-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica T. Lin, Ratawan Ubalee, Chanthap Lon, Sujata Balasubramanian, Worachet Kuntawunginn, Rifat Rahman, Piyaporn Saingam, Thay Kheang Heng, Dav Vy, Savoeun San, Sarath Nuom, Hana Burkly, Nitima Chanarat, Chanudom Ponsa, Lauren Levitz, Christian Parobek, Char Meng Chuor, Sok Somethy, Michele Spring, Charlotte Lanteri, Panita Gosi, Steven R. Meshnick, David L. Saunders, Microscopic Plasmodium falciparum Gametocytemia and Infectivity to Mosquitoes in Cambodia, The Journal of Infectious Diseases, Volume 213, Issue 9, 1 May 2016, Pages 1491–1494, https://doi.org/10.1093/infdis/jiv599
Close - Share Icon Share
Abstract
Although gametocytes are essential for malaria transmission, in Africa many falciparum-infected persons without smear-detectable gametocytes still infect mosquitoes. To see whether the same is true in Southeast Asia, we determined the infectiousness of 119 falciparum-infected Cambodian adults to Anopheles dirus mosquitoes by membrane feeding. Just 5.9% of subjects infected mosquitoes. The 8.4% of patients with smear-detectable gametocytes were >20 times more likely to infect mosquitoes than those without and were the source of 96% of all mosquito infections. In low-transmission settings, targeting transmission-blocking interventions to those with microscopic gametocytemia may have an outsized effect on malaria control and elimination.
Achieving malaria elimination requires an understanding of the local human infectious reservoir to biting mosquitoes. Mosquito feeding studies, whereby anopheline mosquitoes are fed on the blood of infected persons and subsequently dissected for evidence of parasite development, represent a way to measure the infectiousness of human hosts and characterize this reservoir [1]. Such studies have highlighted the importance of sexual stage parasites called gametocytes in human-to-mosquito transmission [2, 3]. Higher gametocytemia more often results in mosquito infection, but persons with low-level gametocytemia not visible with microscopy can also be infective to mosquitoes [2, 4]. Because those with submicroscopic gametocytemia far outnumber those with patent (smear-detectable) gametocytes, it has been postulated that submicroscopic malaria is the source of 20%–50% of all human-to-mosquito transmission when transmission reaches very low levels [5]. Mosquito feeding studies in African children seem to support this assertion, because they have revealed significant proportions of children with submicroscopic gametocytes who infect mosquitoes [6–8]. However, comparable data from low-transmission settings are lacking.
Southeast Asia has been a focus for malaria containment and elimination efforts aimed to prevent the spread of artemisinin-resistant malaria. However, very few mosquito infectivity studies have been done to characterize the human infectious reservoir. As part of a therapeutic efficacy study to assess the transmission-blocking efficacy of primaquine, we conducted mosquito membrane feedings in a cohort of Cambodian adults presenting with uncomplicated P. falciparum malaria. Here, we report on results from the pretreatment feedings to evaluate the importance of gametocytemia in determining infectious potential in this cohort.
METHODS
The study was carried out between 2012 and 2014 in northwestern Cambodia [9]. Study participants were adults aged 18–65 years presenting to Anlong Veng District Hospital with uncomplicated P. falciparum or mixed P. falciparum/P. vivax infection diagnosed with microscopy and confirmed with-time polymerase chain reaction (PCR). Participants provided written informed consent. Ethical approval for the study was obtained from the appropriate US and Cambodian regulatory authorities.
Infectiousness was evaluated via membrane feeding experiments, with mosquitoes harvested 9 and 16 days after feeding to assess for the presence of oocysts and sporozoites, respectively. Anopheles dirus were transported from an insectary in Bangkok to the field site and starved for 6–20 hours before feeding. Venous blood from each subject was drawn into heparin-containing tubes and kept at 37°C until feeding. The median time to mosquito feeding was 46 minutes (range, 1 minute to 5.5 hours), and mosquitoes were fed within 2 hours of blood collection in >80% of patients; 2 mL of blood were fed to approximately 300 colony-reared 5–8-day-old female mosquitoes via an artificial membrane attached to a water-jacketed glass feeder maintained at 37°C, with the goal of yielding approximately 200 engorged (fed) mosquitoes. After 30 minutes, unengorged mosquitoes were removed by aspiration, and the remaining engorged mosquitoes were maintained at 26°C with daily 10% sucrose. Nine days after feeding, mosquito midguts from 50 mosquitoes were examined for the presence of oocysts after dissection in 2% mercurochrome. The number of oocysts per individual mosquito was independently recorded by 2 readers.
Another 50 mosquitoes at both day 9 and day 16 after feeding were saved for malaria detection with PCR, with removal of the mosquito abdomens in those surviving to day 16. Mosquitoes were pooled in groups of 10, preserved in 95% ethanol, and stored at ≤4°C. For DNA extraction, mosquito specimens were manually homogenized using a Kontes Pellet Pestle Cordless Motor and autoclaved plastic pestles in a phosphate-buffered saline/1% saponin solution. A simplified Chelex procedure [10] was used, with solution volumes adapted to pools of 10 mosquitoes. Species-specific real-time quantitative PCR (qPCR) targeting 18s ribosomalRNA was then performed on DNA extracted from 5 pools of 10 mosquitoes. For subjects with mixed P. falciparum/P. vivax infection, individual mosquitoes instead of pooled mosquitoes were saved, and qPCR was performed on sDNA extracted from 30 individual mosquitoes using both P. falciparum and P. vivax primers. PCR evidence of transmission in a given patient was defined as a positive qPCR result at both day 9 and day 16.
Pretreatment gametocyte status for each subject was assessed using both microscopy and reverse-transcriptase PCR (RT-PCR) of the mature gametocyte marker Pfs25. Giemsa-stained thick and thin blood smears were examined by 2 World Health Organization–certified microscopists blinded to each other's results, with counting of gametocytes per 2000 white blood cells. For RT-PCR, RNA was extracted from 2.5 mL of whole blood using the Qiagen PAXgene Blood RNA kit, complementary DNA synthesis was performed, and Pfs25 was amplified using nested PCR, as described elsewhere [11]. Each RNA sample was assayed with an RT-minus control to rule out DNA contamination. PCR products were resolved using gel electrophoresis.
RESULTS
Between December 2012 and February 2014, membrane feeding assays were conducted in 119 adults presenting with uncomplicated falciparum malaria, including 12 with mixed P. falciparum/P. vivax infection. Among these 119 patients, 10 (8.4%) had microscopically patent P. falciparum gametocytemia, with gametocyte counts ranging from 5 to 728/µL (Table 1). All 10 of these patients were also gametocyte positive by Pfs25 RT-PCR. Another 39 patients (33%) had submicroscopic gametocytes detectable only with RT-PCR. This left more than half of patients (70 of 119; 59%) with no detectable gametocytes. Risk factors for patent gametocyte carriage included longer illness before presentation (median, 3 vs 2 days of illness; P = .04) and anemia (hematocrit, 30.5% vs 42.8% in those with or without patent gametocytes, respectively; P = .001) (Supplementary Table 1).
| Patienta . | Gametocyte Count at Microscopy, No./µL . | Gametocyte Positive by RT-PCR of Pfs25 . | Mosquitoes Infected, %b . | Oocysts per Mosquito, Median (Range), No.c . | Mosquito Infection . | |
|---|---|---|---|---|---|---|
| By Midgut Inspectiond . | By qPCR . | |||||
| SN-060e | 728 | Yes | 70 | 42 (1–231) | ✓ | ✓ |
| SN-002e | 705 | Yes | 26 | 1 (1–12) | ✓ | ✓ |
| SN-063 (M)e | 690 | Yes | 87 | … | … | ✓ |
| SN-108e | 342 | Yes | 98 | 149 (48–398) | ✓ | ✓ |
| SN-119 (M)e | 118 | Yes | 10 | … | ✓ | ✓ |
| SN-030 | 66 | Yes | 0 | 0 | … | … |
| SN-006 (M) | 21 | Yes | 0 | 0 | … | … |
| SN-010 | 16 | Yes | 0 | 0 | … | … |
| SN-066 | 7 | Yes | 0 | 0 | … | … |
| SN-007 | 5 | Yes | 0 | 0 | … | … |
| SN-070e | 0 | Yes | 8 | 1 (1–2) | ✓ | ✓ |
| SN-086e | 0 | Yes | ?f | 0 | … | ✓ |
| Patienta . | Gametocyte Count at Microscopy, No./µL . | Gametocyte Positive by RT-PCR of Pfs25 . | Mosquitoes Infected, %b . | Oocysts per Mosquito, Median (Range), No.c . | Mosquito Infection . | |
|---|---|---|---|---|---|---|
| By Midgut Inspectiond . | By qPCR . | |||||
| SN-060e | 728 | Yes | 70 | 42 (1–231) | ✓ | ✓ |
| SN-002e | 705 | Yes | 26 | 1 (1–12) | ✓ | ✓ |
| SN-063 (M)e | 690 | Yes | 87 | … | … | ✓ |
| SN-108e | 342 | Yes | 98 | 149 (48–398) | ✓ | ✓ |
| SN-119 (M)e | 118 | Yes | 10 | … | ✓ | ✓ |
| SN-030 | 66 | Yes | 0 | 0 | … | … |
| SN-006 (M) | 21 | Yes | 0 | 0 | … | … |
| SN-010 | 16 | Yes | 0 | 0 | … | … |
| SN-066 | 7 | Yes | 0 | 0 | … | … |
| SN-007 | 5 | Yes | 0 | 0 | … | … |
| SN-070e | 0 | Yes | 8 | 1 (1–2) | ✓ | ✓ |
| SN-086e | 0 | Yes | ?f | 0 | … | ✓ |
Abbreviations: M, mixed Plasmodium falciparum/Plasmodium vivax infection; qPCR, quantitative polymerase chain reaction; RT-PCR, reverse-transcriptase polymerase chain reaction.
a All patients with either smear-detectable P. falciparum gametocytes or successful mosquito infection, demonstrated either by midgut inspection or with qPCR, are listed. In patients with mixed P. falciparum/P. vivax infection, detection of P. falciparum–specific mosquito infection relied on qPCR of individual mosquitoes.
b The percentage of mosquitoes infected was based on oocyst detection among 50 mosquitoes (for patients with P. falciparum monoinfection) or the proportion of 30 mosquitoes that were qPCR positive at day 9 after feeding (for patients with mixed infections).
c Medians and ranges are given for oocyst-positive mosquitoes only; they were not reported for 2 patients with mixed infection because the species of individual oocysts was not determined.
d In patients with mixed infection, this result was reported as positive if falciparum qPCR–positive mosquitoes were found and oocysts were visualized in midgut dissection.
e These 7 patients were considered infectious in statistical calculations.
f Two of 5 and 5 of 5 pools of mosquitoes were qPCR positive at days 9 and 16 after feeding, respectively, but oocysts were not seen.
| Patienta . | Gametocyte Count at Microscopy, No./µL . | Gametocyte Positive by RT-PCR of Pfs25 . | Mosquitoes Infected, %b . | Oocysts per Mosquito, Median (Range), No.c . | Mosquito Infection . | |
|---|---|---|---|---|---|---|
| By Midgut Inspectiond . | By qPCR . | |||||
| SN-060e | 728 | Yes | 70 | 42 (1–231) | ✓ | ✓ |
| SN-002e | 705 | Yes | 26 | 1 (1–12) | ✓ | ✓ |
| SN-063 (M)e | 690 | Yes | 87 | … | … | ✓ |
| SN-108e | 342 | Yes | 98 | 149 (48–398) | ✓ | ✓ |
| SN-119 (M)e | 118 | Yes | 10 | … | ✓ | ✓ |
| SN-030 | 66 | Yes | 0 | 0 | … | … |
| SN-006 (M) | 21 | Yes | 0 | 0 | … | … |
| SN-010 | 16 | Yes | 0 | 0 | … | … |
| SN-066 | 7 | Yes | 0 | 0 | … | … |
| SN-007 | 5 | Yes | 0 | 0 | … | … |
| SN-070e | 0 | Yes | 8 | 1 (1–2) | ✓ | ✓ |
| SN-086e | 0 | Yes | ?f | 0 | … | ✓ |
| Patienta . | Gametocyte Count at Microscopy, No./µL . | Gametocyte Positive by RT-PCR of Pfs25 . | Mosquitoes Infected, %b . | Oocysts per Mosquito, Median (Range), No.c . | Mosquito Infection . | |
|---|---|---|---|---|---|---|
| By Midgut Inspectiond . | By qPCR . | |||||
| SN-060e | 728 | Yes | 70 | 42 (1–231) | ✓ | ✓ |
| SN-002e | 705 | Yes | 26 | 1 (1–12) | ✓ | ✓ |
| SN-063 (M)e | 690 | Yes | 87 | … | … | ✓ |
| SN-108e | 342 | Yes | 98 | 149 (48–398) | ✓ | ✓ |
| SN-119 (M)e | 118 | Yes | 10 | … | ✓ | ✓ |
| SN-030 | 66 | Yes | 0 | 0 | … | … |
| SN-006 (M) | 21 | Yes | 0 | 0 | … | … |
| SN-010 | 16 | Yes | 0 | 0 | … | … |
| SN-066 | 7 | Yes | 0 | 0 | … | … |
| SN-007 | 5 | Yes | 0 | 0 | … | … |
| SN-070e | 0 | Yes | 8 | 1 (1–2) | ✓ | ✓ |
| SN-086e | 0 | Yes | ?f | 0 | … | ✓ |
Abbreviations: M, mixed Plasmodium falciparum/Plasmodium vivax infection; qPCR, quantitative polymerase chain reaction; RT-PCR, reverse-transcriptase polymerase chain reaction.
a All patients with either smear-detectable P. falciparum gametocytes or successful mosquito infection, demonstrated either by midgut inspection or with qPCR, are listed. In patients with mixed P. falciparum/P. vivax infection, detection of P. falciparum–specific mosquito infection relied on qPCR of individual mosquitoes.
b The percentage of mosquitoes infected was based on oocyst detection among 50 mosquitoes (for patients with P. falciparum monoinfection) or the proportion of 30 mosquitoes that were qPCR positive at day 9 after feeding (for patients with mixed infections).
c Medians and ranges are given for oocyst-positive mosquitoes only; they were not reported for 2 patients with mixed infection because the species of individual oocysts was not determined.
d In patients with mixed infection, this result was reported as positive if falciparum qPCR–positive mosquitoes were found and oocysts were visualized in midgut dissection.
e These 7 patients were considered infectious in statistical calculations.
f Two of 5 and 5 of 5 pools of mosquitoes were qPCR positive at days 9 and 16 after feeding, respectively, but oocysts were not seen.
Based on microscopic detection of oocysts in the mosquito midgut, only 5 (4.2%) of the 119 patients successfully infected ≥1 mosquito of 50 (SN-60, SN-002, SN-108, SN-119 [M, for mixed infection], and SN-070 [M] in Table 1). Another 2 patients were deemed infectious based on PCR positivity of fed mosquitoes (SN-063 [M] and SN-086). Although oocysts were not detected in these cases, multiple mosquitoes/mosquito pools were PCR-positive at both days 9 and 16 after feeding, with robust cycle threshold values in the presence of negative controls. Furthermore, in both patients, subsequent mosquito feeding assays performed after treatment revealed many oocyst-positive mosquitoes at multiple time points (data not shown). Thus, we believe these patients were indeed infectious, but perhaps oocysts had already ruptured or were missed at dissection. Two of the 7 total infectious patients had mixed P. falciparum/P. vivax infections, in which the presence of oocysts could be due to P. falciparum and/or P. vivax infection. However, in both patients, multiple individual mosquitoes were positive with P. falciparum–specific qPCR. In total, 5950 mosquitoes that fed on blood from 119 patients underwent midgut dissection, of which 106 (1.8%) harbored visible falciparum parasites.
We explored the relationship of gametocytemia to mosquito infectivity within the cohort. Of the 7 infectious patients, 5 had patent falciparum gametocytes visible by smear, and 2 harbored submicroscopic gametocytes detectable only with RT-PCR (Table 1). The 5 transmitting patients with patent gametocytemia (SN-060, SN-002, SN-063 [M], SN-108, and SN-119 [M]) represented 50% (5 of 10) of all patients with patent gametocytemia, and each displayed >100 gametocytes/µL on smear (Figure 1). As a group, they infected roughly half of mosquitoes (145 of 250; 58%), and infected mosquitoes harbored a median of 83 oocysts per mosquito (range, 1–398 among the 3 patients without mixed infection). In contrast, the 2 patients with submicroscopic gametocytes who also infected mosquitoes (SN-070 and SN-086) represented just 5.1% (2/39) of the patients with subpatent gametocytes (2 of 39). One of these patients, SN-070, infected 8%of mosquitoes (4 of 50) with 1–2 oocysts per infected mosquito. The proportion of infected mosquitoes could not be determined in SN-086, because oocysts were not seen, and PCR was performed on pools of mosquitoes, of which 2 of 5 pools were positive at day 9 after feeding (Table 1).
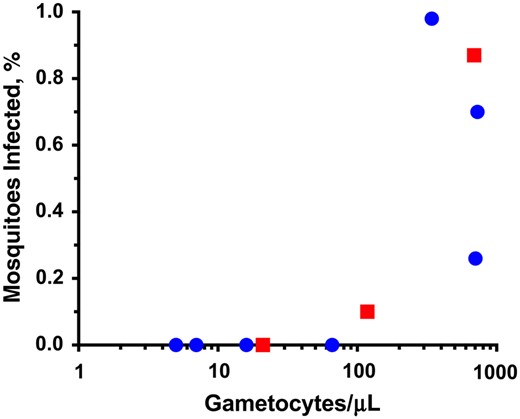
Relationship of smear-detectable gametocytemia to prevalence of mosquito infection. Because of the logarithmic scale, only patients with smear-detectable gametocytes are depicted. Circles represent patients with Plasmodium falciparum monoinfection; squares, those with mixed P. falciparum/P. vivax infection. For patients with mixed infection, the percentage of infected mosquitoes was calculated as the proportion of 30 mosquitoes with positive falciparum-specific quantitative polymerase chain reaction results at day 9 after feeding.
Keeping in mind the relatively small number of cases, we compared the mosquito infectivity of groups of patients based on their gametocyte carriage status to estimate their relative contribution to human-to-mosquito transmission in the cohort. Compared with the 39 patients with submicroscopic gametocytemia detectable only with RT-PCR, the 10 patients with patent gametocytes displayed roughly 10-fold increased infectivity (50% vs 5.1% infected any mosquitoes) and a roughly 100-fold difference in the total number of mosquitoes they infected (102 of 500 vs 4 of 1950 infected mosquitoes with visible oocysts). If microscopy alone were used to determine gametocyte status, then there was >20-fold increased infectivity among those with patent gametocytes (50% vs 1.8%) versus no gametocytes seen on smear. Thus, the one-third of patients with submicroscopic gametocytes contributed to only 4% of overall human-to-mosquito transmission in the cohort (4 of 106 infected mosquitoes), whereas 96% of infected mosquitoes fed on patients with microscopic gametocytemia.
DISCUSSION
We present the first mosquito feeding study to directly measure the contribution of submicroscopic gametocytemia to the infectious reservoir among malaria patients in a low-transmission setting. Widespread use of PCR over the last decade has revealed a large reservoir of persons with submicroscopic parasitemia, raising the question of whether more sensitive diagnostics are needed to detect all those who contribute to the infectious reservoir [8, 12, 13]. To date, this question has only been studied in settings in Africa with relatively intense transmission [6–8]. However, this information is most relevant for informing malaria elimination strategies in areas where transmission has reached very low levels.
In a low-transmission setting in northern Cambodia, we found that a small minority of subjects with malaria—just 5.9% (7 of 119)—were infectious to A. dirus, the main local vector for malaria. Furthermore, the 5 subjects who contributed to 96% of transmission, based on the number of mosquitoes infected, could all be identified by gametocytes visible on a blood smear. This argues against the need for ultrasensitive diagnostics to identify those who contribute the most to ongoing malaria transmission.
These results contrast sharply with findings from Burkina Faso where more than one-third of all comers (not just those with clinically apparent malaria) are infectious to mosquitoes, and submicroscopic gametocyte carriers contribute to nearly half of the infectious reservoir [6, 8] (Supplementary Table 2). Several reasons probably underlie this difference. Most obviously, the overall gametocyte prevalence of 41% by RT-PCR in our study is roughly half that found in Burkina Faso by quantitative nucleic acid sequence-based amplification (67%–93%) [6, 8]. This difference may in part reflect the younger age of subjects in the African studies, where the majority of participants were children, who are more likely to be gametocytemic [3], whereas our study sampled only adults, who more commonly present with falciparum malaria in this setting owing to their work in forested areas. Local transmission intensity itself also seems to shape overall gametocyte carriage rates [14]. As malaria prevalence has decreased in western Cambodia over the last decade, we have noted a drop in patent gametocyte carriage among falciparum-infected subjects enrolled in Armed Forces Research Institute of Medical Sciences clinical trials, from 22% prevalence in 2006–2007 to 15% in 2008–2009 and 8.4% in 2012–2014 in the present study [11]. Finally, the paucity of infectious individuals in our study may relate to differences in vector competence. It has been observed that anopheline mosquitoes from the New World are less efficient vectors of malaria than African ones, partly accounting for lower transmission in South America [15]. Anopheles dirus in Southeast Asia, which have coevolved with both P. vivax and P. falciparum, may exhibit different susceptibility to local P. falciparum strains than the Anopheles gambiae used in most African studies.
Regarding the limitations of membrane feeding assays for measuring infectiousness, the use of insectary-bred mosquitoes may underestimate infectivity when compared with direct feeding, use of wild-caught vectors, or field-based xenodiagnosis. However, this method serves as a practical standard for assessing infectiousness in endemic populations [1]. We also did not perform serum replacement with control serum from malaria-naive individuals as is sometimes done to remove possible transmission-blocking effects of anti-gametocyte immunity, because we were interested in the natural infectiousness of patients.
In conclusion, although malaria elimination strategies in low-transmission settings have focused increasingly on targeting the submicroscopic reservoir of infection, we found that among a group of 119 symptomatic adults in Cambodia, just 5 individuals with gametocytes visible by blood smear contributed to the bulk of human-to- mosquito transmission in the cohort. More studies in low-transmission settings, especially those involving the larger asymptomatic reservoir, are needed to complement our findings and guide malaria elimination efforts.
Notes
Acknowledgments. We thank the study volunteers, as well as the dedicated Cambodian and Thai laboratory, entomology, and clinical support staff who made the study possible.
Disclaimer. The views expressed in this paper are those of the authors and are not to be construed as official or as reflecting the views of the US Department of the Army.
Financial support. This work was supported by the Armed Forces Health Surveillance Center/Global Emerging Infections Surveillance and Response System, Military Infectious Disease Research Program, National Institute of Allergy and Infectious Diseases (K08 AI110651 to J. T. L.), American Society of Tropical Medicine and Hygiene (postdoctoral fellowship in tropical infectious diseases to J. T. L. and Benjamin Kean travel fellowship to Christian Parobek), and Burroughs Wellcome Fund (aforementioned fellowship to J. T. L.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 63rd Annual Meeting of The American Society of Tropical Medicine and Hygiene, New Orleans, Louisiana, 5 November 2014.




