-
PDF
- Split View
-
Views
-
Cite
Cite
Yinon Levy, Yaron Vagima, Avital Tidhar, Ayelet Zauberman, Moshe Aftalion, David Gur, Itay Fogel, Theodor Chitlaru, Yehuda Flashner, Emanuelle Mamroud, Adjunctive Corticosteroid Treatment Against Yersinia pestis Improves Bacterial Clearance, Immunopathology, and Survival in the Mouse Model of Bubonic Plague, The Journal of Infectious Diseases, Volume 214, Issue 6, 15 September 2016, Pages 970–977, https://doi.org/10.1093/infdis/jiw290
Close - Share Icon Share
Abstract
Background. Plague is initiated by Yersinia pestis, a highly virulent bacterial pathogen. In late stages of the infection, bacteria proliferate extensively in the internal organs despite the massive infiltration of neutrophils. The ineffective inflammatory response associated with tissue damage may contribute to the low efficacy of antiplague therapies during late stages of the infection. In the present study, we address the possibility of improving therapeutic efficacy by combining corticosteroid administration with antibody therapy in the mouse model of bubonic plague.
Methods. Mice were subcutaneously infected with a fully virulent Y. pestis strain and treated at progressive stages of the disease with anti–Y. pestis antibodies alone or in combination with the corticosteroid methylprednisolone.
Results. The addition of methylprednisolone to antibody therapy correlated with improved mouse survival, a significant decrease in the amount of neutrophils and matrix metalloproteinase 9 in the tissues, and the mitigation of tissue damage. Interestingly, the combined treatment led to a decrease in the bacterial loads in infected organs.
Conclusions. Corticosteroids induce an unexpectedly effective antibacterial response apart from their antiinflammatory properties, thereby improving treatment efficacy.
The inflammatory response is an important feature of host innate immunity that has evolved to protect the host against invading microorganisms. While a controlled and fine-tuned inflammatory response is beneficial to the host, an excessive response can be harmful, resulting in cumulative tissue damage and organ failure [1–3].
Owing to their broad immune-modulatory and antiinflammatory properties, corticosteroids are widely used to counteract detrimental inflammatory processes [4–7]. However, their use as adjunctive therapy during acute infections is controversial owing to concern over immunosuppression. Yet, several clinical studies have recently shown that, for patients exhibiting acute infections, such as bacterial meningitis, severe pneumonia, and severe sepsis, treatment with corticosteroids had overall beneficial effects [8–12]. Furthermore, in murine models of pulmonary acute lung injury, treatment with corticosteroids improved lung mechanics and reduced the inflammatory response [13–15]. Recently, corticosteroids were also found to modulate inflammation by activating antiinflammatory monocyte subtypes that migrate quickly to the inflamed tissue [16]. All these findings suggest that corticosteroids may counteract uncontrolled inflammatory responses during acute infections.
Bubonic plague, resulting from Yersinia pestis infection following bite by infected fleas, is characterized by the development of buboes, representing grossly enlarged lymph nodes at the site of infection [17]. Without appropriate treatment, bubonic plague can progress to septicemia, secondary pneumonia, or, in rare cases, meningitis [18]. In animal models, high bacterial loads can be found in peripheral organs and in the blood at progressive stages of disease, accompanied by neutrophil accumulation and extensive tissue damage [19]. Notably, at this late stage of the infection, the administration of antiplague therapy affords little remedy [20]. Based on the notion that the inflammatory response influences the progression of the disease, one approach to overcome this therapeutic limitation may be represented by specifically targeting the inflammatory process. Accordingly, in this study, we evaluated the potential of corticosteroids as adjunctive therapy against bubonic plague. The observations documented in this report provide evidence that the addition of a relatively low dose of steroids to anti–Y. pestis antibody therapy is beneficial at late stages of the disease. The combined treatment improved survival rates, significantly reduced acute inflammation, and unexpectedly enhanced bacterial clearance.
MATERIALS AND METHODS
Ethics Statement
This study was performed in accordance with the recommendations for the Care and Use of Laboratory Animals (National Institutes of Health [NIH]). Animal experiments were performed in accordance with Israeli law and were approved by the Institutional Ethics Committee for animal experiments (protocol M-36-09, M-54-10, M-29-15). During the experiments, mice were monitored daily. Humane end points were used in survival studies. Mice exhibiting loss of the righting reflex were euthanized by cervical dislocation.
Animals
Five week-old OF-1 female mice were purchased from Charles River Laboratories (Iffa-Credo, France).
Mouse Infection
Virulent Y. pestis strain Kimberley 53 (Kim53) was grown on brain-heart infusion (BHI) agar plates at 28°C as described elsewhere [21, 22]. Colonies were suspended in saline and serially diluted, and 100 colony-forming units (100 median lethal doses) were injected subcutaneously into the lower right back. The infectious dose was verified by plating diluted bacterial suspensions onto BHI agar.
Treatment of Mice
Rabbit anti-F1 immunoglobulin G (IgG) and anti-LcrV IgG antibodies were purified from hyperimmune sera, as described elsewhere [23]. These antibodies (1–2 mg/mouse in 1 mL of phosphate-buffered saline [PBS]) were injected into the peritoneal cavities of the infected mice (2.5–5 × 105 enzyme-linked immunosorbent assay units/mouse of rabbit anti-F1 IgG and 2000 neutralizing units/mouse of anti-LcrV IgG) 48 or 72 hours after infection. Combined passive and steroid treatment included single doses of methylprednisolone (Mp; 2 mg/Kg Solu-Medrol; Pfizer) injected into the peritoneal cavity 24 hours after infection. Control mice were treated with an equivalent dose of irrelevant antibodies or Mp.
Preparation of Tissues for Evaluation of Inflammation
Blood samples were collected from mice by heart puncture into K2E tubes (BD Microtainer). Hematologic analysis was determined using the Forcyte analyzer (Oxford Science). For fluorescence-activated cell-sorting (FACS) analysis, red blood cells were discarded using red blood cell lysing reagent (Sigma). Spleens and lymph nodes were excised, meshed with PBS containing protease inhibitor cocktail (Sigma), and passed through a cell strainer (Falcon). Cell pellets were fixed in 4% paraformaldehyde, washed, and resuspended in PBS, 3% fetal calf serum, and 2 mM ethylenediaminetetraacetic acid. Filter-sterilized supernatants were used for determination of matrix metalloproteinase 9 (MMP-9) levels.
Flow Cytometric Analysis
Cell suspensions were stained with Ly6G-APC (clone 1A8; BioLegend) and CD11b-PerCP-Cy5.5 (clone M1/70; eBioscience) as previously described [24]. The cells were stained using standard protocols and the appropriate matched isotype control antibodies. Y. pestis was stained with AlexaFluor 488-coupled anti-bacterium antibodies. The analysis was performed on a FACSCalibur flow cytometer with CellQuest Pro (BD Bioscience) software. Compensation was done using single-fluorophore staining of the cell suspensions according to the manufacturer's guidelines.
Quantification of MMP-9 Levels by Zymography
Equal volumes of spleen or inguinal lymph node (ILN) supernatant samples were loaded without boiling onto 10% polyacrylamide gels containing 5 mg/mL gelatin (Sigma, Israel). Following electrophoresis, gels were washed to remove the sodium dodecyl sulfate, incubated in developing buffer (50 mM Tris-HCl, 5 mM CaCl2, 200 mM NaCl, and 0.02% Brij; pH 8.0), and subsequently stained with SeeBand Forte (GeBa, Israel). The MMP-9 levels were measured by calculating the white band intensities, using the ImageJ program (NIH). Samples of HT1080 human fibrosarcoma cell-culture supernatant were included in each gel to identify MMP-9.
Histology and Immunohistochemistry
Formalin-fixed inguinal lymph nodes (ILNs) were embedded in paraffin. Two-micrometer sections were prepared and stained with hematoxylin and eosin. The sections were analyzed using an A1 Axioscope and photographed by a coupled AxioCam ICc3 camera (Zeiss, Germany).
Statistical Analysis
The log-rank test provided by the GraphPad Prism 5 statistical package was used to assess differences in the survival curves of the treated mice. Differences between treatment groups were analyzed by a nonparametric t test (the Mann–Whitney test). Differences with P values of ≤ .05 were considered statistically significant.
RESULTS
Addition of Corticosteroids to Anti–Y. pestis Antibody Therapy Improves Mice Survival
To address the possible beneficial contribution of corticosteroids to antiplague treatment, a murine model of infection was used. Mice were subcutaneously inoculated with a lethal dose of 100 LD50 of the fully virulent Y. pestis strain Kim53, a dose that results in a fully blown form of plague, which culminates with the death of untreated mice 4–5 days after infection. Using this model, experimental animals were treated with anti–Y. pestis antibodies alone or with addition of steroids at progressive stages of the disease. At this time point after infection, untreated animals present high levels of bacteria and neutrophils in peripheral organs and circulation (Supplementary Figures 1 and 2). To avoid oversuppression of host immunity required for effective bacterial clearance, a moderate steroid treatment was designed, using the intermediate-potency corticosteroid Mp, administered once, at a relatively low dose of 2 mg/kg [14]. Corticosteroid treatment preceded the anti–Y. pestis antibody therapy because of the time required to exert their antiinflammatory activity.
The results depicted in Figure 1 show that, while treatment of the infected mice with the anti–Y. pestis antibodies 48 hours after infection could rescue only 25% of the infected animals, the combined therapy increased the survival rates to 75% (P < .05, by the log-rank test). Furthermore, when a second dose of Mp was administered on day 7 of the infection to the mice that received the combined therapy, survival rates further improved, to 87.5% (P < .01, by the log-rank test). Of note, all mice treated with Mp alone died of infection within 5 days, similar to control mice treated with irrelevant antibodies, indicating that the addition of Mp was beneficial only when adjunct to anti–Y. pestis antibodies. The combined treatment, compared with antibody treatment alone, also improved the survival rates when the protective antibodies were administered at a later time point of 72 hours after infection; however, the difference did not reach statistical significance (data not shown). Taken together, these observations clearly indicate that the addition of a low dose of Mp can improve antiplague passive therapy at progressive stages of bubonic plague.
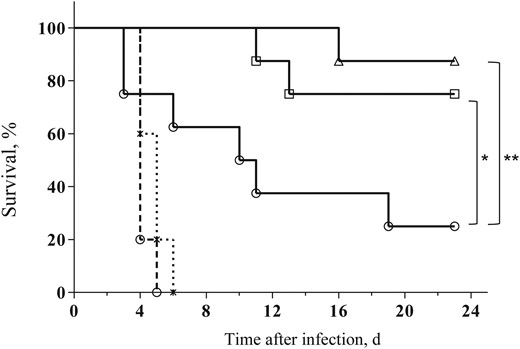
Combined antibody and steroid therapy improves survival of Yersinia pestis–infected mice. Survival of mice (n = 8) infected subcutaneously with 100 median lethal doses (100 colony-forming units) of the fully virulent Y. pestis strain Kim53 and subsequently treated either with anti–Y. pestis antibodies (open circles) or a combination of anti–Y. pestis antibodies and a single dose (open squares) or 2 doses (open triangles) of methylprednisolone (Mp; see “Materials and Methods” section for details). The control animals were treated with normal rabbit immunoglobulin G (dashed line, open circles) or with Mp only (dashed line, asterisks). *P < .05 and **P < .01.
Addition of Corticosteroids to Anti–Y. pestis Antibody Therapy Reduces Inflammation and Tissue Damage During Treatment
It is well established that steroid treatment may affect inflammatory responses associated with infection with various pathogens. Accordingly, to gain insight in the observed beneficial effect of corticosteroid treatment, we first analyzed the magnitude of systemic inflammatory responses during the treatment period. Immediate responses were examined at day 3 after infection, 1 day after the administration of antibody therapy. Late responses were examined on day 7 after infection, 5 days after the beginning of treatment. The results depicted in Figure 2 describe the assessment of the inflammatory process by determining the neutrophil to lymphocyte ratio (NLR) in the circulation of the infected animals, using the Forcyte hematology analyzer. This parameter represents one of the most reliable hematological markers for evaluating the development of systemic inflammation in critically ill patients with severe infections and sepsis [25]. The mean NLR (±SD) for naive OF-1 mice was 0.4 ± 0.14 (Figure 2). Starting from 48 hours after infection, a significant increase in the NLR could be observed, reaching a mean value (±SD) of 1.65 ± 0.34 on day 3 after infection. At that time point, similar NLR values were observed in all 3 treatment groups. Four days later, a significant increase in the NLR values of the mice treated with anti–Y. pestis antibodies alone was observed. The addition of Mp to anti–Y. pestis antibodies not only prevented such an elevation but also resulted in diminished NLR values, compared with those on day 3 after infection. These observations indicate that the addition of Mp to antibody therapy indeed decreased systemic inflammation in treated animals as compared to animals treated with antibody therapy alone.
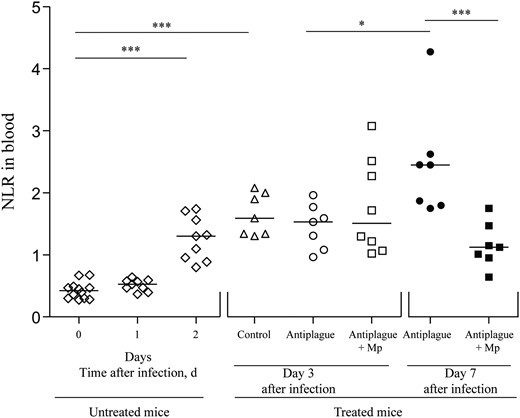
Kinetics of the neutrophil to lymphocyte ratio (NLR) in the blood of Yersinia pestis–infected mice during treatment and recovery. NLRs were measured in the circulation of infected and naive mice (n = 7–12) before antibody therapy (open diamonds) and on days 3 (open symbols) and 7 (closed symbols) after infection. Triangles denote infected mice that were treated with control antibodies, circles denote mice treated with anti–Y. pestis antibodies, and squares denotes mice treated with a combination of methylprednisolone (Mp) and anti–Y. pestis antibodies. Cell measurements in the blood were done using the Forcyte hematology analyzer (see “Materials and Methods” section). Horizontal lines represent the median values. *P < .05 and ***P < .005.
To further confirm that Mp treatment lowers the late inflammatory response, as observed in the circulation, flow cytometry (FACS analysis) was performed to assess the neutrophil levels in the draining ILNs. On day 3 after infection, similar percentages of neutrophils were found in all experimental groups of differentially treated animals. However, on day 7 after infection, the percentage of neutrophils in mice treated with the combined therapy was much lower than that found in mice treated with anti–Y. pestis antibodies alone, reflecting a more balanced inflammatory process (Figure 3A). The total number of neutrophils in the draining ILNs was also reduced in the mice that received combined therapy; however, owing to the high variability of the results, the difference did not reach statistical significance (Figure 3B).
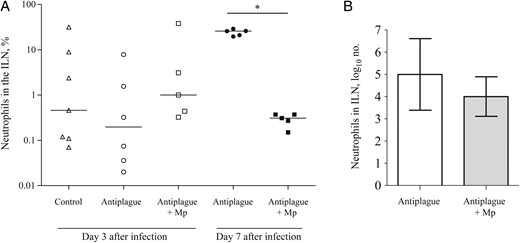
Neutrophil levels in the inguinal lymph nodes (ILNs) of Yersinia pestis–infected mice during treatment and recovery. A, The percentage of neutrophils (Ly6G+CD11b+) in the draining ILNs among mice that were treated with control antibodies (triangles), anti–Y. pestis antibodies (circles), or a combination of methylprednisolone (Mp) and anti–Y. pestis antibodies (squares) on day 3 (open symbols) and day 7 (closed symbols) after infection. Horizontal lines represent the geometric mean values. B, The total number of neutrophils (geometric mean values) in the draining ILNs of mice that were treated with anti–Y. pestis antibodies alone (white bar) or a combination of Mp and anti–Y. pestis antibodies (gray bar) on day 7 after infection The error bars represent 95% confidence intervals. The data represent the results of 2 independent experiments. *P < .05.
The inflammatory response was further evaluated by measuring the MMP-9 level, which represents a good correlate of the extent of inflammatory responses involving neutrophils [26]. This enzyme, secreted from the intracellular granules of neutrophils, facilitates their ability to infiltrate via dense extracellular matrices [27]. MMP-9 levels were measured in tissue homogenates by zymography (see “Materials and Methods” section). On day 3 after infection, comparable MMP-9 levels were measured in the ILNs and spleens of all treatment groups (Figure 4A and 4B, respectively). In contrast, on day 7 after infection, the MMP-9 levels in the mice treated with the combined therapy were significantly lower than those measured in mice treated with anti–Y. pestis antibodies alone (Figure 4).
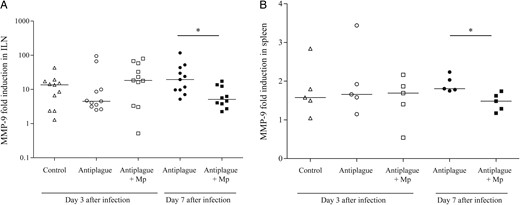
Matrix metalloproteinase 9 (MMP-9) levels in the spleen and inguinal lymph nodes (ILNs) of Yersinia pestis–infected mice during treatment and recovery. The MMP-9 levels were quantified by collagen zymography as described in “Materials and Methods” section. Fold induction of MMP-9 levels (compared to those in naive mice) in the spleen (A) and ILNs (B) on day 3 (open symbols) and day 7 (closed symbols) after infection among mice that were treated with control antibodies (triangles), anti–Y. pestis antibodies (circles), or a combination of methylprednisolone (Mp) and anti–Y. pestis antibodies (squares). Horizontal lines represent the geometric mean values. The data represent the results of 2 (A) and 3 (B) independent experiments. *P < .05.
The impact of the combined treatment on the inflammatory state in the draining ILNs was further evaluated by histological inspection of tissue sections obtained on day 7 after infection. This analysis revealed that 75% of the mice that received antiplague therapy alone had considerable damage to the ILN architecture, compared with mice that received combined therapy. The damaged ILNs from mice that were treated with antibody therapy alone were devoid of lymphocytes, and their volume was filled with neutrophils and cell debris. Lymphatic vessels were clogged with large colonies of Y. pestis surrounded by numerous neutrophils (Figure 5B). In contrast, most ILN sections derived from mice that were treated with the combined therapy appeared to be intact as noninfected ILNs (Figure 5A and 5C).
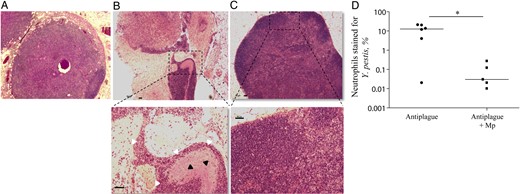
Histological analysis of inguinal lymph node (ILN) sections from mice recovering from Yersinia pestis infection. Hematoxylin and eosin staining of ILN tissue sections from representative naive mice (A), Y. pestis–infected mice treated with anti–Y. pestis antibodies (B), or Y. pestis–infected mice that received the combined treatment (C). Upper panel objective magnification, 2.5× original magnification. The lower panel represents magnification (objective, 10× original magnification) of the dashed rectangle in the upper panel. The black and white arrowheads indicate Y. pestis and neutrophils, respectively. The horizontal bars are equal to 50 μm. D, The percentage of ILN neutrophils (Ly6G+CD11b+) also stained with Y. pestis–specific antibodies among mice that were treated with anti–Y. pestis antibodies alone (closed circles) or with the combined therapy (closed squares) on day 7 after infection. Horizontal lines represent geometric mean values. *P < .05.
Addition of Corticosteroids to Antibody Therapy Lowers Y. pestis Loads in Infected Organs
To directly inspect the interactions between the infective Y. pestis pathogen and the host neutrophils infiltrating the ILNs, FACS analysis was performed on day 7 after infection, using markers enabling the specific detection of neutrophils associated with bacteria. The data depicted in Figure 5D indicate that, in mice treated with anti–Y. pestis antibodies alone, approximately 10% of the ILN neutrophils stained positive for Y. pestis. Interestingly, the combined antiplague therapy was able to reduce the percentage of neutrophil-associated bacteria by >2 orders of magnitude, implying that, in addition to its antiinflammatory activities, Mp may be involved in decreasing the bacterial loads in the infected organs of treated mice.
To address this possibility, the bacterial loads in the ILNs, spleens, and blood were compared between the experimental groups of differentially treated animals (Figure 6). On day 3 after infection, the amount of bacteria in the ILNs of mice treated with anti–Y. pestis antibodies alone was lower than that found in the control animals. The addition of Mp to antiplague therapy further reduced their numbers in the ILNs (Figure 6A). At this time point, the bacterial levels in the spleens and blood of mice treated with anti–Y. pestis antibodies alone or in combination with Mp were significantly lower than those found in control animals, reflecting the expected antibacterial activity of the protective anti–Y. pestis antibodies (Figure 6B and 6C).
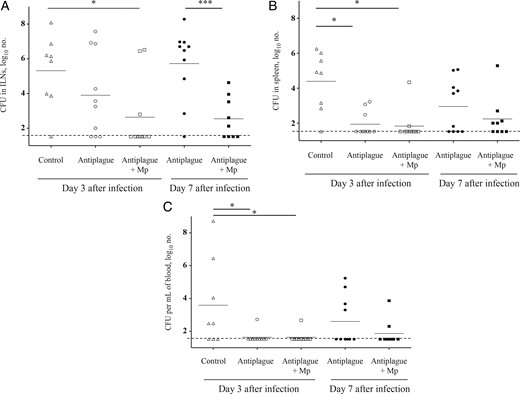
Bacterial load in target tissues of infected mice treated with antiplague or combined therapy. Bacterial colony-forming units (CFU) in the inguinal lymph nodes (ILNs; A), spleens (B), and blood (C) of mice (n = 8–10) infected subcutaneously with 100 median lethal doses (100 CFU) of the fully virulent Y. pestis strain Kim53 and subsequently treated with control antibodies (triangles), anti–Y. pestis antibodies (circles), or combined therapy (squares). Analyses were performed on day 3 (open symbols) and on day 7 (closed symbols) after infection. Small horizontal lines represent the geometric mean values, and the long dashed line indicates the limit of detection. The data represent the results of 3 independent experiments. *P < .05 and ***P < .005.
On day 7 after infection, an increase in the bacterial loads was clearly observed in mice treated with anti–Y. pestis antibodies alone. This observation is in line with the increase in inflammatory parameters observed on day 7 (Figures 2–4). In contrast, in the ILNs of mice treated with the combined therapy, the bacterial counts remained comparable to those found on day 3 after infection, resulting in a significant difference between the 2 treatment groups. While the relevant organs for inspecting the bacterial loads are represented by the ILNs ([28]), we note that similar results were also observed in the spleens and circulation of the treated mice (Figure 6B and 6C). These results clearly show that, in addition to its antiinflammatory activities, Mp also enhanced bacterial clearance, which may explain the improved recovery of the infected animals that ultimately resulted in higher survival rates (Figure 1).
DISCUSSION
Plague is frequently difficult to diagnose owing to the nonspecific initial flu-like symptoms, and accordingly, late diagnosis is often associated with a delay of appropriate treatment. Thus, even aggressive antimicrobial therapy results in significant mortality when it is administered late in infection [20]. The reasons for the inefficacy of delayed antiplague therapy allegedly stem from the inability of the host to control the robust inflammatory response that accompanies the rapid growth and dissemination of this highly virulent pathogen. It is therefore essential to identify means for improving the efficacy of the treatment, especially when it is administered at a later stage of the disease. Steroids are highly potent antiinflammatory agents and may be used for this purpose. Nevertheless, the administration of steroids during infection may increase the sensitivity of the host to the pathogen, as reported by Payne et al in regard to exposure to attenuated Y. pestis strains [29].
We are the first to show that the addition of corticosteroids to anti–Y. pestis antibody therapy has a beneficial effect on survival and recovery from bubonic plague. Using the bubonic plague mouse model, we showed that the addition of a single low dose of the corticosteroid Mp improved the efficacy of anti–Y. pestis antibody therapy administered at progressive stages of the disease, leading to significantly higher survival rates. Furthermore, this combined therapy delayed the onset of mortality among the nonsurviving mice by 8 days, compared with mice receiving only anti–Y. pestis antibodies. This considerable widening of the therapeutic window enabled further treatment of the recovering animals and further improvement of the survival rates (Figure 1). The benefits of the corticosteroid treatments are associated not only with a decrease in the severity of the detrimental inflammatory response (see below) but also, and most significantly, with an increased rate of pathogen clearance, a process that ultimately may be a major factor contributing to the recovery of the infected animals. Notably, the beneficial contribution of adjunctive corticosteroid therapy has also been recently demonstrated in a mouse model of respiratory exposure–induced plague, where it afforded reduced pulmonary inflammation and decreased bacterial loads in the lung (unpublished data).
The beneficial use of corticosteroids as an adjunctive therapy has been previously demonstrated in a limited number of controlled animal studies. For example, in a mouse model of a lethal pandemic influenza A(H1N1) virus infection, dexamethasone treatment ameliorated acute lung infection by reducing lung edema, neutrophil infiltration, and interleukin 6 levels, resulting in increased survival rates of the infected animals at different times, up to 10 days after infection [30]. In a recent study, combined treatment with antibiotics and dexamethasone reduced lung immunopathology and mortality in a mouse model of secondary bacterial pneumonia [15]. In this report, we showed that corticosteroids by themselves do not have a protective effect against Y. pestis and that antibacterial therapy must be adjoined (Figure 1). Moreover, we found that a single low dose of Mp was sufficient to confer improved survival rates. Higher doses of the steroid or multiple doses did not further elevate the survival rates (data not shown). This beneficial effect of a low dose of steroids could prevent undesired complications that might result from long-term or high-dose use of corticosteroids.
In addition to improving the survival rates, the combined steroid and anti–Y. pestis antibody therapy also reduced the inflammatory response, as judged by several inflammation-specific parameters and tissue damage in recovering animals (Figures 2–5). The NLR, which is a reliable parameter for assessing the extent of systemic inflammation in severe infections and sepsis [25], can be used to evaluate the extent of systemic inflammation in the mouse model of bubonic plague. Here, we showed that, 7 days after infection, the various treatment groups exhibited distinct NLR values, in line with the beneficial antiinflammatory effect of the steroid treatment (Figure 2). Future work focusing on characterizing the lymphocyte populations in the course of bubonic plague may provide additional parameters for the earlier assessment of treatment efficacy.
The data showed that the addition of Mp to anti–Y. pestis antibody therapy also leads to diminished inflammation in the target organs as compared to antibody therapy alone. The percentage of ILN-associated neutrophils and the levels of MMP-9 in the ILNs were significantly lower in recovering mice treated with the combined therapy (Figures 3 and 4). Interestingly, the combined treatment resulted in only a slight reduction in the total number of ILN-associated neutrophils as compared to treatment with antibodies alone (Figure 3B). Hence, it appears that the relative number of neutrophils among the total ILN cell population reflect more accurately the severity of the inflammatory state in the affected ILN. Indeed, the integrity of the lymphatic tissue structure in mice treated with Mp (Figure 5C) and the decreased percentage of neutrophils bound to Y. pestis bacilli (Figure 5D) is indicative of reduced inflammation in the draining lymph node and reflects the beneficial contribution of the combined therapy.
Studies addressing the interaction between neutrophils and Y. pestis established that neutrophils are a major target of this pathogen [31]. While early recruitment of neutrophils is essential for limiting the ability of Y. pestis to establish the infection, at progressive stages of plague, even massive infiltration of these phagocytic cells cannot limit bacterial growth and dissemination and may add to tissue damage [19, 24, 32, 33]. Here, we observed that, in convalescing mice treated with anti–Y. pestis antibodies, increased levels of neutrophils and MMP-9 appear to be detrimental because they correlate with elevated bacterial counts in circulation and target tissues (Figure 6). The reasons for this harmful role of tissue-infiltrating neutrophils may stem from the break down of natural tissue barriers by secretion of MMP-9 and other proteolytic enzymes, thereby enabling the pathogen to disseminate into additional tissues. Moreover, the apparent destruction of ILN architecture, mostly observed in mice treated with anti–Y. pestis antibodies only, probably had an adverse effect on the ability of the host to clear the infection, because relevant immune cells were also eliminated by the infiltrating neutrophils. Of note, the beneficial effect of the corticosteroid adjunctive treatment is manifested by a highly significant decrease in the bacterial load in the draining ILNs (Figure 6A), strongly suggesting that the steroid contribution is not merely related to its antiinflammatory potential, but also to potentiation of the clearance mechanism(s) active in the healing process. This correlation between the bacterial burden in the ILNs and the survival rates was recently observed by Guinet et al in a study elucidating the importance of the Pla protease for Y. pestis survival in the mammalian host during bubonic plague [28].
Studies focusing on the identification of the cell populations that mediated the beneficial activity of Mp are complicated by the fact that all immune cells express the corticosteroid receptor [34]. Nonetheless, monocyte-derived macrophages seem to be good candidates because corticosteroids increase their ability to infiltrate infected tissues and to phagocytose apoptotic neutrophils [16, 35]. Monocyte-derived macrophages treated with the antiinflammatory compounds resolvin D1 and resolvin D5 displayed enhanced bacterial engulfment, which correlated with the improved survival of lethally infected mice after treatment with these compounds [36].
In conclusion, we provided proof of concept that mild corticosteroid treatment is beneficial in potentiating postexposure antibody treatment against plague. Elucidation of the mechanisms that enhance bacterial clearance by corticosteroids may provide improved therapeutic tools for the treatment of lethal pathogen infections.
Notes
Disclaimer. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Israel Institute for Biological Research (grant SB/5112-225).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




