-
PDF
- Split View
-
Views
-
Cite
Cite
Lucas Etienne Hermans, Valentina Svicher, Suzan Diepstraten Pas, Romina Salpini, Marta Alvarez, Ziv Ben Ari, Greet Boland, Bianca Bruzzone, Nicola Coppola, Carole Seguin-Devaux, Tomasz Dyda, Federico Garcia, Rolf Kaiser, Sukran Köse, Henrik Krarup, Ivana Lazarevic, Maja M. Lunar, Sarah Maylin, Valeria Micheli, Orna Mor, Simona Paraschiv, Dimitrios Paraskevis, Mario Poljak, Elisabeth Puchhammer-Stöckl, François Simon, Maja Stanojevic, Kathrine Stene-Johansen, Nijaz Tihic, Pascale Trimoulet, Jens Verheyen, Adriana Vince, Nina Weis, Tülay Yalcinkaya, Snjezana Zidovec Lepej, Carlo Perno, Charles A. B. Boucher, Annemarie M. J. Wensing, on behalf of the HEPVIR working group of the European Society for Translational Antiviral Research (ESAR), Combined Analysis of the Prevalence of Drug-Resistant Hepatitis B Virus in Antiviral Therapy–Experienced Patients in Europe (CAPRE), The Journal of Infectious Diseases, Volume 213, Issue 1, 1 January 2016, Pages 39–48, https://doi.org/10.1093/infdis/jiv363
Close - Share Icon Share
Abstract
European guidelines recommend treatment of chronic hepatitis B virus infection (CHB) with the nucleos(t)ide analogs (NAs) entecavir or tenofovir. However, many European CHB patients have been exposed to other NAs, which are associated with therapy failure and resistance. The CAPRE study was performed to gain insight in prevalence and characteristics of NA resistance in Europe.
A survey was performed on genotypic resistance testing results acquired during routine monitoring of CHB patients with detectable serum hepatitis B virus DNA in European tertiary referral centers.
Data from 1568 patients were included. The majority (73.8%) were exposed to lamivudine monotherapy. Drug-resistant strains were detected in 52.7%. The most frequently encountered primary mutation was M204V/I (48.7%), followed by A181T/V (3.8%) and N236T (2.6%). In patients exposed to entecavir (n = 102), full resistance was present in 35.3%. Independent risk factors for resistance were age, viral load, and lamivudine exposure (P < .001).
These findings support resistance testing in cases of apparent NA therapy failure. This survey highlights the impact of exposure to lamivudine and adefovir on development of drug resistance and cross-resistance. Continued use of these NAs needs to be reconsidered at a pan-European level.
Infection with hepatitis B virus (HBV) is among the most prevalent infectious diseases, affecting an estimated 240 million patients globally [1]. Prevalence of chronic infection with HBV (CHB) is generally low in Western, Northern, and Central Europe, where prevalence estimates range between 0.1%–0.7%, but considerably higher in Eastern and Southern European countries, such as Italy (0.2%–4.3%), Romania (5.6%), and Turkey (2.5%–9%) [2, 3].
Since the first clinical trials with lamivudine (LAM) in CHB patients [4], nucleos(t)ide analogs (NAs) have become widely used to treat CHB [5]. NAs target the reverse transcriptase-DNA-polymerase enzyme, thus suppressing viral replication [6]. However, in the presence of antiviral therapy, mutations in the reverse transcriptase (RT) gene can be selected, rendering the virus partially or completely resistant to specific antiviral drugs [7]. Monotherapy with LAM, telbivudine (LdT), and adefovir (ADV) have been shown to be an important risk factor for selection of resistance. In previous studies, drug resistance occurred in 14%–20% of patients treated with LAM per treatment year, and almost 80% of patients harbor LAM-resistant strains after 5 years of treatment [8–10].
Drug resistance and concomitant viremia can have potentially severe clinical consequences, as they can induce progression of liver disease. Furthermore, selection of resistance can induce cross-resistance, affecting future therapeutic options [7]. As the open-reading frames of the RT gene and surface antigen gene (HBsAg) partially overlap, some drug resistance mutations lead to conformational changes in the HBsAg structure that lower the antigenicity of HBsAg [11, 12]. This phenomenon poses a potential public health concern, as these viral strains are less effectively targeted by the neutralizing antibodies induced by conventional HBV vaccines that target HBsAg [13, 14].
Development of HBV drug resistance can be largely avoided since the introduction of entecavir (ETV) and tenofovir (TDF), which were registered in Europe in 2006 and 2009 respectively [15]. When administered as first-line treatment, the risk of developing HBV drug resistance is around 1% in case of ETV after 3 years of treatment [15, 16], and virtually absent in case of TDF [17, 18]. The guidelines of the European Association for Study of the Liver (EASL) recommend ETV or TDF as first-line monotherapy and discourages monotherapy with LAM, ADV, and LdT [5]. In contrast with this recommendation, recent studies have shown that drug prescription practices vary across Europe, and that first-line monotherapy with LAM, ADV, and LdT was still frequently prescribed in several European countries between 2008–2010 [19, 20]. Furthermore, cost-effectiveness studies have argued for the continued use of LAM either as first-line treatment in selected patients or as follow-up after initial suppression with TDF [21–23].
While older NAs continue to be used in European clinical practice, data on the prevalence and patterns of HBV drug resistance, and the relative contribution of different regimens on drug resistance are scarce. This study aims to survey the prevalence and patterns of HBV drug resistance in European patients that experience failure of NA therapy. These data give insight into the impact of different treatment regimens and other risk factors on the development of drug resistance during failure of NA treatment.
METHODS
Study Population
A multicenter survey was performed on genotypic resistance testing results acquired during routine clinical assessments of patients with CHB attending tertiary referral centers in European countries. Patients were eligible if they met all of the following inclusion criteria: CHB with detectable serum HBV DNA, previous exposure to 1 or more nucleos(t)ide analogs, availability of a resistance testing result, and age ≥18 years.
Data Collection and Submission
Patient datasets were collected in the framework of the European Society for translational antiviral research (ESAR). Datasets were submitted from 18 countries (Austria, Bosnia and Herzegovina, Croatia, Denmark, France, Germany, Greece, Israel, Italy, Norway, Luxembourg, the Netherlands, Poland, Romania, Serbia, Slovenia, Spain, and Turkey). In 3 countries (Denmark, France, Italy), data originated from multiple centers. Datasets were uploaded to a central database, and queries were sent to submitters if data quality problems were encountered. Ethical approval for the study was acquired by submitters in cases where national regulations require ethical approval for observational studies. Participating centers or researchers did not receive financial reimbursement for data submission.
Data Characteristics
Clinical data and virological test results obtained during routine clinical testing between January 1998 and August 2012 were collected. Datasets consisted of at least a genotypic-resistance testing result, specification of the type of test that was used, a draw date of the sample and the motivation for resistance testing, patient gender, and center of origin. Clinical information was obtained on serum HBV DNA; hepatitis B surface antigen (HBsAg); hepatitis B e antigen (HBeAg); anti-HBe; serum–alanine aminotransferase (serum-ALT); serological screening for coinfections with human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis D virus (HDV); exposure to at least 1 NA (LAM, LdT, ADV, ETV, TDF); and exposure to (peg–)interferon.
Local laboratories performed genotypic resistance testing using population-based sequence analysis of HBV pol or a line probe assay (INNO-LiPA HBV DR v2 or v3, Innogenetics, Belgium). Sequence data consisted of FASTA files containing nucleic acid sequence information of the RT region of the polymerase gene, covering at least the region of HBV pol between amino acid position 180 and 236. In LiPAs, mutations conferring resistance to ETV (rtT184G, rtM250V, rtS202I) and TDF (rtA194T) were not included prior to August 2008. The ESAR quality control procedure was performed on all submitted sequences. If amino acid substitutions at drug-resistance codons were due to ambiguities consisting of >2 bases per nucleotide position or >1 ambiguities per codon, or if insertions or deletions were present causing a shift in the pol open-reading frame that affected drug-resistance mutation codons, sequences were excluded from the analysis.
Data Analysis
Mutations in the RT region of the polymerase gene at amino acid location rtA181T/V, rtM204V/I/S, rtN236T, and rtM250I/V were interpreted as primary resistance mutations, while rtL80V/I, rtI169T, rtV173L, rtL180M, rtT184A/C/G/S, and rtS202C/G/I were considered secondary or compensatory mutations [24–26]. Mutations linked to resistance to ETV (rtT184G, rtM250I/V, rtS202C/G/I) were not included in some assays prior to the introduction of these compounds.
Sequence alignment and analysis of amino acid substitutions were performed using the publicly available Geno2pheno[HBV] drug-resistance interpretation algorithm [27]. Resistance to antiviral compounds was graded according to the 2012 EASL Clinical Practice guidelines [5]. Intermediate resistance to ETV was defined as the presence of rtM204I or rtM204V + rtL180M. Full resistance to ETV was defined as the presence of rtM204V/I +rtL180M and 1 or more of the following: rtI169T, rtV173L, rtT184A/C/G/S, rtS202C/G/I, or rtM250V. Intermediate resistance to TDF was defined as the presence of rtN236T.
For the purpose of statistical analysis, countries were grouped in geographical regions according to the definitions used by the United Nations Statistic Division (http://unstats.un.org/unsd/default.htm) as follows: Northern Europe (Denmark, Norway), Western Europe (Austria, France, Germany, Luxembourg, the Netherlands), Eastern Europe (Poland, Romania), and Southern Europe (Bosnia and Herzegovina, Croatia, Greece, Italy, Serbia, Slovenia, Spain). Israel and Turkey were grouped with Southern European countries. For time analysis, sample draw dates were categorized in 4 time periods, reflecting the introduction of different NAs in Europe: 1998–2002 (LAM), 2003–2005 (ADV), 2006–2008 (ETV), and 2009–2012 (TDF).
Statistical data were analyzed in SPSS 16.0 (IBM, New York, New York) using Student t test for continuous variables and χ2 test for discrete variables. Dichotomous variables that were significant in univariate analysis (P < .05), as well as categorical values (viral genotype, region of origin, and time period) were included in multivariate analysis.
RESULTS
Study Population
Data of 1648 patients from 18 countries were submitted. Inclusion criteria were not met in 34 cases (2.1%) and sequence quality criteria were not met in 46 cases (2.8%). The remaining 1568 patients were included in the analysis. Of these, 72.1% were male, with a median age of 46 years (interquartile range [IQR]: 35–57). In 20.9% of cases, HIV coinfection was reported. Coinfection with HCV was reported in 3.7% of cases and coinfection with HDV in 1.1% of cases. Genotype D was the most frequently encountered viral genotype (63%), followed by genotype A (26%) (Table 1).
| Parameter . | Data Available (n) . | Units . | 1568 . |
|---|---|---|---|
| Sex | |||
| n = 1523 | Male % (n) | 72.1% (1098) | |
| Age | |||
| n = 1258 | median [IQR] (y) | 46 [35–57] | |
| Resistance | |||
| n = 1568 | resistant % (n) | 52.7% (827) | |
| HBV status | |||
| Log HBV DNA | n = 1341 | Median [IQR] (IU/mL) | 4.8 [3.3–7.1] |
| Serum-ALT | n = 633 | 51 [33–93] | |
| HBeAg | n = 831 | pos % | 52.8% (439) |
| Coinfections | |||
| anti-HIV | n = 611 | pos % (n) | 20.9% (128) |
| anti-HCV | n = 671 | 3.7% (25) | |
| anti-HDV | n = 439 | 1.1% (5) | |
| Detection | n = 1568 | ||
| Sequence analysis | pos % (n) | 54.7% (857) | |
| Line probe assay | 43.4% (681) | ||
| Both | 1.9% (30) | ||
| HBV genotype | n = 887 | ||
| A | pos % (n) | 26.3% (233) | |
| B | 3.2% (28) | ||
| C | 3.9% (35) | ||
| D | 63.1% (560) | ||
| E | 2.1% (19) | ||
| F-H | 1.4% (12) | ||
| Drug history | n = 1317 | ||
| Monotherapy | LAM | 73.8% (972) | |
| ADV | 4.5% (59) | ||
| ETV | 3.8% (50) | ||
| TDF | 1.4% (18) | ||
| LdT | 0.2% (3) | ||
| Dual exposure | LAM+ADV | 8.7% (115) | |
| LAM+ETV | 2.4% (32) | ||
| LAM+TDF | 2.1% (27) | ||
| other | 1.7% (23) | ||
| Triple exposure | LAM+ADV+ETV | 0.5% (6) | |
| LAM+ADV+TDF | 0.4% (5) | ||
| other | 0.5% (6) | ||
| Quadruple exposure | LAM+LdT+ADV+TDF | 0.1% (1) | |
| Parameter . | Data Available (n) . | Units . | 1568 . |
|---|---|---|---|
| Sex | |||
| n = 1523 | Male % (n) | 72.1% (1098) | |
| Age | |||
| n = 1258 | median [IQR] (y) | 46 [35–57] | |
| Resistance | |||
| n = 1568 | resistant % (n) | 52.7% (827) | |
| HBV status | |||
| Log HBV DNA | n = 1341 | Median [IQR] (IU/mL) | 4.8 [3.3–7.1] |
| Serum-ALT | n = 633 | 51 [33–93] | |
| HBeAg | n = 831 | pos % | 52.8% (439) |
| Coinfections | |||
| anti-HIV | n = 611 | pos % (n) | 20.9% (128) |
| anti-HCV | n = 671 | 3.7% (25) | |
| anti-HDV | n = 439 | 1.1% (5) | |
| Detection | n = 1568 | ||
| Sequence analysis | pos % (n) | 54.7% (857) | |
| Line probe assay | 43.4% (681) | ||
| Both | 1.9% (30) | ||
| HBV genotype | n = 887 | ||
| A | pos % (n) | 26.3% (233) | |
| B | 3.2% (28) | ||
| C | 3.9% (35) | ||
| D | 63.1% (560) | ||
| E | 2.1% (19) | ||
| F-H | 1.4% (12) | ||
| Drug history | n = 1317 | ||
| Monotherapy | LAM | 73.8% (972) | |
| ADV | 4.5% (59) | ||
| ETV | 3.8% (50) | ||
| TDF | 1.4% (18) | ||
| LdT | 0.2% (3) | ||
| Dual exposure | LAM+ADV | 8.7% (115) | |
| LAM+ETV | 2.4% (32) | ||
| LAM+TDF | 2.1% (27) | ||
| other | 1.7% (23) | ||
| Triple exposure | LAM+ADV+ETV | 0.5% (6) | |
| LAM+ADV+TDF | 0.4% (5) | ||
| other | 0.5% (6) | ||
| Quadruple exposure | LAM+LdT+ADV+TDF | 0.1% (1) | |
Dual/triple/quadruple exposure: exposure to respectively 2, 3, or 4 NAs, either concurrent or sequential.
Coinfections: detection of antibodies to HCV, HDV, and/or HIV.
Abbreviations: ADV, adefovir; ALT, alanine aminotransferase; ETV, entecavir; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IQR, interquartile range, LAM, lamivudine; LdT, telbivudine; NAs, nucleos(t)ide analogs; TDF, tenofovir.
| Parameter . | Data Available (n) . | Units . | 1568 . |
|---|---|---|---|
| Sex | |||
| n = 1523 | Male % (n) | 72.1% (1098) | |
| Age | |||
| n = 1258 | median [IQR] (y) | 46 [35–57] | |
| Resistance | |||
| n = 1568 | resistant % (n) | 52.7% (827) | |
| HBV status | |||
| Log HBV DNA | n = 1341 | Median [IQR] (IU/mL) | 4.8 [3.3–7.1] |
| Serum-ALT | n = 633 | 51 [33–93] | |
| HBeAg | n = 831 | pos % | 52.8% (439) |
| Coinfections | |||
| anti-HIV | n = 611 | pos % (n) | 20.9% (128) |
| anti-HCV | n = 671 | 3.7% (25) | |
| anti-HDV | n = 439 | 1.1% (5) | |
| Detection | n = 1568 | ||
| Sequence analysis | pos % (n) | 54.7% (857) | |
| Line probe assay | 43.4% (681) | ||
| Both | 1.9% (30) | ||
| HBV genotype | n = 887 | ||
| A | pos % (n) | 26.3% (233) | |
| B | 3.2% (28) | ||
| C | 3.9% (35) | ||
| D | 63.1% (560) | ||
| E | 2.1% (19) | ||
| F-H | 1.4% (12) | ||
| Drug history | n = 1317 | ||
| Monotherapy | LAM | 73.8% (972) | |
| ADV | 4.5% (59) | ||
| ETV | 3.8% (50) | ||
| TDF | 1.4% (18) | ||
| LdT | 0.2% (3) | ||
| Dual exposure | LAM+ADV | 8.7% (115) | |
| LAM+ETV | 2.4% (32) | ||
| LAM+TDF | 2.1% (27) | ||
| other | 1.7% (23) | ||
| Triple exposure | LAM+ADV+ETV | 0.5% (6) | |
| LAM+ADV+TDF | 0.4% (5) | ||
| other | 0.5% (6) | ||
| Quadruple exposure | LAM+LdT+ADV+TDF | 0.1% (1) | |
| Parameter . | Data Available (n) . | Units . | 1568 . |
|---|---|---|---|
| Sex | |||
| n = 1523 | Male % (n) | 72.1% (1098) | |
| Age | |||
| n = 1258 | median [IQR] (y) | 46 [35–57] | |
| Resistance | |||
| n = 1568 | resistant % (n) | 52.7% (827) | |
| HBV status | |||
| Log HBV DNA | n = 1341 | Median [IQR] (IU/mL) | 4.8 [3.3–7.1] |
| Serum-ALT | n = 633 | 51 [33–93] | |
| HBeAg | n = 831 | pos % | 52.8% (439) |
| Coinfections | |||
| anti-HIV | n = 611 | pos % (n) | 20.9% (128) |
| anti-HCV | n = 671 | 3.7% (25) | |
| anti-HDV | n = 439 | 1.1% (5) | |
| Detection | n = 1568 | ||
| Sequence analysis | pos % (n) | 54.7% (857) | |
| Line probe assay | 43.4% (681) | ||
| Both | 1.9% (30) | ||
| HBV genotype | n = 887 | ||
| A | pos % (n) | 26.3% (233) | |
| B | 3.2% (28) | ||
| C | 3.9% (35) | ||
| D | 63.1% (560) | ||
| E | 2.1% (19) | ||
| F-H | 1.4% (12) | ||
| Drug history | n = 1317 | ||
| Monotherapy | LAM | 73.8% (972) | |
| ADV | 4.5% (59) | ||
| ETV | 3.8% (50) | ||
| TDF | 1.4% (18) | ||
| LdT | 0.2% (3) | ||
| Dual exposure | LAM+ADV | 8.7% (115) | |
| LAM+ETV | 2.4% (32) | ||
| LAM+TDF | 2.1% (27) | ||
| other | 1.7% (23) | ||
| Triple exposure | LAM+ADV+ETV | 0.5% (6) | |
| LAM+ADV+TDF | 0.4% (5) | ||
| other | 0.5% (6) | ||
| Quadruple exposure | LAM+LdT+ADV+TDF | 0.1% (1) | |
Dual/triple/quadruple exposure: exposure to respectively 2, 3, or 4 NAs, either concurrent or sequential.
Coinfections: detection of antibodies to HCV, HDV, and/or HIV.
Abbreviations: ADV, adefovir; ALT, alanine aminotransferase; ETV, entecavir; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IQR, interquartile range, LAM, lamivudine; LdT, telbivudine; NAs, nucleos(t)ide analogs; TDF, tenofovir.
Treatment History and Drug-Resistance Profiles
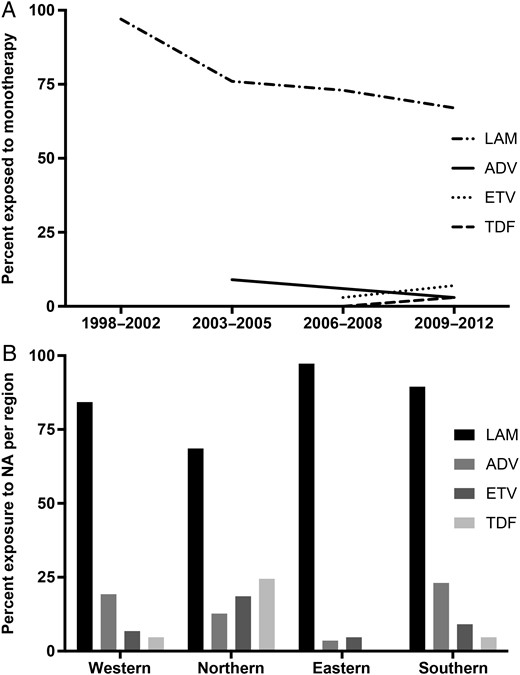
Treatment information was available for 1317 patients. Of these, 1102 (83.7%) were exposed to NA monotherapy, predominantly with LAM (73.8% [972/1317]), followed by ADV (4.5% [59/1317]), ETV (3.8% [50/1317]), TDF (1.4% [18/1317]), and LdT (0.2% [3/1317]). The prevalence of LAM monotherapy was 97% in the earliest time period covered in the analysis (1998–2002) and remained the most frequently encountered regimen (67%) in the most recent time period (2009–2012) (Figure 1A). Exposure to 2 NAs, either simultaneously or consecutively, was present in 197 patients, and most frequently concerned LAM+ADV (8.7% [115/1317]), followed by LAM+ETV (2.4% [32/1317]), LAM+TDF (2.1% [27/1317]), and LAM+LdT (0.9% [12/1317]). Triple exposure was present in 17 cases (1.3%), of which 6 (0.5%) received LAM+ADV+ETV and 5 (0.4%) received LAM+ADV+TDF. Exposure to 4 different NAs (LAM+ADV+TDF+LdT) was present in 1 case. Distribution of NA exposure differed between European regions (Figure 1B).
In 52.7% (827/1568) of patient-derived sequences, 1 or more primary drug-resistance mutations were detected. The most frequently encountered primary mutation was rtM204V/I (48.7% [763/1568]), associated with LAM and LdT use, followed by rtA181T/V (3.8% [60/1568]) and rtN236T (2.6% [40/1568]), conferring resistance to ADV. The mutation rtM204V/I was accompanied by additional mutations in 73.0% (557/763) of cases (Table 2).
| Mutational Pattern . | n = . | % of Mutated Strains . | ||||
|---|---|---|---|---|---|---|
| Full resistance to LAM | ||||||
| rtM204I | 133 | 16.1 | ||||
| … | + rtL80IV | 75 | 9.1 | |||
| … | + rtL180M | 29 | 3.5 | |||
| … | + rtL80IV | + rtL180M | 28 | 3.4 | ||
| rtM204V | 53 | 6.4 | ||||
| … | + rtL180M | 240 | 29.0 | |||
| … | + rtL180M | + rtL80IV | 23 | 2.8 | ||
| … | + rtL80IV | 4 | 0.5 | |||
| rtM204VI | 20 | 2.4 | ||||
| … | + rtL180M | 9 | 1.1 | |||
| … | + rtL180M | + rtL80IV | 5 | 0.6 | ||
| … | + rtL80IV | 2 | 0.2 | |||
| Full resistance to LAM and ETV | ||||||
| rtM204V | + rtL180M | + rtV173L | 63 | 7.6 | ||
| … | … | … | + rtL80IV | 6 | 0.7 | |
| … | … | … | … | + rtM250V | 1 | 0.1 |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtT184ASCG | 21 | 2.5 | ||
| … | … | + rtS202CGI | 6 | 0.7 | ||
| … | … | + rtM250V | 2 | 0.2 | ||
| … | … | + rtI169T | + rtT184ASCG | 2 | 0.2 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| rtM204I | + rtV173L | 5 | 0.6 | |||
| … | … | + rtL180M | 2 | 0.2 | ||
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| … | + rtL180M | + rtL80IV | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtM250V | 1 | 0.1 | ||
| … | + rtT184ASCG | 4 | 0.5 | |||
| rtM204VI | + rtL180M | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL80IV | 1 | 0.1 | |
| … | … | + rtS202CGI | + rtM250V | 1 | 0.1 | |
| … | + rtV173L | 3 | 0.4 | |||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | 2 | 0.2 | |||
| … | + rtN236T | 1 | 0.1 | |||
| … | … | + rtL180M | + rtL80IV | 1 | 0.1 | |
| rtM204V | + rtA181TV | 1 | 0.1 | |||
| … | … | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL180M | 1 | 0.1 | |
| … | + rtN236T | 1 | 0.1 | |||
| rtM204VI | + rtN236T | 1 | 0.1 | |||
| … | … | + rtA181TV | + rtL80IV | 1 | 0.1 | |
| … | … | … | … | + rtL180M | 1 | 0.1 |
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204I | + rtA181TV | + rtL180M | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| rtM204V | + rtN236T | + rtV173L | + rtL180M | 2 | 0.2 | |
| rtM204VI | + rtA181TV | + rtV173L | 1 | 0.1 | ||
| Full resistance to ADV | ||||||
| rtA181TV | 30 | 3.6 | ||||
| …… | + rtN236T | 18 | 2.2 | |||
| …… | + rtL180M | 2 | 0.2 | |||
| rtN236T | 14 | 1.7 | ||||
| Mutational Pattern . | n = . | % of Mutated Strains . | ||||
|---|---|---|---|---|---|---|
| Full resistance to LAM | ||||||
| rtM204I | 133 | 16.1 | ||||
| … | + rtL80IV | 75 | 9.1 | |||
| … | + rtL180M | 29 | 3.5 | |||
| … | + rtL80IV | + rtL180M | 28 | 3.4 | ||
| rtM204V | 53 | 6.4 | ||||
| … | + rtL180M | 240 | 29.0 | |||
| … | + rtL180M | + rtL80IV | 23 | 2.8 | ||
| … | + rtL80IV | 4 | 0.5 | |||
| rtM204VI | 20 | 2.4 | ||||
| … | + rtL180M | 9 | 1.1 | |||
| … | + rtL180M | + rtL80IV | 5 | 0.6 | ||
| … | + rtL80IV | 2 | 0.2 | |||
| Full resistance to LAM and ETV | ||||||
| rtM204V | + rtL180M | + rtV173L | 63 | 7.6 | ||
| … | … | … | + rtL80IV | 6 | 0.7 | |
| … | … | … | … | + rtM250V | 1 | 0.1 |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtT184ASCG | 21 | 2.5 | ||
| … | … | + rtS202CGI | 6 | 0.7 | ||
| … | … | + rtM250V | 2 | 0.2 | ||
| … | … | + rtI169T | + rtT184ASCG | 2 | 0.2 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| rtM204I | + rtV173L | 5 | 0.6 | |||
| … | … | + rtL180M | 2 | 0.2 | ||
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| … | + rtL180M | + rtL80IV | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtM250V | 1 | 0.1 | ||
| … | + rtT184ASCG | 4 | 0.5 | |||
| rtM204VI | + rtL180M | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL80IV | 1 | 0.1 | |
| … | … | + rtS202CGI | + rtM250V | 1 | 0.1 | |
| … | + rtV173L | 3 | 0.4 | |||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | 2 | 0.2 | |||
| … | + rtN236T | 1 | 0.1 | |||
| … | … | + rtL180M | + rtL80IV | 1 | 0.1 | |
| rtM204V | + rtA181TV | 1 | 0.1 | |||
| … | … | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL180M | 1 | 0.1 | |
| … | + rtN236T | 1 | 0.1 | |||
| rtM204VI | + rtN236T | 1 | 0.1 | |||
| … | … | + rtA181TV | + rtL80IV | 1 | 0.1 | |
| … | … | … | … | + rtL180M | 1 | 0.1 |
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204I | + rtA181TV | + rtL180M | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| rtM204V | + rtN236T | + rtV173L | + rtL180M | 2 | 0.2 | |
| rtM204VI | + rtA181TV | + rtV173L | 1 | 0.1 | ||
| Full resistance to ADV | ||||||
| rtA181TV | 30 | 3.6 | ||||
| …… | + rtN236T | 18 | 2.2 | |||
| …… | + rtL180M | 2 | 0.2 | |||
| rtN236T | 14 | 1.7 | ||||
n = % of mutated strains: prevalence of pattern in total of group with primary resistance mutations.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine.
| Mutational Pattern . | n = . | % of Mutated Strains . | ||||
|---|---|---|---|---|---|---|
| Full resistance to LAM | ||||||
| rtM204I | 133 | 16.1 | ||||
| … | + rtL80IV | 75 | 9.1 | |||
| … | + rtL180M | 29 | 3.5 | |||
| … | + rtL80IV | + rtL180M | 28 | 3.4 | ||
| rtM204V | 53 | 6.4 | ||||
| … | + rtL180M | 240 | 29.0 | |||
| … | + rtL180M | + rtL80IV | 23 | 2.8 | ||
| … | + rtL80IV | 4 | 0.5 | |||
| rtM204VI | 20 | 2.4 | ||||
| … | + rtL180M | 9 | 1.1 | |||
| … | + rtL180M | + rtL80IV | 5 | 0.6 | ||
| … | + rtL80IV | 2 | 0.2 | |||
| Full resistance to LAM and ETV | ||||||
| rtM204V | + rtL180M | + rtV173L | 63 | 7.6 | ||
| … | … | … | + rtL80IV | 6 | 0.7 | |
| … | … | … | … | + rtM250V | 1 | 0.1 |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtT184ASCG | 21 | 2.5 | ||
| … | … | + rtS202CGI | 6 | 0.7 | ||
| … | … | + rtM250V | 2 | 0.2 | ||
| … | … | + rtI169T | + rtT184ASCG | 2 | 0.2 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| rtM204I | + rtV173L | 5 | 0.6 | |||
| … | … | + rtL180M | 2 | 0.2 | ||
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| … | + rtL180M | + rtL80IV | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtM250V | 1 | 0.1 | ||
| … | + rtT184ASCG | 4 | 0.5 | |||
| rtM204VI | + rtL180M | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL80IV | 1 | 0.1 | |
| … | … | + rtS202CGI | + rtM250V | 1 | 0.1 | |
| … | + rtV173L | 3 | 0.4 | |||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | 2 | 0.2 | |||
| … | + rtN236T | 1 | 0.1 | |||
| … | … | + rtL180M | + rtL80IV | 1 | 0.1 | |
| rtM204V | + rtA181TV | 1 | 0.1 | |||
| … | … | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL180M | 1 | 0.1 | |
| … | + rtN236T | 1 | 0.1 | |||
| rtM204VI | + rtN236T | 1 | 0.1 | |||
| … | … | + rtA181TV | + rtL80IV | 1 | 0.1 | |
| … | … | … | … | + rtL180M | 1 | 0.1 |
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204I | + rtA181TV | + rtL180M | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| rtM204V | + rtN236T | + rtV173L | + rtL180M | 2 | 0.2 | |
| rtM204VI | + rtA181TV | + rtV173L | 1 | 0.1 | ||
| Full resistance to ADV | ||||||
| rtA181TV | 30 | 3.6 | ||||
| …… | + rtN236T | 18 | 2.2 | |||
| …… | + rtL180M | 2 | 0.2 | |||
| rtN236T | 14 | 1.7 | ||||
| Mutational Pattern . | n = . | % of Mutated Strains . | ||||
|---|---|---|---|---|---|---|
| Full resistance to LAM | ||||||
| rtM204I | 133 | 16.1 | ||||
| … | + rtL80IV | 75 | 9.1 | |||
| … | + rtL180M | 29 | 3.5 | |||
| … | + rtL80IV | + rtL180M | 28 | 3.4 | ||
| rtM204V | 53 | 6.4 | ||||
| … | + rtL180M | 240 | 29.0 | |||
| … | + rtL180M | + rtL80IV | 23 | 2.8 | ||
| … | + rtL80IV | 4 | 0.5 | |||
| rtM204VI | 20 | 2.4 | ||||
| … | + rtL180M | 9 | 1.1 | |||
| … | + rtL180M | + rtL80IV | 5 | 0.6 | ||
| … | + rtL80IV | 2 | 0.2 | |||
| Full resistance to LAM and ETV | ||||||
| rtM204V | + rtL180M | + rtV173L | 63 | 7.6 | ||
| … | … | … | + rtL80IV | 6 | 0.7 | |
| … | … | … | … | + rtM250V | 1 | 0.1 |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtT184ASCG | 21 | 2.5 | ||
| … | … | + rtS202CGI | 6 | 0.7 | ||
| … | … | + rtM250V | 2 | 0.2 | ||
| … | … | + rtI169T | + rtT184ASCG | 2 | 0.2 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| rtM204I | + rtV173L | 5 | 0.6 | |||
| … | … | + rtL180M | 2 | 0.2 | ||
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| … | + rtL180M | + rtL80IV | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtV173L | 1 | 0.1 | |
| … | … | … | … | + rtT184ASCG | 1 | 0.1 |
| … | … | + rtM250V | 1 | 0.1 | ||
| … | + rtT184ASCG | 4 | 0.5 | |||
| rtM204VI | + rtL180M | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL80IV | 1 | 0.1 | |
| … | … | + rtS202CGI | + rtM250V | 1 | 0.1 | |
| … | + rtV173L | 3 | 0.4 | |||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | 2 | 0.2 | |||
| … | + rtN236T | 1 | 0.1 | |||
| … | … | + rtL180M | + rtL80IV | 1 | 0.1 | |
| rtM204V | + rtA181TV | 1 | 0.1 | |||
| … | … | + rtV173L | 1 | 0.1 | ||
| … | … | … | + rtL180M | 1 | 0.1 | |
| … | + rtN236T | 1 | 0.1 | |||
| rtM204VI | + rtN236T | 1 | 0.1 | |||
| … | … | + rtA181TV | + rtL80IV | 1 | 0.1 | |
| … | … | … | … | + rtL180M | 1 | 0.1 |
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204I | + rtA181TV | + rtL180M | + rtS202CGI | 1 | 0.1 | |
| … | … | … | + rtT184ASCG | 1 | 0.1 | |
| rtM204V | + rtN236T | + rtV173L | + rtL180M | 2 | 0.2 | |
| rtM204VI | + rtA181TV | + rtV173L | 1 | 0.1 | ||
| Full resistance to ADV | ||||||
| rtA181TV | 30 | 3.6 | ||||
| …… | + rtN236T | 18 | 2.2 | |||
| …… | + rtL180M | 2 | 0.2 | |||
| rtN236T | 14 | 1.7 | ||||
n = % of mutated strains: prevalence of pattern in total of group with primary resistance mutations.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine.
Of all patients exposed to LAM (n = 1175), resistance was present in 56.6% (665/1175). For ADV, resistance was present in 22.3% (43/193), and for ETV, 35.3% (36/102) (see Figure 2A). In the case of monotherapy without prior exposure to other NAs, rates of drug resistance were highest in patients exposed to LAM (60.1% [584/972]), followed by ADV (18.6% [11/59]). Prevalence rates of drug resistance in patients exposed to LAM remained constant over time, while resistance in patients exposed to ADV and ETV has risen since the introduction of these compounds (see Figure 2B). Data on LdT resistance was inconclusive due to limited exposure to this compound. Of 3 patients reported to have used LdT monotherapy, 2 showed LdT resistance (66.7%). In patients using ETV as monotherapy without prior exposure to other NAs, intermediate resistance to ETV (rtM204V + rtL180M or solitary rtM204I) was encountered in 10% (5/50) of cases. In 1 case of ETV monotherapy (2% [1/50]) viral strains harboring full resistance to ETV were detected. The encountered resistance pattern consisted of rtL180M + rtM204V + rtT184A, associated with prior LAM exposure, although prior LAM exposure was not reported in this patient. The patient did not have other risk factors for previous undisclosed LAM exposure, such as HIV infection.
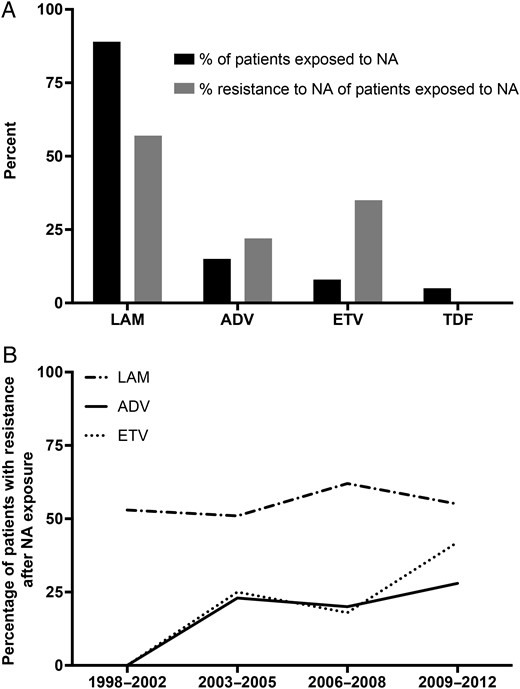
A total of 41 patients were exposed to LAM and ETV. In this subset of patients, resistance to LAM in combination with full resistance to ETV was present in 34.1% of cases (14/41). In the 127 patients treated with both LAM and ADV, resistance to both compounds was not encountered.
Differences were found in the comparative occurrence of rtM204I and rtM204 + rtL180M in viral genotypes A and D, the most prevalent genotypes in the dataset. In genotype A viral strains harboring resistance mutations, prevalence of the rtM204V + rtL180M was 52.5%, compared to 12.5% for rtM204I. In genotype D, the prevalence of rtM204V + rtL180M was 17.5%, compared to 20.2% for rtM204I (Table 3).
| . | % of Mutated Strains . | |||||
|---|---|---|---|---|---|---|
| Mutational Pattern . | . | . | . | . | Genotype A . | Genotype D . |
| Full resistance to LAM | ||||||
| rtM204I | 12.5% | 20.2% | ||||
| … | + rtL180M | 3.3% | 6.5% | |||
| … | … | + rtL80IV | … | 7.2% | ||
| … | + rtL80IV | 4.2% | 8.6% | |||
| rtM204V | … | 1.0% | ||||
| … | + rtL180M | 52.5% | 17.5% | |||
| … | … | + rtL80IV | 0.8% | 3.1% | ||
| … | + rtL80IV | … | 0.3% | |||
| rtM204VI | + rtL180M | … | 0.3% | |||
| Full resistance to LAM and ETV | ||||||
| rtM204I | + rtL180M | + rtV173L | … | 0.3% | ||
| … | … | … | + rtL80IV | … | 0.3% | |
| … | … | … | + rtT184ASCG | … | 0.3% | |
| … | … | … | … | + rtL80IV | 0.8% | … |
| … | + rtV173L | 0.8% | 0.7% | |||
| … | + rtT184ASCG | 2.5% | 0.3% | |||
| rtM204V | + rtL180M | + rtV173L | 12.5% | 6.8% | ||
| … | … | + rtT184ASCG | 1.7% | 5.5% | ||
| … | … | + rtS202CGI | … | 1.4% | ||
| … | … | + rtM250V | … | 0.7% | ||
| … | … | + rtL80IV | + rtV173L | … | 0.3% | |
| … | … | … | … | + rtT184ASCG | … | 0.3% |
| … | … | + rtI169T | + rtT184ASCG | 1.7% | … | |
| … | … | … | + rtV173L | 0.8% | … | |
| … | … | … | … | + rtT184ASCG | 0.8% | … |
| … | + rtV173L | … | 0.7% | |||
| Full resistance to ADV | ||||||
| rtA181TV | 1.7% | 8.6% | ||||
| … | + rtN236T | 1.7% | 4.8% | |||
| rtN236T | 1.7% | 3.4% | ||||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | … | 0.3% | |||
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204V | + rtL180M | + rtA181TV | + rtV173L | … | 0.3% | |
| . | % of Mutated Strains . | |||||
|---|---|---|---|---|---|---|
| Mutational Pattern . | . | . | . | . | Genotype A . | Genotype D . |
| Full resistance to LAM | ||||||
| rtM204I | 12.5% | 20.2% | ||||
| … | + rtL180M | 3.3% | 6.5% | |||
| … | … | + rtL80IV | … | 7.2% | ||
| … | + rtL80IV | 4.2% | 8.6% | |||
| rtM204V | … | 1.0% | ||||
| … | + rtL180M | 52.5% | 17.5% | |||
| … | … | + rtL80IV | 0.8% | 3.1% | ||
| … | + rtL80IV | … | 0.3% | |||
| rtM204VI | + rtL180M | … | 0.3% | |||
| Full resistance to LAM and ETV | ||||||
| rtM204I | + rtL180M | + rtV173L | … | 0.3% | ||
| … | … | … | + rtL80IV | … | 0.3% | |
| … | … | … | + rtT184ASCG | … | 0.3% | |
| … | … | … | … | + rtL80IV | 0.8% | … |
| … | + rtV173L | 0.8% | 0.7% | |||
| … | + rtT184ASCG | 2.5% | 0.3% | |||
| rtM204V | + rtL180M | + rtV173L | 12.5% | 6.8% | ||
| … | … | + rtT184ASCG | 1.7% | 5.5% | ||
| … | … | + rtS202CGI | … | 1.4% | ||
| … | … | + rtM250V | … | 0.7% | ||
| … | … | + rtL80IV | + rtV173L | … | 0.3% | |
| … | … | … | … | + rtT184ASCG | … | 0.3% |
| … | … | + rtI169T | + rtT184ASCG | 1.7% | … | |
| … | … | … | + rtV173L | 0.8% | … | |
| … | … | … | … | + rtT184ASCG | 0.8% | … |
| … | + rtV173L | … | 0.7% | |||
| Full resistance to ADV | ||||||
| rtA181TV | 1.7% | 8.6% | ||||
| … | + rtN236T | 1.7% | 4.8% | |||
| rtN236T | 1.7% | 3.4% | ||||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | … | 0.3% | |||
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204V | + rtL180M | + rtA181TV | + rtV173L | … | 0.3% | |
% of mutated strains: prevalence of pattern in total of group with primary resistance mutations and of same genotype.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine.
| . | % of Mutated Strains . | |||||
|---|---|---|---|---|---|---|
| Mutational Pattern . | . | . | . | . | Genotype A . | Genotype D . |
| Full resistance to LAM | ||||||
| rtM204I | 12.5% | 20.2% | ||||
| … | + rtL180M | 3.3% | 6.5% | |||
| … | … | + rtL80IV | … | 7.2% | ||
| … | + rtL80IV | 4.2% | 8.6% | |||
| rtM204V | … | 1.0% | ||||
| … | + rtL180M | 52.5% | 17.5% | |||
| … | … | + rtL80IV | 0.8% | 3.1% | ||
| … | + rtL80IV | … | 0.3% | |||
| rtM204VI | + rtL180M | … | 0.3% | |||
| Full resistance to LAM and ETV | ||||||
| rtM204I | + rtL180M | + rtV173L | … | 0.3% | ||
| … | … | … | + rtL80IV | … | 0.3% | |
| … | … | … | + rtT184ASCG | … | 0.3% | |
| … | … | … | … | + rtL80IV | 0.8% | … |
| … | + rtV173L | 0.8% | 0.7% | |||
| … | + rtT184ASCG | 2.5% | 0.3% | |||
| rtM204V | + rtL180M | + rtV173L | 12.5% | 6.8% | ||
| … | … | + rtT184ASCG | 1.7% | 5.5% | ||
| … | … | + rtS202CGI | … | 1.4% | ||
| … | … | + rtM250V | … | 0.7% | ||
| … | … | + rtL80IV | + rtV173L | … | 0.3% | |
| … | … | … | … | + rtT184ASCG | … | 0.3% |
| … | … | + rtI169T | + rtT184ASCG | 1.7% | … | |
| … | … | … | + rtV173L | 0.8% | … | |
| … | … | … | … | + rtT184ASCG | 0.8% | … |
| … | + rtV173L | … | 0.7% | |||
| Full resistance to ADV | ||||||
| rtA181TV | 1.7% | 8.6% | ||||
| … | + rtN236T | 1.7% | 4.8% | |||
| rtN236T | 1.7% | 3.4% | ||||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | … | 0.3% | |||
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204V | + rtL180M | + rtA181TV | + rtV173L | … | 0.3% | |
| . | % of Mutated Strains . | |||||
|---|---|---|---|---|---|---|
| Mutational Pattern . | . | . | . | . | Genotype A . | Genotype D . |
| Full resistance to LAM | ||||||
| rtM204I | 12.5% | 20.2% | ||||
| … | + rtL180M | 3.3% | 6.5% | |||
| … | … | + rtL80IV | … | 7.2% | ||
| … | + rtL80IV | 4.2% | 8.6% | |||
| rtM204V | … | 1.0% | ||||
| … | + rtL180M | 52.5% | 17.5% | |||
| … | … | + rtL80IV | 0.8% | 3.1% | ||
| … | + rtL80IV | … | 0.3% | |||
| rtM204VI | + rtL180M | … | 0.3% | |||
| Full resistance to LAM and ETV | ||||||
| rtM204I | + rtL180M | + rtV173L | … | 0.3% | ||
| … | … | … | + rtL80IV | … | 0.3% | |
| … | … | … | + rtT184ASCG | … | 0.3% | |
| … | … | … | … | + rtL80IV | 0.8% | … |
| … | + rtV173L | 0.8% | 0.7% | |||
| … | + rtT184ASCG | 2.5% | 0.3% | |||
| rtM204V | + rtL180M | + rtV173L | 12.5% | 6.8% | ||
| … | … | + rtT184ASCG | 1.7% | 5.5% | ||
| … | … | + rtS202CGI | … | 1.4% | ||
| … | … | + rtM250V | … | 0.7% | ||
| … | … | + rtL80IV | + rtV173L | … | 0.3% | |
| … | … | … | … | + rtT184ASCG | … | 0.3% |
| … | … | + rtI169T | + rtT184ASCG | 1.7% | … | |
| … | … | … | + rtV173L | 0.8% | … | |
| … | … | … | … | + rtT184ASCG | 0.8% | … |
| … | + rtV173L | … | 0.7% | |||
| Full resistance to ADV | ||||||
| rtA181TV | 1.7% | 8.6% | ||||
| … | + rtN236T | 1.7% | 4.8% | |||
| rtN236T | 1.7% | 3.4% | ||||
| Full resistance to LAM and ADV | ||||||
| rtM204I | + rtA181TV | … | 0.3% | |||
| Full resistance to LAM, ETV, and ADV | ||||||
| rtM204V | + rtL180M | + rtA181TV | + rtV173L | … | 0.3% | |
% of mutated strains: prevalence of pattern in total of group with primary resistance mutations and of same genotype.
Abbreviations: ADV, adefovir; ETV, entecavir; LAM, lamivudine.
Immune Escape Variants
The mutational pattern sI195M + sD164E in the S gene occurs as a result of nucleotide substitutions underlying the rtV173L +rtM204V mutational pattern in the overlapping open reading frame of the pol gene. This pattern was present in 9.7% (42/431) of all resistant strains of which a sequence of pol was available. The presence of this mutational pattern was associated with higher viral loads with a mean difference of 1.4 log (95% confidence interval [CI], .7–2.2; P < .001) compared to patients with any other resistance mutation(s). This correlation remained significant after correction for antiviral regimen, age, viral genotype, and time and region of sample collection (P < .05). The second investigated mutational pattern in the S gene is the introduction of a stop codon, sW172*, which can occur as a result of selection of the rtA181T ADV-associated resistance mutation. This pattern was present in 3.0% (13/431) of sequences in which resistance mutations were found. There was no significant difference in viral load between groups with and without this mutational pattern.
Factors Associated With Drug-Resistance Detection
In univariate analysis, factors positively associated with the presence of drug resistance mutations were age, log serum HBV DNA, exposure to LAM or LdT, and viral genotype D. Also, the use of a line probe assay (LiPA) was associated with the presence of resistance mutations. Negatively associated factors were exposure to ADV, ETV, and TDF, as well as viral genotypes B, C, and E. Rates of HBV drug resistance did not differ significantly between HBV monoinfected and HBV/HIV coinfected patients (Table 4).
| . | Resistance . | No Resistance . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Exp(B) (95% CI) . | P Value . | |||
| General | ||||||
| age | 49.3 | 43.4 | … | <.001 | 1.03 (1.01–1.05) | <.001 |
| gender (% male) | 73.9% | 70.1% | NS | |||
| HBV status | ||||||
| log HBV DNA | 5.4 | 4.8 | … | <.001 | 1.20 (1.08–1.34) | <.001 |
| serum-ALT | 88.7 | 112.9 | NS | |||
| HBeAg + | 55.7% | 49.5% | NS | |||
| detection with LiPA | 48.5% | 39.5% | 1.4 (1.2–1.8) | <.001 | ||
| Coinfections | ||||||
| HCV | 3.1% | 4.3% | NS | |||
| HDV | 0.0% | 2.2% | NS | |||
| HIV | 20.7% | 21.1% | NS | |||
| Treatment exposure | ||||||
| LAM | 95.3% | 81.6% | 4.5 (3.0–6.8) | <.001 | 2.91 (1.31–6.46) | <.001 |
| ADV | 12.1% | 17.9% | 0.6 (.5–.9) | <.05 | NS | |
| LdT | 2.2% | 0.5% | 4.3 (1.2–14.7) | <.05 | NS | |
| ETV | 5.2% | 11.0% | 0.4 (.3–.7) | <.001 | NS | |
| TDF | 3.0% | 6.9% | 0.4 (.2–.7) | <.05 | NS | |
| Interferon | 35.4% | 38.0% | NS | |||
| Genotype | ||||||
| A | 27.8% | 24.8% | NS | 5.88 (1.03–33.52) | <.05 | |
| B | 0.9% | 5.3% | 0.2 (.1–.5) | <.001 | NS | |
| C | 1.4% | 6.4% | 0.2 (.1–.5) | <.001 | NS | |
| D | 67.7% | 58.8% | 1.5 (1.1–1.9) | <.05 | NS | |
| E | 1.2% | 3.1% | 0.4 (.1–1.0) | <.05 | NS | |
| F | 0.2% | 0.4% | NS | |||
| G | 0.5% | 0.7% | NS | |||
| H | 0.2% | 0.7% | NS | |||
| Geographical origin | ||||||
| Western Europe | 18.4% | 28.3% | 0.6 (.4–.7) | <.001 | 0.45 (.21–.97) | <.05 |
| Northern Europe | 3.6% | 9.7% | 0.4 (.2–.5) | <.001 | 0.25 (.10–.61) | <.05 |
| Eastern Europe | 37.7% | 20.8% | 2.3 (1.8–2.9) | <.001 | NS | |
| Southern Europe | 40.3% | 41.2% | NS | (reference) | ||
| Year of analysis | ||||||
| 1998–2002 | 10.8% | 11.1% | NS | NS | ||
| 2003–2005 | 11.5% | 12.6% | NS | 4.57 (1.84–11.32) | <.001 | |
| 2006–2008 | 41.0% | 35.9% | 1.2 (1.0–1.5) | <.05 | 1.94 (1.07–3.52) | <.05 |
| 2009–2012 | 36.8% | 40.5% | NS | (reference) | ||
| . | Resistance . | No Resistance . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Exp(B) (95% CI) . | P Value . | |||
| General | ||||||
| age | 49.3 | 43.4 | … | <.001 | 1.03 (1.01–1.05) | <.001 |
| gender (% male) | 73.9% | 70.1% | NS | |||
| HBV status | ||||||
| log HBV DNA | 5.4 | 4.8 | … | <.001 | 1.20 (1.08–1.34) | <.001 |
| serum-ALT | 88.7 | 112.9 | NS | |||
| HBeAg + | 55.7% | 49.5% | NS | |||
| detection with LiPA | 48.5% | 39.5% | 1.4 (1.2–1.8) | <.001 | ||
| Coinfections | ||||||
| HCV | 3.1% | 4.3% | NS | |||
| HDV | 0.0% | 2.2% | NS | |||
| HIV | 20.7% | 21.1% | NS | |||
| Treatment exposure | ||||||
| LAM | 95.3% | 81.6% | 4.5 (3.0–6.8) | <.001 | 2.91 (1.31–6.46) | <.001 |
| ADV | 12.1% | 17.9% | 0.6 (.5–.9) | <.05 | NS | |
| LdT | 2.2% | 0.5% | 4.3 (1.2–14.7) | <.05 | NS | |
| ETV | 5.2% | 11.0% | 0.4 (.3–.7) | <.001 | NS | |
| TDF | 3.0% | 6.9% | 0.4 (.2–.7) | <.05 | NS | |
| Interferon | 35.4% | 38.0% | NS | |||
| Genotype | ||||||
| A | 27.8% | 24.8% | NS | 5.88 (1.03–33.52) | <.05 | |
| B | 0.9% | 5.3% | 0.2 (.1–.5) | <.001 | NS | |
| C | 1.4% | 6.4% | 0.2 (.1–.5) | <.001 | NS | |
| D | 67.7% | 58.8% | 1.5 (1.1–1.9) | <.05 | NS | |
| E | 1.2% | 3.1% | 0.4 (.1–1.0) | <.05 | NS | |
| F | 0.2% | 0.4% | NS | |||
| G | 0.5% | 0.7% | NS | |||
| H | 0.2% | 0.7% | NS | |||
| Geographical origin | ||||||
| Western Europe | 18.4% | 28.3% | 0.6 (.4–.7) | <.001 | 0.45 (.21–.97) | <.05 |
| Northern Europe | 3.6% | 9.7% | 0.4 (.2–.5) | <.001 | 0.25 (.10–.61) | <.05 |
| Eastern Europe | 37.7% | 20.8% | 2.3 (1.8–2.9) | <.001 | NS | |
| Southern Europe | 40.3% | 41.2% | NS | (reference) | ||
| Year of analysis | ||||||
| 1998–2002 | 10.8% | 11.1% | NS | NS | ||
| 2003–2005 | 11.5% | 12.6% | NS | 4.57 (1.84–11.32) | <.001 | |
| 2006–2008 | 41.0% | 35.9% | 1.2 (1.0–1.5) | <.05 | 1.94 (1.07–3.52) | <.05 |
| 2009–2012 | 36.8% | 40.5% | NS | (reference) | ||
Resistance/no resistance: prevalence or median of the variable in the group with/without primary drug resistance.
Reference category: In multivariate analysis, only variables with univariate significance (P < .05), genotype A–E, and all other categorical variables were included. For geographical origin, “Southern Europe” was selected as reference category. For year of analysis, “2009–2012” was used as reference category.
Coinfections: detection of antibodies to HCV, HDV, and/or HIV.
Abbreviations: ADV, adefovir; ALT, alanine transaminase; CI, confidence interval; ETV, entecavir; Exp(B), exponentiation of B coefficient; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; LAM, lamivudine; LdT, telbivudine; LiPA, line probe assay; TDF, tenofovir.
| . | Resistance . | No Resistance . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Exp(B) (95% CI) . | P Value . | |||
| General | ||||||
| age | 49.3 | 43.4 | … | <.001 | 1.03 (1.01–1.05) | <.001 |
| gender (% male) | 73.9% | 70.1% | NS | |||
| HBV status | ||||||
| log HBV DNA | 5.4 | 4.8 | … | <.001 | 1.20 (1.08–1.34) | <.001 |
| serum-ALT | 88.7 | 112.9 | NS | |||
| HBeAg + | 55.7% | 49.5% | NS | |||
| detection with LiPA | 48.5% | 39.5% | 1.4 (1.2–1.8) | <.001 | ||
| Coinfections | ||||||
| HCV | 3.1% | 4.3% | NS | |||
| HDV | 0.0% | 2.2% | NS | |||
| HIV | 20.7% | 21.1% | NS | |||
| Treatment exposure | ||||||
| LAM | 95.3% | 81.6% | 4.5 (3.0–6.8) | <.001 | 2.91 (1.31–6.46) | <.001 |
| ADV | 12.1% | 17.9% | 0.6 (.5–.9) | <.05 | NS | |
| LdT | 2.2% | 0.5% | 4.3 (1.2–14.7) | <.05 | NS | |
| ETV | 5.2% | 11.0% | 0.4 (.3–.7) | <.001 | NS | |
| TDF | 3.0% | 6.9% | 0.4 (.2–.7) | <.05 | NS | |
| Interferon | 35.4% | 38.0% | NS | |||
| Genotype | ||||||
| A | 27.8% | 24.8% | NS | 5.88 (1.03–33.52) | <.05 | |
| B | 0.9% | 5.3% | 0.2 (.1–.5) | <.001 | NS | |
| C | 1.4% | 6.4% | 0.2 (.1–.5) | <.001 | NS | |
| D | 67.7% | 58.8% | 1.5 (1.1–1.9) | <.05 | NS | |
| E | 1.2% | 3.1% | 0.4 (.1–1.0) | <.05 | NS | |
| F | 0.2% | 0.4% | NS | |||
| G | 0.5% | 0.7% | NS | |||
| H | 0.2% | 0.7% | NS | |||
| Geographical origin | ||||||
| Western Europe | 18.4% | 28.3% | 0.6 (.4–.7) | <.001 | 0.45 (.21–.97) | <.05 |
| Northern Europe | 3.6% | 9.7% | 0.4 (.2–.5) | <.001 | 0.25 (.10–.61) | <.05 |
| Eastern Europe | 37.7% | 20.8% | 2.3 (1.8–2.9) | <.001 | NS | |
| Southern Europe | 40.3% | 41.2% | NS | (reference) | ||
| Year of analysis | ||||||
| 1998–2002 | 10.8% | 11.1% | NS | NS | ||
| 2003–2005 | 11.5% | 12.6% | NS | 4.57 (1.84–11.32) | <.001 | |
| 2006–2008 | 41.0% | 35.9% | 1.2 (1.0–1.5) | <.05 | 1.94 (1.07–3.52) | <.05 |
| 2009–2012 | 36.8% | 40.5% | NS | (reference) | ||
| . | Resistance . | No Resistance . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Exp(B) (95% CI) . | P Value . | |||
| General | ||||||
| age | 49.3 | 43.4 | … | <.001 | 1.03 (1.01–1.05) | <.001 |
| gender (% male) | 73.9% | 70.1% | NS | |||
| HBV status | ||||||
| log HBV DNA | 5.4 | 4.8 | … | <.001 | 1.20 (1.08–1.34) | <.001 |
| serum-ALT | 88.7 | 112.9 | NS | |||
| HBeAg + | 55.7% | 49.5% | NS | |||
| detection with LiPA | 48.5% | 39.5% | 1.4 (1.2–1.8) | <.001 | ||
| Coinfections | ||||||
| HCV | 3.1% | 4.3% | NS | |||
| HDV | 0.0% | 2.2% | NS | |||
| HIV | 20.7% | 21.1% | NS | |||
| Treatment exposure | ||||||
| LAM | 95.3% | 81.6% | 4.5 (3.0–6.8) | <.001 | 2.91 (1.31–6.46) | <.001 |
| ADV | 12.1% | 17.9% | 0.6 (.5–.9) | <.05 | NS | |
| LdT | 2.2% | 0.5% | 4.3 (1.2–14.7) | <.05 | NS | |
| ETV | 5.2% | 11.0% | 0.4 (.3–.7) | <.001 | NS | |
| TDF | 3.0% | 6.9% | 0.4 (.2–.7) | <.05 | NS | |
| Interferon | 35.4% | 38.0% | NS | |||
| Genotype | ||||||
| A | 27.8% | 24.8% | NS | 5.88 (1.03–33.52) | <.05 | |
| B | 0.9% | 5.3% | 0.2 (.1–.5) | <.001 | NS | |
| C | 1.4% | 6.4% | 0.2 (.1–.5) | <.001 | NS | |
| D | 67.7% | 58.8% | 1.5 (1.1–1.9) | <.05 | NS | |
| E | 1.2% | 3.1% | 0.4 (.1–1.0) | <.05 | NS | |
| F | 0.2% | 0.4% | NS | |||
| G | 0.5% | 0.7% | NS | |||
| H | 0.2% | 0.7% | NS | |||
| Geographical origin | ||||||
| Western Europe | 18.4% | 28.3% | 0.6 (.4–.7) | <.001 | 0.45 (.21–.97) | <.05 |
| Northern Europe | 3.6% | 9.7% | 0.4 (.2–.5) | <.001 | 0.25 (.10–.61) | <.05 |
| Eastern Europe | 37.7% | 20.8% | 2.3 (1.8–2.9) | <.001 | NS | |
| Southern Europe | 40.3% | 41.2% | NS | (reference) | ||
| Year of analysis | ||||||
| 1998–2002 | 10.8% | 11.1% | NS | NS | ||
| 2003–2005 | 11.5% | 12.6% | NS | 4.57 (1.84–11.32) | <.001 | |
| 2006–2008 | 41.0% | 35.9% | 1.2 (1.0–1.5) | <.05 | 1.94 (1.07–3.52) | <.05 |
| 2009–2012 | 36.8% | 40.5% | NS | (reference) | ||
Resistance/no resistance: prevalence or median of the variable in the group with/without primary drug resistance.
Reference category: In multivariate analysis, only variables with univariate significance (P < .05), genotype A–E, and all other categorical variables were included. For geographical origin, “Southern Europe” was selected as reference category. For year of analysis, “2009–2012” was used as reference category.
Coinfections: detection of antibodies to HCV, HDV, and/or HIV.
Abbreviations: ADV, adefovir; ALT, alanine transaminase; CI, confidence interval; ETV, entecavir; Exp(B), exponentiation of B coefficient; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; LAM, lamivudine; LdT, telbivudine; LiPA, line probe assay; TDF, tenofovir.
Prevalence of resistance was not equally distributed between geographical regions. Prevalence was lower in Western and Northern European countries and higher in Eastern European countries.
In multivariate analysis, risk factors for resistance were age (P < .001), HBV DNA (P < .001), lamivudine use (P < .001), and viral genotype A (P < .05). Sample collection in time periods 2003–2005 (P < .001) and 2006–2008 (P < .05) was also independently associated with a higher risk of resistance. Northern and Western European sample origin was negatively associated with the presence of resistance (P < .05).
DISCUSSION
In this largest-to-date European survey of 1568 NA-experienced CHB patients in whom resistance testing was performed, drug resistance was observed in half of the cases. Drug resistance was frequently encountered in patients exposed to monotherapy with LAM and ADV. Several cases of intermediate drug resistance to ETV and 1 case of full resistance to ETV were detected in patients receiving ETV without prior exposure to other NAs.
This survey included resistance testing results that were acquired during routine clinical assessments. In most European clinical settings, resistance testing is performed in cases of therapy failure where HBV drug resistance is suspected or needs to be ruled out. The prevalence of HBV drug resistance in this group of patients may be different than in the overall treated CHB population with detectable viremia during treatment. The result should therefore be interpreted as an estimate of the likelihood of encountering drug resistance when genotypic resistance testing is performed.
This study demonstrates the extensive influence of LAM monotherapy on the presence of resistance. In patients exposed to LAM monotherapy, drug resistance was encountered in the majority of cases. In most cases, the pattern of resistance involved the rtM204V mutation. This mutation was frequently accompanied by secondary or compensatory mutations. In the case of therapy failure after exposure to both LAM and ETV, viral strains harbored LAM-associated mutational patterns conferring full cross-resistance to ETV in one-third of cases, which is in concordance with long term follow-up data on cumulative probability of ETV resistance in patients previously treated with LAM [16]. As these mutations are indicative of prolonged viremia under LAM monotherapy [28], this finding suggests that prescription of ETV after prolonged LAM therapy failure still occurs in clinical practice.
Monotherapy with LAM was still the most frequently prescribed treatment regimen in the most recent time period covered by this survey. While discouraged by recent EASL guidelines, this practice is in line with recently proposed treatment strategies using LAM monotherapy as a first-line treatment and employing TDF only as a rescue therapy if LAM resistance develops [21–23, 29]. Despite these proposed strategies, a recent review of cost-effectiveness studies concluded that monotherapy with ETV or TDF is more cost effective than treatment with LAM or ADV and should also be recommended for low- and middle-income countries [30]. In addition, in those patients for whom TDF use is contraindicated due to renal impairment, prolonged LAM exposure can generate cross-resistance to ETV and thereby eliminate the last remaining therapeutic option.
The LAM-associated rtM204V + rtV173L drug resistance pattern can lead to corresponding changes in the S gene (sI195M + sD164E), resulting in the production of immune escape variants [11]. Patients infected with strains harboring this mutational pattern had significantly higher viral loads. This effect is most likely due to the presence of compensatory mutations in pol that restore HBV replicative capacity, which is significantly impaired in the presence of a solitary rtM204V mutation [31–33]. Another possible explanation for high viral loads in these patients is that immune escape variant viral strains are less effectively neutralized by the immune system [11].
In this study, resistance to ETV was detected in 12% of patients with (reported) first-line ETV monotherapy (n = 50). In only 1 case (2%), the mutational pattern conferred full resistance to ETV, which is similar to that reported in previous studies [12, 34]. Although there was no indication of previous LAM use in these patients, we cannot fully exclude the possibility of prior undisclosed treatment with LAM. As HBV can persist for long periods of time as covalently closed circular DNA (cccDNA), even during treatment and HBsAg seroclearance [35], the cccDNA reservoir can act as an archive of drug-resistant viral strains [36]. Previous studies have shown that therapy failure of ETV is more likely to occur in patients previously carrying LAM-resistant viral strains even when resistance was no longer detectable after LAM interruption [37, 38]. Another possible explanation for the presence of these resistance mutations is transmission of resistant viral strains, which has been described previously in different settings [39, 40].
In this survey, independent risk factors for the presence of drug resistance in cases of NA treatment failure were age, viral load, previous use of LAM, and viral genotype A. The correlation between age and drug resistance may reflect treatment duration, which could not be separately assessed in this survey. The correlation between viral genotype A and drug resistance, which was not significant in univariate analysis, was just below the significance threshold of P > .05 with a wide 95% confidence interval. The significance of this finding is therefore unclear. Although this study was not set up as a geographically representative study, regional and temporal differences in the prevalence of drug resistance were encountered. In multivariate analysis correcting for confounding factors such as drug exposure and viral genotype, the risk of drug resistance in patients with apparent NA therapy failure was lower in Western and Northern Europe and higher in Eastern Europe when compared to Southern Europe, where the prevalence of resistance was comparable to that of the overall study population. These differences may reflect long duration of exposure to older compounds due to unavailability of ETV and TDF in some countries. Indeed, in this dataset the prevalence of lamivudine use in Eastern Europe was higher than in other regions. This is confirmed by a recent survey of prescription practices in Europe [20]. Other possible factors influencing the differences in prevalence of resistance across European regions include regional differences in clinical use of resistance testing in the follow-up of cases of apparent NA therapy failure, or general differences in patient care. Analysis of the impact of time period on the presence of drug resistance revealed that a higher risk of drug resistance was present in cases of therapy failure detected between 2003 and 2008. This likely reflects long durations of therapy with older NAs before the availability of ETV and TDF.
Comparison of conventional Sanger sequencing and line probe assay results demonstrated a higher prevalence of resistance in samples analyzed using LiPA. Previous studies have demonstrated a higher sensitivity or false-positive rate of LiPA compared to Sanger sequencing for the detection of drug-resistance mutations [41, 42]. However, in multivariate analysis, detection with LiPA was not related to a higher rate of drug resistance. This suggests that the higher rate of resistance detected with LiPA was confounded by higher rates or longer duration of LAM exposure in centers and specific time periods in which LiPA was used.
In this study, CHB patients with HIV coinfection had a similar risk of harboring drug-resistant HBV as monoinfected patients. A previous study on NA treatment in HBV/HIV-coinfected patients has demonstrated that high rates of virological response for TDF are similar in mono- and coinfected patients [43].
One limitation of this study relates to the pooling of data from countries that differ in certain aspects of clinical practice. Heterogeneity might exist in clinical motives for performance of genotypic resistance testing and in the local availability of different antiviral compounds. However, pooled analysis of European data offers the opportunity to provide a more comprehensive view of the clinical correlates and characteristics of HBV drug resistance. Another limitation is that this study only included population-based sequencing results and LiPA results, as these assays are performed in routine clinical practice. The relevance of minority drug-resistant variants may be clarified in future research.
In conclusion, this survey highlights that in recent years, LAM monotherapy is associated with the majority of cases of drug resistance development in Europe. It also shows that LAM-associated drug resistance mutations conferring cross-resistance to ETV are frequently present in patients with ETV-therapy failure. This supports the view that continued LAM use needs to be reconsidered at a pan-European level. Furthermore, the high prevalence of drug resistance in this survey supports the continued use of genotypic testing in cases of apparent therapy failure, particularly in patients previously exposed to LAM, ADV, and LdT. The detection of resistance mutations in patients reportedly using ETV as a first-line therapy also supports the use of genotypic resistance testing in this subset of patients.
Notes
Acknowledgments. We thank the following members of the Danish Database for Hepatitis B and C (DANHEP): Peer Brehm Christensen, Mette Rye Clausen, Jan Gerstoft, Alex Lund Laursen, Suzanne Lunding, Axel Møller, and Poul Schlichting.
Financial support. I. L. received a grant from the Ministry of Education, Science and Technological Development, Republic of Serbia (grant no. 175073), during the conduct of the study. S. P. received a grant from project BESTHOPE (grant no. 4-003/2012) (UEFISCDI), during the conduct of the study. N. W. received a grant from The Danish Council for Independent Research (grant no. 12-127717), during the conduct of the study, and received fees for advisory board participation, lectures, and chairing meetings from Abbvie, Bristol-Myers Squibb (BMS), GlaxoSmithKline, Janssen, MSD, and Medivir. A. M. J. W. received consultancy fees, travel and/or research grants from BMS, Gilead, Janssen, MSD, and Viiv Healthcare. None of these were related to this work. D. P. received funding by the Hellenic Scientific Society for the study of AIDS and STDs, during the conduct of the study. V. S. and C. F. P. received educational and research grants from BMS and Gilead, during the conduct of this study.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: 10th European meeting on HIV and Hepatitis, Barcelona, Spain, 28–30 March 2012; 11th European meeting on HIV and Hepatitis, Rome, Italy, 21 March 2013.
L. E. H. and V. S. contributed equally to this work.




