-
PDF
- Split View
-
Views
-
Cite
Cite
Pierre Tonnerre, Nathalie Gérard, Pierre-Jean Gavlovsky, Simon Mazalrey, Maryvonne Hourmant, Mary-Luce Cheneau, Anne Cesbron-Gautier, Karine Renaudin, Céline Bressollette-Bodin, Béatrice Charreau, MICA Mutant A5.1 Influences BK Polyomavirus Reactivation and Associated Nephropathy After Kidney Transplantation, The Journal of Infectious Diseases, Volume 214, Issue 5, 1 September 2016, Pages 807–816, https://doi.org/10.1093/infdis/jiw168
Close - Share Icon Share
Abstract
Background. BK polyomavirus (BKPyV) frequently reactivates in kidney transplant recipients during immunosuppressive therapy and triggers BKPyV-associated nephropathy and graft rejection. Determining effective risk factors for BKPyV reactivation is required to achieve efficient prevention.
Methods. This study investigated the role of major histocompatibility complex (MHC) class I–related chain A (MICA) in BKPyV reactivation in a cohort of 144 transplant donor/recipient pairs, including recipients with no reactivation (controllers) and those with mild (virurics) or severe (viremics) BKPyV reactivation after graft receipt.
Results. We show that, in the kidney, MICA is predominantly expressed in tubule epithelial cells, the natural targets of BKPyV, questioning a role for MICA in the immune control of BKPyV infection. Focusing on MICA genotype, we found a lower incidence of BKPyV reactivation in recipients of a renal graft from a donor carrying the MICA A5.1 mutant, which encodes a truncated nonconventional MICA. We established that a mismatch for MICA A5.1 between transplant donor and recipient is critical for BKPyV reactivation and BKPyV-associated nephropathy. Functionally, we found that a low prevalence of BKPyV reactivation was associated with elevated anti-MICA sensitization and reduced plasma level of soluble MICA in recipients, 2 potential effector mechanisms.
Discussions. These findings identify the MHC-related MICA as an immunogenetic factor that may functionally influence anti-BKPyV immune responses and infection outcomes.
The seroprevalence of BK polyomavirus (BKPyV) is very high (up to 87%) in healthy individuals [1]. Although not associated with disease in immunocompetent individuals, BKPyV is frequently reactivated in kidney transplant recipients during receipt of an immunosuppressive regimen and currently poses a major challenge to transplantation [2]. BKPyV replication is typically found in renal tubular epithelial cells [3–5]. Symptomatic reactivation may result in BKPyV-associated nephropathy and graft loss and in late-onset hemorrhagic cystitis [6, 7]. Immune and genetic factors controlling BKPyV reactivation remain elusive.
MICA is expressed in solid-organ transplants and plays a role in alloimmunity by inducing anti-MICA alloantibodies [8, 9]. MICA is also a key player in innate and adaptive immunity and contributes to the control of viral infection as a ligand for the activating natural killer (NK) cell receptor NKG2D [10, 11]. MICA is highly polymorphic, and up to 102 alleles have been described so far [12]. The A5.1 variant results in a frameshift mutation that generates a premature stop codon that truncates the MICA transmembrane domain and cytoplasmic tail [13]. This feature subsequently affects MICA subcellular localization [14], membrane anchorage [15], and surface expression [16] and the release of soluble MICA (sMICA) molecules [17]. The MICA A5.1 genetic variant associates with 9 alleles—MICA*008, *023, *028, *053, *058, *070, *073, *080, and *082—with MICA*008 by far the most frequent allele of diverse populations worldwide (27%–40% of individuals carry ≥1 MICA*008 allele) [18–20]. Regarding the immune response, the functional consequences of carrying the MICA*008/A5.1 allele as compared to full-length wild-type (WT) MICA alleles remain poorly understood.
Allotransplantation poses the challenges of a possible mismatch for MICA between the transplant donor and recipient. The immune impact of MICA mismatch in kidney transplantation remains mostly uncovered. Our previous study demonstrated that a mismatch between donor (D) and recipient (R) for the MICA A5.1 mutation could be a risk factor for long-term transplant survival, owing to enhanced anti-MICA sensitization of kidney transplant recipients [16]. Here, we hypothesized that structural changes featuring MICA A5.1 proteins could affect BKPyV reactivation after transplantation through atypical modulation of antiviral immune responses. The present study examined the expression and subcellular localization of MICA in kidney biopsy specimens and cells and retrospectively investigated MICA genotypes and the presence of anti-MICA antibodies and sMICA in a cohort of 144 kidney transplant donor/recipient pairs according to the occurrence and severity of BKPyV reactivation after graft receipt.
METHODS
Study Approval
The observational retrospective study was performed according to the guidelines of the local ethics committee (CCPRB, CHU de Nantes, France). Consent was obtained from participants before their inclusion in this study, to collect and store cells, sera, and DNA (DIVAT BioCollection INSERM, French Health Minister Project N°02G55).
Subjects and Samples
To investigate the impact of MICA A5.1 mutation on recipient BKPyV reactivation, 144 patients who underwent kidney transplantation between 2010 and 2012 at ITUN (CHU de Nantes, France) were included in the study. Recipients that were followed up for at least 1 year to monitor for reactivation of BKPyV were included. To allow matching analyses, only donor/recipient pairs with genomic DNA available for MICA typing were selected. HLA and MICA antibody testing in sera obtained before and after transplantation was performed at the Laboratoire HLA, EFS Pays de la Loire, Nantes, France, using the Luminex Bead Array technique (One Lambda, Canoga Park, California), for detection of HLA or MICA antibody (LABScreen Mixed), and for the identification of HLA and MICA single antigen (LABScreen Single antigen). Antibodies were detected by the fluorescent signal for each bead coated with HLA or MICA antigen, normalized to the value measured with the negative control serum. MFI values >500 were considered as positive.
BKPyV Monitoring After Transplantation
During the period studied, urine specimens were collected and whole-blood specimens were collected into ethylenediaminetetraacetic acid (EDTA)–lined tubes after transplantation for BKPyV reactivation monitoring. If the viruria level was >107 copies/mL or viremia testing had positive results, monthly monitoring was performed. Based on the international recommendations [21], suspected BKPyV-associated nephropathy was defined by persistent and high-level BKPyV viremia (defined as a viremia level of >104 copies/mL at >2 consecutive measurements), and confirmed BKPyV-associated nephropathy was defined by positive immunostaining of a renal biopsy specimen (AC anti-SV40 LT). In case of a confirmed BKPyV viremia level of >104 copies/mL, doses of mycophenolate mofetil (Cellcept, Roche Pharmaceuticals, Basel, Switzerland) or enteric-coated mycophenolate sodium (Myfortic, Novartis Pharma) and/or tacrolimus (Prograf, Astellas Pharma, Tokyo, Japan) were progressively decreased or treatment was interrupted according to the response to the first reduction in immunosuppression and the presence of BKPyV-associated nephropathy. In case of BKPyV-associated nephropathy, a minimal level of immunosuppression was maintained with a low dose of tacrolimus or a switch to low-dose cyclosporine A.
For BKPyV nucleic acid testing, DNA was extracted from whole blood, using the MagNAPure instrument and the MagNA Pure LC Total Nucleic Acid Isolation Kit. Urines samples were boiled, centrifuged, and diluted at 1:10 with phosphate-buffered saline (PBS). Viral DNA detection and quantification were performed using an in-house quantitative real-time polymerase chain reaction, as we previously described [22]. BKPyV viruria and viremia lower limits of quantification were 10 000 copies/mL of urine and 500 copies/mL of whole blood, respectively.
MICA A5.1 Genotyping
MICA typing of transplant donors and recipients was performed as we previously described [16, 23]. Briefly, MICA exon 5 DNA was amplified with the following primers: MICA5-F 5′-CCTTTTTTTCAGGGAAAGTGC-3’ and MICA5-R 5′-CCTTACCATCTCCAGAAACTGC-3’. DNA sequencing was performed (Sequencing Core Facility INSERM/SFR Santé F. Bonamy, Nantes, France) using a 48-capillary AB3730 automatic system (Applied Biosystems, Foster City, California) and analyzed using ChromasPro 1.5 software (Digital River, Shannon, Ireland).
Immunofluorescence
Consent was obtained to collect and use renal biopsy specimens and/or kidney explants for research purposes. Section of frozen kidney biopsy specimens from 6 donors were immunostained according to the following method. Eight-micrometer-thick sections were dried, fixed in acetone, and permeabilized in 0.5% saponin. After rehydration in PBS, tissue sections were blocked with 4% donkey or goat serum for 1 hour before incubation with monoclonal anti-MICA/B antibodies (D-8, 10 µg/mL, Santa Cruz Biotechnology, Heidelberg, Germany) at 4°C for 2 hours, followed by incubation for 2 hours with anti-mouse Alexa 488–labeled secondary antibodies and 4’, 6’-diamidino-2-phenylindole for nuclear staining (DAPI, Invitrogen). Specimens were analyzed by confocal fluorescence microscopy (Nikon A1 RSi, Tokyo, Japan) and quantified using the Fiji open source software [24].
Cell Culture and Flow Cytometry
Primary cultures of vascular endothelial cells (human aortic endothelial cells) were isolated and cultured as we previously described [16]. Primary cultures of human renal proximal tubule endothelial cells (American Tissue Culture Collection, LGC Standards, Molsheim, France), human epithelial cells (HEK293), and a CHO cell line were grown using an epithelial cell growth kit (ATCC) or Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (HEK293 and CHO). For phenotype analysis, 1 × 105–2 × 105 cells/sample were harvested using trypsin/EDTA, washed twice, and incubated for 30 minutes with anti-MICA antibodies (BamOmab, Tubingen, Germany). After 3 washes, cells were incubated with Alexa 488–labeled goat anti-mouse F(ab’)2 immunoglobulin G (IgG; Jackson Laboratory) at 4°C for 30 minutes. Negative controls were evaluated using an istotype-matched IgG control. Fluorescence was measured on 10 000 cells/sample, using LSR II (BD Biosciences, Franklin Lakes, New Jersey) and analyzed using FlowJo software (Tree Star). Results shown are representative of at least 3 independent experiments.
Enzyme-Linked Immunoassay (ELISA) for sMICA
Quantification of sMICA was done using a sandwich ELISA from Immatics (Tubingen, Germany), using recipients’’ sera harvested before transplantation and at months 6 and 12 after transplantation. Briefly, coating was performed overnight with capture anti-MICA monoclonal antibody (5 µg/mL), serum samples were diluted 1:3 in 7.5% bovine serum albumin–PBS, recombinant MICA*004 was used as standard, and anti-MICA/B antibody (1 µg/mL) was used as a sandwich monoclonal antibody. Experiments were performed in triplicate according to the manufacturer's recommendations.
Data Analysis
Statistical analysis of the association between donors/recipients of MICA WT or A5.1 genotype mismatch and BKV infection or MICA alloimmunity was performed using Pearson χ2 or Fisher exact tests. Quantitative data are expressed as means ± standard errors of the mean and were compared using the nonparametric Mann–Whitney test or the Kruskal–Wallis test (with the Dunn multiple comparisons post hoc test) if there were >2 conditions. The cumulative incidence for BKPyV-associated nephropathy, based on MICA genotype D/R combination, was assessed using the log-rank test. Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, California). A P value of < .05 was considered statistically significant.
RESULTS
Predominant Expression for MICA in Renal Tubule Epithelial Cells, the Natural Targets of BKPyV Infection
Because, no exhaustive description of MICA expression in the kidney was available [25], we sought to examine its basal cellular localization in normal kidneys. Figure 1 shows that MICA is most predominantly expressed by epithelial cells in renal tubules and by some vascular endothelial cells. Expression in the tubule epithelium seems higher in intensity than in the endothelium, where glomerular and arterial endothelial cells were stained (Figure 1A). Among renal tubules, both proximal and distal tubular epithelial cells were stained. Sustained MICA expression on tubular epithelial cells was further confirmed by flow cytometry performed on human renal proximal epithelial cells (Figure 1B). In tubules, MICA staining was concentrated at the apical side of epithelial cells, revealing a yet unknown polarity for MICA in renal cells (Supplementary Figure 1). Since epithelial tubular cells are natural targets for BKPyV infection and replication [26] and because reactivation was of donor origin [27], we postulated that, in kidney transplants, MICA polymorphisms may yield changes in MICA proteins that could affect the effectiveness of the response to and outcome of BKPyV infection. We focused on the MICA A5.1 mutant, which, in contrast to full-length WT MICA alleles, encodes a truncated MICA protein (Figure 1C) [14, 15]. Consistent with our previous results [16], the MICA A5.1 mutation was associated with elevated MICA expression at the cell surface (Figure 1D).

Major histocompatibility complex class I–related chain A (MICA) expression and localization in control kidney. A, Representative immunostaining for MICA (green) in normal kidney, showing MICA expression in a glomerule renal tubules, and vascular endothelium. Nuclei were stained with DAPI (blue). B, Flow cytometry comparing MICA expression on human renal proximal tubule epithelial cells (HRPTECs) and the renal cell line HEK293. The CHO cell line was used as a negative control. C, A schematic representation of exons and protein domain distribution for full-length MICA wild type (WT) and the MICA A5.1 mutant. D, A representative analysis comparing MICA levels on cultured primary endothelial cells isolated from homozygous MICA WT (WT/WT), heterozygous (WT/A5.1), or homozygous MICA A5.1 (A5.1/A5.1) donors.
Study Cohort
Our cohort study included 144 kidney transplant donor/recipient pairs, with grafting occurring between 2010 and 2012 and at least 1-year of follow-up monitoring for BKPyV reactivation after transplantation. Kidney transplant recipients comprised 57 patients without BKPyV reactivation (BKPyV controllers), 35 patients with mild BKPyV infection (positive viruria and negative viremia test results), and 52 recipients with severe BKPyV infection (positive viruria and positive viremia test results). In the viremia group, 14 patients (26.92%) had histologically confirmed BKPyV-associated nephropathy on renal biopsy. Banked donor and recipient biological samples (serum and DNA) were used for MICA genotyping, anti-MICA sensitization analysis, and quantification of sMICA. Characteristics of the cohort are summarized in Table 1. There was no significant difference in recipient or donor ages, percentages of males, or HLA mismatches. The majority of patients received similar preconditioning and maintenance therapies. The mean follow-up periods were 29, 36, and 38 months in the viremic, viruric only, and controller groups, respectively.
Demographic and Clinical Characteristics of Donors and Recipients, by BK Polyomavirus Status
| Characteristic . | BKPyV Controllers (n = 57) . | BKPyV Virurics (n = 35) . | BKPyV Viremics (n = 52) . | P Valuea . |
|---|---|---|---|---|
| Donors | ||||
| Age, y | 52 (34–77) | 49 (18–77) | 56.5 (17–78) | .0628b |
| Sex | ||||
| Male | 37 (64.91) | 20 (57.14) | 27 (51.92) | .3839 |
| Female, no. | 20 | 15 | 25 | |
| Recipients | ||||
| Age, y | 51 (25–74) | 52 (31–79) | 60 (31–81) | .0618b |
| Sex | ||||
| Male | 38 (66.66) | 22 (62.85) | 29 (55.76) | .4992 |
| Female, no. | 19 | 13 | 23 | |
| Total HLA-A, -B, -DR mismatches, no. | 4 (0–6) | 4 (0–5) | 3 (0–5) | .1020b |
| Postgraft follow-up time, mo | 38 (12–60) | 36 (12–60) | 29 (12–54) | .1922b |
| BKPyV reactivation tests, by specimen, no. | ||||
| Urine | 5 (2–10) | 5 (2–13) | 7 (3–15) | <.0001b |
| Whole blood | 5 (2–10) | 6 (2–13) | 7 (3–18) | <.0001b |
| Time to postgraft BKPyV reactivation diagnosis,c mo | NA | 6 (1–49) | 5 (1–17) | .857d |
| Maximum BKPyV load, by specimen, log copies/mL | ||||
| Urine | NA | 5.2 (2.6–9.6) | 9.45 (3.6–10.8) | <.0001d |
| Whole blood | NA | NA | 4.3 (2.0–7.2) | NA |
| BKPyV-associated nephropathy | None | None | 14 (26.92) | NA |
| Immunosuppressive drugs | ||||
| Induction | ||||
| ATG-Fresenius or Thymoglobuline | 28 (49.12) | 17 (48.57) | 28 (53.84) | .8496 |
| Basiliximab (Simulect) | 29 (50.87) | 18 (51.42) | 24 (46.15) | |
| Initial and maintenance | ||||
| Corticosteroids | 40 (70.17) | 23 (65.71) | 39 (75.00) | .6399 |
| Mycophenolate mofetil (Cellcept) | 52 (91.22) | 34 (97.14) | 50 (96.15) | .3869 |
| Tacrolimus | 54 (94.73) | 34 (97.14) | 50 (96.15) | .9694 |
| Cyclosporine A or others | 2 (3.50) | 1 (2.85) | 2 (3.84) | |
| Antiviral prophylaxis | ||||
| Valganciclovir (Rovalcyte) | 38 (66.66) | 21 (60.00) | 37 (71.15) | .5568 |
| Ganciclovir | 1 (1.75) | 1 (2.85) | None | 1.0000 |
| Characteristic . | BKPyV Controllers (n = 57) . | BKPyV Virurics (n = 35) . | BKPyV Viremics (n = 52) . | P Valuea . |
|---|---|---|---|---|
| Donors | ||||
| Age, y | 52 (34–77) | 49 (18–77) | 56.5 (17–78) | .0628b |
| Sex | ||||
| Male | 37 (64.91) | 20 (57.14) | 27 (51.92) | .3839 |
| Female, no. | 20 | 15 | 25 | |
| Recipients | ||||
| Age, y | 51 (25–74) | 52 (31–79) | 60 (31–81) | .0618b |
| Sex | ||||
| Male | 38 (66.66) | 22 (62.85) | 29 (55.76) | .4992 |
| Female, no. | 19 | 13 | 23 | |
| Total HLA-A, -B, -DR mismatches, no. | 4 (0–6) | 4 (0–5) | 3 (0–5) | .1020b |
| Postgraft follow-up time, mo | 38 (12–60) | 36 (12–60) | 29 (12–54) | .1922b |
| BKPyV reactivation tests, by specimen, no. | ||||
| Urine | 5 (2–10) | 5 (2–13) | 7 (3–15) | <.0001b |
| Whole blood | 5 (2–10) | 6 (2–13) | 7 (3–18) | <.0001b |
| Time to postgraft BKPyV reactivation diagnosis,c mo | NA | 6 (1–49) | 5 (1–17) | .857d |
| Maximum BKPyV load, by specimen, log copies/mL | ||||
| Urine | NA | 5.2 (2.6–9.6) | 9.45 (3.6–10.8) | <.0001d |
| Whole blood | NA | NA | 4.3 (2.0–7.2) | NA |
| BKPyV-associated nephropathy | None | None | 14 (26.92) | NA |
| Immunosuppressive drugs | ||||
| Induction | ||||
| ATG-Fresenius or Thymoglobuline | 28 (49.12) | 17 (48.57) | 28 (53.84) | .8496 |
| Basiliximab (Simulect) | 29 (50.87) | 18 (51.42) | 24 (46.15) | |
| Initial and maintenance | ||||
| Corticosteroids | 40 (70.17) | 23 (65.71) | 39 (75.00) | .6399 |
| Mycophenolate mofetil (Cellcept) | 52 (91.22) | 34 (97.14) | 50 (96.15) | .3869 |
| Tacrolimus | 54 (94.73) | 34 (97.14) | 50 (96.15) | .9694 |
| Cyclosporine A or others | 2 (3.50) | 1 (2.85) | 2 (3.84) | |
| Antiviral prophylaxis | ||||
| Valganciclovir (Rovalcyte) | 38 (66.66) | 21 (60.00) | 37 (71.15) | .5568 |
| Ganciclovir | 1 (1.75) | 1 (2.85) | None | 1.0000 |
Data are median (range) or no. (%) of subjects, unless otherwise indicated. See Methods for a description of the study groups.
Abbreviations: BKPyV, BK polyomavirus; NA, not applicable.
a By the Pearson χ2 or Fisher exact tests, unless otherwise indicated.
b By the Kruskal–Wallis test.
c Corresponds to the time to the first positive viruria test.
d By the Mann–Whitney test.
Demographic and Clinical Characteristics of Donors and Recipients, by BK Polyomavirus Status
| Characteristic . | BKPyV Controllers (n = 57) . | BKPyV Virurics (n = 35) . | BKPyV Viremics (n = 52) . | P Valuea . |
|---|---|---|---|---|
| Donors | ||||
| Age, y | 52 (34–77) | 49 (18–77) | 56.5 (17–78) | .0628b |
| Sex | ||||
| Male | 37 (64.91) | 20 (57.14) | 27 (51.92) | .3839 |
| Female, no. | 20 | 15 | 25 | |
| Recipients | ||||
| Age, y | 51 (25–74) | 52 (31–79) | 60 (31–81) | .0618b |
| Sex | ||||
| Male | 38 (66.66) | 22 (62.85) | 29 (55.76) | .4992 |
| Female, no. | 19 | 13 | 23 | |
| Total HLA-A, -B, -DR mismatches, no. | 4 (0–6) | 4 (0–5) | 3 (0–5) | .1020b |
| Postgraft follow-up time, mo | 38 (12–60) | 36 (12–60) | 29 (12–54) | .1922b |
| BKPyV reactivation tests, by specimen, no. | ||||
| Urine | 5 (2–10) | 5 (2–13) | 7 (3–15) | <.0001b |
| Whole blood | 5 (2–10) | 6 (2–13) | 7 (3–18) | <.0001b |
| Time to postgraft BKPyV reactivation diagnosis,c mo | NA | 6 (1–49) | 5 (1–17) | .857d |
| Maximum BKPyV load, by specimen, log copies/mL | ||||
| Urine | NA | 5.2 (2.6–9.6) | 9.45 (3.6–10.8) | <.0001d |
| Whole blood | NA | NA | 4.3 (2.0–7.2) | NA |
| BKPyV-associated nephropathy | None | None | 14 (26.92) | NA |
| Immunosuppressive drugs | ||||
| Induction | ||||
| ATG-Fresenius or Thymoglobuline | 28 (49.12) | 17 (48.57) | 28 (53.84) | .8496 |
| Basiliximab (Simulect) | 29 (50.87) | 18 (51.42) | 24 (46.15) | |
| Initial and maintenance | ||||
| Corticosteroids | 40 (70.17) | 23 (65.71) | 39 (75.00) | .6399 |
| Mycophenolate mofetil (Cellcept) | 52 (91.22) | 34 (97.14) | 50 (96.15) | .3869 |
| Tacrolimus | 54 (94.73) | 34 (97.14) | 50 (96.15) | .9694 |
| Cyclosporine A or others | 2 (3.50) | 1 (2.85) | 2 (3.84) | |
| Antiviral prophylaxis | ||||
| Valganciclovir (Rovalcyte) | 38 (66.66) | 21 (60.00) | 37 (71.15) | .5568 |
| Ganciclovir | 1 (1.75) | 1 (2.85) | None | 1.0000 |
| Characteristic . | BKPyV Controllers (n = 57) . | BKPyV Virurics (n = 35) . | BKPyV Viremics (n = 52) . | P Valuea . |
|---|---|---|---|---|
| Donors | ||||
| Age, y | 52 (34–77) | 49 (18–77) | 56.5 (17–78) | .0628b |
| Sex | ||||
| Male | 37 (64.91) | 20 (57.14) | 27 (51.92) | .3839 |
| Female, no. | 20 | 15 | 25 | |
| Recipients | ||||
| Age, y | 51 (25–74) | 52 (31–79) | 60 (31–81) | .0618b |
| Sex | ||||
| Male | 38 (66.66) | 22 (62.85) | 29 (55.76) | .4992 |
| Female, no. | 19 | 13 | 23 | |
| Total HLA-A, -B, -DR mismatches, no. | 4 (0–6) | 4 (0–5) | 3 (0–5) | .1020b |
| Postgraft follow-up time, mo | 38 (12–60) | 36 (12–60) | 29 (12–54) | .1922b |
| BKPyV reactivation tests, by specimen, no. | ||||
| Urine | 5 (2–10) | 5 (2–13) | 7 (3–15) | <.0001b |
| Whole blood | 5 (2–10) | 6 (2–13) | 7 (3–18) | <.0001b |
| Time to postgraft BKPyV reactivation diagnosis,c mo | NA | 6 (1–49) | 5 (1–17) | .857d |
| Maximum BKPyV load, by specimen, log copies/mL | ||||
| Urine | NA | 5.2 (2.6–9.6) | 9.45 (3.6–10.8) | <.0001d |
| Whole blood | NA | NA | 4.3 (2.0–7.2) | NA |
| BKPyV-associated nephropathy | None | None | 14 (26.92) | NA |
| Immunosuppressive drugs | ||||
| Induction | ||||
| ATG-Fresenius or Thymoglobuline | 28 (49.12) | 17 (48.57) | 28 (53.84) | .8496 |
| Basiliximab (Simulect) | 29 (50.87) | 18 (51.42) | 24 (46.15) | |
| Initial and maintenance | ||||
| Corticosteroids | 40 (70.17) | 23 (65.71) | 39 (75.00) | .6399 |
| Mycophenolate mofetil (Cellcept) | 52 (91.22) | 34 (97.14) | 50 (96.15) | .3869 |
| Tacrolimus | 54 (94.73) | 34 (97.14) | 50 (96.15) | .9694 |
| Cyclosporine A or others | 2 (3.50) | 1 (2.85) | 2 (3.84) | |
| Antiviral prophylaxis | ||||
| Valganciclovir (Rovalcyte) | 38 (66.66) | 21 (60.00) | 37 (71.15) | .5568 |
| Ganciclovir | 1 (1.75) | 1 (2.85) | None | 1.0000 |
Data are median (range) or no. (%) of subjects, unless otherwise indicated. See Methods for a description of the study groups.
Abbreviations: BKPyV, BK polyomavirus; NA, not applicable.
a By the Pearson χ2 or Fisher exact tests, unless otherwise indicated.
b By the Kruskal–Wallis test.
c Corresponds to the time to the first positive viruria test.
d By the Mann–Whitney test.
Recipients of a Renal Graft Carrying the MICA A5.1 Mutation Have a Lower Risk of BKPyV Reactivation
First, we examined the frequency of recipients and associated transplant donors carrying none (WT/WT), 1 (WT/A5.1), or 2 (A5.1/A5.1) MICA A5.1 alleles according to the occurrence of BKPyV reactivation and infection severity. In recipients, we found a roughly similar distribution of the 3 genotypes among the groups (Figure 2A). A significantly higher prevalence of donors carrying at least 1 MICA A5.1 allele was observed in transplant recipients without BKPyV reactivation (77.19%) as compared to recipients with BKPyV viremia (49.99%; P = .0119; Figure 2B). These data suggest that intragraft MICA expression may have a significant influence on BKPyV reactivation after transplantation and may indicate a protective role for MICA A5.1.
![Influence of the MICA A5.1 mutation among transplant donors and recipients on BK polyomavirus (BKPyV) reactivation. Percentages of recipients and donors carrying none (homozygous wild type [WT]), 1 (heterozygous), or 2 (homozygous A5.1) MICA A5.1 alleles in BKPyV controller (−), viruric (+), and viremic (++) patients. Bars represent percentages of recipients (A) and donors (B) in each group. Abbreviation: NS, not significant.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/214/5/10.1093_infdis_jiw168/2/m_jiw16802.jpeg?Expires=1750053744&Signature=HRcckaHTiTBpmkF3T6Cf4us2P5yYYQAuyRQ1UzVFgOP1iGlQQ79OUd2NTOVZQu8VTNFcc3EMEKOV1Rz24zTkyodc-cAt85i7fqIq77V2108acgXEJVuEWwRrh2k7NaFSOALajket2Z8onvPpy9BMucY4pq0Oad48usXUy8aHFZxVbiuHoEsW8RD2ADJhYFOkx3cQnGy5TevQsQGaxDWnOszBgShgcfJLiyMoLPP~YslwqLFUrDIY447JRZ4RKX37KOA4jJK13NSoOBSI3mpFEjbZvzKU4S7XManAg7DZvQVEY-YUL5WZr~-B6t-F~dSU0-hHKcAxLYjnyT2dADDKMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Influence of the MICA A5.1 mutation among transplant donors and recipients on BK polyomavirus (BKPyV) reactivation. Percentages of recipients and donors carrying none (homozygous wild type [WT]), 1 (heterozygous), or 2 (homozygous A5.1) MICA A5.1 alleles in BKPyV controller (−), viruric (+), and viremic (++) patients. Bars represent percentages of recipients (A) and donors (B) in each group. Abbreviation: NS, not significant.
Mismatch for MICA A5.1 Variant Between Transplant Donor and Recipient Is Critical for BKPyV Reactivation and BKPyV-Associated Nephropathy
We then investigated the effect of a mismatch for MICA A5.1 between donor (D) and recipient (R) pairs on BKPyV reactivation. Four combinations were examined, including the 2 well-matched pairs DWT/RWT and DA5.1/RA5.1 and the 2 mismatched combinations DWT/RA5.1 and DA5.1/RWT (Figure 3A). A net increase in the percentage of DWT/RA5.1 pairs was found among recipients with BKPyV viremia (26.9%) as compared to BKPyV controllers (10.50%), with an intermediate frequency among recipients with mild infection (BKPyV viruria, 14.3%). Distribution for DWT/RWT exhibited a similar increase (12.3% among controllers, 14.3% among viruric-only individuals, and 23.1% among viremic individuals). Interestingly, in the subgroup of viremic patients who developed polyomavirus–associated nephropathy (n = 14), the DWT/RA5.1 and DWT/RWT combinations were found together in 57.2% of patients. On the other hand, an opposite gradual decrease in the percentage of the DA5.1/RWT combination among severe (11.5%) and mild (17.1%) BKPyV infections, compared with BKPyV-negative recipients (31.6%), suggests that this combination is associated with a lower risk of BKPyV reactivation. Again, in our cohort, BKPyV-associated nephropathy was rarely associated with the DA5.1/RWT combination (1 case BKPyV-associated nephropathy of 14 [7.1%]), further supporting the concept that this combination could be protective. The predominant well-matched DA5.1/RA5.1 combination showed only few variations among the groups.
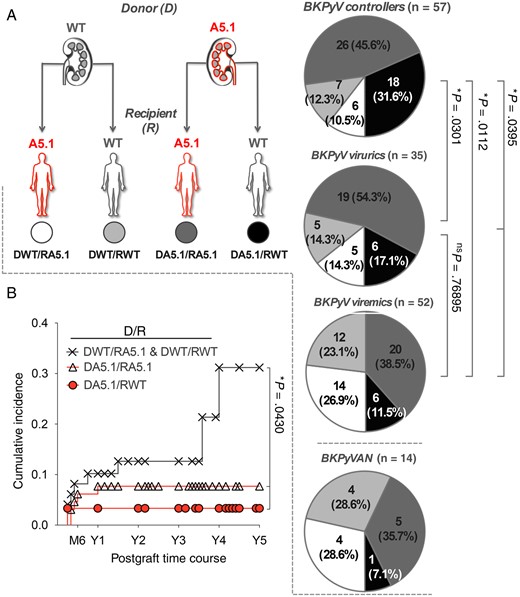
Influence of donor/recipient MICA A5.1 mismatch on BK polyomavirus (BKPyV) reactivation. A, According to both donor (D) and recipient (R) MICA genotypes, D/R pairs were classified into 4 groups as follows: DWT/RA5.1 (white panel), DWT/RWT (light gray panel), DA5.1/RA5.1 (dark gray panel), and DA5.1/RWT (black panel). The distribution of the 4 D/R combinations among BKPyV controllers (−), viruric patients (+), viremic patients (++), and patients with BKPyV-associated nephropathy (BKPyVAN) are presented. “A5.1” refers to both heterozygous and homozygous individuals for the MICA A5.1 mutation. “WT” refers to individuals homozygous for MICA wild-type alleles. B, Cumulative incidence of BKPyV-associated nephropathy 5 years after transplantation, according to MICA A5.1 D/R combination.
Subsequent univariate analysis showed a significant association between the combinations DWT/RA5.1 and DWT/RWT and a higher risk for BKPyV-associated nephropathy, with a cumulative incidence of 31.19% at 5 years, compared with 7.69% for DA5.1/RA5.1 and 3.33% for DA5.1/RWT (P = .0430; Figure 3B). Our data also indicate that DWT/RA5.1 and DWT/RWT were associated with the occurrence of BKPyV-associated nephropathy after the first year following transplantation.
Anti-MICA Sensitization in Transplant Recipients Is Associated With a Low Prevalence of BKPyV Reactivation
The humoral response against MICA was examined in the present cohort in regard to MICA A5.1 mismatch and virus reactivation. The frequency of MICA-immunized recipients was found to be significantly higher in recipients with no BKPyV reactivation (24.55%) versus recipients with severe BKPyV reactivation (9.60%; P = .0463; Figure 4A). Antibodies were mostly de novo anti-MICA antibodies induced in response to kidney transplantation. Elevated titers of anti-MICA antibodies were also associated with an absence of BKPyV reactivation (P = .0025; Figure 4B). Again, intermediate values in both antibody frequency and titer were found in patients with mild BKPyV reactivation (ie, viruria only), suggesting a link between MICA immunization and BKPyV reactivation. Among BKPyV controllers, the level of de novo antibodies was significantly greater in the DA5.1/RWT group, compared with levels for the DWT/RA5.1 (P = .0447) and DA5.1/RA5.1 (P = .0091) combinations (Figure 4C). Next, the time course of anti-MICA antibodies revealed that MICA immunization reaches a peak early during the first month after transplantation and then progressively decreases, stabilizing after 1 year (Figure 4D).
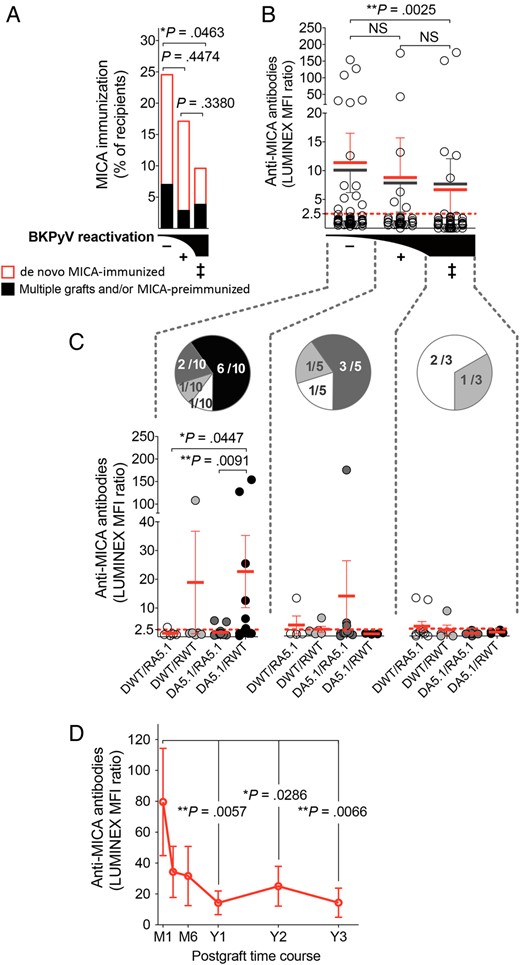
Frequency, strength, and time course of anti–major histocompatibility complex (MHC) class I–related chain A (MICA) immunity in kidney transplant recipients. A, Percentages of recipients with anti-MICA antibodies in BK polyomavirus (BKPyV) controller (−), viruric (+), and viremic (++) patients. Presence of preformed (black) vs de novo postgraft-induced (red) anti-MICA antibodies is indicated. Bars represent percentages in each group. B and C, Relative titers (expressed as median fluorescence intensity ratios from Luminex analyses; means ± standard errors of the mean are indicated) of anti-MICA antibodies, according to BKPyV infection (B) and MICA A5.1 D/R mismatch (C). In panel B, black bars refer to the analysis of all patients, whereas red bars refer to patients with only de novo MICA immunization. In panel C, only recent recipients of transplants with de novo MICA immunization were included. The number of recipients in the groups is indicated in sector graphs. D, Time course of the anti-MICA response in transplant recipients with MICA immunization in the cohort (n = 25); statistical analysis was performed by comparing data to values at month 1. Abbreviation: NS, not significant.
To further investigate the possible contribution of recipient immunization status, the frequency and titer of anti-HLA class I and class II antibodies were also examined (Supplementary Figure 2). Sustained BKPyV reactivation was associated in our cohort with a higher frequency of anti-HLA class I sensitization (69.22%) in comparison with mild (39.99%) or no (45.61%) virus reactivation (P = .0047 and P = .0240, respectively). Although not statistically significant, a similar trend toward more anti-HLA class II sensitization associated with worse BKPyV evolution was also observed.
sMICA Levels Are Reduced in Transplant Recipients With No BKPyV Reactivation
A finely tuned balance between levels of sMICA and anti-MICA antibodies is critical for an effective control of tumors [28, 29]. Therefore, we wondered whether the increase in anti-MICA antibodies might be correlated with alterations in sMICA levels in kidney transplant recipients. Using ELISA, we quantified sMICA levels in plasma samples from kidney transplant recipients collected before (month 0) and 6 months after transplantation. Although roughly similar levels of sMICA were present in all patients at month 0 (mean levels, 171.7, 172.9, and 170.8 pg/mL for controller, viruric only, and viremic patients, respectively; Supplementary Figure 3), a significant decrease in sMICA levels was observed in the BKPyV controller group at month 6 after graft, compared with month 0 values (P = .0182), whereas no significant changes were found in kidney transplant recipients with moderate or sustained virus reactivation (Figure 5A). Moreover, our data show that, among BKPyV controllers, the significant decrease in sMICA levels at month 6 was restricted to recipients in the DA5.1/RWT combination (P = .0068). To further investigate the relationship between sMICA levels and anti-MICA immunity, we first distinguished patients with no variation or an increase of sMICA levels after transplantation from those with postgraft decreases in sMICA levels and then evaluated the proportion of patients with detectable anti-MICA antibodies (Figure 5B). Patients with anti-MICA were significantly predominant among the recipients, with a postgraft decrease in sMICA levels (15 of 50 [30%]), compared with recipients with increased or unchanged sMICA levels (3 of 32 [9.4%]; P = .0314), emphasizing a possible duality between anti-MICA antibodies and circulating sMICA that may contribute to the control of BKPyV reactivation.
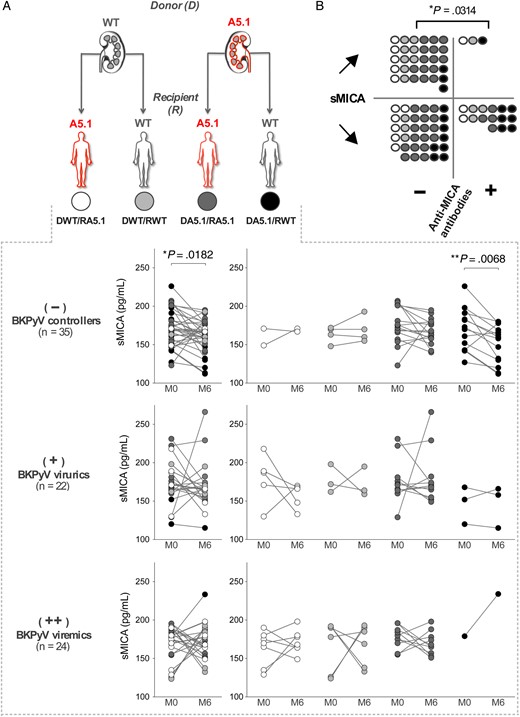
Plasma levels of soluble major histocompatibility complex (MHC) class I–related chain A (sMICA) in transplant recipients. A, Plasma levels of sMICA were quantified at months 0 and 6 after transplantation, using a dedicated enzyme-linked immunosorbent assay, in BK polyomavirus (BKPyV) controller (−), viruric (+), and viremic (++) patients. Concentrations of sMICA according to donor/recipient mismatch are indicated. B, Comparison of variations in sMICA plasma levels between months 0 and 6 (“↗,”increase; ”↘,” decrease), according to the MICA immunization status of the recipients (“−” no immunization; “+,”immunization).
DISCUSSION
Although a reduction in T-cell immunosuppression is effective to restore immune control of BKPyV replication, this approach must be balanced against the increased risk of kidney transplant rejection. Determination of the risk factors for BKPyV reactivation after transplantation is required to develop preventive measures against BKPyV-associated nephropathy and graft rejection. Our first question was to investigate the distribution of MICA proteins in the kidney, and our findings revealed strong expression of MICA in the kidney, with predominance in tubular epithelial cells, the natural cell target for BKPyV [30–32]. These data support the idea that allele-dependent changes in MICA structure in tubule epithelial cells may affect, via its interaction with NKG2D, immune antiviral NK and T-cell responses [33] [34, 35], as well as antigraft immunity. We found that MICA also polarizes on the apical side of tubule epithelium. Whether MICA polarization in renal tubular epithelial cells may change with respect to the MICA allele, such as MICA*008/A5.1, and be involved in pathologies remains to be explored.
An important finding of this study was a higher prevalence of donors carrying at least 1 MICA A5.1 allele among transplant recipients with no BKPyV reactivation as compared to recipients with BKPyV viremia (P = .0148), supporting the idea that MICA A5.1 could be a protective allele against BKPyV infection. Consistent with this hypothesis, the genotyping of donor/recipient pairs identified MICA A5.1 mismatch as a critical factor for BKPyV reactivation. Recipients carrying a WT MICA genotype transplanted with a kidney from a donor carrying the A5.1 MICA variant have a lower risk of reactivating BKPyV and developing BKPyV-associated nephropathy. In contrast, combinations involving a donor carrying a WT MICA genotype (DWT/RA5.1 and DWT/RWT) compose a high-risk group.
Only limited and still conflicting data are available on the role of HLA matching in the pathogenesis of BKPyV in transplant recipients [36–38]. Similarly the implication of individual HLA antigens in the course of BKPyV infection remains preliminary [39]. Interestingly, a protective role of the KIR3DS1 genotype in BKPyV was reported, which sustains the involvement of both NK cells and immunogenetics [34].
Confirming results of our previous study [16], in this cohort we observed that a DA5.1/RWT mismatch correlates with increased anti-MICA immunity in controllers. Our findings raise the interesting question of whether anti-MICA antibodies directed against donor MICA molecules expressed on tubule epithelial cells could be protective by eliminating infected cells. There are some possible explanations of the specific targeting of MICA*008/A5.1 on infected cells by antibodies. First, MICA A5.1 displays specific features that avoid its downregulation by viral proteins such as HCMV UL142 [17] and may similarly permit escape from regulation by BKPyV. Second, a minimal threshold could be required for antibody-mediated activation of complement or cell cytotoxicity, and it can be speculated that only MICA A5.1 reaches the level needed. Finally, another possibility is that MICA A5.1 is associated with changes in MICA polarization on tubular epithelial cells and unmasks epitopes for antibody binding and subsequent killing. Moreover, the early and transient presence of these antibodies in kidney transplant recipients (Figure 4D) may ensure a timely control of infection by preventing further BKPyV replication.
In contrast, sustained reactivation correlates with a higher prevalence of anti-HLA antibodies. It could be argued that HLA sensitization may promote humoral rejection and the subsequent implementation of immunosuppression creating conditions for BKPyV replication [40]. In contrast to anti-HLA, monitoring of the anti-MICA response is not routinely performed. Thus, early and transient MICA sensitization, as observed in our cohort, should not, in contrary to HLA, induce changes in immunosuppressive regimen favoring virus replication.
A drop in sMICA level could be temporally linked to the rise in anti-MICA antibodies in the low-risk group of patients. Thus, another role for anti-MICA antibodies could be to neutralize circulating sMICA. In several tumors, sMICA is released by proteolysis that results in systemic downregulation of NKG2D on NK and CD8+ T cells and evasion of immune recognition [28]. The level of sMICA has a diagnostic value in cancer [41]. Human immunodeficiency virus type 1 (HIV-1) also indirectly suppresses NK cell recognition of HIV-1–infected CD4+ T cells by enhancing sMICA release [42, 43]. Thus, reduced sMICA levels in patients in whom BKPyV is not reactivated may help to maintain a sustained level of NKG2D activation required for virus control by NK and T cells [34, 35]. Although our findings suggest an association between sMICA and anti-MICA antibodies, further investigations are required to clarify whether immune complexes were formed in vivo and cleared from the circulation.
Our study has several limitations that should be considered for a balanced interpretation of the results. First, the investigation was done in a relatively small cohort, particularly when we considered the various combinations of donors and recipients. In addition, analysis of a nonrelated allele, as well as an investigation of the impact of the immunosuppressive regimen, might have helped validate the data.
To conclude, we propose MICA A5.1 D/R matching as a critical factor to consider to avoid BKPyV reactivation. Here, we identified the MICA A5.1 mutation as a good prognostic factor for BKPyV reactivation when carried by the transplant donor and particularly when the recipient does not carry the mutation. In contrast, combinations involving MICA WT donors were associated with severe BKPyV infection (based on a high viruria level and the presence of viremia) and BKPyV-associated nephropathy. Further basic and prospective clinical studies are needed to define whether selective matching between transplant donor and recipient could prevent BKPyV reactivation and BKPyV-associated nephropathy and to elucidate the molecular mechanisms involved.
Notes
Acknowledgments. We thank the MicroPicell imaging core facility (Philippe Hulin and Steven Nedellec; SFR Santé F. Bonamy, University of Nantes) for confocal microscopy and data analysis.
P. T., N. G., P.-J. G., A. C.-G., C. B.-B., and B. C. conceived and designed the experiments. P. T., N. G., P.-J. G., S. M., and M.-L. C. performed the experiments. P. T., N. G., P.-J. G., A. C.-G., C. B.-B., B. C., and K. R. analyzed the data. S. M., A. C.-G., M. H., K. R., and C. B.-B. contributed reagents, materials, and analysis tools. P. T., A. C.-G., C. B.-B., and B. C. wrote the paper.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the IHU-Cesti, LabEx IGO, and LabEx Transplantex projects, which received French government financial support, managed by the National Research Agency, via Investment Into The Future programs ANR-10-IBHU-005, ANR-11-LABX-0016-01, and ANR-11-LABX-0070); Nantes Metropole and the Pays de la Loire Region (support to the IHU-Cesti project); the Fondation Centaure (support to the French Transplantation Research Network); the Agence de la Biomédecine; and the IHU-Cesti (fellowship to P.-J. G.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Present affiliation: Gastrointestinal Unit, Massachusetts General Hospital and Harvard Medical School, Boston.
C. B.-B. and B. C. contributed equally to this work.




