-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel L. Winer, James P. Hughes, Qinghua Feng, Joshua E. Stern, Long Fu Xi, Laura A. Koutsky, Incident Detection of High-Risk Human Papillomavirus Infections in a Cohort of High-Risk Women Aged 25–65 Years, The Journal of Infectious Diseases, Volume 214, Issue 5, 1 September 2016, Pages 665–675, https://doi.org/10.1093/infdis/jiw074
Close - Share Icon Share
Abstract
Background. The risk of incident high-risk human papillomavirus (HR-HPV) infection associated with recent sexual behaviors is undefined in mid-adult women (defined as women aged 25–65 years).
Methods. Triannually, 420 female online daters aged 25–65 years submitted vaginal specimens for HPV testing and completed health and sexual behavior questionnaires. The cumulative incidence of and risk factors for incident HR-HPV detection were estimated by Kaplan–Meier and Cox proportional hazards methods.
Results. The 12-month cumulative incidence of HR-HPV detection was 25.4% (95% confidence interval [CI], 21.3%–30.1%). Current hormonal contraceptive use was positively associated with incident HR-HPV detection. Lifetime number of male sex partners was also positively associated but only among women not recently sexually active with male partners. In analysis that adjusted for hormonal contraceptive use and marital status, women reporting multiple male partners or male partners who were new, casual, or had ≥1 concurrent partnership had a hazard of incident HR-HPV detection that was 2.81 times (95% CI, 1.38–5.69 times) that for women who reported no male sex partners in the past 6 months. Thus, among women with multiple male partners or male partners who were new, casual, or had ≥1 concurrent partnership, approximately 64% of incident HR-HPV infections were attributable to one of those partners.
Conclusions. Among high-risk mid-adult women with recent new male partners, multiple male partners, or male partners who were casual or had ≥1 concurrent partnership, about two thirds of incident HR-HPV detections are likely new acquisitions, whereas about one third of cases are likely redetections of prior infections.
(See the editorial commentary by Ermel and Fife on pages 657–8.)
Among women, the incidence of genital human papillomavirus (HPV) infection peaks around 25 years of age and declines with increasing age [1–4]. Whereas the risk of infection increases with each new male sex partner acquired during adolescence and young adulthood [5], the risk of new infection from new partners acquired in mid-adulthood (defined as ages 25–65 years) is unclear. Clarifying susceptibility to new high-risk HPV (HR-HPV) infections could inform whether vaccinating high-risk subgroups of women >26 years of age (for whom prophylactic HPV vaccines are not currently licensed in the United States [1]) might be beneficial. In addition, data on the relative likelihood of new acquisition versus redetection of prior infection could inform clinician-patient psychosocial counseling on the source of infection for mid-adult women for whom HR-HPV is newly detected during routine cervical cancer screening. With frequencies of divorce, dating, and remarriage in mid-adulthood increasing [6, 7], the risk of newly acquired HR-HPV infection and subsequent cervical neoplasia might be greater now than among cohorts of mid-adult women in previous decades. In general, however, mid-adult women with recent new partners have been underrepresented in HPV studies.
Previously, we reported baseline results from a cohort study targeting mid-adult women likely to report recent new male sex partners [8]. Measures of both recent and cumulative sexual behavior were associated with prevalent HR-HPV infection. Although cross-sectional, these data suggest that mid-adult women are susceptible to new infections from new partners. The goals of the present longitudinal analysis were to better understand how new acquisition and redetection of prior infection contribute to new HR-HPV detection in mid-adult women with new partners. Therefore, we characterized risk factors for newly detected HR-HPV infections and defined the proportion of newly detected infections attributable to recent partners.
METHODS
From 2007 to 2010, Internet-based recruitment methods were used to enroll 25–65-year-old female online daters into a longitudinal study of genital HPV infections. Recruitment procedures and eligibility criteria were described previously [8]. All subjects provided written informed consent. The protocol was approved by the University of Washington Institutional Review Board.
Women were enrolled in 2 phases (March 2007–June 2008 and June 2008–May 2010). In both, participants were mailed kits containing demographic, sexual behavior, and health history questionnaires and materials for self-collecting vaginal samples for HPV DNA testing, at 4-month intervals [8]. In phase 1, women returned information and specimens for up to 2 kits. In phase 2, a subset of phase 1 women were reenrolled up to 1 year later and invited to return information and specimens for 2 additional kits. Additional women were newly enrolled into phase 2 and completed up to 4 kits.
Vaginal samples were tested for HPV DNA, using polymerase chain reaction (PCR)–based methods described previously [9]. HPV DNA and human β-globin (an internal control for sample sufficiency) were amplified simultaneously. PCR products were dotted onto nylon filters and probed with biotin-labeled HPV generic and β-globin probes. Specimens determined to be HPV positive by generic probe or β-globin negative by dot blot were typed using the Roche Linear Array HPV genotyping test (Roche Molecular Systems, Alameda, California), which detects 37 HPV types, including the following 19 types classified as carcinogenic or probably/possibly carcinogenic: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39 [10, 11]. Samples that were β-globin negative during the initial dot blot step and HPV negative by the Roche assay were considered insufficient.
Analyses were restricted to women who returned a baseline sample and ≥1 follow-up sample sufficient for HPV testing. The cumulative incidence of newly detected type-specific HR-HPV was estimated by Kaplan–Meier methods. Incident detection was defined as detection of a type during follow up that was not detected at baseline. The time at risk was calculated from baseline to first detection of type-specific HPV (using the midpoint between the incident positive test result and collection of the previous sample), or the last sample. Separate models were constructed to estimate the cumulative incidence of any HR-HPV detection (woman-level analysis) and detection of each HR-HPV type (infection-level analyses). Incidence rates were calculated by dividing the number of new detections by number of women-years at risk.
Marginal Cox proportional hazards models were used to determine risk factors for incident HR-HPV detection. The time to event was measured from the baseline sample to first detection of each HR-HPV type or the last available sample, with each woman contributing at-risk time for each of 19 types. Analyses were stratified according to HR-HPV type, with the assumption of common relative hazards across types while allowing the baseline hazards to vary. Robust variance estimates were used to account for within-subject correlation. Scaled Schoenfeld residuals [12] were used to test for proportionality of hazards over time; when the proportionality assumption of the Cox model was violated, a variable-by-time interaction term was tested.
Separate models were constructed for (1) all women, (2) women not sexually active with male partners within 6 months of sample collection, and (3) women sexually active with male partners within 6 months of sample collection. A woman could contribute at-risk time to >1 model if, for example, she was sexually active with a male partner within 6 months of the 4-month follow-up sample but not within 6 months of the 8-month sample.
Age at first intercourse was included as a fixed risk factor. Time-dependent risk factors included age, marital status, smoking history, abnormal Papanicolaou test history, current hormonal contraceptive use, menopausal status (restricted to women ≥45 years of age), sex with ≥1 male partner in the prior 6 months, and lifetime number of male sex partners. Variables that were statistically significant (P < .05) in univariate analyses were entered into final multivariate models.
Among women reporting sex with ≥1 male partner in the prior 6 months, we also evaluated male partner/partnership characteristics, including reports of new partners, casual (versus regular) partners, younger partners, partners with other concurrent partnerships, partners met online, condom use, and circumcision status. If multiple partners were reported in the past 6 months, the characteristic was summarized across partners/partnerships. Report of ≥2 male partners in the prior 6 months was also evaluated. Of these 8 variables, we used those found to be statistically significant (ie, those with a P value of <.05) in univariate analyses (hereafter called “high-risk sexual behaviors”) to create a composite variable reflecting the number of high-risk sexual behaviors in the prior 6 months (0, 1, or ≥2). The composite variable was included in the multivariate model.
Separately, we evaluated a 3-level composite variable for all women to compare the incidence of new HR-HPV detection across increasing risk levels of recent sexual behavior, from not recently sexually active to sexually active with ≥1 high-risk behavior. We evaluated potential confounding by the time-dependent risk factors described above (excluding individual 6-month sexual behavior variables). Variables that changed any of the composite variable point estimates by >10% were retained in the final model. We used the adjusted hazard ratios to calculate the risk of new HR-HPV detection attributable to recent sex without any high-risk behaviors and to sex with ≥1 recent high-risk behavior.
Given the 2-phase design, a subset of women had a gap of up to 1 year between 2 follow-up samples. Therefore, we conducted sensitivity analyses for all analyses described above to determine whether the results differed when women with sample gaps of >8 months were excluded.
Statistical analyses were performed using Stata, version 12.1 (StataCorp, College Station, Texas).
RESULTS
We enrolled 521 age-eligible women [8]. Two (0.4%) with insufficient baseline samples were excluded. Of the remaining 519 women, 421 (81.1%) returned ≥1 follow-up sample. Women who did versus those who did not return ≥1 follow-up sample were similar with respect to age and lifetime number of partners (data not shown). One additional woman was excluded because her 1 follow-up sample was insufficient. Therefore, analyses were restricted to 420 women. At enrollment, participants had a mean age (±standard deviation [SD]) of 35.7 ± 9.6 years and reported a median lifetime number of 11 male sex partners (interquartile range, 6–20.5 partners). Half reported new or multiple male partners within 6 months of enrollment (Figure 1 and Supplementary Table). Mean follow-up duration (±SD) was 12.5 ± 5.0 months, the mean interval (±SD) between sample collection was 5.1 ± 1.4 months, and 299 (71.2%) returned 4 samples. Seventy-four women (17.6%) had a gap of >8 months between 2 samples.
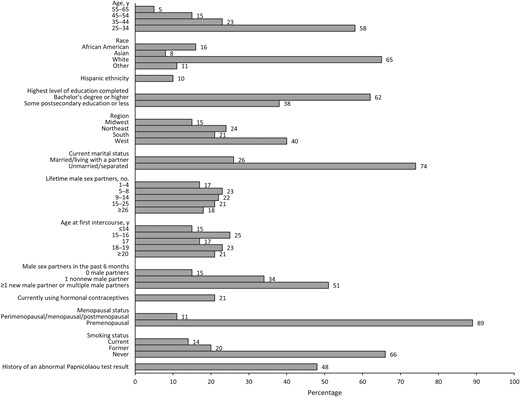
Baseline demographic, health, and sexual history characteristics of 420 women aged 25–65 years who date online. Additional baseline characteristics are included as part of the Supplementary Table.
The 12-month cumulative incidence of HR-HPV detection was 25.4% (95% confidence interval [CI], 21.3%–30.1%; Figure 2). The types with the highest 12-month cumulative incidence were HPV-53 (6.5%; 95% CI, 4.4%–9.7%) and HPV-16 (4.3%; 95% CI, 2.6%–7.1%). The 12-month cumulative incidence of HPV-16 or HPV-18 (high-risk types included in all HPV vaccines) was 6.1% (95% CI, 4.1%–9.0%). Of the 5 additional HR-HPV types in the nonavalent vaccine (31, 33, 45, 52, and 58) [13], the 12-month cumulative incidence was highest for HPV-52 (3.9%; 95% CI, 2.3–6.6; Table 1).
Type-Specific Cumulative Incidence and Incidence Rates of Newly Detected High-Risk Human Papillomavirus (HPV) Among 420 Women Aged 25–65 Years Who Date Online
| HPV Type(s) . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rate per 100 Person-Years (95% CI) . | 12-mo Cumulative Incidence (95% CI) . |
|---|---|---|---|---|
| Any high-risk typea | 170 | 7914 | 29.5 (24.4–35.6) | 25.4 (21.3–30.1) |
| 16 or 18 | 27 | 417 | 6.5 (4.4–9.4) | 6.1 (4.1–9.0) |
| 16 | 18 | 396 | 4.5 (2.9–7.2) | 4.3 (2.6–7.1) |
| 18 | 10 | 407 | 2.5 (1.3–4.6) | 2.5 (1.3–4.7) |
| 26 | 2 | 433 | 0.5 (.1–1.8) | 0.5 (.1–1.9) |
| 31 | 9 | 419 | 2.1 (1.1–4.1) | 2.1 (1.1–4.3) |
| 33 | 3 | 432 | 0.7 (.2–2.2) | 0.7 (.2–2.2) |
| 35 | 5 | 422 | 1.2 (.5–2.8) | 1.0 (.4–2.6) |
| 39 | 6 | 410 | 1.5 (.7–3.3) | 1.7 (.8–3.9) |
| 45 | 7 | 425 | 1.6 (.8–3.5) | 1.4 (.6–3.3) |
| 51 | 15 | 405 | 3.7 (2.2–6.2) | 4.1 (2.5–6.7) |
| 52 | 15 | 404 | 3.7 (2.2–6.2) | 3.9 (2.3–6.6) |
| 53 | 26 | 390 | 6.7 (4.5–9.8) | 6.5 (4.4–9.7) |
| 56 | 8 | 421 | 1.9 (1.0–3.8) | 1.8 (.9–3.7) |
| 58 | 5 | 417 | 1.2 (.5–2.9) | 1.3 (.6–3.2) |
| 59 | 11 | 415 | 2.7 (1.5–4.8) | 2.1 (1.1–4.2) |
| 66 | 8 | 412 | 1.9 (1.0–3.9) | 1.9 (.9–4.0) |
| 68 | 5 | 423 | 1.2 (.5–2.8) | 1.3 (.5–3.1) |
| 73 | 9 | 423 | 2.1 (1.1–4.1) | 2.0 (1.0–4.1) |
| 82 | 6 | 430 | 1.4 (.6–3.1) | 1.5 (.7–3.3) |
| IS39 | 2 | 433 | 0.5 (.1–1.8) | 0.6 (0.1–2.3) |
| HPV Type(s) . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rate per 100 Person-Years (95% CI) . | 12-mo Cumulative Incidence (95% CI) . |
|---|---|---|---|---|
| Any high-risk typea | 170 | 7914 | 29.5 (24.4–35.6) | 25.4 (21.3–30.1) |
| 16 or 18 | 27 | 417 | 6.5 (4.4–9.4) | 6.1 (4.1–9.0) |
| 16 | 18 | 396 | 4.5 (2.9–7.2) | 4.3 (2.6–7.1) |
| 18 | 10 | 407 | 2.5 (1.3–4.6) | 2.5 (1.3–4.7) |
| 26 | 2 | 433 | 0.5 (.1–1.8) | 0.5 (.1–1.9) |
| 31 | 9 | 419 | 2.1 (1.1–4.1) | 2.1 (1.1–4.3) |
| 33 | 3 | 432 | 0.7 (.2–2.2) | 0.7 (.2–2.2) |
| 35 | 5 | 422 | 1.2 (.5–2.8) | 1.0 (.4–2.6) |
| 39 | 6 | 410 | 1.5 (.7–3.3) | 1.7 (.8–3.9) |
| 45 | 7 | 425 | 1.6 (.8–3.5) | 1.4 (.6–3.3) |
| 51 | 15 | 405 | 3.7 (2.2–6.2) | 4.1 (2.5–6.7) |
| 52 | 15 | 404 | 3.7 (2.2–6.2) | 3.9 (2.3–6.6) |
| 53 | 26 | 390 | 6.7 (4.5–9.8) | 6.5 (4.4–9.7) |
| 56 | 8 | 421 | 1.9 (1.0–3.8) | 1.8 (.9–3.7) |
| 58 | 5 | 417 | 1.2 (.5–2.9) | 1.3 (.6–3.2) |
| 59 | 11 | 415 | 2.7 (1.5–4.8) | 2.1 (1.1–4.2) |
| 66 | 8 | 412 | 1.9 (1.0–3.9) | 1.9 (.9–4.0) |
| 68 | 5 | 423 | 1.2 (.5–2.8) | 1.3 (.5–3.1) |
| 73 | 9 | 423 | 2.1 (1.1–4.1) | 2.0 (1.0–4.1) |
| 82 | 6 | 430 | 1.4 (.6–3.1) | 1.5 (.7–3.3) |
| IS39 | 2 | 433 | 0.5 (.1–1.8) | 0.6 (0.1–2.3) |
Abbreviation: CI, confidence interval.
a Defined as positivity for any of the following 19 carcinogenic, probably carcinogenic, or possibly carcinogenic types: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39.
Type-Specific Cumulative Incidence and Incidence Rates of Newly Detected High-Risk Human Papillomavirus (HPV) Among 420 Women Aged 25–65 Years Who Date Online
| HPV Type(s) . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rate per 100 Person-Years (95% CI) . | 12-mo Cumulative Incidence (95% CI) . |
|---|---|---|---|---|
| Any high-risk typea | 170 | 7914 | 29.5 (24.4–35.6) | 25.4 (21.3–30.1) |
| 16 or 18 | 27 | 417 | 6.5 (4.4–9.4) | 6.1 (4.1–9.0) |
| 16 | 18 | 396 | 4.5 (2.9–7.2) | 4.3 (2.6–7.1) |
| 18 | 10 | 407 | 2.5 (1.3–4.6) | 2.5 (1.3–4.7) |
| 26 | 2 | 433 | 0.5 (.1–1.8) | 0.5 (.1–1.9) |
| 31 | 9 | 419 | 2.1 (1.1–4.1) | 2.1 (1.1–4.3) |
| 33 | 3 | 432 | 0.7 (.2–2.2) | 0.7 (.2–2.2) |
| 35 | 5 | 422 | 1.2 (.5–2.8) | 1.0 (.4–2.6) |
| 39 | 6 | 410 | 1.5 (.7–3.3) | 1.7 (.8–3.9) |
| 45 | 7 | 425 | 1.6 (.8–3.5) | 1.4 (.6–3.3) |
| 51 | 15 | 405 | 3.7 (2.2–6.2) | 4.1 (2.5–6.7) |
| 52 | 15 | 404 | 3.7 (2.2–6.2) | 3.9 (2.3–6.6) |
| 53 | 26 | 390 | 6.7 (4.5–9.8) | 6.5 (4.4–9.7) |
| 56 | 8 | 421 | 1.9 (1.0–3.8) | 1.8 (.9–3.7) |
| 58 | 5 | 417 | 1.2 (.5–2.9) | 1.3 (.6–3.2) |
| 59 | 11 | 415 | 2.7 (1.5–4.8) | 2.1 (1.1–4.2) |
| 66 | 8 | 412 | 1.9 (1.0–3.9) | 1.9 (.9–4.0) |
| 68 | 5 | 423 | 1.2 (.5–2.8) | 1.3 (.5–3.1) |
| 73 | 9 | 423 | 2.1 (1.1–4.1) | 2.0 (1.0–4.1) |
| 82 | 6 | 430 | 1.4 (.6–3.1) | 1.5 (.7–3.3) |
| IS39 | 2 | 433 | 0.5 (.1–1.8) | 0.6 (0.1–2.3) |
| HPV Type(s) . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rate per 100 Person-Years (95% CI) . | 12-mo Cumulative Incidence (95% CI) . |
|---|---|---|---|---|
| Any high-risk typea | 170 | 7914 | 29.5 (24.4–35.6) | 25.4 (21.3–30.1) |
| 16 or 18 | 27 | 417 | 6.5 (4.4–9.4) | 6.1 (4.1–9.0) |
| 16 | 18 | 396 | 4.5 (2.9–7.2) | 4.3 (2.6–7.1) |
| 18 | 10 | 407 | 2.5 (1.3–4.6) | 2.5 (1.3–4.7) |
| 26 | 2 | 433 | 0.5 (.1–1.8) | 0.5 (.1–1.9) |
| 31 | 9 | 419 | 2.1 (1.1–4.1) | 2.1 (1.1–4.3) |
| 33 | 3 | 432 | 0.7 (.2–2.2) | 0.7 (.2–2.2) |
| 35 | 5 | 422 | 1.2 (.5–2.8) | 1.0 (.4–2.6) |
| 39 | 6 | 410 | 1.5 (.7–3.3) | 1.7 (.8–3.9) |
| 45 | 7 | 425 | 1.6 (.8–3.5) | 1.4 (.6–3.3) |
| 51 | 15 | 405 | 3.7 (2.2–6.2) | 4.1 (2.5–6.7) |
| 52 | 15 | 404 | 3.7 (2.2–6.2) | 3.9 (2.3–6.6) |
| 53 | 26 | 390 | 6.7 (4.5–9.8) | 6.5 (4.4–9.7) |
| 56 | 8 | 421 | 1.9 (1.0–3.8) | 1.8 (.9–3.7) |
| 58 | 5 | 417 | 1.2 (.5–2.9) | 1.3 (.6–3.2) |
| 59 | 11 | 415 | 2.7 (1.5–4.8) | 2.1 (1.1–4.2) |
| 66 | 8 | 412 | 1.9 (1.0–3.9) | 1.9 (.9–4.0) |
| 68 | 5 | 423 | 1.2 (.5–2.8) | 1.3 (.5–3.1) |
| 73 | 9 | 423 | 2.1 (1.1–4.1) | 2.0 (1.0–4.1) |
| 82 | 6 | 430 | 1.4 (.6–3.1) | 1.5 (.7–3.3) |
| IS39 | 2 | 433 | 0.5 (.1–1.8) | 0.6 (0.1–2.3) |
Abbreviation: CI, confidence interval.
a Defined as positivity for any of the following 19 carcinogenic, probably carcinogenic, or possibly carcinogenic types: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and IS39.
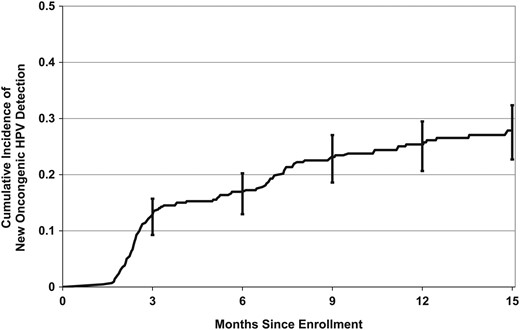
Cumulative incidence of detecting a new high-risk human papillomavirus (HPV) type that was not present at baseline. Data are for 420 women aged 25–65 years. Error bars represent 95% confidence intervals.
Among all women, lifetime number of male partners, current hormonal contraceptive use, and recent sex with male partners were each positively associated with incident HR-HPV in univariate analyses (Table 2). In the multivariate model, lifetime number of male partners (adjusted hazard ratio for ≥9 vs 1–4 partners, 2.56; 95% CI, 1.15–5.69) and hormonal contraceptive use (adjusted hazard ratio vs nonusers, 1.82; 95% CI, 1.17–2.83) remained significant independent predictors of incident HR-HPV.
Hazard Ratios (HRs) for the Associations Between Selected Risk Factors and Incident High-Risk Human Papillomavirus Detection Among 420 Women Aged 25–65 Years
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 103 | 4453 | Reference (1.0) | … | … | … |
| 35–44 | 43 | 1868 | 1.01 (.61–1.68) | … | … | … |
| 45–54 | 21 | 1129 | 0.81 (.45–1.47) | … | … | … |
| 55–65 | 3 | 464 | 0.28 (.07–1.21) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 24 | 1166 | Reference (1.0) | … | … | … |
| 15–16 | 49 | 2022 | 1.18 (.57–2.44) | … | … | … |
| 17 | 30 | 1235 | 1.14 (.53–2.47) | … | … | … |
| 18–19 | 44 | 1798 | 1.19 (.59–2.42) | … | … | … |
| ≥20 | 22 | 1666 | 0.65 (.30–1.42) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 126 | 5429 | Reference (1.0) | … | … | … |
| Married or living with a partner | 42 | 2416 | 0.75 (.48–1.17) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 990 | Reference (1.0) | 9 | 942 | Reference (1.0) |
| 5–8 | 34 | 1696 | 2.28 (.93–5.62) | 31 | 1602 | 1.98 (.79–4.94) |
| ≥9 | 127 | 5085 | 2.92 (1.32–6.47) | 117 | 4781 | 2.56 (1.15–5.69) |
| Currently using hormonal contraceptives | ||||||
| No | 105 | 5945 | Reference (1.0) | 104 | 5787 | Reference (1.0) |
| Yes | 53 | 1574 | 1.94 (1.25–3.01) | 53 | 1538 | 1.82 (1.17–2.83) |
| Smoking status | ||||||
| Never | 97 | 4871 | Reference (1.0) | … | … | … |
| Former | 52 | 2095 | 1.30 (.80–2.11) | … | … | … |
| Current | 21 | 941 | 1.14 (.55–2.36) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 85 | 4076 | Reference (1.0) | … | … | … |
| Yes | 78 | 3659 | 1.02 (.67–1.54) | … | … | … |
| Male sex partners in the past 6 mo, no. | ||||||
| 0 | 17 | 1632 | Reference (1.0) | 16 | 1527 | Reference (1.0) |
| ≥1 | 152 | 6199 | 2.24 (1.16–4.30) | 141 | 5798 | 1.88 (.95–3.72) |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 568 | Reference (1.0) | … | … | … |
| Perimenopausal or menopausal or postmenopausal | 11 | 1002 | 0.48 (.18–1.30) | … | … | … |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 103 | 4453 | Reference (1.0) | … | … | … |
| 35–44 | 43 | 1868 | 1.01 (.61–1.68) | … | … | … |
| 45–54 | 21 | 1129 | 0.81 (.45–1.47) | … | … | … |
| 55–65 | 3 | 464 | 0.28 (.07–1.21) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 24 | 1166 | Reference (1.0) | … | … | … |
| 15–16 | 49 | 2022 | 1.18 (.57–2.44) | … | … | … |
| 17 | 30 | 1235 | 1.14 (.53–2.47) | … | … | … |
| 18–19 | 44 | 1798 | 1.19 (.59–2.42) | … | … | … |
| ≥20 | 22 | 1666 | 0.65 (.30–1.42) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 126 | 5429 | Reference (1.0) | … | … | … |
| Married or living with a partner | 42 | 2416 | 0.75 (.48–1.17) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 990 | Reference (1.0) | 9 | 942 | Reference (1.0) |
| 5–8 | 34 | 1696 | 2.28 (.93–5.62) | 31 | 1602 | 1.98 (.79–4.94) |
| ≥9 | 127 | 5085 | 2.92 (1.32–6.47) | 117 | 4781 | 2.56 (1.15–5.69) |
| Currently using hormonal contraceptives | ||||||
| No | 105 | 5945 | Reference (1.0) | 104 | 5787 | Reference (1.0) |
| Yes | 53 | 1574 | 1.94 (1.25–3.01) | 53 | 1538 | 1.82 (1.17–2.83) |
| Smoking status | ||||||
| Never | 97 | 4871 | Reference (1.0) | … | … | … |
| Former | 52 | 2095 | 1.30 (.80–2.11) | … | … | … |
| Current | 21 | 941 | 1.14 (.55–2.36) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 85 | 4076 | Reference (1.0) | … | … | … |
| Yes | 78 | 3659 | 1.02 (.67–1.54) | … | … | … |
| Male sex partners in the past 6 mo, no. | ||||||
| 0 | 17 | 1632 | Reference (1.0) | 16 | 1527 | Reference (1.0) |
| ≥1 | 152 | 6199 | 2.24 (1.16–4.30) | 141 | 5798 | 1.88 (.95–3.72) |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 568 | Reference (1.0) | … | … | … |
| Perimenopausal or menopausal or postmenopausal | 11 | 1002 | 0.48 (.18–1.30) | … | … | … |
Abbreviation: CI, confidence interval.
a The following variables were included in the multivariate model: hormonal contraceptive use, lifetime number of male sex partners, and male sex partners in the past 6 months.
b Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable.
c Restricted to women ≥45 years of age.
Hazard Ratios (HRs) for the Associations Between Selected Risk Factors and Incident High-Risk Human Papillomavirus Detection Among 420 Women Aged 25–65 Years
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 103 | 4453 | Reference (1.0) | … | … | … |
| 35–44 | 43 | 1868 | 1.01 (.61–1.68) | … | … | … |
| 45–54 | 21 | 1129 | 0.81 (.45–1.47) | … | … | … |
| 55–65 | 3 | 464 | 0.28 (.07–1.21) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 24 | 1166 | Reference (1.0) | … | … | … |
| 15–16 | 49 | 2022 | 1.18 (.57–2.44) | … | … | … |
| 17 | 30 | 1235 | 1.14 (.53–2.47) | … | … | … |
| 18–19 | 44 | 1798 | 1.19 (.59–2.42) | … | … | … |
| ≥20 | 22 | 1666 | 0.65 (.30–1.42) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 126 | 5429 | Reference (1.0) | … | … | … |
| Married or living with a partner | 42 | 2416 | 0.75 (.48–1.17) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 990 | Reference (1.0) | 9 | 942 | Reference (1.0) |
| 5–8 | 34 | 1696 | 2.28 (.93–5.62) | 31 | 1602 | 1.98 (.79–4.94) |
| ≥9 | 127 | 5085 | 2.92 (1.32–6.47) | 117 | 4781 | 2.56 (1.15–5.69) |
| Currently using hormonal contraceptives | ||||||
| No | 105 | 5945 | Reference (1.0) | 104 | 5787 | Reference (1.0) |
| Yes | 53 | 1574 | 1.94 (1.25–3.01) | 53 | 1538 | 1.82 (1.17–2.83) |
| Smoking status | ||||||
| Never | 97 | 4871 | Reference (1.0) | … | … | … |
| Former | 52 | 2095 | 1.30 (.80–2.11) | … | … | … |
| Current | 21 | 941 | 1.14 (.55–2.36) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 85 | 4076 | Reference (1.0) | … | … | … |
| Yes | 78 | 3659 | 1.02 (.67–1.54) | … | … | … |
| Male sex partners in the past 6 mo, no. | ||||||
| 0 | 17 | 1632 | Reference (1.0) | 16 | 1527 | Reference (1.0) |
| ≥1 | 152 | 6199 | 2.24 (1.16–4.30) | 141 | 5798 | 1.88 (.95–3.72) |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 568 | Reference (1.0) | … | … | … |
| Perimenopausal or menopausal or postmenopausal | 11 | 1002 | 0.48 (.18–1.30) | … | … | … |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 103 | 4453 | Reference (1.0) | … | … | … |
| 35–44 | 43 | 1868 | 1.01 (.61–1.68) | … | … | … |
| 45–54 | 21 | 1129 | 0.81 (.45–1.47) | … | … | … |
| 55–65 | 3 | 464 | 0.28 (.07–1.21) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 24 | 1166 | Reference (1.0) | … | … | … |
| 15–16 | 49 | 2022 | 1.18 (.57–2.44) | … | … | … |
| 17 | 30 | 1235 | 1.14 (.53–2.47) | … | … | … |
| 18–19 | 44 | 1798 | 1.19 (.59–2.42) | … | … | … |
| ≥20 | 22 | 1666 | 0.65 (.30–1.42) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 126 | 5429 | Reference (1.0) | … | … | … |
| Married or living with a partner | 42 | 2416 | 0.75 (.48–1.17) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 990 | Reference (1.0) | 9 | 942 | Reference (1.0) |
| 5–8 | 34 | 1696 | 2.28 (.93–5.62) | 31 | 1602 | 1.98 (.79–4.94) |
| ≥9 | 127 | 5085 | 2.92 (1.32–6.47) | 117 | 4781 | 2.56 (1.15–5.69) |
| Currently using hormonal contraceptives | ||||||
| No | 105 | 5945 | Reference (1.0) | 104 | 5787 | Reference (1.0) |
| Yes | 53 | 1574 | 1.94 (1.25–3.01) | 53 | 1538 | 1.82 (1.17–2.83) |
| Smoking status | ||||||
| Never | 97 | 4871 | Reference (1.0) | … | … | … |
| Former | 52 | 2095 | 1.30 (.80–2.11) | … | … | … |
| Current | 21 | 941 | 1.14 (.55–2.36) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 85 | 4076 | Reference (1.0) | … | … | … |
| Yes | 78 | 3659 | 1.02 (.67–1.54) | … | … | … |
| Male sex partners in the past 6 mo, no. | ||||||
| 0 | 17 | 1632 | Reference (1.0) | 16 | 1527 | Reference (1.0) |
| ≥1 | 152 | 6199 | 2.24 (1.16–4.30) | 141 | 5798 | 1.88 (.95–3.72) |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 568 | Reference (1.0) | … | … | … |
| Perimenopausal or menopausal or postmenopausal | 11 | 1002 | 0.48 (.18–1.30) | … | … | … |
Abbreviation: CI, confidence interval.
a The following variables were included in the multivariate model: hormonal contraceptive use, lifetime number of male sex partners, and male sex partners in the past 6 months.
b Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable.
c Restricted to women ≥45 years of age.
In women reporting no male sex partners within the prior 6 months, lifetime number of partners and current hormonal contraceptive use were each positively associated with incident HR-HPV in univariate analyses (Table 3). No incident HR-HPV cases were detected in women reporting 1–4 partners, yielding a hazard ratio of infinity for ≥5 partners (exact unadjusted 95% CI, 1.02–∞). After adjustment for lifetime number of partners, incident HR-HPV detection was 4.16 (95% CI, 1.27–13.63) times more likely in women reporting current hormonal contraceptive use than in nonusers. The proportional hazards assumption was violated for age and abnormal Papanicolaou test history, indicating that the effects of these variables on HR-HPV detection incidence were not constant over time. Tests of variable-by-time interaction terms were not statistically significant, however (data not shown); therefore, only overall hazard ratios are reported. Menopausal status was not evaluated because only 1 outcome was observed in women who were ≥45 years of age and not recently sexually active.
Hazard Ratios (HRs) for the Associations Between Select Risk Factors and Incident High-Risk Human Papillomavirus (HPV) Detection Among 110 Women Aged 25–65 Years Who Reported No Male Sex Partners in the Past 6 Months
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, yb | ||||||
| 25–34 | 8 | 709 | Reference (1.0) | … | … | … |
| 35–44 | 8 | 486 | 1.66 (.42–6.57) | … | … | … |
| 45–54 | 1 | 246 | 0.35 (.05–2.43) | … | … | … |
| 55–65 | 0 | 190 | 0 (0–2.19)c | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 1 | 125 | Reference (1.0) | … | … | … |
| 15–16 | 6 | 472 | 1.53 (.23–10.15) | … | … | … |
| 17 | 1 | 172 | 0.68 (.05–9.25) | … | … | … |
| 18–19 | 8 | 373 | 2.50 (.32–19.69) | … | … | … |
| ≥20 | 1 | 490 | 0.24 (.02–3.14) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 14 | 1368 | Reference (1.0) | … | … | … |
| Married or living with a partner | 2 | 256 | 0.77 (.19–3.16) | … | … | … |
| Lifetime male sex partners, no.d | ||||||
| 1–4 | 0 | 317 | Reference (1.0) | 0 | 317 | Reference (1.0) |
| ≥5 | 17 | 1277 | ∞ (1.02–∞c) | 16 | 1210 | ∞ (1.02–∞c) |
| Currently using hormonal contraceptives | ||||||
| No | 11 | 1407 | Reference (1.0) | 11 | 1387 | Reference (1.0) |
| Yes | 5 | 140 | 4.66 (1.33–16.38) | 5 | 140 | 4.16 (1.27–13.63) |
| Smoking status | ||||||
| Never | 10 | 1066 | Reference (1.0) | … | … | … |
| Former | 6 | 421 | 1.70 (.44–6.57) | … | … | … |
| Current | 1 | 145 | 0.80 (.10–6.52) | … | … | … |
| Ever had an abnormal Papanicolaou test resultb | ||||||
| No | 9 | 885 | Reference (1.0) | … | … | … |
| Yes | 8 | 676 | 1.18 (.33–4.21) | … | … | … |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, yb | ||||||
| 25–34 | 8 | 709 | Reference (1.0) | … | … | … |
| 35–44 | 8 | 486 | 1.66 (.42–6.57) | … | … | … |
| 45–54 | 1 | 246 | 0.35 (.05–2.43) | … | … | … |
| 55–65 | 0 | 190 | 0 (0–2.19)c | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 1 | 125 | Reference (1.0) | … | … | … |
| 15–16 | 6 | 472 | 1.53 (.23–10.15) | … | … | … |
| 17 | 1 | 172 | 0.68 (.05–9.25) | … | … | … |
| 18–19 | 8 | 373 | 2.50 (.32–19.69) | … | … | … |
| ≥20 | 1 | 490 | 0.24 (.02–3.14) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 14 | 1368 | Reference (1.0) | … | … | … |
| Married or living with a partner | 2 | 256 | 0.77 (.19–3.16) | … | … | … |
| Lifetime male sex partners, no.d | ||||||
| 1–4 | 0 | 317 | Reference (1.0) | 0 | 317 | Reference (1.0) |
| ≥5 | 17 | 1277 | ∞ (1.02–∞c) | 16 | 1210 | ∞ (1.02–∞c) |
| Currently using hormonal contraceptives | ||||||
| No | 11 | 1407 | Reference (1.0) | 11 | 1387 | Reference (1.0) |
| Yes | 5 | 140 | 4.66 (1.33–16.38) | 5 | 140 | 4.16 (1.27–13.63) |
| Smoking status | ||||||
| Never | 10 | 1066 | Reference (1.0) | … | … | … |
| Former | 6 | 421 | 1.70 (.44–6.57) | … | … | … |
| Current | 1 | 145 | 0.80 (.10–6.52) | … | … | … |
| Ever had an abnormal Papanicolaou test resultb | ||||||
| No | 9 | 885 | Reference (1.0) | … | … | … |
| Yes | 8 | 676 | 1.18 (.33–4.21) | … | … | … |
Analysis was restricted to women reporting no sex with male partners in the 6 months prior to HPV assessment. Women could enter and exit the analysis multiple times if their report of male partners in the prior 6 months varied among follow-up HPV assessments.
Abbreviation: CI, confidence interval.
a Hormonal contraceptive use was adjusted for lifetime number of male sex partners. Lifetime number of male sex partners was unadjusted because we are not aware of exact methods for calculating adjusted CIs.
b A test of scaled Shoenfeld residuals indicated that the proportional hazards assumption was violated. However, no statistically significant variable by time interaction was observed. Therefore, only 1 overall HR is reported.
c Exact unadjusted CI. We are not aware of exact methods for calculating adjusted CIs.
d Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable. In this model, lifetime number of partners was further collapsed into a dichotomous variable (1–4 and ≥5) because of 0 outcomes among women with 1–4 partners.
Hazard Ratios (HRs) for the Associations Between Select Risk Factors and Incident High-Risk Human Papillomavirus (HPV) Detection Among 110 Women Aged 25–65 Years Who Reported No Male Sex Partners in the Past 6 Months
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, yb | ||||||
| 25–34 | 8 | 709 | Reference (1.0) | … | … | … |
| 35–44 | 8 | 486 | 1.66 (.42–6.57) | … | … | … |
| 45–54 | 1 | 246 | 0.35 (.05–2.43) | … | … | … |
| 55–65 | 0 | 190 | 0 (0–2.19)c | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 1 | 125 | Reference (1.0) | … | … | … |
| 15–16 | 6 | 472 | 1.53 (.23–10.15) | … | … | … |
| 17 | 1 | 172 | 0.68 (.05–9.25) | … | … | … |
| 18–19 | 8 | 373 | 2.50 (.32–19.69) | … | … | … |
| ≥20 | 1 | 490 | 0.24 (.02–3.14) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 14 | 1368 | Reference (1.0) | … | … | … |
| Married or living with a partner | 2 | 256 | 0.77 (.19–3.16) | … | … | … |
| Lifetime male sex partners, no.d | ||||||
| 1–4 | 0 | 317 | Reference (1.0) | 0 | 317 | Reference (1.0) |
| ≥5 | 17 | 1277 | ∞ (1.02–∞c) | 16 | 1210 | ∞ (1.02–∞c) |
| Currently using hormonal contraceptives | ||||||
| No | 11 | 1407 | Reference (1.0) | 11 | 1387 | Reference (1.0) |
| Yes | 5 | 140 | 4.66 (1.33–16.38) | 5 | 140 | 4.16 (1.27–13.63) |
| Smoking status | ||||||
| Never | 10 | 1066 | Reference (1.0) | … | … | … |
| Former | 6 | 421 | 1.70 (.44–6.57) | … | … | … |
| Current | 1 | 145 | 0.80 (.10–6.52) | … | … | … |
| Ever had an abnormal Papanicolaou test resultb | ||||||
| No | 9 | 885 | Reference (1.0) | … | … | … |
| Yes | 8 | 676 | 1.18 (.33–4.21) | … | … | … |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, yb | ||||||
| 25–34 | 8 | 709 | Reference (1.0) | … | … | … |
| 35–44 | 8 | 486 | 1.66 (.42–6.57) | … | … | … |
| 45–54 | 1 | 246 | 0.35 (.05–2.43) | … | … | … |
| 55–65 | 0 | 190 | 0 (0–2.19)c | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 1 | 125 | Reference (1.0) | … | … | … |
| 15–16 | 6 | 472 | 1.53 (.23–10.15) | … | … | … |
| 17 | 1 | 172 | 0.68 (.05–9.25) | … | … | … |
| 18–19 | 8 | 373 | 2.50 (.32–19.69) | … | … | … |
| ≥20 | 1 | 490 | 0.24 (.02–3.14) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 14 | 1368 | Reference (1.0) | … | … | … |
| Married or living with a partner | 2 | 256 | 0.77 (.19–3.16) | … | … | … |
| Lifetime male sex partners, no.d | ||||||
| 1–4 | 0 | 317 | Reference (1.0) | 0 | 317 | Reference (1.0) |
| ≥5 | 17 | 1277 | ∞ (1.02–∞c) | 16 | 1210 | ∞ (1.02–∞c) |
| Currently using hormonal contraceptives | ||||||
| No | 11 | 1407 | Reference (1.0) | 11 | 1387 | Reference (1.0) |
| Yes | 5 | 140 | 4.66 (1.33–16.38) | 5 | 140 | 4.16 (1.27–13.63) |
| Smoking status | ||||||
| Never | 10 | 1066 | Reference (1.0) | … | … | … |
| Former | 6 | 421 | 1.70 (.44–6.57) | … | … | … |
| Current | 1 | 145 | 0.80 (.10–6.52) | … | … | … |
| Ever had an abnormal Papanicolaou test resultb | ||||||
| No | 9 | 885 | Reference (1.0) | … | … | … |
| Yes | 8 | 676 | 1.18 (.33–4.21) | … | … | … |
Analysis was restricted to women reporting no sex with male partners in the 6 months prior to HPV assessment. Women could enter and exit the analysis multiple times if their report of male partners in the prior 6 months varied among follow-up HPV assessments.
Abbreviation: CI, confidence interval.
a Hormonal contraceptive use was adjusted for lifetime number of male sex partners. Lifetime number of male sex partners was unadjusted because we are not aware of exact methods for calculating adjusted CIs.
b A test of scaled Shoenfeld residuals indicated that the proportional hazards assumption was violated. However, no statistically significant variable by time interaction was observed. Therefore, only 1 overall HR is reported.
c Exact unadjusted CI. We are not aware of exact methods for calculating adjusted CIs.
d Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable. In this model, lifetime number of partners was further collapsed into a dichotomous variable (1–4 and ≥5) because of 0 outcomes among women with 1–4 partners.
In women reporting male sex partners within the prior 6 months, lifetime number of partners, current hormonal contraceptive use, report of recent new or casual partners, report of recent partners with ≥1 concurrent partnerships, and report of ≥2 recent partners were each positively associated with incident HR-HPV detection in univariate analyses (Table 4). The final multivariate model included lifetime number of partners, hormonal contraceptive use, and a composite variable measuring recent high-risk sexual behavior. Current hormonal contraceptive use (adjusted hazard ratio versus nonusers, 1.65; 95% CI, 1.05–2.59) remained an independent predictor of incident HR-HPV detection. Compared with women reporting a recent male sex partner but no high-risk sexual behaviors, those with 1 recent high-risk behavior (new or casual partners, partners with ≥1 concurrent partnership, or ≥2 partners; adjusted hazard ratio, 2.06; 95% CI, 1.17–3.64) or ≥2 recent high-risk sexual behaviors (adjusted hazard ratio, 2.53; 95% CI, 1.49–4.30) were more likely to have incident HR-HPV detected.
Hazard Ratios (HRs) for the Associations Between Select Risk Factors and Incident High-Risk Human Papillomavirus (HPV) Detection Among 362 Women Aged 25–65 Years Who Report ≥1 Male Sex Partner in the Past 6 Months
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 94 | 3688 | Reference (1.0) | … | … | … |
| 35–44 | 35 | 1369 | 0.98 (.59–1.63) | … | … | … |
| 45–54 | 20 | 883 | 0.89 (.48–1.66) | … | … | … |
| 55–65 | 3 | 260 | 0.46 (.11–1.88) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 23 | 1034 | Reference (1.0) | … | … | … |
| 15–16 | 42 | 1515 | 1.27 (.59–2.76) | … | … | … |
| 17 | 29 | 1057 | 1.21 (.55–2.65) | … | … | … |
| 18–19 | 36 | 1409 | 1.17 (.57–2.37) | … | … | … |
| ≥20 | 21 | 1157 | 0.82 (.37–1.84) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 112 | 3999 | Reference (1.0) | … | … | … |
| Married or living with a partner | 39 | 2138 | 0.65 (.41–1.03) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 660 | Reference (1.0) | 9 | 625 | Reference (1.0) |
| 5–8 | 31 | 1303 | 1.90 (.77–4.72) | 28 | 1240 | 1.46 (.58–3.71) |
| ≥9 | 112 | 4139 | 2.19 (1.00–4.81) | 104 | 3933 | 1.57 (.70–3.53) |
| Currently using hormonal contraceptives | ||||||
| No | 93 | 4483 | Reference (1.0) | 93 | 4400 | Reference (1.0) |
| Yes | 48 | 1412 | 1.71 (1.08–2.70) | 48 | 1398 | 1.65 (1.05–2.59) |
| Smoking status | ||||||
| Never | 86 | 3721 | Reference (1.0) | … | … | … |
| Former | 46 | 1674 | 1.22 (.73–2.04) | … | … | … |
| Current | 20 | 796 | 1.09 (.51–2.31) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 76 | 3157 | Reference (1.0) | … | … | … |
| Yes | 69 | 2932 | 0.97 (.63–1.50) | … | … | … |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 433 | Reference (1.0) | … | … | … |
| Perimenopausal, menopausal, or postmenopausal | 10 | 692 | 0.47 (.18–1.27) | … | … | … |
| Sexual behaviors in the past 6 mo | ||||||
| New male sex partnersd | ||||||
| No | 62 | 3523 | Reference (1.0) | … | … | … |
| Yes | 89 | 2626 | 1.90 (1.25–2.88) | … | … | … |
| Casual male sex partnersd | ||||||
| No | 68 | 3848 | Reference (1.0) | … | … | … |
| Yes | 81 | 2305 | 1.97 (1.31–2.97) | … | … | … |
| Younger male sex partnersd | ||||||
| No | 77 | 3680 | Reference (1.0) | … | … | … |
| Yes | 75 | 2519 | 1.45 (.96–2.17) | … | … | … |
| Male sex partner with ≥1 concurrent partnershipd | ||||||
| No or unknown | 77 | 4073 | Reference (1.0) | … | … | … |
| Yes | 75 | 2095 | 1.87 (1.22–2.87) | … | … | … |
| Male partner whom the subject met onlined | ||||||
| No | 65 | 3217 | Reference (1.0) | … | … | … |
| Yes | 87 | 2966 | 1.45 (.95–2.19) | … | … | … |
| Condom use with male sex partnerse | ||||||
| Always | 128 | 4795 | Reference (1.0) | … | … | … |
| Not always | 22 | 1282 | 1.57 (.93–2.63) | … | … | … |
| Circumcision status of male partnersf | ||||||
| Uncircumcised or unknown | 46 | 1516 | Reference (1.0) | … | … | … |
| Circumcised | 106 | 4640 | 0.77 (.48–1.23) | … | … | … |
| ≥2 male sex partners | ||||||
| No | 65 | 3928 | Reference (1.0) | … | … | … |
| Yes | 87 | 2271 | 2.30 (1.52–3.47) | … | … | … |
| Composite high-riskg sexual behavior variable | ||||||
| 0 high-risk behaviors | 27 | 2253 | Reference (1.0) | 26 | 2135 | Reference (1.0) |
| 1 high-risk behavior | 34 | 1336 | 2.08 (1.19–3.63) | 32 | 1219 | 2.06 (1.17–3.64) |
| ≥2 high-risk behaviors | 91 | 2611 | 2.85 (1.73–4.70) | 83 | 2443 | 2.53 (1.49–4.30) |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 94 | 3688 | Reference (1.0) | … | … | … |
| 35–44 | 35 | 1369 | 0.98 (.59–1.63) | … | … | … |
| 45–54 | 20 | 883 | 0.89 (.48–1.66) | … | … | … |
| 55–65 | 3 | 260 | 0.46 (.11–1.88) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 23 | 1034 | Reference (1.0) | … | … | … |
| 15–16 | 42 | 1515 | 1.27 (.59–2.76) | … | … | … |
| 17 | 29 | 1057 | 1.21 (.55–2.65) | … | … | … |
| 18–19 | 36 | 1409 | 1.17 (.57–2.37) | … | … | … |
| ≥20 | 21 | 1157 | 0.82 (.37–1.84) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 112 | 3999 | Reference (1.0) | … | … | … |
| Married or living with a partner | 39 | 2138 | 0.65 (.41–1.03) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 660 | Reference (1.0) | 9 | 625 | Reference (1.0) |
| 5–8 | 31 | 1303 | 1.90 (.77–4.72) | 28 | 1240 | 1.46 (.58–3.71) |
| ≥9 | 112 | 4139 | 2.19 (1.00–4.81) | 104 | 3933 | 1.57 (.70–3.53) |
| Currently using hormonal contraceptives | ||||||
| No | 93 | 4483 | Reference (1.0) | 93 | 4400 | Reference (1.0) |
| Yes | 48 | 1412 | 1.71 (1.08–2.70) | 48 | 1398 | 1.65 (1.05–2.59) |
| Smoking status | ||||||
| Never | 86 | 3721 | Reference (1.0) | … | … | … |
| Former | 46 | 1674 | 1.22 (.73–2.04) | … | … | … |
| Current | 20 | 796 | 1.09 (.51–2.31) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 76 | 3157 | Reference (1.0) | … | … | … |
| Yes | 69 | 2932 | 0.97 (.63–1.50) | … | … | … |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 433 | Reference (1.0) | … | … | … |
| Perimenopausal, menopausal, or postmenopausal | 10 | 692 | 0.47 (.18–1.27) | … | … | … |
| Sexual behaviors in the past 6 mo | ||||||
| New male sex partnersd | ||||||
| No | 62 | 3523 | Reference (1.0) | … | … | … |
| Yes | 89 | 2626 | 1.90 (1.25–2.88) | … | … | … |
| Casual male sex partnersd | ||||||
| No | 68 | 3848 | Reference (1.0) | … | … | … |
| Yes | 81 | 2305 | 1.97 (1.31–2.97) | … | … | … |
| Younger male sex partnersd | ||||||
| No | 77 | 3680 | Reference (1.0) | … | … | … |
| Yes | 75 | 2519 | 1.45 (.96–2.17) | … | … | … |
| Male sex partner with ≥1 concurrent partnershipd | ||||||
| No or unknown | 77 | 4073 | Reference (1.0) | … | … | … |
| Yes | 75 | 2095 | 1.87 (1.22–2.87) | … | … | … |
| Male partner whom the subject met onlined | ||||||
| No | 65 | 3217 | Reference (1.0) | … | … | … |
| Yes | 87 | 2966 | 1.45 (.95–2.19) | … | … | … |
| Condom use with male sex partnerse | ||||||
| Always | 128 | 4795 | Reference (1.0) | … | … | … |
| Not always | 22 | 1282 | 1.57 (.93–2.63) | … | … | … |
| Circumcision status of male partnersf | ||||||
| Uncircumcised or unknown | 46 | 1516 | Reference (1.0) | … | … | … |
| Circumcised | 106 | 4640 | 0.77 (.48–1.23) | … | … | … |
| ≥2 male sex partners | ||||||
| No | 65 | 3928 | Reference (1.0) | … | … | … |
| Yes | 87 | 2271 | 2.30 (1.52–3.47) | … | … | … |
| Composite high-riskg sexual behavior variable | ||||||
| 0 high-risk behaviors | 27 | 2253 | Reference (1.0) | 26 | 2135 | Reference (1.0) |
| 1 high-risk behavior | 34 | 1336 | 2.08 (1.19–3.63) | 32 | 1219 | 2.06 (1.17–3.64) |
| ≥2 high-risk behaviors | 91 | 2611 | 2.85 (1.73–4.70) | 83 | 2443 | 2.53 (1.49–4.30) |
Analysis was restricted to women reporting sex with a male partner in the 6 months prior to oncogenic HPV assessment. Women could enter and exit the analysis multiple times if report of male partners in the prior 6 months varied among follow-up oncogenic HPV assessments.
Abbreviation: CI, confidence interval.
a The following variables were included in the multivariate model: lifetime number of male sex partners, hormonal contraceptive use, and the composite 6-month sexual behavior variable.
b Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable.
c Restricted to women ≥45 years of age.
d The variable was coded as “yes” if at least 1 partner/partnership during the past 6 months fit the characteristic of interest.
e The variable was coded as “always” if the subject reported always using condoms with all male partners during the past 6 months. If a subject reported not always using condoms with at least 1 male partner, the variable was coded as “not always.” Otherwise, if condom use data were missing for at least 1 partner, the variable was set to missing.
f If the circumcision status of 1 partner was reported as uncircumcised or unknown, the variable was coded as “uncircumcised or unknown.” If all partners were reported as circumcised, the variable was coded as “circumcised.”
g Six-month sexual behavior variables that were statistically significant in univariate analyses (including reports of new male partners, reports of casual male partners, reports of partners with ≥1 concurrent partnership, and reports of ≥2 male partners) were classified as “high-risk” and used to create the composite 6-month sexual behavior variable.
Hazard Ratios (HRs) for the Associations Between Select Risk Factors and Incident High-Risk Human Papillomavirus (HPV) Detection Among 362 Women Aged 25–65 Years Who Report ≥1 Male Sex Partner in the Past 6 Months
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 94 | 3688 | Reference (1.0) | … | … | … |
| 35–44 | 35 | 1369 | 0.98 (.59–1.63) | … | … | … |
| 45–54 | 20 | 883 | 0.89 (.48–1.66) | … | … | … |
| 55–65 | 3 | 260 | 0.46 (.11–1.88) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 23 | 1034 | Reference (1.0) | … | … | … |
| 15–16 | 42 | 1515 | 1.27 (.59–2.76) | … | … | … |
| 17 | 29 | 1057 | 1.21 (.55–2.65) | … | … | … |
| 18–19 | 36 | 1409 | 1.17 (.57–2.37) | … | … | … |
| ≥20 | 21 | 1157 | 0.82 (.37–1.84) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 112 | 3999 | Reference (1.0) | … | … | … |
| Married or living with a partner | 39 | 2138 | 0.65 (.41–1.03) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 660 | Reference (1.0) | 9 | 625 | Reference (1.0) |
| 5–8 | 31 | 1303 | 1.90 (.77–4.72) | 28 | 1240 | 1.46 (.58–3.71) |
| ≥9 | 112 | 4139 | 2.19 (1.00–4.81) | 104 | 3933 | 1.57 (.70–3.53) |
| Currently using hormonal contraceptives | ||||||
| No | 93 | 4483 | Reference (1.0) | 93 | 4400 | Reference (1.0) |
| Yes | 48 | 1412 | 1.71 (1.08–2.70) | 48 | 1398 | 1.65 (1.05–2.59) |
| Smoking status | ||||||
| Never | 86 | 3721 | Reference (1.0) | … | … | … |
| Former | 46 | 1674 | 1.22 (.73–2.04) | … | … | … |
| Current | 20 | 796 | 1.09 (.51–2.31) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 76 | 3157 | Reference (1.0) | … | … | … |
| Yes | 69 | 2932 | 0.97 (.63–1.50) | … | … | … |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 433 | Reference (1.0) | … | … | … |
| Perimenopausal, menopausal, or postmenopausal | 10 | 692 | 0.47 (.18–1.27) | … | … | … |
| Sexual behaviors in the past 6 mo | ||||||
| New male sex partnersd | ||||||
| No | 62 | 3523 | Reference (1.0) | … | … | … |
| Yes | 89 | 2626 | 1.90 (1.25–2.88) | … | … | … |
| Casual male sex partnersd | ||||||
| No | 68 | 3848 | Reference (1.0) | … | … | … |
| Yes | 81 | 2305 | 1.97 (1.31–2.97) | … | … | … |
| Younger male sex partnersd | ||||||
| No | 77 | 3680 | Reference (1.0) | … | … | … |
| Yes | 75 | 2519 | 1.45 (.96–2.17) | … | … | … |
| Male sex partner with ≥1 concurrent partnershipd | ||||||
| No or unknown | 77 | 4073 | Reference (1.0) | … | … | … |
| Yes | 75 | 2095 | 1.87 (1.22–2.87) | … | … | … |
| Male partner whom the subject met onlined | ||||||
| No | 65 | 3217 | Reference (1.0) | … | … | … |
| Yes | 87 | 2966 | 1.45 (.95–2.19) | … | … | … |
| Condom use with male sex partnerse | ||||||
| Always | 128 | 4795 | Reference (1.0) | … | … | … |
| Not always | 22 | 1282 | 1.57 (.93–2.63) | … | … | … |
| Circumcision status of male partnersf | ||||||
| Uncircumcised or unknown | 46 | 1516 | Reference (1.0) | … | … | … |
| Circumcised | 106 | 4640 | 0.77 (.48–1.23) | … | … | … |
| ≥2 male sex partners | ||||||
| No | 65 | 3928 | Reference (1.0) | … | … | … |
| Yes | 87 | 2271 | 2.30 (1.52–3.47) | … | … | … |
| Composite high-riskg sexual behavior variable | ||||||
| 0 high-risk behaviors | 27 | 2253 | Reference (1.0) | 26 | 2135 | Reference (1.0) |
| 1 high-risk behavior | 34 | 1336 | 2.08 (1.19–3.63) | 32 | 1219 | 2.06 (1.17–3.64) |
| ≥2 high-risk behaviors | 91 | 2611 | 2.85 (1.73–4.70) | 83 | 2443 | 2.53 (1.49–4.30) |
| Variable . | Incident Detections, No. . | Person-Years at Risk, No. . | Univariate HR (95% CI) . | Incident Detections, No. . | Person-Years at Risk, No. . | Multivariate HR (95% CI)a . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 25–34 | 94 | 3688 | Reference (1.0) | … | … | … |
| 35–44 | 35 | 1369 | 0.98 (.59–1.63) | … | … | … |
| 45–54 | 20 | 883 | 0.89 (.48–1.66) | … | … | … |
| 55–65 | 3 | 260 | 0.46 (.11–1.88) | … | … | … |
| Age at first intercourse, y | ||||||
| ≤14 | 23 | 1034 | Reference (1.0) | … | … | … |
| 15–16 | 42 | 1515 | 1.27 (.59–2.76) | … | … | … |
| 17 | 29 | 1057 | 1.21 (.55–2.65) | … | … | … |
| 18–19 | 36 | 1409 | 1.17 (.57–2.37) | … | … | … |
| ≥20 | 21 | 1157 | 0.82 (.37–1.84) | … | … | … |
| Marital status | ||||||
| Unmarried or separated | 112 | 3999 | Reference (1.0) | … | … | … |
| Married or living with a partner | 39 | 2138 | 0.65 (.41–1.03) | … | … | … |
| Lifetime male sex partners, no.b | ||||||
| 1–4 | 9 | 660 | Reference (1.0) | 9 | 625 | Reference (1.0) |
| 5–8 | 31 | 1303 | 1.90 (.77–4.72) | 28 | 1240 | 1.46 (.58–3.71) |
| ≥9 | 112 | 4139 | 2.19 (1.00–4.81) | 104 | 3933 | 1.57 (.70–3.53) |
| Currently using hormonal contraceptives | ||||||
| No | 93 | 4483 | Reference (1.0) | 93 | 4400 | Reference (1.0) |
| Yes | 48 | 1412 | 1.71 (1.08–2.70) | 48 | 1398 | 1.65 (1.05–2.59) |
| Smoking status | ||||||
| Never | 86 | 3721 | Reference (1.0) | … | … | … |
| Former | 46 | 1674 | 1.22 (.73–2.04) | … | … | … |
| Current | 20 | 796 | 1.09 (.51–2.31) | … | … | … |
| Ever had an abnormal Papanicolaou test result | ||||||
| No | 76 | 3157 | Reference (1.0) | … | … | … |
| Yes | 69 | 2932 | 0.97 (.63–1.50) | … | … | … |
| Menopausal statusc | ||||||
| Premenopausal | 13 | 433 | Reference (1.0) | … | … | … |
| Perimenopausal, menopausal, or postmenopausal | 10 | 692 | 0.47 (.18–1.27) | … | … | … |
| Sexual behaviors in the past 6 mo | ||||||
| New male sex partnersd | ||||||
| No | 62 | 3523 | Reference (1.0) | … | … | … |
| Yes | 89 | 2626 | 1.90 (1.25–2.88) | … | … | … |
| Casual male sex partnersd | ||||||
| No | 68 | 3848 | Reference (1.0) | … | … | … |
| Yes | 81 | 2305 | 1.97 (1.31–2.97) | … | … | … |
| Younger male sex partnersd | ||||||
| No | 77 | 3680 | Reference (1.0) | … | … | … |
| Yes | 75 | 2519 | 1.45 (.96–2.17) | … | … | … |
| Male sex partner with ≥1 concurrent partnershipd | ||||||
| No or unknown | 77 | 4073 | Reference (1.0) | … | … | … |
| Yes | 75 | 2095 | 1.87 (1.22–2.87) | … | … | … |
| Male partner whom the subject met onlined | ||||||
| No | 65 | 3217 | Reference (1.0) | … | … | … |
| Yes | 87 | 2966 | 1.45 (.95–2.19) | … | … | … |
| Condom use with male sex partnerse | ||||||
| Always | 128 | 4795 | Reference (1.0) | … | … | … |
| Not always | 22 | 1282 | 1.57 (.93–2.63) | … | … | … |
| Circumcision status of male partnersf | ||||||
| Uncircumcised or unknown | 46 | 1516 | Reference (1.0) | … | … | … |
| Circumcised | 106 | 4640 | 0.77 (.48–1.23) | … | … | … |
| ≥2 male sex partners | ||||||
| No | 65 | 3928 | Reference (1.0) | … | … | … |
| Yes | 87 | 2271 | 2.30 (1.52–3.47) | … | … | … |
| Composite high-riskg sexual behavior variable | ||||||
| 0 high-risk behaviors | 27 | 2253 | Reference (1.0) | 26 | 2135 | Reference (1.0) |
| 1 high-risk behavior | 34 | 1336 | 2.08 (1.19–3.63) | 32 | 1219 | 2.06 (1.17–3.64) |
| ≥2 high-risk behaviors | 91 | 2611 | 2.85 (1.73–4.70) | 83 | 2443 | 2.53 (1.49–4.30) |
Analysis was restricted to women reporting sex with a male partner in the 6 months prior to oncogenic HPV assessment. Women could enter and exit the analysis multiple times if report of male partners in the prior 6 months varied among follow-up oncogenic HPV assessments.
Abbreviation: CI, confidence interval.
a The following variables were included in the multivariate model: lifetime number of male sex partners, hormonal contraceptive use, and the composite 6-month sexual behavior variable.
b Lifetime number of male partners was initially categorized into quintiles (1–4, 5–8, 9–14, 15–25, and ≥26); in all analyses, however, similar risks of incident detection among categories of >5 to 8 partners were observed. Therefore, a post hoc decision was made to collapse lifetime number of male partners into a 3-level variable.
c Restricted to women ≥45 years of age.
d The variable was coded as “yes” if at least 1 partner/partnership during the past 6 months fit the characteristic of interest.
e The variable was coded as “always” if the subject reported always using condoms with all male partners during the past 6 months. If a subject reported not always using condoms with at least 1 male partner, the variable was coded as “not always.” Otherwise, if condom use data were missing for at least 1 partner, the variable was set to missing.
f If the circumcision status of 1 partner was reported as uncircumcised or unknown, the variable was coded as “uncircumcised or unknown.” If all partners were reported as circumcised, the variable was coded as “circumcised.”
g Six-month sexual behavior variables that were statistically significant in univariate analyses (including reports of new male partners, reports of casual male partners, reports of partners with ≥1 concurrent partnership, and reports of ≥2 male partners) were classified as “high-risk” and used to create the composite 6-month sexual behavior variable.
In the full cohort, compared with women reporting no recent male sex partners, those reporting a recent partner and no high-risk sexual behaviors were not statistically significantly more likely to have incident HR-HPV detected (adjusted hazard ratio, 1.19; 95% CI, .53–2.68), after adjustment for current hormonal contraceptive use and marital status; Table 5). Women reporting ≥1 high-risk sexual behavior were 2.81 (95% CI, 1.38–5.69) times as likely as those not recently sexually active to have incident HR-HPV detected. Thus, among mid-adult women with recent new, multiple, or high-risk male partners, the risk of incident HR-HPV attributable to one of those partners was estimated to be 64.4%.
Hazard Ratios (HRs) for the Association Between Recent Sexual Behaviors and Incident High-Risk Human Papillomavirus (HPV) Detection in All 420 Women Aged 25–65 Years
| Variablea . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rateb per 100 Person-Years (95% CI) . | Unadjusted HR (95% CI) . | Adjusted HR (95% CI)c . |
|---|---|---|---|---|---|
| Not sexually active with a male partner | 17 | 1632 | 1.04 (.65–1.68) | Reference (1.0) | Reference (1.0) |
| Sexually active with no high-riskd behavior | 27 | 2253 | 1.20 (.82–1.75) | 1.11 (.52–2.37) | 1.19 (.53–2.68) |
| Sexually active with ≥1 high-riskd behavior | 125 | 3946 | 3.17 (2.66–3.77) | 2.87 (1.49–5.55) | 2.81 (1.38–5.69) |
| Variablea . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rateb per 100 Person-Years (95% CI) . | Unadjusted HR (95% CI) . | Adjusted HR (95% CI)c . |
|---|---|---|---|---|---|
| Not sexually active with a male partner | 17 | 1632 | 1.04 (.65–1.68) | Reference (1.0) | Reference (1.0) |
| Sexually active with no high-riskd behavior | 27 | 2253 | 1.20 (.82–1.75) | 1.11 (.52–2.37) | 1.19 (.53–2.68) |
| Sexually active with ≥1 high-riskd behavior | 125 | 3946 | 3.17 (2.66–3.77) | 2.87 (1.49–5.55) | 2.81 (1.38–5.69) |
Abbreviation: CI, confidence interval.
a Composite variable for sexual risk behaviors in past 6 mo.
b Average type-specific incidence rate.
c Adjusted for current hormonal contraceptive use and marital status.
d High-risk behaviors were defined as behaviors that were statistically significantly associated with incident high-risk HPV detection in women reporting ≥1 male partner in the past 6 months, as follows: reports of new male partners, reports of casual male partners, reports of partners with ≥1 concurrent partnership, and reports of ≥2 male partners (Table 4).
Hazard Ratios (HRs) for the Association Between Recent Sexual Behaviors and Incident High-Risk Human Papillomavirus (HPV) Detection in All 420 Women Aged 25–65 Years
| Variablea . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rateb per 100 Person-Years (95% CI) . | Unadjusted HR (95% CI) . | Adjusted HR (95% CI)c . |
|---|---|---|---|---|---|
| Not sexually active with a male partner | 17 | 1632 | 1.04 (.65–1.68) | Reference (1.0) | Reference (1.0) |
| Sexually active with no high-riskd behavior | 27 | 2253 | 1.20 (.82–1.75) | 1.11 (.52–2.37) | 1.19 (.53–2.68) |
| Sexually active with ≥1 high-riskd behavior | 125 | 3946 | 3.17 (2.66–3.77) | 2.87 (1.49–5.55) | 2.81 (1.38–5.69) |
| Variablea . | Incident Detections, No. . | Person-Years at Risk, No. . | Incidence Rateb per 100 Person-Years (95% CI) . | Unadjusted HR (95% CI) . | Adjusted HR (95% CI)c . |
|---|---|---|---|---|---|
| Not sexually active with a male partner | 17 | 1632 | 1.04 (.65–1.68) | Reference (1.0) | Reference (1.0) |
| Sexually active with no high-riskd behavior | 27 | 2253 | 1.20 (.82–1.75) | 1.11 (.52–2.37) | 1.19 (.53–2.68) |
| Sexually active with ≥1 high-riskd behavior | 125 | 3946 | 3.17 (2.66–3.77) | 2.87 (1.49–5.55) | 2.81 (1.38–5.69) |
Abbreviation: CI, confidence interval.
a Composite variable for sexual risk behaviors in past 6 mo.
b Average type-specific incidence rate.
c Adjusted for current hormonal contraceptive use and marital status.
d High-risk behaviors were defined as behaviors that were statistically significantly associated with incident high-risk HPV detection in women reporting ≥1 male partner in the past 6 months, as follows: reports of new male partners, reports of casual male partners, reports of partners with ≥1 concurrent partnership, and reports of ≥2 male partners (Table 4).
Results for all analyses were similar when excluding women with sample gaps of >8 months, although magnitudes of association for recent sexual behavior variables were generally stronger (data not shown).
DISCUSSION
By 1 year of follow-up, one quarter of the women in our high-risk cohort tested positive for a new HR-HPV type that was not detected at enrollment. While differences in assays (including HPV types targeted), sampling sites and intervals, and study populations complicate cross-study comparisons, the incidence of HR-HPV detection (29.5 cases per 100 women-years) was considerably higher than in previous mid-adult cohorts that did not target women with recent new sex partners (ranging from 5 to 15 per 100 women-years) [3, 14–18]. Similarly, the incidence of detection of vaccine types HPV-16 or HPV-18 was significantly higher (6.5 cases per 100 women-years) than in a lower-risk cohort of 3730 women aged 24–45 year years (median lifetime number of sex partners, 2) enrolled in the placebo arm of an international quadrivalent HPV vaccine trial (2.6 case per 100 women-years) [19].
While half of the women in our high-risk cohort reported new or multiple male partners within 6 months of enrollment, 15% reported no recent partners. Therefore, we were able to compare predictors of newly detected HR-HPV infections among women who were and those who were not recently sexually active. Lifetime number of male sex partners was strongly positively associated with incident detection but only among women without recent partners. In these women, incident HR-HPV detection likely represented redetection of prior infection. Viral levels in persistent HPV infections can fluctuate below assay detection thresholds, contributing to intermittent detection [20]. Furthermore, there is biologic evidence that HPV can enter a latent state in the basal cells of the cervical epithelium, with potential for reactivation [21, 22]. Age-related immune suppression may contribute to reactivation of previously acquired infections [23].
Among sexually active women, adjustment for recent high-risk sexual behavior attenuated the association between lifetime number of partners and incident HR-HPV detection. Recent sexual risk behaviors positively associated with incident HR-HPV detection included reports of new or casual male partners, nonmonogamous partners, and multiple partners. When these behaviors were combined into a composite variable, we observed a dose-response relationship, whereby compared with woman sexually active with 1 male partner who was not new, casual, or thought to be nonmonogamous, the likelihood of HR-HPV positivity increased with 1 and ≥2 high-risk characteristics, respectively. Previous studies showed that both lifetime [16, 19, 23] and recent [3, 15, 16, 19, 23] male sex partners were positively associated with incident HPV detection in mid-adult women, but some considered only 2 HPV assessment points separated by 2–7 years [23, 24] or defined “recent” as partners acquired at any point during follow-up [3, 23, 25]. Among studies including both lifetime and recent male partners in multivariate analysis, most [16, 23, 24] but not all [25] showed that associations with lifetime number of partners remained after adjustment for recent new partners, but none specifically evaluated these risk factors in a cohort restricted to currently sexually active women. To our knowledge, only 1 previous study of mid-adult women considered characteristics of male partners or partnerships as risk factors for incident HPV infection; consistent with our findings, Gonzalez et al [23] reported that, among 45–75-year-old women in Costa Rica, those reporting a male partner with other concurrent partnerships were more likely to test newly positive for HPV 5–6 years after an HPV-negative baseline visit than women reporting a male partner thought to be monogamous.
We previously reported a 1.4-fold increased likelihood of prevalent HR-HPV associated with meeting recent male sex partners via the Internet [8]. In the present analysis in the same cohort, the univariate magnitude of association between reports of recent online partners and incident HR-HPV detection was similar (a 1.5-fold increase) but not statistically significant and not as strong as associations observed for other recent sexual behavior characteristics. Therefore, our results do not support a unique increased risk of HPV acquisition in mid-adult women associated with meeting partners online.
By creating 3 sexual behavior risk categories, we were able to estimate the risk of incident HR-HPV detection attributable to recent sexual activity. Our data suggest that among mid-adult women with recent new, multiple, or risky male partners (eg, casual partners or nonmonogamous partners), about two thirds of newly detected HR-HPV infections are attributable to one of these recent partners, whereas about one third are attributable to redetection of prior infection. In contrast, the risk of incident HR-HPV detection in women with 1 nonnew, non–high-risk partner was not significantly elevated, compared with the risk among sexually inactive women, suggesting that unmeasured male partner behavior is unlikely to explain a significant fraction of newly detected HR-HPV in mid-adult women. Rositch et al [16] performed a similar analysis among a low-risk cohort of 35–60-year-old women in Baltimore, Maryland. Similar to our study, compared with women not recently sexually active, the risk of incident HPV detection was elevated in women with recent new partners but not in women with nonnew partners. Despite a strong (5.6-fold) relative risk of incident HPV detection associated with new partners, only 10% of women in the Baltimore cohort reported a new partner during follow-up. Therefore, only 27% of incident infections in the cohort could be attributed to new partners. Interestingly, however, among those with new partners, the risk of incident HPV detection attributable to one of those partners was higher than the risk attributable to new, multiple, or high-risk partners in our study (82% vs 64%). Given likely correlations between past and recent risky sexual behavior [8], the highest-risk women in our study were more likely to have been previously infected with HR-HPV (and at higher risk for redetection of prior infection) than the highest risk women in the Baltimore cohort. Therefore, it is not surprising that the risk attributable to recent high-risk behavior was lower in our study.
The likelihood of risky behaviors to correlate over time also makes it difficult to disentangle risk factors for incident versus prevalent HR-HPV infections. Thus, targeted vaccination of mid-adult women at high-risk for new acquisition but low-risk of previous exposure to vaccine-type HPV is challenging [1, 4] (although with the introduction of the nonavalent HPV vaccine [13], the odds of having been exposed to all vaccine types is significantly reduced). In our cohort, although the individual sexual behaviors associated with prevalent [8] versus incident HR-HPV infection differed somewhat, we observed similar dose-response relationships between increasing level of risky recent sexual behavior and both prevalent and incident HR-HPV infection.
Most studies reported a declining incidence of HPV infection with age [3, 17–19, 25] (with some reporting a secondary smaller peak in older women [3, 25]). In our study, incidence was similar in 25–34 and 35–44 year old women and did not decline appreciably until age 55–65 years. (With <5% of our cohort ≥55 years, however, we were unable to detect a statistically significant decline.) Whereas lifetime numbers of male sex partners increased with increasing age, women aged 55–65 years were less likely than younger women to report recent new or multiple partners (data not shown), offering a plausible explanation for the observed age-related HR-HPV incidence patterns.
Current hormonal contraceptive use was positively associated with incident HR-HPV detection. The magnitude of the association was strongest in women who were not recently sexually active. The relationship between hormonal contraceptive use and HPV infection has been inconsistent [26–35]. Residual confounding by past or recent unmeasured sexual behavior is a possible explanation for our findings. Biologic mechanisms for a role of estrogen and/or progesterone in enhancing the likelihood of new acquisition and/or persistent HPV infection (eg, via increased oncogene expression [36, 37] or decreased host immune response [34, 38]) have also been proposed. Evidence to date indicates that hormonal contraceptives are more likely to affect HPV persistence than new acquisition [34], suggesting that any biologic explanation for our findings is more likely due to an effect on increased likelihood of maintaining low-level, intermittently detected persistent infection than to an effect on increased likelihood of new acquisition.
Study limitations should be noted. First, we could not distinguish new HR-HPV acquisition from redetection of prior infection. While HPV serology measurements may provide additional information on the likelihood of new versus redetected infection, serology is an imperfect measure of past HPV infection [39–41]. Second, in analyses restricted to women without recent partners, the small number of incident HR-HPV detections may have limited our ability to detect statistical significance for modest associations. Third, intervals between sample collections varied, and given the 2-phase design, 18% of participants had a gap of >8 months between 2 samples. Sensitivity analyses excluding these women yielded similar results for all analyses, although magnitudes of association for recent sexual behavior variables were generally stronger. Some incident infections detected after an extended visit gap could have occurred prior to the 6-month sexual behavior assessment period, thus attenuating the associations observed in the full cohort. Fourth, the study population was a convenience sample and likely not representative of all mid-adult women seeking new sex partners. Finally, our results may not generalize to lower-risk cohorts of mid-adult women with fewer partners. For example, the median lifetime number of partners reported by 25–44-year-old women (10 partners) was considerably higher than among similarly aged women in the population-based 2006–2008 US National Survey of Family Growth (3.6 partners) [42].
In conclusion, our results indicate that mid-adult women are at risk for newly acquired HR-HPV infections and that the risk is positively associated with recent high-risk sexual behavior. At the same time, a significant portion of newly detected HR-HPV infections in mid-adulthood are likely due to redetection of prior infection (even in women with new exposures). Our results suggest that, while prophylactic HPV vaccination in mid-adulthood is likely to provide some protection against new HR-HPV acquisition in high-risk women, the benefit may be limited. Our results further suggest that, in women without recent sexual risk behavior, most newly detected HR-HPV is due to redetection of prior infection. These results may be reassuring to women in monogamous sexual partnerships who test positive for HR-HPV during routine cervical cancer screening and useful for clinicians who counsel women with positive results. Future studies should seek to quantify the risks of cervical precancer or cancer associated with HR-HPV infections that are newly acquired in mid-adulthood versus those that represent reactivation or intermittent persistent detection.
Notes
Financialsupport. This work was supported by the Developmental Awards Program of the National Institute of Allergy and Infectious Diseases Sexually Transmitted Infections and Topical Microbicide Cooperative Research Centers (grant AI 31448 to the University of Washington) and by a National Institute of Allergy and Infectious Diseases K01 award (grant AI 079270 to R. L. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 27th International Papillomavirus Conference, Berlin, Germany, 17–22 September 2011.




