figure 1A). Viral titers were similar in all arms at day 3. This finding indicates that RBV monotherapy at high concentrations delays CHIKV replication but does not result in complete suppression. The EC50 for RBV over the 3-day study was 142.70 µg/mL, as estimated by the full mathematical model. The model fit and parameter estimates are listed in Table 1. Vero cell cytotoxicity was not observed with RBV therapy during the 3-day study (data not shown).
Median Estimate Parameter Values From the Monotherapy Studies With Ribavirin (RBV) or Interferon (IFN) Alfa
| Drug . | Model Fit . | Parameter, Mean ± SD . | ||||
|---|---|---|---|---|---|---|
| r2a . | kturn,b per d . | EC50c . | Hd . | POPMAX,e Log10 PFU/mL . | kdeath,f per d . | |
| RBV | 0.88 | 0.85 ± 0.39 | 142.70 ± 126.13 | 4.28 ± 1.14 | 8.54 ± 8.38 | 0.37 ± 0.54 |
| IFN alfa | 0.98 | 0.87 ± 3.04 | 2571.41 ± 860.98 | 1.24 ± 1.43 | 8.37 ± 8.45 | 0.37 ± 0.23 |
| Drug . | Model Fit . | Parameter, Mean ± SD . | ||||
|---|---|---|---|---|---|---|
| r2a . | kturn,b per d . | EC50c . | Hd . | POPMAX,e Log10 PFU/mL . | kdeath,f per d . | |
| RBV | 0.88 | 0.85 ± 0.39 | 142.70 ± 126.13 | 4.28 ± 1.14 | 8.54 ± 8.38 | 0.37 ± 0.54 |
| IFN alfa | 0.98 | 0.87 ± 3.04 | 2571.41 ± 860.98 | 1.24 ± 1.43 | 8.37 ± 8.45 | 0.37 ± 0.23 |
Abbreviation: PFU, plaque-forming units.
ar2 is coefficient of determination that quantifies goodness of fit.
bkturn is the first-order viral production rate constant for chikungunya virus.
c EC50 is the drug concentration of drug at which viral production rate constants are reduced by half. Data for RBV are in μg/mL, and data for IFN alfa are in IU/mL.
dH represents the Hill constant.
e POPMAX is the maximal amount of total viral burden.
fkdeath is the first-order rate of disintegration of infectious viral particle.
Median Estimate Parameter Values From the Monotherapy Studies With Ribavirin (RBV) or Interferon (IFN) Alfa
| Drug . | Model Fit . | Parameter, Mean ± SD . | ||||
|---|---|---|---|---|---|---|
| r2a . | kturn,b per d . | EC50c . | Hd . | POPMAX,e Log10 PFU/mL . | kdeath,f per d . | |
| RBV | 0.88 | 0.85 ± 0.39 | 142.70 ± 126.13 | 4.28 ± 1.14 | 8.54 ± 8.38 | 0.37 ± 0.54 |
| IFN alfa | 0.98 | 0.87 ± 3.04 | 2571.41 ± 860.98 | 1.24 ± 1.43 | 8.37 ± 8.45 | 0.37 ± 0.23 |
| Drug . | Model Fit . | Parameter, Mean ± SD . | ||||
|---|---|---|---|---|---|---|
| r2a . | kturn,b per d . | EC50c . | Hd . | POPMAX,e Log10 PFU/mL . | kdeath,f per d . | |
| RBV | 0.88 | 0.85 ± 0.39 | 142.70 ± 126.13 | 4.28 ± 1.14 | 8.54 ± 8.38 | 0.37 ± 0.54 |
| IFN alfa | 0.98 | 0.87 ± 3.04 | 2571.41 ± 860.98 | 1.24 ± 1.43 | 8.37 ± 8.45 | 0.37 ± 0.23 |
Abbreviation: PFU, plaque-forming units.
ar2 is coefficient of determination that quantifies goodness of fit.
bkturn is the first-order viral production rate constant for chikungunya virus.
c EC50 is the drug concentration of drug at which viral production rate constants are reduced by half. Data for RBV are in μg/mL, and data for IFN alfa are in IU/mL.
dH represents the Hill constant.
e POPMAX is the maximal amount of total viral burden.
fkdeath is the first-order rate of disintegration of infectious viral particle.
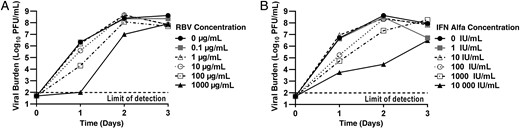
Antiviral activity of ribavirin (RBV) and interferon (IFN) alfa as monotherapy against chikungunya virus (CHIKV). Vero cells were inoculated with CHIKV at a multiplicity of infection of 0.0001 and different concentrations of RBV (A) or IFN alfa (B) were added immediately after infection. Extracellular viral burden, reported as log10 plaque forming units (PFU)/mL, from cell culture supernatants was analyzed daily using a plaque assay.
IFN Alfa Monotherapy Against CHIKV
IFN alfa monotherapy was also assessed for antiviral activity against CHIKV. IFN alfa at concentrations of 1 IU/mL and 10 IU/mL had no inhibitory effect on CHIKV replication, but a dose-response relationship was observed at concentrations of ≥100 IU/mL at day 1 after infection (Figure 1B), with an EC50 value of 173 IU/mL. However, IFN alfa was not able to provide continued viral suppression and the antiviral effect was lost in the 100 IU/mL and 1000 IU/mL treatment arms by days 2 and 3, respectively. EC50 values were 5593 IU/mL at day 2 and 9285 IU/mL at day 3. Concentrations of 10 000 IU/mL inhibited CHIKV relative to the control at all time points, but viral titers steadily increased in this treatment arm throughout the 3 days, peaking at 106.5 PFU/mL (Figure 1B). These findings are similar to those described above for RBV, as IFN alfa delayed but did not completely suppress CHIKV replication. The mathematical model yielded an EC50 estimate of 2571.41 IU/mL for IFN alfa over the entire study duration (Table 1). Cytotoxicity was not observed in Vero cells treated with IFN alfa (data not shown).
RBV and IFN Alfa Combination Therapy Against CHIKV
RBV and IFN alfa were evaluated against CHIKV as combination therapy to determine whether enhanced viral suppression could be achieved using 2 compounds simultaneously. The antiviral activity of the combination regimens was evaluated at day 2 after infection, as this time point resulted in peak viral production in the control arm. RBV and IFN alfa monotherapy suppressed viral replication by 2.4 log10 and 3.4 log10, respectively, relative to the control (Figure 2). Enhanced antiviral activity was largely observed when high concentrations of RBV (≥100 µg/mL) and IFN alfa (100 IU/mL) were present. For example, 1000 µg/mL of RBV in combination with 100 IU/mL of IFN alfa inhibited viral production by 4.9 log10 as compared to the control, providing an extra 2.5- and 1.5-log10 suppression relative to the maximal effect achieved by RBV and IFN alfa alone. The highest concentrations evaluated (1000 µg/mL RBV plus 10 000 IU/mL IFN alfa) almost completely suppressed viral production, resulting in viral titers at the assay limit of detection (102 PFU/mL).
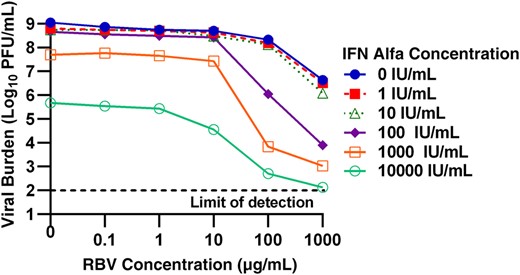
Antiviral activity of combination of ribavirin (RBV) and interferon (IFN) alfa against chikungunya virus (CHIKV). Vero cells were inoculated with CHIKV at a multiplicity of infection of 0.0001, and different concentrations of IFN alfa and RBV were added immediately after infection. Extracellular viral burdens, reported as log10 plaque-forming units (PFU)/mL, from cell culture supernatants were analyzed at day 2 after infection via a plaque assay.
The Greco URSA model was fit to the data shown in Figure 2 to identify the drug-drug interaction (ie, synergy, additivity, or antagonism) for antiviral effect between RBV and IFN alfa. The model fit the data well, resulting in an r2 of 0.947 (Table 2). EC50 values reported by the Greco URSA model were similar to those observed in monotherapy studies with values equivalent to 833 µg/mL for RBV and 5761 IU/mL for IFN alfa at day 2 after infection. α, the drug interaction parameter, was very high, yielding a value of 265.5 (95% CI, 85.88–445.1). These data indicate that the combination of RBV and IFN alfa are highly synergistic for inhibition of infectious CHIKV.
| Parameter . | Final Estimate . | Units . |
|---|---|---|
| r2a | 0.947 | … |
| Econb | 8.809 | Log10 PFU/mL |
| EC50 RBVc | 833.0 | µg/mL |
| m1d | 1.074 | … |
| EC50IFN alfae | 5761.0 | IU/mL |
| m2f | 0.6219 | … |
| αg | 265.5 (85.88–445.1h) | … |
| Parameter . | Final Estimate . | Units . |
|---|---|---|
| r2a | 0.947 | … |
| Econb | 8.809 | Log10 PFU/mL |
| EC50 RBVc | 833.0 | µg/mL |
| m1d | 1.074 | … |
| EC50IFN alfae | 5761.0 | IU/mL |
| m2f | 0.6219 | … |
| αg | 265.5 (85.88–445.1h) | … |
Abbreviations: IFN, interferon; PFU, plaque-forming units; RBV, ribavirin.
ar2 is coefficient of determination that quantifies goodness of fit.
bEcon is a measure of effect (or response) of the control.
cEC50 RBV is the concentration of drug resulting in half maximal effect of RBV.
dm1 is the Hill constant for RBV.
e EC50 IFN α is the concentration of drug resulting in half maximal effect of IFN alfa.
fm2 is the Hill constant for IFN alfa.
gα is the interaction parameter.
h 95% confidence interval.
| Parameter . | Final Estimate . | Units . |
|---|---|---|
| r2a | 0.947 | … |
| Econb | 8.809 | Log10 PFU/mL |
| EC50 RBVc | 833.0 | µg/mL |
| m1d | 1.074 | … |
| EC50IFN alfae | 5761.0 | IU/mL |
| m2f | 0.6219 | … |
| αg | 265.5 (85.88–445.1h) | … |
| Parameter . | Final Estimate . | Units . |
|---|---|---|
| r2a | 0.947 | … |
| Econb | 8.809 | Log10 PFU/mL |
| EC50 RBVc | 833.0 | µg/mL |
| m1d | 1.074 | … |
| EC50IFN alfae | 5761.0 | IU/mL |
| m2f | 0.6219 | … |
| αg | 265.5 (85.88–445.1h) | … |
Abbreviations: IFN, interferon; PFU, plaque-forming units; RBV, ribavirin.
ar2 is coefficient of determination that quantifies goodness of fit.
bEcon is a measure of effect (or response) of the control.
cEC50 RBV is the concentration of drug resulting in half maximal effect of RBV.
dm1 is the Hill constant for RBV.
e EC50 IFN α is the concentration of drug resulting in half maximal effect of IFN alfa.
fm2 is the Hill constant for IFN alfa.
gα is the interaction parameter.
h 95% confidence interval.
Simulation of RBV Plus IFN Alfa Antiviral Activity and Experimental Validation
The best-fit model parameter estimates (Table 2) were used to predict the antiviral activity of a standard clinical regimen of RBV plus IFN alfa against CHIKV. Human PK profiles associated with oral administration of RBV at 600 mg twice daily [11, 12] and a single injection of IFN alfa at 18 million IU [13, 14] were used for the simulation. Computer simulations predicted that the standard clinical regimen of RBV plus IFN alfa combination therapy would yield a 2.5-log10 reduction in viral burden after 24 hours. We examined the validity of the simulation output by assessing the antiviral activity at clinically relevant exposures of RBV and IFN alfa as combination therapy, using the in vitro HFIM system. The experimental results showed that RBV plus IFN alfa therapy resulted in a 2.1-log10 reduction in viral burden as compared to the control 24 hours after treatment (Figure 3). These findings demonstrate that the model predictions were reliable in predicting the antiviral activity of the combination regimen. Moreover, the results show that the clinically available RBV plus IFN alfa therapy has the potential to reduce CHIKV burden by at least 99% during the first 24 hours of treatment.
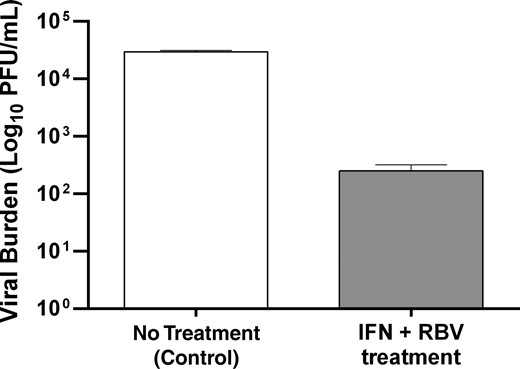
Antiviral activity of ribavirin (RBV) and interferon (IFN) alfa as combination therapy against chikungunya virus (CHIKV) 24 hours after infection, using the hollow fiber infection model system. Hollow fiber cartridges were inoculated with 108 Vero cells and CHIKV at a multiplicity of infection of 10−6. The clinical 24 hours exposures of IFN alfa (32 166 IU/mL) and RBV (0.3603 µg/mL) were administered as a continuous infusion immediately after infection. Extracellular viral burden from supernatants harvested from the hollow fiber cartridges 24 hours after therapy was quantified by a plaque assay. Abbreviation: PFU, plaque forming units.
DISCUSSION
CHIKV is becoming a significant public health problem in the tropics and subtropics throughout the globe. Clinical symptoms of viral infection, including painful and debilitating arthritis, can become chronic and persist for years, severely reducing the quality of life in affected patients. Antiviral therapy has the potential to reduce or eliminate the clinical manifestations associated with CHIKV infections. However, these therapies do not yet exist, and treatment for CHIKV infection is strictly supportive in a clinical setting. Thus, there is a great need for new therapeutic strategies for the treatment of CHIKV infection. The goal of the present study is to evaluate the antiviral activity of 2 broad-spectrum antiviral agents, RBV and IFN alfa, as monotherapy and combination therapy against CHIKV, in an attempt to identify a therapeutic regimen for CHIKV infection.
RBV and IFN alfa are attractive therapeutic candidates for the treatment of CHIKV infection because both have broad-spectrum antiviral activity and are FDA approved for human clinical use. Previous studies have explored the clinical potential of RBV for the treatment of CHIKV infection. In 1 small clinical study, 10 CHIKV-infected patients with prolonged arthritis symptoms were treated with 200 mg of RBV twice daily for a week [18]. All RBV-treated patients exhibited improvement in joint pain and joint and soft-tissue swelling was decreased in 80% of these individuals. Although these findings suggest that RBV may be effective at reducing CHIKV disease manifestations in humans, the direct antiviral activity of RBV against CHIKV in man is still unclear.
Our preclinical evaluations demonstrated that RBV monotherapy delays the production of infectious CHIKV but does not completely suppress it (Figure 1A). Furthermore, high concentrations of RBV were required to achieve substantial antiviral effect, with EC50 and EC90 values of 142.70 µg/mL and 238.35 µg/mL, respectively, for the 3-day study. These concentrations fall outside of the therapeutic window for RBV and are considered toxic in humans. For example, the clinical dose of RBV is 600 mg twice daily (based on HCV dosing recommendations) and yields a maximal 24-hour AUC exposure of 48 mg·h/L [11]. The EC50 value for RBV against CHIKV is associated with a 24-hour AUC exposure of 3425 mg·h/L (142.70 µg/mL × 24 hours), and the EC90 is equivalent to an exposure of 5720 mg·h/L (238.35 µg/mL × 24 hours). These exposures are 71-fold and 119-fold higher, respectively, than the standard clinical dosage regimen and are not attainable in people because of toxicity. Thus, RBV as monotherapy is not a suitable treatment regimen for CHIKV infection in humans.
Similarly, IFN alfa monotherapy was effective against CHIKV at high concentrations (Figure 1B). IFN alfa levels of at least 100 IU/mL suppress CHIKV production at day 1 after therapy, but 10 000 IU/mL were needed to sustain antiviral activity through day 3. The EC50 for IFN alfa was 2571.41 IU/mL, and the EC90 was 15 063.51 IU/mL determined over the 3-day study. The maximal clinical dose of IFN alfa is 36 million IU daily, and this regimen has a reported mean 24-hour AUC exposure of 3072 IU h/mL [14]. However, 24-hour AUC exposures associated with the EC50 and EC90 values are 61 714 IU h/mL and 361 524 IU h/mL, respectively, which are 20-fold and 118-fold higher, respectively, than clinical exposures. Higher clinical doses cannot be administered to patients, owing to toxicity. Thus, these studies demonstrate that IFN alfa as monotherapy is only effective at supratherapeutic doses and would not be appropriate for CHIKV treatment.
Combination chemotherapy with ≥2 antiviral agents is an attractive therapeutic strategy to provide enhanced antiviral activity. This strategy has been used for the treatment of HCV and human immunodeficiency virus (HIV) infections. Previous studies have shown that combination therapy with RBV and IFN alfa are synergistic for CHIKV suppression in vitro [19]. Synergistic interactions between RBV and IFN alfa may allow for the use of lower concentrations of each drug in combination without compromising antiviral activity. Our results show that RBV and IFN alfa as combination chemotherapy are highly synergistic, resulting in enhanced viral inhibition as compared to monotherapy treatment arms and an α (the drug interaction parameter) of 265.5 (Table 2). These findings suggest that combination therapy has potential to be a therapeutic strategy for CHIKV, as it may be possible to achieve sufficient levels of viral inhibition at physiologic exposures when RBV and IFN alfa are administered together. To examine this hypothesis, we performed computer simulations to predict the degree of viral inhibition when a standard clinical regimen of RBV (oral delivery of 600 mg twice daily) and IFN alfa (a single injection of 18 million IU) were administered simultaneously. The mean human PK parameters for RBV and IFN alfa were used in the simulation. Simulation outputs predicted a 2.5-log10 reduction in viral burden after 24 hours of therapy. This prediction was validated experimentally, using the HFIM system. Thus, our findings suggest that RBV plus IFN alfa therapy at standard clinical regimens could reduce CHIKV levels by 99% 24 hours after therapy. Considering that our experiments and simulations were conducted in the absence of an immune response, our predictions may underreport antiviral activity, with further inhibition likely with a functioning immune system.
The objective of these studies was to explore the potential of a currently approved and available therapeutic regimen for the treatment of CHIKV infection, a disease for which antiviral therapy does not exist. Despite the promising results described, there are limitations to our study. Our antiviral screen was conducted with only the vaccine strain of CHIKV, as this strain has reduced biosafety requirements as compared to other clinical strains. Future studies will evaluate the antiviral activity of the RBV plus IFN alfa combination regimen against clinically relevant CHIKV isolates. Also, for RBV plus IFN alfa therapy to be successful clinically, the PK/PD relationships for both compounds against CHIKV must be understood to identify the PD index that is most closely linked with viral suppression. This information is critical to the design of optimal dosage regimens. This is currently an active area of research in our laboratory.
In conclusion, our results show that RBV and IFN alfa have antiviral activity against CHIKV and display synergistic effects when used as combination chemotherapy. This study provides evidence that the standard clinical regimen of RBV plus IFN alfa suppresses CHIKV replication and, thus, may serve as a potential therapeutic strategy for infected patients. However, further studies should be performed to determine the effectiveness of this regimen in a clinical setting.
Notes
Financial support. This work was supported by the Institute for Therapeutic Innovation, University of Florida; and by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (grant P41-EB001978).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




