-
PDF
- Split View
-
Views
-
Cite
Cite
Soren Gantt, Jackson Orem, Elizabeth M. Krantz, Rhoda Ashley Morrow, Stacy Selke, Meei-Li Huang, Joshua T. Schiffer, Keith R. Jerome, Annet Nakaganda, Anna Wald, Corey Casper, Lawrence Corey, Prospective Characterization of the Risk Factors for Transmission and Symptoms of Primary Human Herpesvirus Infections Among Ugandan Infants, The Journal of Infectious Diseases, Volume 214, Issue 1, 1 July 2016, Pages 36–44, https://doi.org/10.1093/infdis/jiw076
Close - Share Icon Share
Abstract
Background. Human herpesvirus (HHV) infections are common during infancy. Primary infections are frequently asymptomatic and best studied prospectively by using direct viral detection.
Methods. Oropharyngeal swab specimens were collected weekly from Ugandan newborn infants, their mothers, and other children in the household. Blood specimens were collected every 4 months. Samples were tested for herpes simplex virus (HSV) types 1 and 2, Epstein-Barr virus (EBV), cytomegalovirus (CMV), HHV-6A, HHV-6B, and HHV-8, using quantitative polymerase chain reaction.
Results. Thirty-two infants, 32 mothers, and 49 other household children were followed for a median of 57 weeks. Seventeen mothers had human immunodeficiency virus type 1 (HIV) infection; no infants acquired HIV-1. The 12-month incidence of postnatal infection was 76% for HHV-6B, 59% for CMV, 47% for EBV, 8% for HSV-1, and 0% for HHV-8. The quantity of oropharyngeal shedding by contacts was associated with HHV-6A or HHV-6B transmission. Maternal HIV-1 infection was associated with EBV transmission, while breastfeeding and younger child contacts were associated with CMV transmission. Except for HSV-1, primary HHV infections were subclinical.
Conclusions. By capturing exposures and acquisition events, we found that the incidence and risk factors of infection vary by HHV type. HSV-1 infection, unlike other HHV infections, caused acute clinical illness in these infants.
Virtually every child worldwide becomes infected with several members of the human herpesvirus (HHV) family within a few years of birth [1–4]. These viruses include herpes simplex virus type 1 (HSV-1), HSV-2, varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), HHV-6A, HHV-6B, HHV-7, and HHV-8. HHV infections are lifelong, and in addition to directly causing pathology in large numbers of individuals, they also form an important part of the human virome that may affect subsequent immune responses to infectious pathogens and allergens [5, 6]. With the exception of VZV, which is frequently transmitted by aerosol, infection with HHVs results from direct mucosal inoculation; most HHV infections in children are acquired via the oral route [7].
Children in sub-Saharan Africa and other resource-poor settings acquire multiple HHV infections during a particularly short period [1, 2, 4, 8, 9]. Furthermore, African children and those born to human immunodeficiency virus type 1 (HIV)–infected women may develop sequelae of HHV infections not often seen outside of the region [9–12]. We therefore undertook a comprehensive evaluation of primary infection with multiple HHVs in a cohort of HIV-1–exposed and unexposed African infants.
METHODS
Study Cohort and Data Collection
All study procedures were approved by the relevant human subjects protection committees in Kampala, Uganda; Seattle, Washington; and Vancouver, Canada. Pregnant women were recruited from the prenatal clinics at Mulago Hospital in Kampala from 2008 to 2009. Eligibility required providing informed consent, having a documented HIV-1 status, the presence of at least 1 other preschool-aged (“secondary”) child living in the home, and having a stable residence nearby. HIV-1–infected women were overrepresented to examine the effect of HIV-1 infection on HHV shedding and the risk of primary infection of the child. Seven women were enrolled from a cohort with HHV-8 detected in the oropharynx [13].
As previously described [13], study personnel collected oropharyngeal swab specimens and data from the infants followed from birth and their household contacts weekly. Blood specimens were collected from infants at 6 weeks of age and every 4 months thereafter. A standardized weekly questionnaire captured symptoms and information about potential exposures to virus (Supplementary Methods).
Sample Processing and Viral Testing
Oropharyngeal swab specimens and plasma samples were tested for HSV-1, HSV-2, EBV, CMV, HHV-6A, HHV-6B, and HHV-8, using real-time quantitative polymerase chain reaction (qPCR) as previously described [14–19]. The cutoffs for a positive test for swab specimens were 3 copies/reaction (approximately 150 copies/mL of swab buffer) and 1 copy/reaction (50 copies/mL) for plasma. Typing was attempted for every sample with detectable HSV, and HHV-6 typing was performed for ≥1 sample from each subject with at least 2 positive results. Serologic testing for CMV (immunoglobulin M [IgM] and immunoglobulin G [IgG]) and EBV (IgM and IgG against the viral capsid antigen and IgG against nuclear antigen) was performed using enzyme-linked immunosorbent assay kits (Wampole [Alere], Boucherville, Quebec), and serologic testing for HSV-1 and HSV-2 was performed using type-specific Western blot [20]. Antibodies to HHV-6A, HHV-6B, or HHV-8 were not measured.
Definitions of Primary Infection
Primary infection was defined as the first episode of oral viral shedding and/or viremia following birth, using specific criteria that were developed to best fit the observed qPCR data for each HHV [3] (Supplementary Methods). Serologic testing was used to validate these criteria for HSV-1, HSV-2, CMV, and EBV infections.
Statistical Analyses
The cumulative incidence of primary infection with each virus was calculated using Kaplan–Meier methods. Risk factors for primary infection were assessed by fitting marginal Cox proportional hazards models for each virus simultaneously [21]. Details regarding parameterization of exposures and multivariable modeling appear in the Supplementary Methods. To examine seasonal trends of HHV infections, an exact χ2 goodness-of-fit test was used. To determine whether primary infection with each virus was associated with symptoms, 2 complementary approaches, a nested case-control design and a case-crossover design, were used as detailed in the Supplementary Methods. P values of <.05 were considered statistically significant.
RESULTS
Study Subjects and Samples
One hundred thirteen participants, including 32 women, their 32 newborn infants, and 49 secondary children residing in their households, were enrolled (Table 1). Seventeen mothers and 4 secondary children were HIV-1 infected. CD4+ T-cell counts were available for 9 mothers (53%) at enrollment (median, 441 cells/mm3; range, 385–885 cells/mm3). All mothers received antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV-1, according to World Health Organization recommendations, during the study period. No infant became infected with HIV-1 during the study. Median follow-up was 57 weeks (range, 3–119 weeks), during which 1914 oropharyngeal swab specimens and 141 plasma specimens from primary children were tested for quantification of HHV DNA; the HHV DNA load was also measured in an additional 1507 swab specimens from mothers and 1588 swab specimens from secondary children.
Cohort Characteristics and Behavioral Risk Factors for Primary Human Herpesvirus Infection
| Characteristics of Primary Childrena . | Maternal HIV-1 Status . | ||
|---|---|---|---|
| Negative . | Positive . | Overall . | |
| Primary children | |||
| Evaluated, no. | 15 | 17 | 32 |
| Male sex | 6 (40) | 10 (59) | 16 (50) |
| Oropharyngeal samples collected weekly, no. | 57 (32–75) | 58 (4–117) | 58 (4–117) |
| Secondary children | |||
| Evaluated, no. | 22 | 27 | 49 |
| Age, y | 3.9 (1.6–6.3) | 3.6 (0.3–6.5) | 3.8 (0.3–6.5) |
| HIV-1 infected | 0 (0) | 4 (15) | 4 (8) |
| HIV-1 status unknown | 0 (0) | 7 (26) | 7 (14) |
| No. per family | |||
| 1 | 11 (73) | 10 (59) | 21 (66) |
| 2 | 1 (7) | 4 (24) | 5 (16) |
| 3 | 3 (20) | 3 (18) | 6 (19) |
| Behavioral exposureb | |||
| Breastfeeding during study | |||
| None | 0 (0) | 4 (24) | 4 (12) |
| Weaned during study | 3 (20) | 11 (65) | 14 (44) |
| Breastfed for entire study | 12 (80) | 2 (12) | 14 (44) |
| Breastfeeding duration,c wks | 45 (25–78) | 14 (1–31) | 21 (1–78) |
| Visits with saliva-sharing behavior,d % | 62 (0–100) | 21 (0–87) | 50 (0–100) |
| Characteristics of Primary Childrena . | Maternal HIV-1 Status . | ||
|---|---|---|---|
| Negative . | Positive . | Overall . | |
| Primary children | |||
| Evaluated, no. | 15 | 17 | 32 |
| Male sex | 6 (40) | 10 (59) | 16 (50) |
| Oropharyngeal samples collected weekly, no. | 57 (32–75) | 58 (4–117) | 58 (4–117) |
| Secondary children | |||
| Evaluated, no. | 22 | 27 | 49 |
| Age, y | 3.9 (1.6–6.3) | 3.6 (0.3–6.5) | 3.8 (0.3–6.5) |
| HIV-1 infected | 0 (0) | 4 (15) | 4 (8) |
| HIV-1 status unknown | 0 (0) | 7 (26) | 7 (14) |
| No. per family | |||
| 1 | 11 (73) | 10 (59) | 21 (66) |
| 2 | 1 (7) | 4 (24) | 5 (16) |
| 3 | 3 (20) | 3 (18) | 6 (19) |
| Behavioral exposureb | |||
| Breastfeeding during study | |||
| None | 0 (0) | 4 (24) | 4 (12) |
| Weaned during study | 3 (20) | 11 (65) | 14 (44) |
| Breastfed for entire study | 12 (80) | 2 (12) | 14 (44) |
| Breastfeeding duration,c wks | 45 (25–78) | 14 (1–31) | 21 (1–78) |
| Visits with saliva-sharing behavior,d % | 62 (0–100) | 21 (0–87) | 50 (0–100) |
Data are no. (%) of subjects or median value (range), unless otherwise indicated.
Abbreviation: HIV-1, human immunodeficiency virus type 1.
a No primary children acquired HIV-1 during study follow-up.
b Data regarding breastfeeding and saliva-sharing behaviors were captured each week, using a standardized questionnaire. Saliva-sharing behaviors between mothers and primary children represent a composite of any of the following behaviors reported during a study week: premastication of food given to primary child, kissing primary child on the mouth, sharing drinking or eating utensils with primary child, and rubbing saliva into a bite or wound.
c Among mothers who weaned during study.
d Percentage of visits with each behavior, calculated per household.
Cohort Characteristics and Behavioral Risk Factors for Primary Human Herpesvirus Infection
| Characteristics of Primary Childrena . | Maternal HIV-1 Status . | ||
|---|---|---|---|
| Negative . | Positive . | Overall . | |
| Primary children | |||
| Evaluated, no. | 15 | 17 | 32 |
| Male sex | 6 (40) | 10 (59) | 16 (50) |
| Oropharyngeal samples collected weekly, no. | 57 (32–75) | 58 (4–117) | 58 (4–117) |
| Secondary children | |||
| Evaluated, no. | 22 | 27 | 49 |
| Age, y | 3.9 (1.6–6.3) | 3.6 (0.3–6.5) | 3.8 (0.3–6.5) |
| HIV-1 infected | 0 (0) | 4 (15) | 4 (8) |
| HIV-1 status unknown | 0 (0) | 7 (26) | 7 (14) |
| No. per family | |||
| 1 | 11 (73) | 10 (59) | 21 (66) |
| 2 | 1 (7) | 4 (24) | 5 (16) |
| 3 | 3 (20) | 3 (18) | 6 (19) |
| Behavioral exposureb | |||
| Breastfeeding during study | |||
| None | 0 (0) | 4 (24) | 4 (12) |
| Weaned during study | 3 (20) | 11 (65) | 14 (44) |
| Breastfed for entire study | 12 (80) | 2 (12) | 14 (44) |
| Breastfeeding duration,c wks | 45 (25–78) | 14 (1–31) | 21 (1–78) |
| Visits with saliva-sharing behavior,d % | 62 (0–100) | 21 (0–87) | 50 (0–100) |
| Characteristics of Primary Childrena . | Maternal HIV-1 Status . | ||
|---|---|---|---|
| Negative . | Positive . | Overall . | |
| Primary children | |||
| Evaluated, no. | 15 | 17 | 32 |
| Male sex | 6 (40) | 10 (59) | 16 (50) |
| Oropharyngeal samples collected weekly, no. | 57 (32–75) | 58 (4–117) | 58 (4–117) |
| Secondary children | |||
| Evaluated, no. | 22 | 27 | 49 |
| Age, y | 3.9 (1.6–6.3) | 3.6 (0.3–6.5) | 3.8 (0.3–6.5) |
| HIV-1 infected | 0 (0) | 4 (15) | 4 (8) |
| HIV-1 status unknown | 0 (0) | 7 (26) | 7 (14) |
| No. per family | |||
| 1 | 11 (73) | 10 (59) | 21 (66) |
| 2 | 1 (7) | 4 (24) | 5 (16) |
| 3 | 3 (20) | 3 (18) | 6 (19) |
| Behavioral exposureb | |||
| Breastfeeding during study | |||
| None | 0 (0) | 4 (24) | 4 (12) |
| Weaned during study | 3 (20) | 11 (65) | 14 (44) |
| Breastfed for entire study | 12 (80) | 2 (12) | 14 (44) |
| Breastfeeding duration,c wks | 45 (25–78) | 14 (1–31) | 21 (1–78) |
| Visits with saliva-sharing behavior,d % | 62 (0–100) | 21 (0–87) | 50 (0–100) |
Data are no. (%) of subjects or median value (range), unless otherwise indicated.
Abbreviation: HIV-1, human immunodeficiency virus type 1.
a No primary children acquired HIV-1 during study follow-up.
b Data regarding breastfeeding and saliva-sharing behaviors were captured each week, using a standardized questionnaire. Saliva-sharing behaviors between mothers and primary children represent a composite of any of the following behaviors reported during a study week: premastication of food given to primary child, kissing primary child on the mouth, sharing drinking or eating utensils with primary child, and rubbing saliva into a bite or wound.
c Among mothers who weaned during study.
d Percentage of visits with each behavior, calculated per household.
Incidence of Primary Infections
Three primary children had congenital infection (2 children with CMV and another with HHV-8) and were excluded from subsequent analyses related to those viruses. The number of postnatal primary infections was 20 for CMV, 19 for EBV, 9 for HSV (8 were due to HSV-1, and the other was due to HSV-2, at 6 weeks of age), 24 for HHV-6 (23 were due to HHV-6B, and 1 was due to both HHV-6A and HHV-6B), and 0 for HHV-8. The cumulative incidence of postnatal infection with each virus is shown in Figure 1 and Supplementary Table 1.
![Cumulative incidence of primary infection with different human herpesviruses (HHVs). A, Postnatal infections occurring in the first 18 months of life for primary children are shown. The cumulative incidence of postnatal infection at 6 months was 55.7% (95% confidence interval [CI], 39.2%–73.6%) for HHV-6B, 48.2% (95% CI, 32.0%–67.4%) for cytomegalovirus (CMV), 12.9% (95% CI, 5.1%–30.9%) for Epstein-Barr virus (EBV), 0% for herpes simplex virus 1 (HSV-1), and 0% for HHV-8. The cumulative incidence of postnatal infection at 12 months was 76.2% (95% CI, 60.0%–89.4%) for HHV-6B, 59.3% (95% CI, 42.2%–77.1%) for CMV, 47.4% (95% CI, 31.3%–66.6%) for EBV, 8.0% (95% CI, 2.1%–28.5%) for HSV-1, and 0% for HHV-8. B and C, Stratified data showing infants born to human immunodeficiency virus type 1 (HIV-1)–uninfected women (B) and HIV-1–infected women (C).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/214/1/10.1093_infdis_jiw076/3/m_jiw07601.jpeg?Expires=1749836423&Signature=gmAuXNr73nEiKeAErZEXiZlcje65NnY4xqcjsMRa6sWNcmvwHDPsLX3ShYEW7EVh5ZiZVsI6iosN~hOIlhun3RCAEeGJCIOEwrqkO9NZcYknZKBERA1wJmY80CXHnsPBOfZd74SWf9LZD7tRUXT7Ehmwno6LMJOYYiYhXFL29-BvF3U8Hw-tgd3x4~tmsT10AQsFnskt0U9N5gxVl6hiBtVN9JpBhJsFQX3ajAbrpjLFkl6IdmCw2UWcatmlNa89u559dKg7Nga6jucuuoifsmAfWfJ5GjxIN6iRPemwaxNiS4psynyklxMjMDgMEhrM-442XrfH3BC4Hik3Ro2ZXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Cumulative incidence of primary infection with different human herpesviruses (HHVs). A, Postnatal infections occurring in the first 18 months of life for primary children are shown. The cumulative incidence of postnatal infection at 6 months was 55.7% (95% confidence interval [CI], 39.2%–73.6%) for HHV-6B, 48.2% (95% CI, 32.0%–67.4%) for cytomegalovirus (CMV), 12.9% (95% CI, 5.1%–30.9%) for Epstein-Barr virus (EBV), 0% for herpes simplex virus 1 (HSV-1), and 0% for HHV-8. The cumulative incidence of postnatal infection at 12 months was 76.2% (95% CI, 60.0%–89.4%) for HHV-6B, 59.3% (95% CI, 42.2%–77.1%) for CMV, 47.4% (95% CI, 31.3%–66.6%) for EBV, 8.0% (95% CI, 2.1%–28.5%) for HSV-1, and 0% for HHV-8. B and C, Stratified data showing infants born to human immunodeficiency virus type 1 (HIV-1)–uninfected women (B) and HIV-1–infected women (C).
Prolonged oral shedding was common following primary infection, although the frequency and quantity varied by HHV (Figure 2). Among those with plasma available, viremia was detected in 17 of 20 infants with primary CMV infection, 0 of 1 with HHV-6A and HHV-6B, 3 of 23 with HHV-6B only, 8 of 16 with EBV, 2 of 7 with HSV-1, and 1 of 1 with HSV-2. Among those with subsequent oropharyngeal swab specimens available, 3 infants had HHV DNA detected in plasma prior to meeting criteria for primary infection by oropharyngeal shedding: 1 infant had CMV detected in plasma 2 weeks before, 1 infant with HHV-6B detected in plasma 2 weeks before, and 1 infant had HHV-6B detected in plasma 1 week before onset of shedding. Of plasma samples with detectable CMV DNA, 44% were the first plasma sample collected at or after acquisition; of samples with detectable HHV-6B DNA, 50% were the first sample collected; of samples with detectable EBV, 44% were the first sample collected; and of samples with detectable HSV-1 or HSV-2, all were the first sample collected.
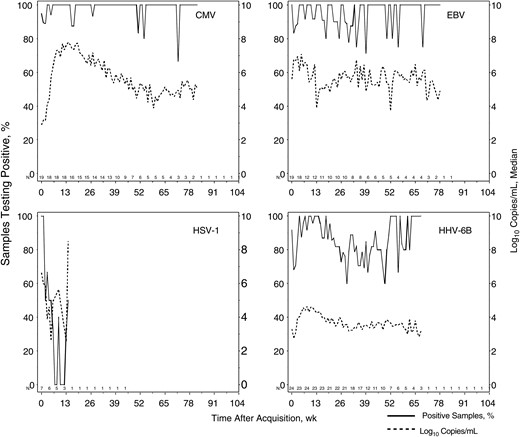
Temporal frequency and quantity of oropharyngeal shedding with different human herpesviruses (HHVs) by primary children. All infants with documented primary infection during the study who had at least 1 sample collected after infection are shown. The median log10 number of copies per milliliter was computed only among samples in which that virus was detected. The number of infants providing data at each week varied and is shown just above the x-axes; data are not shown for weeks when only a single infant provided data. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HSV-1, herpes simplex virus 1.
Among primary infections confirmed by virologic analysis, antibody seroconversion was seen in 17 of 19 CMV infections (90%), 11 of 14 EBV infections (79%), and all 4 HSV-1 infections. Seroconversion occurred at a median of 1.7 months (interquartile range [IQR], 1.4–2.5 months), 4.5 months (IQR, 3.2–5.7 month), and 1.8 months (IQR, 0.8–2.5 months) after the defined time of acquisition for CMV, EBV, and HSV-1, respectively. Primary infections without documented seroconversion were limited to children who had a single blood sample collected <2 months after acquisition or who acquired infection before 4 months of age, when maternal antibody is typically detectable. Conversely, results of all antibody tests conducted at ≥8 months of age were negative among infants without primary infection by qPCR criteria, with the exception of CMV test results for 2 infants and EBV test results for 1.
Exposures From Household Contacts
Breastfeeding and salivary sharing behaviors are depicted in Table 1. Of the 32 mothers, 28 (88%) reported breastfeeding on ≥1 visit, with 14 (50%) breastfeeding for the duration of their follow-up. HIV-1–infected women breastfed less frequently than HIV-1–uninfected women (P < .001). Of the 17 HIV-1–infected women, 13 (76%) breastfed the primary child; 2 women breastfed throughout the study, while the remaining 11 women breastfed for a median of 14 weeks (range, 1–31 weeks). During at least 1 visit, most mothers (84%) reported saliva-sharing behaviors with their infants (ie, premastication of food given to primary child, kissing primary child on the mouth, sharing drinking or eating utensils with primary child, and rubbing saliva into a bite or wound); such behaviors were reported for 48% of weekly visits. Fewer HIV-1–infected mothers (13 of 17 [76%]) reported saliva-sharing behaviors with their infants (37% of weeks), as compared with HIV-1–uninfected mothers (14 of 15 [93%]; 61% of weeks), although this difference was not statistically significant (P = .10).
Oropharyngeal shedding of HHVs by mothers and secondary children is shown in Figure 3. While infants were at risk for infection, the frequency and median quantity of HHVs detected in oropharyngeal swab specimens from mothers were as follows: 363 of 766 swab specimens (47%) and 2.8 log10 copies/mL (range, 2.2–5.7 log10 copies/mL) for HHV-6A or HHV-6B, 58 of 852 swab specimens (7%) and 2.6 log10 copies/mL (range, 2.2–3.9 log10 copies/mL) for CMV, 802 of 1222 swab specimens (66%) and 4.9 log10 copies/mL (range, 2.2–9. log10 copies/mL) for EBV, 85 of 1492 swab specimens (6%) and 4.3 log10 copies/mL (range, 2.2–7.5 log10 copies/mL) for HSV-1 or HSV-2, and 229 of 1456 swab specimens (16%) and 4.3 log10 copies/mL (range, 2.2–6.8 log10 copies/mL) for HHV-8.
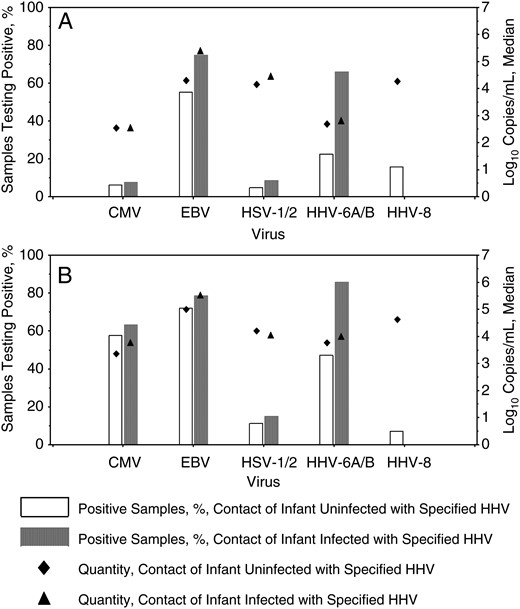
Oropharyngeal human herpesvirus (HHV) shedding by household contacts, stratified by acquisition of infection in primary children. A, HHV detected in oropharyngeal swab specimens from mothers. B, HHV detected in oropharyngeal swab specimens from secondary children. Only samples collected while the primary infants were at risk for acquisition of each virus were included. Bars represent the percentage of samples that were positive, and diamonds represent the median log10 number of copies per milliliter detected among positive samples. Open bars/diamonds correspond to household contacts of infants who did not become infected with the specified HHV, and shaded bars/triangles correspond to household contacts of infants who became infected with the specified HHV. Typing was unable to distinguish HHV-6A from HHV-6B and herpes simplex virus type 1 (HSV-1) from HSV-2 in all contact samples. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus.
While infants were at risk for infection, the frequency and median quantity of HHVs detected in oropharyngeal swab specimens from secondary children were as follows: 531 of 810 swab specimens (66%) and 3.9 log10 copies/mL (range, 2.3–6.5 log10 copies/mL) for HHV-6A or HHV-6B, 569 of 945 swab specimens (60%) and 3.5 log10 copies/mL (range, 2.2–6.7 log10 copies/mL) for CMV, 990 of 1318 swab specimens (75%) and 5.3 log10 copies/mL (range, 2.2–9.2 log10 copies/mL) for EBV, 193 of 1569 swab specimens (12%) and 4.2 log10 copies/mL (range, 2.2–8.2 log10 copies/mL) for HSV-1 or HSV-2, and 108 of 1538 swab specimens (7%) and 4.6 log10 copies/mL (range, 2.2–7.3 log10 copies/mL) for HHV-8.
The number of women with at least 1 positive swab specimen was 29 (91%) for HHV-6A or HHV-6B (of those with typed samples, 9 mothers had only HHV-6B detected, 1 had both HHV-6A and HHV-6B detected, and none had only HHV-6A detected), 17 (57%) for CMV, 32 (100%) for EBV, 25 (78%) for HSV-1 or HSV-2 (of those with typed samples, 21 mothers had only HSV-1 detected, 3 had both HSV-1 and HSV-2 detected, and none had only HSV-2 detected), and 13 (42%) for HHV-8. The number of secondary children with at least 1 positive swab specimen was 38 (84%) for HHV-6A or HHV-6B (of those with typed samples, 11 secondary children had only HHV-6B detected, and none had HHV-6A detected), 38 (95%) for CMV, 46 (100%) for EBV, 34 (72%) for HSV-1 or HSV-2 (of those with typed samples, 27 secondary children had only HSV-1 detected, 3 had both HSV-1 and HSV-2 detected, and none had only HSV-2), and 11 (24%) for HHV-8 (Figure 3).
Oropharyngeal shedding of EBV was more frequent in HIV-1–infected mothers as compared to HIV-1–uninfected mothers (83% vs 54% of swab specimens; P = .003). Shedding of HHV-6A or HHV-6B was similar among HIV-1–infected and HIV-1–uninfected mothers (50% and 45% of swab specimens, respectively; P = .76). Shedding by HIV-1–infected and HIV-1–uninfected mothers was relatively infrequent for CMV (9% and 5% of swab specimens, respectively; P = .25), HSV-1 or HSV-2 (7% and 5% of swab specimens, respectively; P = .29), and HHV-8 (24% and 8% of swab specimens, respectively; P = .14). Excluding the 7 women who were enrolled from a cohort with HHV-8 detected in the oropharynx, shedding of HHV-8 was infrequent in both HIV-1–infected women (16%) and HIV-1–uninfected women (8%; P = .51).
Risk Factors for Primary Infections
Risk factors for primary infection differed between HHVs (Table 2). In univariable analysis, earlier acquisition of CMV was associated with younger age of secondary children living in the household (hazard ratio [HR], 1.4 for each year younger; 95% confidence interval [CI], 1.1–1.9; P = .01). After adjustment for maternal HIV-1 infection status and level of viral shedding by household contacts, however, breastfeeding was the strongest risk factor for primary CMV infection (HR, 5.0; 95% CI, 1.2–21.1; P = .03). Primary CMV infection remained associated with younger age of secondary children in multivariable analyses, and there was a strong trend toward increased risk with maternal HIV-1 infection (HR, 3.0; 95% CI, 1.0–9.1; P = .05). Maternal HIV-1 infection and quantity of EBV in maternal saliva conferred a higher risk for EBV acquisition, while breastfeeding of the primary child appeared protective against EBV acquisition, in the univariable analysis. However, in adjusted analyses, only maternal HIV-1 infection remained a significant risk factor for EBV acquisition (HR, 7.2; 95% CI, 2.4–22.2; P < .001). Primary HHV-6A or HHV-6B infection was strongly associated with the quantity of virus shed by household contacts, both in univariable and multivariable analyses (HR, 1.3 for each log10 increase in the number of HHV-6A or HHV-6B copies/mL saliva; 95% CI, 1.1–1.5; P < .001). In multivariable analyses, we observed a trend toward a higher risk of HHV-6A or HHV-6B acquisition in female primary children (HR, 2.3; 95% CI, 1.0–5.3; P = .054). We also noted a borderline increased risk of primary infection with oropharyngeal shedding of high HSV-1 or HSV-2 copy numbers by household contacts (HR, 3.4; 95% CI, .9–13.2; P = .07); however, too few primary HSV infections occurred for multivariable modeling. Acquisition of each HHV appeared to be independent; no association was observed between prior HHV infection and risk of acquiring an additional HHV (Table 2). No seasonality was observed for primary infection with any of the viruses studied (Supplementary Figure 1).
| Covariate . | Cytomegalovirus . | Epstein-Barr Virus . | HHV-6A or HHV-6Ba . | HSV-1 or HSV-2a . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P Values . | Adjusted HRb (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRc (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRd (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | |
| Female sex | 0.9 (.4–2.2) | .90 | 0.9 (.4–2.2) | .82 | 0.9 (.4–2.1) | .77 | 1.0 (.4–2.6) | .96 | 1.6 (.7–3.5) | .25 | 2.3 (1.0–5.3) | .054 | 1.1 (.3–4.3) | .85 |
| Age of secondary childrene | 1.4 (1.1–1.9) | .01 | 1.4 (1.0–2.0) | .04 | 1.1 (.8–1.4) | .65 | 0.8 (.5–1.2) | .36 | 1.0 (.8–1.2) | .84 | 0.9 (.7–1.2) | .54 | 1.2 (.8–1.8) | .38 |
| Maternal HIV-1 infection | 1.0 (.4–2.3) | .97 | 3.0 (1.0–9.1) | .05 | 6.7 (2.4–18.9) | <.001 | 7.2 (2.4–22.2) | <.001 | 0.8 (.4–1.7) | .55 | 0.6 (.3–1.4) | .25 | 0.7 (.2–3.5) | .70 |
| Maternal shedding quantityf | 0.3 (.1–2.2) | .26 | 0.4 (.1–3.3) | .43 | 1.3 (1.0–1.6) | .02 | 1.0 (.8–1.3) | .88 | 1.5 (1.0–2.1) | .04 | 1.5 (1.0–2.1) | .04 | 1.8 (.4–7.2) | .42 |
| Secondary children shedding quantityf | 1.0 (.8–1.2) | .99 | 1.0 (.8–1.3) | .69 | 1.0 (.9–1.2) | .62 | 1.0 (.9–1.2) | .71 | 1.2 (1.0–1.4) | .02 | 1.2 (1.1–1.4) | .003 | 4.9 (.4–54.3) | .19 |
| Household shedding quantityf | 1.0 (.8–1.2) | .91 | 1.0 (.8–1.3) | .73 | 1.1 (1.0–1.2) | .24 | 1.0 (.9–1.1) | .83 | 1.3 (1.1–1.5) | .001 | 1.3 (1.1–1.5) | <.001 | 3.4 (.9–13.2) | .07 |
| Breastfeedingg | 3.1 (.9–10.8) | .07 | 5.0 (1.2–21.1) | .03 | 0.2 (.1–.6) | .002 | 0.8 (.2–4.4) | .81 | 1.2 (.5–2.5) | .69 | 1.2 (.3–4.8) | .79 | 2.5 (.6–11.1) | .23 |
| Saliva sharing behaviors with primary childg | 1.4 (.5–3.5) | .54 | 1.4 (.5–4.0) | .52 | 0.5 (.2–1.1) | .10 | 1.1 (.4–2.9) | .88 | 1.2 (.6–2.5) | .67 | 1.2 (.6–2.5) | .62 | 1.0 (.2–3.8) | .98 |
| Any prior HHV infection | 1.2 (.4–3.6) | .71 | 1.2 (.5–3.1) | .73 | 1.8 (.4–9.3) | .46 | 1.3 (.3–5.1) | .69 | 2.0 (.8–5.1) | .16 | 1.2 (.4–3.9) | .73 | … | … |
| Covariate . | Cytomegalovirus . | Epstein-Barr Virus . | HHV-6A or HHV-6Ba . | HSV-1 or HSV-2a . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P Values . | Adjusted HRb (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRc (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRd (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | |
| Female sex | 0.9 (.4–2.2) | .90 | 0.9 (.4–2.2) | .82 | 0.9 (.4–2.1) | .77 | 1.0 (.4–2.6) | .96 | 1.6 (.7–3.5) | .25 | 2.3 (1.0–5.3) | .054 | 1.1 (.3–4.3) | .85 |
| Age of secondary childrene | 1.4 (1.1–1.9) | .01 | 1.4 (1.0–2.0) | .04 | 1.1 (.8–1.4) | .65 | 0.8 (.5–1.2) | .36 | 1.0 (.8–1.2) | .84 | 0.9 (.7–1.2) | .54 | 1.2 (.8–1.8) | .38 |
| Maternal HIV-1 infection | 1.0 (.4–2.3) | .97 | 3.0 (1.0–9.1) | .05 | 6.7 (2.4–18.9) | <.001 | 7.2 (2.4–22.2) | <.001 | 0.8 (.4–1.7) | .55 | 0.6 (.3–1.4) | .25 | 0.7 (.2–3.5) | .70 |
| Maternal shedding quantityf | 0.3 (.1–2.2) | .26 | 0.4 (.1–3.3) | .43 | 1.3 (1.0–1.6) | .02 | 1.0 (.8–1.3) | .88 | 1.5 (1.0–2.1) | .04 | 1.5 (1.0–2.1) | .04 | 1.8 (.4–7.2) | .42 |
| Secondary children shedding quantityf | 1.0 (.8–1.2) | .99 | 1.0 (.8–1.3) | .69 | 1.0 (.9–1.2) | .62 | 1.0 (.9–1.2) | .71 | 1.2 (1.0–1.4) | .02 | 1.2 (1.1–1.4) | .003 | 4.9 (.4–54.3) | .19 |
| Household shedding quantityf | 1.0 (.8–1.2) | .91 | 1.0 (.8–1.3) | .73 | 1.1 (1.0–1.2) | .24 | 1.0 (.9–1.1) | .83 | 1.3 (1.1–1.5) | .001 | 1.3 (1.1–1.5) | <.001 | 3.4 (.9–13.2) | .07 |
| Breastfeedingg | 3.1 (.9–10.8) | .07 | 5.0 (1.2–21.1) | .03 | 0.2 (.1–.6) | .002 | 0.8 (.2–4.4) | .81 | 1.2 (.5–2.5) | .69 | 1.2 (.3–4.8) | .79 | 2.5 (.6–11.1) | .23 |
| Saliva sharing behaviors with primary childg | 1.4 (.5–3.5) | .54 | 1.4 (.5–4.0) | .52 | 0.5 (.2–1.1) | .10 | 1.1 (.4–2.9) | .88 | 1.2 (.6–2.5) | .67 | 1.2 (.6–2.5) | .62 | 1.0 (.2–3.8) | .98 |
| Any prior HHV infection | 1.2 (.4–3.6) | .71 | 1.2 (.5–3.1) | .73 | 1.8 (.4–9.3) | .46 | 1.3 (.3–5.1) | .69 | 2.0 (.8–5.1) | .16 | 1.2 (.4–3.9) | .73 | … | … |
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus type 1; HR, hazard ratio; HSV, herpes simplex virus.
a Typing was unable to distinguish HHV-6A from HHV-6B and HSV-1 from HSV-2 in all contact samples.
b All estimates were adjusted for maternal HIV-1 status and breastfeeding. Maternal HIV-1 status and breastfeeding estimates were also adjusted for household mean log10 copy.
c Maternal HIV-1 status estimate was adjusted for household mean log10 copy. All other estimates were adjusted for maternal HIV-1 status.
d Viral exposure estimates were adjusted for maternal HIV-1 status and saliva sharing. All other estimates were adjusted for maternal HIV-1 status, household mean log10 copy, and saliva sharing.
e Modeled as the average age of all secondary children in the household at each visit. Estimates represent the HR for each year younger.
f HRs for viral exposures represent the HR for a 1-unit increase in the mean log10 virus copies (negatives are counted as zeros), using a 28-day window for all viruses except HSV-1 or HSV-2, which uses the entire covariate history.
g Measured in the 28 days before each visit.
| Covariate . | Cytomegalovirus . | Epstein-Barr Virus . | HHV-6A or HHV-6Ba . | HSV-1 or HSV-2a . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P Values . | Adjusted HRb (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRc (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRd (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | |
| Female sex | 0.9 (.4–2.2) | .90 | 0.9 (.4–2.2) | .82 | 0.9 (.4–2.1) | .77 | 1.0 (.4–2.6) | .96 | 1.6 (.7–3.5) | .25 | 2.3 (1.0–5.3) | .054 | 1.1 (.3–4.3) | .85 |
| Age of secondary childrene | 1.4 (1.1–1.9) | .01 | 1.4 (1.0–2.0) | .04 | 1.1 (.8–1.4) | .65 | 0.8 (.5–1.2) | .36 | 1.0 (.8–1.2) | .84 | 0.9 (.7–1.2) | .54 | 1.2 (.8–1.8) | .38 |
| Maternal HIV-1 infection | 1.0 (.4–2.3) | .97 | 3.0 (1.0–9.1) | .05 | 6.7 (2.4–18.9) | <.001 | 7.2 (2.4–22.2) | <.001 | 0.8 (.4–1.7) | .55 | 0.6 (.3–1.4) | .25 | 0.7 (.2–3.5) | .70 |
| Maternal shedding quantityf | 0.3 (.1–2.2) | .26 | 0.4 (.1–3.3) | .43 | 1.3 (1.0–1.6) | .02 | 1.0 (.8–1.3) | .88 | 1.5 (1.0–2.1) | .04 | 1.5 (1.0–2.1) | .04 | 1.8 (.4–7.2) | .42 |
| Secondary children shedding quantityf | 1.0 (.8–1.2) | .99 | 1.0 (.8–1.3) | .69 | 1.0 (.9–1.2) | .62 | 1.0 (.9–1.2) | .71 | 1.2 (1.0–1.4) | .02 | 1.2 (1.1–1.4) | .003 | 4.9 (.4–54.3) | .19 |
| Household shedding quantityf | 1.0 (.8–1.2) | .91 | 1.0 (.8–1.3) | .73 | 1.1 (1.0–1.2) | .24 | 1.0 (.9–1.1) | .83 | 1.3 (1.1–1.5) | .001 | 1.3 (1.1–1.5) | <.001 | 3.4 (.9–13.2) | .07 |
| Breastfeedingg | 3.1 (.9–10.8) | .07 | 5.0 (1.2–21.1) | .03 | 0.2 (.1–.6) | .002 | 0.8 (.2–4.4) | .81 | 1.2 (.5–2.5) | .69 | 1.2 (.3–4.8) | .79 | 2.5 (.6–11.1) | .23 |
| Saliva sharing behaviors with primary childg | 1.4 (.5–3.5) | .54 | 1.4 (.5–4.0) | .52 | 0.5 (.2–1.1) | .10 | 1.1 (.4–2.9) | .88 | 1.2 (.6–2.5) | .67 | 1.2 (.6–2.5) | .62 | 1.0 (.2–3.8) | .98 |
| Any prior HHV infection | 1.2 (.4–3.6) | .71 | 1.2 (.5–3.1) | .73 | 1.8 (.4–9.3) | .46 | 1.3 (.3–5.1) | .69 | 2.0 (.8–5.1) | .16 | 1.2 (.4–3.9) | .73 | … | … |
| Covariate . | Cytomegalovirus . | Epstein-Barr Virus . | HHV-6A or HHV-6Ba . | HSV-1 or HSV-2a . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P Values . | Adjusted HRb (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRc (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | Adjusted HRd (95% CI) . | P Values . | Unadjusted HR (95% CI) . | P Values . | |
| Female sex | 0.9 (.4–2.2) | .90 | 0.9 (.4–2.2) | .82 | 0.9 (.4–2.1) | .77 | 1.0 (.4–2.6) | .96 | 1.6 (.7–3.5) | .25 | 2.3 (1.0–5.3) | .054 | 1.1 (.3–4.3) | .85 |
| Age of secondary childrene | 1.4 (1.1–1.9) | .01 | 1.4 (1.0–2.0) | .04 | 1.1 (.8–1.4) | .65 | 0.8 (.5–1.2) | .36 | 1.0 (.8–1.2) | .84 | 0.9 (.7–1.2) | .54 | 1.2 (.8–1.8) | .38 |
| Maternal HIV-1 infection | 1.0 (.4–2.3) | .97 | 3.0 (1.0–9.1) | .05 | 6.7 (2.4–18.9) | <.001 | 7.2 (2.4–22.2) | <.001 | 0.8 (.4–1.7) | .55 | 0.6 (.3–1.4) | .25 | 0.7 (.2–3.5) | .70 |
| Maternal shedding quantityf | 0.3 (.1–2.2) | .26 | 0.4 (.1–3.3) | .43 | 1.3 (1.0–1.6) | .02 | 1.0 (.8–1.3) | .88 | 1.5 (1.0–2.1) | .04 | 1.5 (1.0–2.1) | .04 | 1.8 (.4–7.2) | .42 |
| Secondary children shedding quantityf | 1.0 (.8–1.2) | .99 | 1.0 (.8–1.3) | .69 | 1.0 (.9–1.2) | .62 | 1.0 (.9–1.2) | .71 | 1.2 (1.0–1.4) | .02 | 1.2 (1.1–1.4) | .003 | 4.9 (.4–54.3) | .19 |
| Household shedding quantityf | 1.0 (.8–1.2) | .91 | 1.0 (.8–1.3) | .73 | 1.1 (1.0–1.2) | .24 | 1.0 (.9–1.1) | .83 | 1.3 (1.1–1.5) | .001 | 1.3 (1.1–1.5) | <.001 | 3.4 (.9–13.2) | .07 |
| Breastfeedingg | 3.1 (.9–10.8) | .07 | 5.0 (1.2–21.1) | .03 | 0.2 (.1–.6) | .002 | 0.8 (.2–4.4) | .81 | 1.2 (.5–2.5) | .69 | 1.2 (.3–4.8) | .79 | 2.5 (.6–11.1) | .23 |
| Saliva sharing behaviors with primary childg | 1.4 (.5–3.5) | .54 | 1.4 (.5–4.0) | .52 | 0.5 (.2–1.1) | .10 | 1.1 (.4–2.9) | .88 | 1.2 (.6–2.5) | .67 | 1.2 (.6–2.5) | .62 | 1.0 (.2–3.8) | .98 |
| Any prior HHV infection | 1.2 (.4–3.6) | .71 | 1.2 (.5–3.1) | .73 | 1.8 (.4–9.3) | .46 | 1.3 (.3–5.1) | .69 | 2.0 (.8–5.1) | .16 | 1.2 (.4–3.9) | .73 | … | … |
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus type 1; HR, hazard ratio; HSV, herpes simplex virus.
a Typing was unable to distinguish HHV-6A from HHV-6B and HSV-1 from HSV-2 in all contact samples.
b All estimates were adjusted for maternal HIV-1 status and breastfeeding. Maternal HIV-1 status and breastfeeding estimates were also adjusted for household mean log10 copy.
c Maternal HIV-1 status estimate was adjusted for household mean log10 copy. All other estimates were adjusted for maternal HIV-1 status.
d Viral exposure estimates were adjusted for maternal HIV-1 status and saliva sharing. All other estimates were adjusted for maternal HIV-1 status, household mean log10 copy, and saliva sharing.
e Modeled as the average age of all secondary children in the household at each visit. Estimates represent the HR for each year younger.
f HRs for viral exposures represent the HR for a 1-unit increase in the mean log10 virus copies (negatives are counted as zeros), using a 28-day window for all viruses except HSV-1 or HSV-2, which uses the entire covariate history.
g Measured in the 28 days before each visit.
Symptoms Associated With Primary Infection
As expected, irritability and runny nose were reported for all 32 infants during study follow-up, while fever, diarrhea, rash, and cough were each reported for 31 of the 32 infants (Supplementary Figures 2–7). Mouth sores were reported for half of the infants, and seizures were reported for 4 infants. Overall, fever and diarrhea were each reported on 15% of weeks, irritability on 18%, rash on 28%, cough on 39%, and runny nose on 50%, while mouth sores were only reported on 2% and seizures on <1%.
Primary infections with HSV-1 were most strongly associated with clinical illness (Supplementary Tables 2–8). From 1 week before to 1 week after HSV-1 acquisition, infants were more likely to be irritable (odds ratio [OR], 6.7; 95% CI, 1.7–∞; P = .03), using the nested case-control analysis (Supplementary Tables 2 and 3). The case-crossover analysis similarly showed an association of HSV-1 acquisition with irritability (OR, 9.2; 95% CI, 2.3–∞; P = .02; Supplementary Tables 4 and 8). Both analyses suggested a trend toward fever (OR, 5.3 [P = .06] for the case-control analysis; OR, 5.1 [P = .14] for the case-crossover analysis), paracetamol use (OR, 5.3, P = .06 for the case-control analysis; OR, 6.4, P = .08 for the case-crossover analysis), and mouth sores (OR, 3.0, P = .38 for the case-control analysis; OR, 5.2, P = .17 for the case-crossover analysis) being more common at the time of primary HSV-1 infection (Supplementary Tables 3 and 8). Primary HHV-6B infection was not associated with symptoms in the case-control analysis (Supplementary Table 3), but in the case-crossover design there was greater paracetamol use (P = .03) and a trend of more frequent fever (P = .05; Supplementary Table 7). Neither CMV nor EBV primary infection was associated with any symptom. When stratifying by maternal HIV-1 infection status in the case-crossover analysis, we found no statistically significant difference in the clinical presentation of any primary HHV infection by maternal HIV-1 status, although most symptoms of HSV-1 infection occurred among HIV-1–exposed infants (Supplementary Tables 5–8).
DISCUSSION
In this study, we have comprehensively evaluated and compared primary infection with multiple HHVs in a cohort of African infants, beginning at birth. Our findings largely support prior observations but extend the precision and detail of the epidemiologic and clinical aspects of primary infection with each of these viruses in young children. We found that most Ugandan infants acquired several HHV infections during the first year of life. HHV-6B and CMV were acquired earliest, followed by EBV and then HSV-1; although HHV-8 infection is endemic in Uganda, we found no postnatal HHV-8 acquisition during infancy. In addition, the design of this study allowed a unique evaluation of the intensity of the infants' exposures to multiple HHVs. Risk factors for primary infection differed by virus, and the level of oropharyngeal shedding by contacts appeared to be important only for transmission of HHV-6A or HHV-6B and HSV-1 or HSV-2.
The incidence of HHV-6B infection was similar to what has been described in North American and Japanese children [3, 22, 23]. As has been observed in one North American study, girls appeared to have a higher risk of HHV-6 infection [3]. All but 1 primary HHV-6 infection in our Ugandan cohort was due to HHV-6B, as has been found in children in resource-rich regions, but this finding differs from that from one of 2 other studies performed in Africa, which suggested that most childhood infections were due to HHV-6A [8, 24].
Maternal HIV-1 infection was the dominant risk factor for EBV acquisition, independently of maternal shedding. Among HIV-1–unexposed infants in our cohort, EBV infection was uncommon before 6 months of life. In contrast, EBV infection began within the first month of life of HIV-1–exposed infants. Such early EBV infections have been observed before among HIV-1–exposed uninfected infants [12, 25] and suggest that maternal HIV-1 infection might increase the risk of transmission through impaired transfer of EBV-specific maternal antibody [26, 27]. Further studies are needed to determine whether maternal antibody is protective against EBV [28, 29]. If so, a vaccine that elicited sufficient levels of EBV-specific antibody might confer sterilizing immunity [30]. While EBV is routinely detected in breast milk [31, 32] and appears to be infectious to B cells [33], our data reinforce previous reports that breast milk is not a major source of EBV transmission [34, 35]. In contrast, rapid acquisition of CMV began from the time of birth among both HIV-1–exposed and HIV-1–unexposed infants and, as expected, was strongly associated with breastfeeding [36]. Maternal HIV-1 status was not associated with primary HHV-6A or HHV-6B infection or primary HSV-1 or HSV-2 infection.
The lack of primary HHV-8 infections was unexpected because oropharyngeal shedding of HHV-8 by contacts was similar in frequency and quantity to that of HSV in this cohort. Although congenital HHV-8 infection is reportedly rare [37, 38], we observed one apparent case. Serologic studies of HHV-8 in regions where infection is endemic have documented infections in young children, although uncommonly during infancy [38–41]. The basis for differences in attack rates between HHVs among infants with similar exposures is unknown but may reflect virus-specific differences in infectivity or host defense mechanisms.
Although primary EBV infection has been reported to cause symptoms among young children in some studies [12, 42], this was not observed in our cohort or in others [43]. Neither were symptoms attributable to primary CMV infection. Symptoms were associated with primary HSV-1 infection but were often nonspecific; oral lesions were not consistently observed. HHV-6B was not found to be associated with roseola [3], perhaps because of the high background rates of fever and rash reported among infants at all time points in this cohort. Delayed symptoms, such as those due to infectious mononucleosis or other immune-mediated manifestations of infection, could have been missed by our analysis [44].
Strengths of this study include the prospective design, with enrollment at birth, and the precise determination of the week of infection by means of direct viral detection by sensitive molecular methods. Determination of primary infection by virologic analysis was typically made several weeks before seroconversion. A unique feature of this study was the quantitative surveillance of exposure to virus shed by family members, in addition to other risk factors for transmission. Finally, we designed this study to quantify the incidence and risk factors for primary infection with multiple HHVs simultaneously in the same infant cohort.
Several limitations should also be noted. Although 72 postnatal primary HHV infections were captured, the number of primary infections for each virus was relatively small. The findings from this cohort may not be generalizable to those for infants in high-resource populations. We purposefully oversampled women with HIV-1 infection, to understand the influence of maternal HIV-1 on HHV acquisition in infants. We also oversampled women with oral HHV-8 shedding, in an attempt to increase the chance of observing infant infections, given that the dynamics of early childhood acquisition of HHV-8 are not well understood. Contact shedding was not measured in breast milk or urine. Fathers, school-aged children, and contacts outside the household were not sampled, which could have led to missing other exposures. Typing was unable to distinguish HHV-6A from HHV-6B and HSV-1 from HSV-2 in all samples. The 28-day windows that were selected to define time-dependent exposures were somewhat arbitrary, as data to guide appropriate time frames are not available.
In conclusion, we characterized the risk factors for transmission and the natural history of primary infection due to several HHVs among African infants in extensive detail. Most primary HHV infections were asymptomatic in this cohort of African infants. The incidence and associated risk factors differed by virus. We have shown that breastfeeding is not a significant risk factor for EBV infection, a conclusion strengthened by demonstrating the association between breastfeeding and CMV transmission in the same mother-infant pairs. We also showed that exposure to oropharyngeal shedding of HHV-6A or HHV-6B and, likely, HSV-1 or HSV-2 by household contacts is the major risk factor for those infections in infants. Reported saliva-sharing behaviors were not found to be associated with acquisition of any HHV. Finally, we found that maternal HIV-1 infection is independently associated with a higher incidence of EBV infection, which may indicate a protective role for maternal antibody. Our findings suggest that efforts to prevent primary infection with HHVs need to be focused on early infancy.
Notes
Acknowledgments. We thank all of the study participants and their family members, as well as Zaam Nalule, Ruth Nakuya, Immaculate Mbarusha, Isma Lubega, Amalia Magaret, Anne Cent, Erica Sessle, Warren Phipps, James Ferrenberg, Ari Bitton, and Lisa Bunts.
Financial support. This work was supported by the National Institutes of Health Roadmap KL2 Clinical Scholar Training Program (grant KL2-RR025015-01 to S. G.), the University of Washington Center for AIDS Research (new investigator awards P30-AI027757 [to S. G.], P01-AI 030731 [to L. C., A. W., R. A., S. S., and M.-L. H.], R01-CA138165 [to C. C.], and P30-CA015704 [to C. C. and L. C.], and the Child & Family Research Institute Salary Award [to S. G.]).
Potential conflicts of interest. A. W. reports receiving personal fees from Aicuris, Amgen, Eisai, UpToDate, and Admedus and support from Agenus, Genentech, Genocea, Gilead, and Vical. C. Casper reports receiving grants, personal fees, and nonfinancial support from Janssen Pharmaceuticals and grants and nonfinancial support from GSK and TempTime. L. C. reports receiving personal fees from Immune Design and has a patent null licensed. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 14th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, Maryland, 12–13 November 2013; Pediatric Academic Societies, Vancouver, Canada, 3–6 May 2014; 40th Annual International Herpesvirus Workshop, Boise, Idaho, 25–29 July 2015; 15th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, Maryland, 26–27 October 2015; International HHV-6 & 7 Conference, Boston, Massachusetts, 9–11 November 2015.
C. C. and L. C. contributed equally to this work.




