-
PDF
- Split View
-
Views
-
Cite
Cite
Saurabh Bhattacharya, Oindrilla Mukherjee, Asish K. Mukhopadhyay, Rukhsana Chowdhury, A Conserved Helicobacter pylori Gene, HP0102, Is Induced Upon Contact With Gastric Cells and Has Multiple Roles in Pathogenicity, The Journal of Infectious Diseases, Volume 214, Issue 2, 15 July 2016, Pages 196–204, https://doi.org/10.1093/infdis/jiw139
Close - Share Icon Share
Abstract
Contact with host cells is recognized as a signal capable of triggering expression of bacterial genes important for host pathogen interaction. Adherence of Helicobacter pylori to the gastric epithelial cell line AGS strongly upregulated expression of a gene, HP0102, in the adhered bacteria in all strains examined, including several Indian clinical isolates. The gene is highly conserved and ubiquitously present in all 69 sequenced H. pylori genomes at the same genomic locus, as well as in 15 Indian clinical isolates. The gene is associated with 2 distinct phenotypes related to pathogenicity. In AGS cell–adhered H. pylori, it has a role in upregulation of cagA expression from a specific σ28-RNAP promoter and consequent induction of the hummingbird phenotype in the infected AGS cells. Furthermore, HP0102 has a role in chemotaxis and a ΔHP0102 mutant exhibited low acid-escape response that might account for the poor colonization efficiency of the mutant.
Helicobacter pylori, a gram-negative, microaerophilic bacterium, colonizes the gastric mucosa of at least half the world's population [1]. Most H. pylori–infected individuals have chronic-active superficial gastritis throughout their lives, and a significant fraction progresses to peptic ulcer, mucosa-associated lymphoid tissue lymphoma, or even gastric cancer [2–4]. The clinical outcomes of H. pylori infection may be attributed to 2 major factors, the cytotoxin-associated gene CagA and vacuolating cytotoxin VacA, and to a host of accessory virulence determinants [5]. CagA is translocated to the host cell cytoplasm through a type IV secretion system and is phosphorylated by the Src family of tyrosine kinases [5]. CagA can interfere with cell signaling pathways, resulting in altered spreading, migration, and adhesion of epithelial cells, commonly known as the hummingbird phenotype [5]. VacA is a secretory protein that exerts multiple effects on host cells and can induce apoptosis [5].
Many bacterial pathogens have evolved to recognize environmental factors characteristic of the host physiological site of infection as signals for correct spatiotemporal expression of pathogenicity elements [6–10]. Consistent with this common theme, acidic pH and salt concentration have been reported to induce expression of the major virulence gene, cagA, in certain H. pylori strains [11–14]. In addition to environmental parameters, contact with eukaryotic cells is also recognized as a stimulus that modulates virulence gene expression in a number of pathogens [15–23]. The H. pylori virulence genes, cagA and vacA, are strongly induced in bacteria following adherence to epithelial cell lines [22, 23].
Successful infection of a host by a pathogen depends not only on classical virulence factors like toxins but also, very importantly, on efficient colonization of appropriate host niches by the pathogen. Colonization of the stomach by H. pylori is a complex process, which depends on a wide range of bacterial and host factors. Motility of H. pylori is considered to be necessary for colonization of the stomach and enhances pathological outcomes in humans [24]. Bacteria generate propulsion by flagellar rotation but also need to modulate the direction of flagellar rotation to control their trajectory [25, 26]. The chemotaxis system couples environmental sensing to the control of flagellar rotation to enable bacteria to move toward chemoattractants or away from chemorepellents [27]. H. pylori uses chemotaxis to escape the acidic conditions of the gastric lumen and colonize the gastric epithelium. In this context, chemotaxis is considered an essential colonization factor for H. pylori, and chemotaxis mutants have been shown to be colonization defective [28, 29]. The chemotaxis signaling system in H. pylori is similar in many ways to the chemotaxis signaling system of other bacteria [30, 31]. However, in recent studies new members of chemotaxis regulators have been identified that are unique to the family of Epsilonproteobacteria, suggesting that this group of bacteria might have evolved additional regulators for controlling chemotaxis [32].
A study using a genome-saturating library of H. pylori transposon mutants in a mouse infection model identified 223 genes that encode potential colonization factors [28]. One of these genes, HP0102, encodes a predicted glycosyltransferase and was reported to be important for colonization. In this study, we demonstrate that expression of the HP0102 gene is strongly induced following adherence to AGS cells and is necessary for upregulation of cagA expression following adherence to AGS cells. We also demonstrate that HP0102 is necessary for normal chemotaxis and acid-escape response of H. pylori.
MATERIALS AND METHODS
All experiments were performed following standard procedures and are described in the Supplementary Data.
RESULTS
Expression of the HP0102 Gene Is Upregulated in H. pylori Following Adherence to AGS Cells
HP0102 Sequence Conservation Analysis in Helicobacter pylori Indian Clinical Isolates
| H. pylori Strain . | Isolated From Patients With . | cag PAI . | HP0102 Sequence Conservation,a % . | HP0102 Gene Sequence Accession No. . | |
|---|---|---|---|---|---|
| DU . | NUD . | ||||
| I-121 | Yes | No | Present | 96 | KT932667 |
| 135 (1) | Yes | No | Present | 96 | KT932666 |
| I-30 | Yes | No | Present | 96 | KT884537 |
| I-90 | Yes | No | Present | 96 | KT884534 |
| I-230 | Yes | No | Present | 96 | KT884538 |
| San52 | No | Yes | Present | 96 | KT884542 |
| San77 | No | Yes | Present | 96 | KT884535 |
| San10 | No | Yes | Present | 95 | KT884539 |
| San64 | No | Yes | Present | 95 | KT884540 |
| 162 | Yes | No | Present | 95 | KT884533 |
| Osc51 | No | Yes | Present | 95 | KT884545 |
| I-10 | Yes | No | Present | 94 | KT884541 |
| Osc30 | No | Yes | Present | 94 | KT884543 |
| 152 1(A) | Yes | No | Present | 94 | KT884536 |
| 155 1(A) | Yes | No | Present | 94 | KT884544 |
| H. pylori Strain . | Isolated From Patients With . | cag PAI . | HP0102 Sequence Conservation,a % . | HP0102 Gene Sequence Accession No. . | |
|---|---|---|---|---|---|
| DU . | NUD . | ||||
| I-121 | Yes | No | Present | 96 | KT932667 |
| 135 (1) | Yes | No | Present | 96 | KT932666 |
| I-30 | Yes | No | Present | 96 | KT884537 |
| I-90 | Yes | No | Present | 96 | KT884534 |
| I-230 | Yes | No | Present | 96 | KT884538 |
| San52 | No | Yes | Present | 96 | KT884542 |
| San77 | No | Yes | Present | 96 | KT884535 |
| San10 | No | Yes | Present | 95 | KT884539 |
| San64 | No | Yes | Present | 95 | KT884540 |
| 162 | Yes | No | Present | 95 | KT884533 |
| Osc51 | No | Yes | Present | 95 | KT884545 |
| I-10 | Yes | No | Present | 94 | KT884541 |
| Osc30 | No | Yes | Present | 94 | KT884543 |
| 152 1(A) | Yes | No | Present | 94 | KT884536 |
| 155 1(A) | Yes | No | Present | 94 | KT884544 |
Abbreviations: DU, duodenal ulcer; NUD, nonulcer dyspepsia; PAI, pathogenicity island.
a Sequence conservation analysis was done considering the HP0102 gene sequence of H. pylori strain 26695 as a query.
HP0102 Sequence Conservation Analysis in Helicobacter pylori Indian Clinical Isolates
| H. pylori Strain . | Isolated From Patients With . | cag PAI . | HP0102 Sequence Conservation,a % . | HP0102 Gene Sequence Accession No. . | |
|---|---|---|---|---|---|
| DU . | NUD . | ||||
| I-121 | Yes | No | Present | 96 | KT932667 |
| 135 (1) | Yes | No | Present | 96 | KT932666 |
| I-30 | Yes | No | Present | 96 | KT884537 |
| I-90 | Yes | No | Present | 96 | KT884534 |
| I-230 | Yes | No | Present | 96 | KT884538 |
| San52 | No | Yes | Present | 96 | KT884542 |
| San77 | No | Yes | Present | 96 | KT884535 |
| San10 | No | Yes | Present | 95 | KT884539 |
| San64 | No | Yes | Present | 95 | KT884540 |
| 162 | Yes | No | Present | 95 | KT884533 |
| Osc51 | No | Yes | Present | 95 | KT884545 |
| I-10 | Yes | No | Present | 94 | KT884541 |
| Osc30 | No | Yes | Present | 94 | KT884543 |
| 152 1(A) | Yes | No | Present | 94 | KT884536 |
| 155 1(A) | Yes | No | Present | 94 | KT884544 |
| H. pylori Strain . | Isolated From Patients With . | cag PAI . | HP0102 Sequence Conservation,a % . | HP0102 Gene Sequence Accession No. . | |
|---|---|---|---|---|---|
| DU . | NUD . | ||||
| I-121 | Yes | No | Present | 96 | KT932667 |
| 135 (1) | Yes | No | Present | 96 | KT932666 |
| I-30 | Yes | No | Present | 96 | KT884537 |
| I-90 | Yes | No | Present | 96 | KT884534 |
| I-230 | Yes | No | Present | 96 | KT884538 |
| San52 | No | Yes | Present | 96 | KT884542 |
| San77 | No | Yes | Present | 96 | KT884535 |
| San10 | No | Yes | Present | 95 | KT884539 |
| San64 | No | Yes | Present | 95 | KT884540 |
| 162 | Yes | No | Present | 95 | KT884533 |
| Osc51 | No | Yes | Present | 95 | KT884545 |
| I-10 | Yes | No | Present | 94 | KT884541 |
| Osc30 | No | Yes | Present | 94 | KT884543 |
| 152 1(A) | Yes | No | Present | 94 | KT884536 |
| 155 1(A) | Yes | No | Present | 94 | KT884544 |
Abbreviations: DU, duodenal ulcer; NUD, nonulcer dyspepsia; PAI, pathogenicity island.
a Sequence conservation analysis was done considering the HP0102 gene sequence of H. pylori strain 26695 as a query.
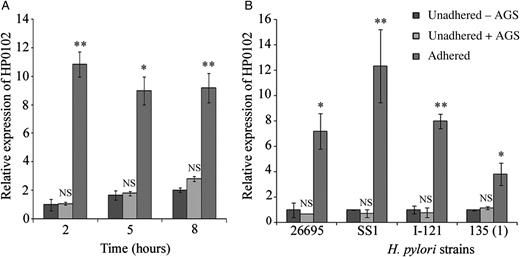
Induction of the HP0102 gene in AGS-adhered Helicobacter pylori strains. A, RNA was extracted from H. pylori strain G27 (A) or different H. pylori strains (B) after adherence to AGS cells (adhered) and from unadhered H. pylori incubated under identical conditions without cell line (unadhered - AGS) or collected from the supernatants of infected AGS cells (unadhered + AGS). HP0102 expression was estimated by quantitative reverse transcription polymerase chain reaction analysis. RNA was extracted 2, 5, and 8 hours after adherence of strain G27 to AGS cells and 2 hours after adherence of the other strains. Relative expression levels were calculated as fold induction of gene expression in adhered bacteria, considering the expression in unadhered bacteria grown without AGS at 2 hours arbitrarily as 1. Bar diagram represents the mean value ± SD of at least 3 experiments. The statistical significance of differences (P values) in expression of the HP0102 gene between the unadhered (−AGS) and adhered H. pylori cells were obtained by the Student t test and are indicated. No statistical significance was obtained in differences of HP0102 expression between unadhered (−AGS) bacteria and unadhered (+AGS) bacteria. *P ≤ .05 and **P ≤ .01. Abbreviation: NS, not significant.
Parenthetically, no difference in HP0102 expression was observed between unadhered bacteria grown without cell line and those isolated from the supernatant of infected AGS monolayers (Figure 1), suggesting that HP0102 induction required direct contact of the bacteria with the AGS cells and was not due to a component secreted by the cell line.
Prevalence and Conservation of HP0102 Gene in H. pylori Strains
To detect the prevalence of the HP0102 gene in H. pylori strains, the gene sequence of strain 26695 was used as a query to search for homologous sequences in H. pylori genomes (available at: http://blast.ncbi.nlm.nih.gov/Blast.cgi). The gene was present in all 69 H. pylori strains whose genome sequences were available, and a high degree of sequence conservation of the gene (>93%) was observed among the different strains (Supplementary Data). Fifteen additional H. pylori clinical strains isolated from India (Table 1) were also examined for the presence of the HP0102 gene by polymerase chain reaction (PCR) analysis, using primers flanking the gene. Sequencing of the PCR products revealed a high degree of conservation of the gene sequence (Table 1). These results indicated that the HP0102 gene is almost ubiquitously present and highly conserved in H. pylori strains. Furthermore, an HP0102 homolog was present in at least 11 different Helicobacter species examined (Supplementary Data). To investigate the function of HP0102, a H. pylori G27ΔHP0102 strain was constructed and analyzed. Expression of the genes HP0103 and HP0101, located upstream and downstream, respectively, of HP0102, was comparable between G27ΔHP0102 and the parental G27 strain (Supplementary Data), indicating that the mutation in HP0102 did not affect expression of the neighboring genes.
Role of HP0102 in Induction of the Hummingbird Phenotype in AGS Cells
H. pylori G27 wild-type (WT) and G27ΔHP0102 strains were added to AGS monolayers at different multiplicities of infection (MOIs), and the number of adhered bacteria was enumerated at different times after adherence (Supplementary Data). With increasing MOIs, the number of adhered bacteria increased progressively. No significant difference in the number of AGS-adhered bacteria was observed between G27WT and ΔHP0102 strains at any MOI during the 24-hour period of examination (Supplementary Data), indicating that HP0102 might have no role in the adherence of H. pylori to gastric cells. However, a remarkable difference in morphology was observed between AGS cells infected with the WT strain and those infected with the ΔHP0102 strain.
![Morphology of AGS cells following adherence of Helicobacter pylori strains. AGS monolayers (uninfected) were incubated with H. pylori strains G27 (wild type [WT]), ΔHP0102, and ΔHP0102 complemented with the HP0102 gene (ΔHP0102/HP0102) at different multiplicities of infection (MOIs) for 5 hours, washed to remove unadhered bacteria, and observed on an inverted microscope in phase contrast at 20× original magnification. The number of elongated cells were counted and expressed as the percentage of the total cells in each sample. Bar diagram represents the mean value ± SD of 3 experiments. The statistical significance of the differences (P values) in number of elongated cells between WT and the ΔHP0102 mutant or complemented strain (ΔHP0102/HP0102) is indicated. *P ≤ .05 and **P ≤ .01. Abbreviation: NS, not significant.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/214/2/10.1093_infdis_jiw139/2/m_jiw13902.jpeg?Expires=1749978313&Signature=ivDBJlbq9ZMC6lWIB00-E3~zUQ1KRbkAU7SVM7ME5~OMVBHHeaTC-6pnzq-Ai5HVRwQPicCds0jq3A6yUR0rAnLjXheiki54p7~QZ--EkZdbUWOg7K5PSdmQZH7tdVK6wMUOpwXZEl5EVO6MnyO5Ot1J6AYyb1R8RCD1-Ur4KpqHwKL76E-OtRHzy~mXuj8aasdNULEC5-2f~zBVvnm0l~sOZCad-aC2BY-AlKDsNV1vaRuuY5E2PcB9B-xgbKR8A34o0Mcc8NpHq1IbUtCfkoAE4c~7i8aFmplzUq7qznIEJ2e~~Xt1ZMI~tblxUenNniCABTLmwM96sHhLV4Fovw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Morphology of AGS cells following adherence of Helicobacter pylori strains. AGS monolayers (uninfected) were incubated with H. pylori strains G27 (wild type [WT]), ΔHP0102, and ΔHP0102 complemented with the HP0102 gene (ΔHP0102/HP0102) at different multiplicities of infection (MOIs) for 5 hours, washed to remove unadhered bacteria, and observed on an inverted microscope in phase contrast at 20× original magnification. The number of elongated cells were counted and expressed as the percentage of the total cells in each sample. Bar diagram represents the mean value ± SD of 3 experiments. The statistical significance of the differences (P values) in number of elongated cells between WT and the ΔHP0102 mutant or complemented strain (ΔHP0102/HP0102) is indicated. *P ≤ .05 and **P ≤ .01. Abbreviation: NS, not significant.
One of the consequences of H. pylori infection is induction of the proinflammatory signaling cascades that ultimately lead to host cell secretion of interleukin 8 (IL-8) [5]. Consistent with the decreased scattering and elongation induced in AGS cells by the G27ΔHP0102 strain, the amount of IL-8 detected in culture supernatants of the G27ΔHP0102-infected AGS cells was lower than that in supernatants of AGS cells infected by the G27WT bacteria (Supplementary Data). Complementation of the G27ΔHP0102 strain with the full-length HP0102 gene restored IL-8 secretion to WT levels (Supplementary Data). Of note, there was a significant difference in IL-8 production between uninfected and infected AGS cell cultures (Supplementary Data). Since the hummingbird phenotype and IL-8 secretion are associated with production and subsequent translocation of CagA from adhered H. pylori to the gastric cells, cagA expression and translocation was next examined in AGS-adhered G27WT and G27ΔHP0102 cells.
Role of HP0102 in cagA Induction in AGS-Adhered H. pylori
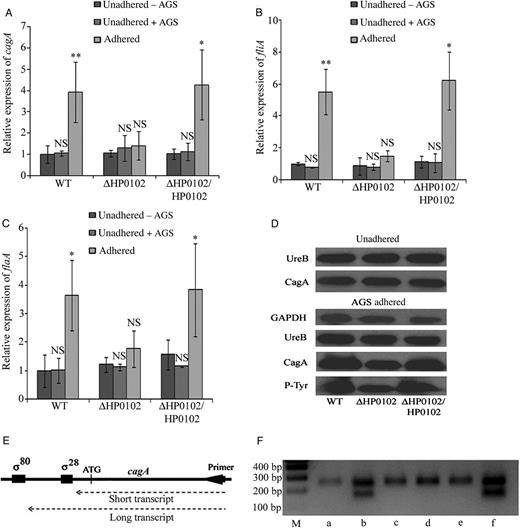
Gene expression and Western blot analysis in AGS-adhered and unadhered Helicobacter pylori strains. Expression of cagA (A), fliA (B), and flaA (C) was estimated in unadhered and AGS-adhered G27 wild-type (WT), ΔHP0102 mutant, and ΔHP0102/HP0102 complemented strains by quantitative reverse transcription polymerase chain reaction (PCR) analysis. Transcript levels are expressed relative to that in the unadhered WT strain, arbitrarily taken as 1. Results represent the average of at least 3 independent experiments. Error bars represent SD. The statistical significance (P values) of the differences in gene expression between the unadhered (−AGS) and adhered H. pylori cells were obtained by the Student t test and are indicated. No statistical significance was obtained in differences in gene expression between unadhered (−AGS) bacteria and unadhered (+AGS) bacteria. D, Western blot analysis of CagA in unadhered WT and H. pylori–infected AGS cultures. UreB was used as an internal control. Phosphorylated CagA was estimated in H. pylori–infected AGS cultures, using P-Tyr antibody, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. E, Schematic depicting transcripts initiating from the σ80 and σ28 promoters of cagA, using the rapid amplification of complementary DNA ends (RACE) primers. F, 5′ RACE PCR analysis of cagA transcripts in unadhered WT (a), AGS-adhered WT (b), unadhered ΔHP0102 mutant (c), AGS-adhered ΔHP0102 mutant (d), unadhered ΔHP0102/HP0102 strain (e), and AGS-adhered ΔHP0102/HP0102 strain (f). *P ≤ .05 and **P ≤ .01. Abbreviations: M, molecular size markers; NS, not significant.
Western blot analysis indicated that the level of CagA was higher in the G27WT strain than in the G27ΔHP0102 strain following adherence to AGS cells (Figure 3D). The relative difference in CagA levels between AGS-adhered WT and ΔHP0102 was reflected in the levels of phosphorylated CagA in infected AGS cells, indicating that the activity of the type IV secretion system responsible for translocating CagA remains unaffected in the ΔHP0102 mutant (Figure 3D). Results obtained with the complemented ΔHP0102/HP0102 strain were similar to those for the WT strain (Figure 3D).
HP0102 Influences cagA Expression by Regulating Expression of fliA
We have previously demonstrated that a σ28-RNAP–dependent promoter located downstream of the annotated σ80-RNAP promoter of cagA is activated following adherence of H. pylori to AGS cells and that cagA transcripts are initiated from both promoters in AGS-adhered H. pylori [23]. To examine whether HP0102 has a role in activation of the σ28-RNAP–dependent promoter of cagA, expression of fliA, encoding σ28, was examined in the WT, ΔHP0102, and ΔHP0102/HP0102 strains. fliA expression increased about 5.5-fold upon adherence of the G27WT strain to AGS cells, but no upregulation was observed in the AGS-adhered ΔHP0102 strain (Figure 3B). fliA expression in unadhered G27WT and ΔHP0102 strains was comparable, indicating that HP0102 might have a role in upregulation of fliA expression, specifically in AGS-adhered H. pylori. The complemented strain behaved essentially like the WT strain.
To examine the status of functional σ28 in the WT strain and the ΔHP0102 mutant, expression of flaA, known to be dependent on σ28 [33, 34], was compared between the 2 strains. flaA expression increased about 4-fold following adherence of the WT strain and the ΔHP0102/HP0102 strain to AGS cells. However, no upregulation was observed in the AGS-adhered ΔHP0102 strains (Figure 3C). Thus, functional σ28 increased following adherence to AGS cells, and the induction was dependent on HP0102.
Because we previously identified a σ28-RNAP–dependent promoter in addition to the σ80-RNAP–dependent promoter of cagA expression [23], we next used 5′ rapid amplification of complementary DNA ends (RACE) PCR to determine which promoter was active in unadhered and AGS-adhered G27WT and ΔHP0102 strains. A single transcript likely to have originated from the σ80 promoter was detected in unadhered WT and ΔHP0102 strains. A shorter transcript whose size suggested that it might have originated from the σ28 promoter was detected in the AGS-adhered WT and ΔHP0102/HP0102 strains but not in the AGS-adhered ΔHP0102 strain (Figure 3F).
To determine whether σ28 also has a role in upregulation of HP0102 following adherence to AGS cells, HP0102 expression was examined in unadhered and AGS-adhered H. pylori ΔfliA [23]. No difference in HP0102 expression was observed between the WT and ΔfliA strains (Supplementary Data), indicating that σ28 has no role in upregulation of HP0102 in AGS-adhered H. pylori.
Role of HP0102 in Motility and Chemotaxis
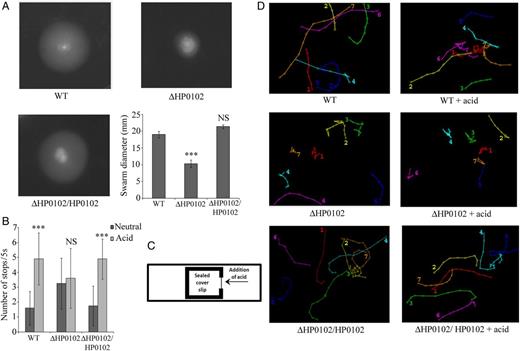
Motility and chemotaxis. A, Swarm plate assay of Helicobacter pylori G27 wild-type (WT), ΔHP0102 mutant, and ΔHP0102/HP0102 complemented strains. Bar diagram represents the average ± standard deviation (SD) swarm diameter of 3 experiments. The statistical significance of the differences (P values) in swarm diameter between the WT and ΔHP0102 mutant or ΔHP0102/HP0102 complemented strains are indicated. B, Swimming of G27WT, ΔHP0102, and ΔHP0102/HP0102 strains in liquid medium before (neutral) and after addition of acid was observed by video microscopy, and the number of stops of individual bacteria (n = 20) during a 5-second period was counted. The bar diagram represents the mean value ± SD of 3 experiments. The statistical significance of the differences (P values) in the number of stops before and after addition of acid in the different strains is indicated. C, Illustration of a wet mount chemotaxis assay (Supplementary Data). D, Analysis of the swimming trajectories of WT, ΔHP0102, and ΔHP0102/HP0102 strains. Using MTrackJ software, the 2D trajectories of individual bacterial cell were determined. Each trajectory is represented by a number, and the position of the number represents the final point of the trajectory. See Supplementary Data and Supplementary Data for H. pylori G27WT before and after addition of acid, Supplementary Data and Supplementary Data for the ΔHP0102 mutant before and after addition of acid, and Supplementary Data and Supplementary Data for the ΔHP0102/HP0102 complemented strain before and after addition of acid, respectively. ***P ≤ .001. Abbreviation: NS, not significant.
Chemotaxis Per Se Does Not Affect cagA Expression Following Adherence to AGS Cells
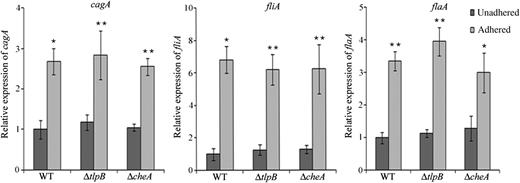
Induction of cagA, fliA, and flaA in chemotaxis mutants of Helicobacter pylori. Expression of cagA, fliA, and flaA in unadhered and AGS-adhered wild-type (WT), ΔtlpB, and ΔcheA strains was examined by quantitative reverse transcription polymerase chain reaction analysis. Transcript levels are expressed relative to that in the unadhered WT strain, arbitrarily taken as 1. The bar diagram represents the mean value ± standard deviation (SD) of at least 3 experiments. Error bars represent SDs. The statistical significance (P values) of the differences in gene expression between AGS-adhered and unadhered H. pylori strains are indicated. *P ≤ .05 and **P ≤ .01.
Role of HP0102 in H. pylori Strain 26695
The role of HP0102 in regulating cagA expression in AGS-adhered H. pylori was examined in another strain, 26695. Similar to H. pylori strain G27, expression of HP0102 was upregulated >7-fold in strain 26695 following adherence to AGS cells (Figure 1B). Adherence of 26695ΔHP0102 to AGS cells resulted in much less cell scattering and reduced IL-8 production as compared to the 26695WT strain (Supplementary Data). Furthermore, similar to H. pylori G27, expression of cagA, fliA, and flaA were upregulated in AGS-adhered H. pylori 26695 WT but not in the ΔHP0102 mutant (Supplementary Data). 5’ RACE analysis was performed to elucidate the transcription start site of cagA, and the results were similar to that obtained for strain G27 (Supplementary Data). Since strain 26695 is nonmotile because of a mutation in fliP [37], the role of HP0102 in chemotaxis could not be examined in this strain.
Expression of HP0102 and cagA Is Upregulated in H. pylori Following Adherence to Primary Gastric Cell Culture
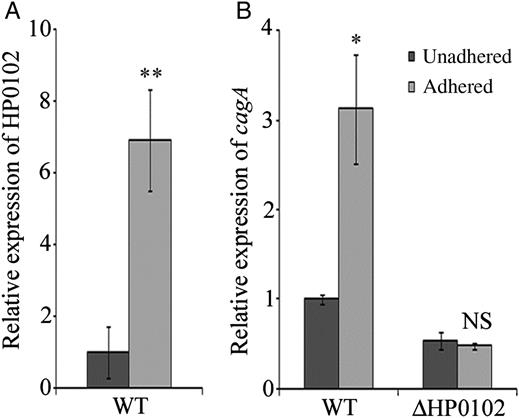
Induction of HP0102 and cagA upon infection of primary gastric cells. A, RNA was extracted from Helicobacter pylori G27 2 hours after adherence to primary gastric cells (adhered) or from H. pylori incubated under identical conditions without cell line (unadhered), and HP0102 expression was estimated by quantitative reverse transcription polymerase chain reaction analysis. B, Expression of cagA was estimated in AGS-adhered or unadhered H. pylori G27 wild type (WT) and ΔHP0102. Relative expression levels were calculated as fold induction of gene expression in adhered bacteria, considering the expression in unadhered bacteria arbitrarily as 1. Bar diagram represents the means ± SD of at least 3 experiments. The statistical significance of differences (P values) in gene expression between the unadhered and adhered H. pylori cells was obtained by the Student t test and are indicated. *P ≤ .05 and **P ≤ .01. Abbreviation: NS, not significant.
DISCUSSION
The ubiquitous presence and high degree of conservation of the HP0102 gene in all 84 H. pylori strains, including 15 Indian isolates, examined, as well as upregulation of expression of the gene following adherence to gastric cells suggested that the gene might have important functions. Indeed, it has been demonstrated in this study that HP0102 has a role in 2 different processes: (1) induction of the hummingbird phenotype in AGS cells following H. pylori adherence and (2) chemotaxis and acid-escape response.
One of the most important virulence factors of H. pylori is CagA that is translocated from adhered bacteria to the gastric cells and produces a characteristic scattering and elongation [5]. We have earlier demonstrated that contact with host cells rapidly upregulated expression of cagA in AGS-adhered H. pylori strains [22, 23]. In our endeavor to identify factors necessary for host cell contact–dependent cagA upregulation, we have reported that cagA expression was specifically upregulated in AGS-adhered H. pylori from a σ28-RNAP–dependent alternative promoter of cagA [23].
Interestingly, cagA transcription start site identification by 5’ RACE indicated that HP0102 has a role in activation of the σ28-RNAP–dependent promoter following H. pylori adherence to AGS cells, since transcripts originating from the σ28 promoter of cagA were detected in the AGS-adhered WT strain but not in the ΔHP0102 mutant strain. This may be due to higher σ28 levels in the adhered WT strains as compared to the ΔHP0102 mutant, as suggested by the observation that expression of the σ28-dependent flaA was significantly higher in the AGS-adhered WT strain than in the ΔHP0102 mutant. Thus, HP0102 has a role in increasing the concentration of functional σ28 in AGS-adhered H. pylori. We have recently reported that another factor, the flagellar hook length control protein FliK exerts a similar effect [23]. Similar to HP0102, H. pylori fliK expression was upregulated following adherence to AGS cells, and a ΔfliK mutant, defective in hook basal body assembly, had lower levels of free σ28 than the WT strain following adherence to AGS cells; consequently, expression from the σ28-dependent promoter of cagA was not observed in the AGS-adhered ΔfliK mutant [23]. Thus, in both the ΔfliK and ΔHP0102 mutants, after adherence to AGS cells, levels of active σ28 was lower than in the WT strain; consequently, induction of σ28-dependent transcription of cagA following adherence to AGS cells was not observed. Expression of fliK was, however, not affected in the ΔHP0102 mutant, and the induction of fliK reported in the WT strain following AGS adherence was also observed in the ΔHP0102 mutant (data not shown). Thus, we have now identified 2 factors, FliK and HP0102, that have nonoverlapping roles in cagA expression and are required for upregulation of cagA, specifically in host cell–associated H. pylori. Further studies of ΔfliK and ΔHP0102 infection in mice models are required to establish the role of the gene products in H. pylori pathogenesis.
Recent studies of H. pylori clinical isolates from 2 diverse geographical locations, Colombia [38] and Portugal [39], have suggested a strong association between the presence of a DNA motif (AATAAGATA) upstream of the cagA open reading frame (ORF) and high levels of cagA expression and disease severity. The motif is included within the σ28 RNAP promoter upstream of the cagA ORF [23]. In this context, it might be important to consider that the mere presence of this motif in a H. pylori strain is not sufficient to drive cagA expression to high levels, but it is also important to determine the functional status of the factors FliK and HP0102 to predict whether CagA upregulation would occur following infection with a particular strain. A study to examine the correlation between cagA upregulation, fliK, and HP0102 status in a large number of H. pylori strains isolated from individuals with different clinical symptoms has recently been initiated in our laboratory. In addition, further work is ongoing to determine the correlation between HP0102 and other fundamental processes in H. pylori.
HP0102 has a role in another phenotype important for H. pylori pathogenicity—chemotaxis. ΔHP0102 mutants have normal flagella and are motile, but they exhibit aberrant flagellar rotation. As compared to the WT strain, the ΔHP0102 mutant switches flagellar rotation more frequently even under standard culture conditions. When exposed to acid, the WT strain shows a definite directional movement away from the acid, but the mutant continues to switch flagellar rotation randomly and hence has a poor acid-escape response. H. pylori has 4 chemoreceptors, of which 1, TlpB, is required for chemorepulsive responses to acid [35]. HP0102 is encoded in an operon downstream from tlpB (HP0103). The expression of tlpB was similar between WT and ΔHP0102 strains (Supplementary Data), but whether HP0102 can influence the activity of TlpB either directly or indirectly needs further investigation.
It is intriguing that a glycosyltransferase, HP0102, mediates the diverse effects reported in this study. Glycosyltransferases have been reported to affect virulence, motility, and biofilm formation in pathogens such as Pseudomonas syringae, Xanthomonas citri, and Streptococcus species [40–44], although the molecular basis of these effects is as yet largely unknown. Based on the results reported in this study, we hypothesize that the primary effect of HP0102 is on the upregulation of fliA (σ28) expression. H. pylori has evolved to be able co-opt σ28 to increase cagA expression following adherence to host cells. This integration presumably ensures that 2 essential virulence determinants, motility/chemotaxis and CagA, are coordinately expressed.
Notes
Acknowledgments. We thank all members of the laboratory, for cooperation and helpful discussions; Kalidas Paul, for suggestions and excellent technical support; D. Scott Merrell, Uniformed Services University of Health Sciences (Bethesda, MD), for the generous gift of H. pylori G27 strain; and Dr Apurba K. Pal and Dr Kingshuk K. Dhar, R. G. Kar Medical College and Hospital, for human gastric biopsy materials.
Financial support. The work was supported by the Council of Scientific and Industrial Research–Indian Institute of Chemical Biology (grant number BSC0119) and the Council of Scientific and Industrial Research (research fellowship to S. B.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




