-
PDF
- Split View
-
Views
-
Cite
Cite
Myron J. Levin, Kenneth E. Schmader, Lei Pang, Angela Williams-Diaz, Gary Zerbe, Jennifer Canniff, Michael J. Johnson, Yupanqui Caldas, Alice Cho, Nancy Lang, Shu-Chih Su, Janie Parrino, Zoran Popmihajlov, Adriana Weinberg, Cellular and Humoral Responses to a Second Dose of Herpes Zoster Vaccine Administered 10 Years After the First Dose Among Older Adults, The Journal of Infectious Diseases, Volume 213, Issue 1, 1 January 2016, Pages 14–22, https://doi.org/10.1093/infdis/jiv480
Close - Share Icon Share
Abstract
Herpes zoster vaccine (ZV) was administered as a second dose to 200 participants ≥70 years old who had received a dose of ZV ≥10 years previously (NCT01245751).
Varicella zoster virus (VZV) antibody titers (measured by a VZV glycoprotein–based enzyme-linked immunosorbent assay [gpELISA]) and levels of interferon γ (IFN-γ) and interleukin 2 (IL-2; markers of VZV-specific cell-mediated immunity [CMI], measured by means of ELISPOT analysis) in individuals aged ≥70 years who received a booster dose of ZV were compared to responses of 100 participants aged 50–59 years, 100 aged 60–69 years, and 200 aged ≥70 years who received their first dose of ZV. The study was powered to demonstrate noninferiority of the VZV antibody response at 6 weeks in the booster-dose group, compared with the age-matched first-dose group.
Antibody responses were similar at baseline and after vaccination across all age and treatment groups. Both baseline and postvaccination VZV-specific CMI were lower in the older age groups. Peak gpELISA titers and their fold rise from baseline generally correlated with higher baseline and postvaccination VZV-specific CMI. IFN-γ and IL-2 results for subjects ≥70 years old were significantly higher at baseline and after vaccination in the booster-dose group, compared with the first-dose group, indicating that a residual effect of ZV on VZV-specific CMI persisted for ≥10 years and was enhanced by the booster dose.
These findings support further investigation of ZV administration in early versus later age and of booster doses for elderly individuals at an appropriate interval after initial immunization against HZ.
NCT01245751.
(See the editorial commentary by Schmid on pages 1–2.)
The herpes zoster (HZ) vaccine (ZV) prevents or attenuates HZ in people ≥50 years of age [1, 2]. This effect correlates with the boost in varicella zoster virus (VZV)–specific antibody and T-cell immunity (CMI) that follows vaccination [3–5], and ZV partially reverses the decline in VZV-specific CMI that characterizes aging [3, 6]. VZV-specific CMI is considered a mechanistic correlate of protection against HZ [7, 8]. In many viral infections, CD8+ T cells are the main effectors of viral clearance, while CD4+ T cells have mainly a helper role. However, in herpesvirus infections, CD4+ T cells have both helper and effector roles and are a major focus of VZV-specific immune defense studies [9–11]. Previous publications characterized the central memory CD4+ T-cell response to ZV by using proliferation assays and the effector CD4+ T-cell response by using interferon γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays [3, 6, 12]. Less is known about the CD4+ effector memory T cells that have the ability to rapidly upregulate cytokine production and other effector mechanisms upon encountering their cognate antigen and that may play a critical role in controlling the early stages of VZV reactivation. Terminally differentiated effector responses to ZV constitute another T-cell subset of interest, particularly in elderly individuals [13]. Differentiated effectors initially produce high levels of cytokines but eventually become exhausted and lose their ability to participate in antiviral infections [14]. The exhausted T-cell population increases with aging [15].
During aging there is a progressive decline in immune responsiveness to vaccination and a shortening of the duration of vaccine-induced immunity [16]. This characteristic of immune senescence is apparent in studies of vaccines administered to elderly individuals, such as those targeting influenza virus and pneumococcus [17–19]. The licensed ZV (Zostavax, Merck & Co., Inc., Kenilworth, New Jersey) exemplifies this pattern. In the pivotal trial, a single dose of ZV showed a 51.3% efficacy in preventing HZ and a 66.7% efficacy in preventing postherpetic neuralgia in adults ≥60 years old [1]. That trial had a mean observation period of 3.13 years (the longest period was 4.9 years, for 1 subject). Subsequent observation of >14 200 participants for an additional 3.3–7.8 years demonstrated a decline in efficacy, although the vaccine-induced protection remained significant for at least 5 years [20]. Further follow-up to evaluate long-term persistence, which included years 7–11 after ZV receipt, demonstrated a further decline in efficacy to 21.1% for preventing HZ and to 35.4% for preventing postherpetic neuralgia [21]. As an initial step in investigating the potential for reversing this decline in efficacy, we determined that a booster dose of ZV administered to adults ≥70 years of age elicits a VZV antibody response that is noninferior to that of ZV administered as a first dose, and we compared the cellular and humoral immune responses to this booster dose of ZV in older vaccinees to those in younger vaccinees (NCT01245751).
METHODS
Population
Subjects were enrolled into one of 4 study groups (Figure 1). Group 1 was 200 subjects ≥70 years old who received ZV ≥10 years previously, group 2 was 200 subjects ≥70 years old who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 was 100 subjects 60 to <70 years old who never received ZV, and group 4 was 100 subjects ≥50 to <60 years of age who never received ZV.
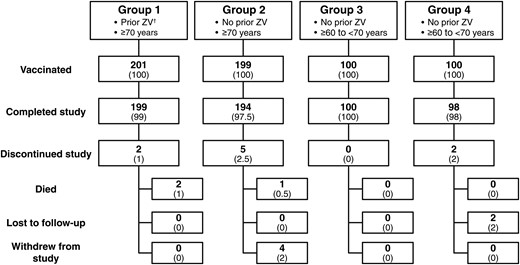
Disposition of study participants, by group. Data are no. (%) of participants. Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV. †ZV (ZOSTAVAXTM) received ≥10 years previously.
Inclusion Criteria
Subjects either had a history of varicella or had resided in the United States for ≥30 years with no history of HZ. Subjects were afebrile (oral temperature, <38.0°C) on the day of vaccination. Any underlying chronic illness was stable. Subjects signed informed consent for the protocol that was approved by the institutional review boards at the University of Colorado Anschutz Medical Campus and Duke University Health System.
Exclusion Criteria
Exclusion criteria comprised the following: prior history of HZ, history of hypersensitivity reaction to any vaccine component, receipt of immunoglobulin or blood products during the 5 months prior to vaccination and throughout the study, vaccination with any live virus or inactivated vaccine within 4 weeks before or after receiving ZV, recent receipt of immunosuppressive therapy or suspected immune dysfunction or immune suppressing illness, or receipt of systemic antiherpesvirus therapy during the study period. No prior ZV was permitted for subjects in groups 2–4.
Vaccine
Subjects received a single subcutaneous injection of ZV (dose, approximately 0.65 mL) in the deltoid region of the nondominant arm on day 1.
Sample Acquisition
Blood samples were obtained immediately before vaccination and at weeks 1, 6, and 52 after ZV receipt.
Safety Assessment
All subjects were followed for safety. A detailed safety profile through week 6 after ZV receipt was assessed with a vaccine report card that recorded injection-site and systemic adverse experiences, elevated temperatures, and rashes. These were reviewed with site personnel at the week 1 and week 6 visits. Serious adverse events were queried by telephone at weeks 12 and 26 after ZV receipt and at week 52 visit. Serious adverse experiences were collected for all study subjects through week 52 after ZV receipt regardless of reported relationship to the study vaccine.
Immunologic End Points
VZV-Specific Antibody Assay
The VZV glycoprotein–based enzyme-linked immunosorbent assay (gpELISA; performed at PPD Vaccines and Biologics, Richmond, VA) detects immunoglobulin G antibodies to purified VZV glycoproteins from MRC-5 cells infected with VZV, using methods described elsewhere [22]. VZV glycoproteins or uninfected MRC-5 lysates were adsorbed to polystyrene microtiter wells. Experimental, control, and standard curve serum samples were incubated in the wells in duplicate (Supplementary Materials). The change in the optical density was calculated as the difference between the average OD of the 2 VZV antigen-coated wells and that of the 2 MRC-5 antigen-coated control wells. Antibody quantitation by gpELISA, in units per milliliter, was achieved by comparing the change in OD in samples to the OD on a standard curve.
VZV-Specific IFN-γ and IL-2 ELISPOT Assays
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Sigma Diagnostics), collected, and frozen as previously described [23]. Cells frozen at the Duke University Health System site were sent to Denver, Colorado, in liquid nitrogen dewars. Dual-color IFN-γ and IL-2 FluoroSPOT (ELISPOT) assays were performed using MabTech Fluorospot kits per the manufacturer's instructions (Supplementary Materials). Results were reported as the mean number of spot-forming cells (SFCs)/106 PBMCs in VZV-stimulated wells, after subtracting the mean number of SFCs in control wells containing medium. An assay control of PBMCs from a single leukopack was included in each run.
Statistical Analysis
Geometric mean titers (GMTs) and geometric mean fold-rises from baseline were calculated for gpELISA findings, by treatment group and visit, and the geometric mean counts (GMCs) yielded by the IFN-γ, IL-2, and IFN-γ/IL-2 ELISPOT assays were similarly calculated. The 95% confidence intervals were computed on the basis of the t distribution. Adverse events (number and incidence) were summarized by treatment group.
RESULTS
Demographic Characteristics
Six hundred subjects were enrolled into the 4 groups indicated in Figure 1. The first 2 groups comprised individuals who were at least 70 years old and received either a dose of ZV ≥10 years after a previous dose (group 1) or their first dose of ZV (group 2). Group 3 and group 4 were 60–69 years old and 50–59 years old, respectively. They received their first dose of ZV. Only 9 of 600 subjects did not complete the study. Table 1 describes the demographic composition of the participants, who were predominantly white non-Hispanic and female. The mean and median ages for groups 1 and 2 were similar, as were the age distribution in groups between ages 70–90 years.
| Characteristic . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . | Overall (n = 600) Participants, No. (%) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 94 (46.8) | 105 (52.8) | 35 (35.0) | 23 (23.0) | 257 (42.8) |
| Female | 107 (53.2) | 94 (52.8) | 65 (65.0) | 77 (77.0) | 343 (57.2) |
| Age, y | |||||
| 50–54 | 0 | 0 | 0 | 37 (37.0) | 37 (6.2) |
| 55–59 | 0 | 0 | 0 | 63 (63.0) | 63 (10.5) |
| 60–64 | 0 | 0 | 57 (57.0) | 0 | 57 (9.5) |
| 65–69 | 0 | 0 | 43 (43.0) | 0 | 43 (7.2) |
| 70–74 | 65 (31.3) | 92 (46.2) | 0 | 0 | 155 (25.8) |
| 75–79 | 81 (40.3) | 52 (26.1) | 0 | 0 | 133 (22.2) |
| 80–84 | 45 (22.4) | 37 (18.6) | 0 | 0 | 82 (13.7) |
| 85–89 | 10 (5.0) | 17 (8.5) | 0 | 0 | 27 (4.5) |
| 90–94 | 2 (1.0) | 1 (0.5) | 0 | 0 | 3 (0.5) |
| Mean ± SD | 77.1 ± 4.5 | 76.3 ± 5.1 | 64.1 ± 3.1 | 55.5 ± 2.8 | 71.1 |
| Median | 76.0 | 75.0 | 63.0 | 56.0 | 73.0 |
| Race | |||||
| Native American or Asian | 0 (0.0) | 4 (2.0) | 1 (1.0) | 0 (0.0) | 2 (0.3) |
| African American | 1 (0.5) | 5 (2.5) | 8 (8.0) | 6 (6.0) | 20 (3.3) |
| White | 200 (99.5) | 188 (94.5) | 90 (90.0) | 93 (93.0) | 571 (95.2) |
| Ethnicity | |||||
| Hispanic/Latino | 2 (1.0) | 2 (1.0) | 0 (0.0) | 4 (4.0) | 8 (1.3) |
| Not Hispanic/Latino | 198 (98.5) | 196 (98.5) | 100 (100.0) | 96 (96.0) | 590 (98.3) |
| Characteristic . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . | Overall (n = 600) Participants, No. (%) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 94 (46.8) | 105 (52.8) | 35 (35.0) | 23 (23.0) | 257 (42.8) |
| Female | 107 (53.2) | 94 (52.8) | 65 (65.0) | 77 (77.0) | 343 (57.2) |
| Age, y | |||||
| 50–54 | 0 | 0 | 0 | 37 (37.0) | 37 (6.2) |
| 55–59 | 0 | 0 | 0 | 63 (63.0) | 63 (10.5) |
| 60–64 | 0 | 0 | 57 (57.0) | 0 | 57 (9.5) |
| 65–69 | 0 | 0 | 43 (43.0) | 0 | 43 (7.2) |
| 70–74 | 65 (31.3) | 92 (46.2) | 0 | 0 | 155 (25.8) |
| 75–79 | 81 (40.3) | 52 (26.1) | 0 | 0 | 133 (22.2) |
| 80–84 | 45 (22.4) | 37 (18.6) | 0 | 0 | 82 (13.7) |
| 85–89 | 10 (5.0) | 17 (8.5) | 0 | 0 | 27 (4.5) |
| 90–94 | 2 (1.0) | 1 (0.5) | 0 | 0 | 3 (0.5) |
| Mean ± SD | 77.1 ± 4.5 | 76.3 ± 5.1 | 64.1 ± 3.1 | 55.5 ± 2.8 | 71.1 |
| Median | 76.0 | 75.0 | 63.0 | 56.0 | 73.0 |
| Race | |||||
| Native American or Asian | 0 (0.0) | 4 (2.0) | 1 (1.0) | 0 (0.0) | 2 (0.3) |
| African American | 1 (0.5) | 5 (2.5) | 8 (8.0) | 6 (6.0) | 20 (3.3) |
| White | 200 (99.5) | 188 (94.5) | 90 (90.0) | 93 (93.0) | 571 (95.2) |
| Ethnicity | |||||
| Hispanic/Latino | 2 (1.0) | 2 (1.0) | 0 (0.0) | 4 (4.0) | 8 (1.3) |
| Not Hispanic/Latino | 198 (98.5) | 196 (98.5) | 100 (100.0) | 96 (96.0) | 590 (98.3) |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
| Characteristic . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . | Overall (n = 600) Participants, No. (%) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 94 (46.8) | 105 (52.8) | 35 (35.0) | 23 (23.0) | 257 (42.8) |
| Female | 107 (53.2) | 94 (52.8) | 65 (65.0) | 77 (77.0) | 343 (57.2) |
| Age, y | |||||
| 50–54 | 0 | 0 | 0 | 37 (37.0) | 37 (6.2) |
| 55–59 | 0 | 0 | 0 | 63 (63.0) | 63 (10.5) |
| 60–64 | 0 | 0 | 57 (57.0) | 0 | 57 (9.5) |
| 65–69 | 0 | 0 | 43 (43.0) | 0 | 43 (7.2) |
| 70–74 | 65 (31.3) | 92 (46.2) | 0 | 0 | 155 (25.8) |
| 75–79 | 81 (40.3) | 52 (26.1) | 0 | 0 | 133 (22.2) |
| 80–84 | 45 (22.4) | 37 (18.6) | 0 | 0 | 82 (13.7) |
| 85–89 | 10 (5.0) | 17 (8.5) | 0 | 0 | 27 (4.5) |
| 90–94 | 2 (1.0) | 1 (0.5) | 0 | 0 | 3 (0.5) |
| Mean ± SD | 77.1 ± 4.5 | 76.3 ± 5.1 | 64.1 ± 3.1 | 55.5 ± 2.8 | 71.1 |
| Median | 76.0 | 75.0 | 63.0 | 56.0 | 73.0 |
| Race | |||||
| Native American or Asian | 0 (0.0) | 4 (2.0) | 1 (1.0) | 0 (0.0) | 2 (0.3) |
| African American | 1 (0.5) | 5 (2.5) | 8 (8.0) | 6 (6.0) | 20 (3.3) |
| White | 200 (99.5) | 188 (94.5) | 90 (90.0) | 93 (93.0) | 571 (95.2) |
| Ethnicity | |||||
| Hispanic/Latino | 2 (1.0) | 2 (1.0) | 0 (0.0) | 4 (4.0) | 8 (1.3) |
| Not Hispanic/Latino | 198 (98.5) | 196 (98.5) | 100 (100.0) | 96 (96.0) | 590 (98.3) |
| Characteristic . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . | Overall (n = 600) Participants, No. (%) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 94 (46.8) | 105 (52.8) | 35 (35.0) | 23 (23.0) | 257 (42.8) |
| Female | 107 (53.2) | 94 (52.8) | 65 (65.0) | 77 (77.0) | 343 (57.2) |
| Age, y | |||||
| 50–54 | 0 | 0 | 0 | 37 (37.0) | 37 (6.2) |
| 55–59 | 0 | 0 | 0 | 63 (63.0) | 63 (10.5) |
| 60–64 | 0 | 0 | 57 (57.0) | 0 | 57 (9.5) |
| 65–69 | 0 | 0 | 43 (43.0) | 0 | 43 (7.2) |
| 70–74 | 65 (31.3) | 92 (46.2) | 0 | 0 | 155 (25.8) |
| 75–79 | 81 (40.3) | 52 (26.1) | 0 | 0 | 133 (22.2) |
| 80–84 | 45 (22.4) | 37 (18.6) | 0 | 0 | 82 (13.7) |
| 85–89 | 10 (5.0) | 17 (8.5) | 0 | 0 | 27 (4.5) |
| 90–94 | 2 (1.0) | 1 (0.5) | 0 | 0 | 3 (0.5) |
| Mean ± SD | 77.1 ± 4.5 | 76.3 ± 5.1 | 64.1 ± 3.1 | 55.5 ± 2.8 | 71.1 |
| Median | 76.0 | 75.0 | 63.0 | 56.0 | 73.0 |
| Race | |||||
| Native American or Asian | 0 (0.0) | 4 (2.0) | 1 (1.0) | 0 (0.0) | 2 (0.3) |
| African American | 1 (0.5) | 5 (2.5) | 8 (8.0) | 6 (6.0) | 20 (3.3) |
| White | 200 (99.5) | 188 (94.5) | 90 (90.0) | 93 (93.0) | 571 (95.2) |
| Ethnicity | |||||
| Hispanic/Latino | 2 (1.0) | 2 (1.0) | 0 (0.0) | 4 (4.0) | 8 (1.3) |
| Not Hispanic/Latino | 198 (98.5) | 196 (98.5) | 100 (100.0) | 96 (96.0) | 590 (98.3) |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Safety
The vaccine was generally well tolerated in all groups (Table 2). As many as 57% of subjects in groups 1 and 2 and 75% of subjects in groups 3 and 4 reported ≥1 adverse experience through week 52. Most of the adverse events were injection site reactions (pain and erythema) occurring 1–5 days after ZV receipt. Only 6 subjects reported injection-site adverse experiences from day 6 to week 6 after ZV receipt. The higher rate of adverse events in groups 3 and 4 was primarily related to a higher rate of injection site reactions. Vaccine-related non–injection site reactions occurred in 3%–8% of subjects ≤6 weeks after ZV receipt. Serious adverse events through week 52 were more common in the older age groups, but no event in any group was considered vaccine related. Overall, the incidence and categories of adverse events were similar between subjects in group 1 and those in group 2.
| Category . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . |
|---|---|---|---|---|
| ≥1 AE | 115 (57) | 109 (55) | 75 (75) | 74 (74) |
| Injection-site AE | 69 (34) | 61 (31) | 56 (56) | 57 (57) |
| Non–injection site AE | 82 (41) | 74 (37) | 38 (38) | 40 (40) |
| Vaccine-related non–injection site AE | 7 (3.5) | 6 (3) | 4 (4) | 8 (8) |
| Serious AE | 29 (14) | 29 (15) | 6 (6) | 2 (2) |
| Vaccine-related serious AE | 0 | 0 | 0 | 0 |
| Category . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . |
|---|---|---|---|---|
| ≥1 AE | 115 (57) | 109 (55) | 75 (75) | 74 (74) |
| Injection-site AE | 69 (34) | 61 (31) | 56 (56) | 57 (57) |
| Non–injection site AE | 82 (41) | 74 (37) | 38 (38) | 40 (40) |
| Vaccine-related non–injection site AE | 7 (3.5) | 6 (3) | 4 (4) | 8 (8) |
| Serious AE | 29 (14) | 29 (15) | 6 (6) | 2 (2) |
| Vaccine-related serious AE | 0 | 0 | 0 | 0 |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
| Category . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . |
|---|---|---|---|---|
| ≥1 AE | 115 (57) | 109 (55) | 75 (75) | 74 (74) |
| Injection-site AE | 69 (34) | 61 (31) | 56 (56) | 57 (57) |
| Non–injection site AE | 82 (41) | 74 (37) | 38 (38) | 40 (40) |
| Vaccine-related non–injection site AE | 7 (3.5) | 6 (3) | 4 (4) | 8 (8) |
| Serious AE | 29 (14) | 29 (15) | 6 (6) | 2 (2) |
| Vaccine-related serious AE | 0 | 0 | 0 | 0 |
| Category . | Group 1 (n = 201) Participants, No. (%) . | Group 2 (n = 199) Participants, No. (%) . | Group 3 (n = 100) Participants, No. (%) . | Group 4 (n = 100) Participants, No. (%) . |
|---|---|---|---|---|
| ≥1 AE | 115 (57) | 109 (55) | 75 (75) | 74 (74) |
| Injection-site AE | 69 (34) | 61 (31) | 56 (56) | 57 (57) |
| Non–injection site AE | 82 (41) | 74 (37) | 38 (38) | 40 (40) |
| Vaccine-related non–injection site AE | 7 (3.5) | 6 (3) | 4 (4) | 8 (8) |
| Serious AE | 29 (14) | 29 (15) | 6 (6) | 2 (2) |
| Vaccine-related serious AE | 0 | 0 | 0 | 0 |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
gpELISA Responses
Table 3 displays the primary end point, gpELISA-detected antibody level, in each study group obtained per protocol. At baseline, there was no significant difference in the antibody GMTs in subjects ≥70 years old between those receiving a booster dose and those receiving a first dose of ZV, and the GMTs in the younger age groups did not differ significantly from the GMTs of the older subjects. All groups developed an increase in GMT at week 1 and a further increase in GMT at week 6 after vaccination. The GMTs at week 6 did not differ by age group. While the GMT in each group at week 52 was higher than baseline, this difference was not significant.
Antibody (Ab) Responses Determined by a Varicella Zoster Virus Glycoprotein–Based Enzyme-Linked Immunosorbent Assay
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | |
| GMT | ||||||||
| Day 1 | 201 | 248 (220–280) | 199 | 254 (224–289) | 100 | 212 (174–259) | 98 | 260 (218–310) |
| Week 1 | 200 | 309 (278–344) | 197 | 285 (251–324) | 100 | 298 (251–355) | 96 | 349 (296–410) |
| Week 6 | 198 | 389 (351–431) | 195 | 369 (331–412) | 98 | 403 (345–469) | 97 | 445 (385–514) |
| Week 52 | 182 | 282 (251–316) | 171 | 283 (248–322) | 89 | 271 (226–325) | 91 | 292 (248–343) |
| GMFR | ||||||||
| Week 1 | 200 | 1.2 (1.2–1.3) | 197 | 1.1 (1.1–1.2) | 100 | 1.4 (1.3–1.5) | 96 | 1.4 (1.3–1.5) |
| Week 6 | 198 | 1.5 (1.3–1.6) | 195 | 1.5 (1.4–1.6) | 98 | 1.9 (1.7–2.1) | 97 | 1.7 (1.6–1.9) |
| Week 52 | 182 | 1.1 (1.1–1.2) | 171 | 1.1 (1.0–1.2) | 89 | 1.2 (1.1–1.3) | 91 | 1.2 (1.1–1.3) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | |
| GMT | ||||||||
| Day 1 | 201 | 248 (220–280) | 199 | 254 (224–289) | 100 | 212 (174–259) | 98 | 260 (218–310) |
| Week 1 | 200 | 309 (278–344) | 197 | 285 (251–324) | 100 | 298 (251–355) | 96 | 349 (296–410) |
| Week 6 | 198 | 389 (351–431) | 195 | 369 (331–412) | 98 | 403 (345–469) | 97 | 445 (385–514) |
| Week 52 | 182 | 282 (251–316) | 171 | 283 (248–322) | 89 | 271 (226–325) | 91 | 292 (248–343) |
| GMFR | ||||||||
| Week 1 | 200 | 1.2 (1.2–1.3) | 197 | 1.1 (1.1–1.2) | 100 | 1.4 (1.3–1.5) | 96 | 1.4 (1.3–1.5) |
| Week 6 | 198 | 1.5 (1.3–1.6) | 195 | 1.5 (1.4–1.6) | 98 | 1.9 (1.7–2.1) | 97 | 1.7 (1.6–1.9) |
| Week 52 | 182 | 1.1 (1.1–1.2) | 171 | 1.1 (1.0–1.2) | 89 | 1.2 (1.1–1.3) | 91 | 1.2 (1.1–1.3) |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMFR, geometric mean fold-rise from baseline; GMT, geometric mean titer.
Antibody (Ab) Responses Determined by a Varicella Zoster Virus Glycoprotein–Based Enzyme-Linked Immunosorbent Assay
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | |
| GMT | ||||||||
| Day 1 | 201 | 248 (220–280) | 199 | 254 (224–289) | 100 | 212 (174–259) | 98 | 260 (218–310) |
| Week 1 | 200 | 309 (278–344) | 197 | 285 (251–324) | 100 | 298 (251–355) | 96 | 349 (296–410) |
| Week 6 | 198 | 389 (351–431) | 195 | 369 (331–412) | 98 | 403 (345–469) | 97 | 445 (385–514) |
| Week 52 | 182 | 282 (251–316) | 171 | 283 (248–322) | 89 | 271 (226–325) | 91 | 292 (248–343) |
| GMFR | ||||||||
| Week 1 | 200 | 1.2 (1.2–1.3) | 197 | 1.1 (1.1–1.2) | 100 | 1.4 (1.3–1.5) | 96 | 1.4 (1.3–1.5) |
| Week 6 | 198 | 1.5 (1.3–1.6) | 195 | 1.5 (1.4–1.6) | 98 | 1.9 (1.7–2.1) | 97 | 1.7 (1.6–1.9) |
| Week 52 | 182 | 1.1 (1.1–1.2) | 171 | 1.1 (1.0–1.2) | 89 | 1.2 (1.1–1.3) | 91 | 1.2 (1.1–1.3) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | Participants, No. . | Ab Level, U/mL (95% CI) . | |
| GMT | ||||||||
| Day 1 | 201 | 248 (220–280) | 199 | 254 (224–289) | 100 | 212 (174–259) | 98 | 260 (218–310) |
| Week 1 | 200 | 309 (278–344) | 197 | 285 (251–324) | 100 | 298 (251–355) | 96 | 349 (296–410) |
| Week 6 | 198 | 389 (351–431) | 195 | 369 (331–412) | 98 | 403 (345–469) | 97 | 445 (385–514) |
| Week 52 | 182 | 282 (251–316) | 171 | 283 (248–322) | 89 | 271 (226–325) | 91 | 292 (248–343) |
| GMFR | ||||||||
| Week 1 | 200 | 1.2 (1.2–1.3) | 197 | 1.1 (1.1–1.2) | 100 | 1.4 (1.3–1.5) | 96 | 1.4 (1.3–1.5) |
| Week 6 | 198 | 1.5 (1.3–1.6) | 195 | 1.5 (1.4–1.6) | 98 | 1.9 (1.7–2.1) | 97 | 1.7 (1.6–1.9) |
| Week 52 | 182 | 1.1 (1.1–1.2) | 171 | 1.1 (1.0–1.2) | 89 | 1.2 (1.1–1.3) | 91 | 1.2 (1.1–1.3) |
Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMFR, geometric mean fold-rise from baseline; GMT, geometric mean titer.
ELISPOT Responses
VZV-specific CMI was evaluated on the basis of IFN-γ and IL-2 secretion by SFCs (Tables 4 and 5 and Supplementary Tables 1–3). Both IFN-γ and IL-2 are T-helper type 1 (Th1) cytokines, but the kinetics of their responses to live vaccines suggest that IFN-γ primarily identifies effector T cells, which rapidly respond to the viral challenge with a peak at 7 days after immunization, while levels of IL-2–producing T cells increase slowly, which is typical for memory T cells. Based on IFN-γ and IL-2 production, the following categories were assigned: total effector cells, measured on the basis of SFCs secreting IFN-γ, with or without secretion of IL-2; effector memory cells, measured on the basis of SFCs secreting IFN-γ and IL-2; total memory cells, measured on the basis of SFCs secreting IL-2, with or without secretion of IFN-γ; central memory cells, measured on the basis of SFCs secreting IL-2 but not IFN-γ; and differentiated effector cells, measured on the basis of SFCs secreting IFN-γ but not IL-2. At baseline, counts of VZV-specific T-cell subsets—total effector, effector-memory, total memory, central memory, and differentiated effector cells—were highest in the 50–59-year old group and generally decreased with increasing age. However, although the ≥70-year-old group that received ZV for the first time (group 2) had significantly lower responses than the 60–69-year-old group (group 3) in 18 of 20 comparisons, including all CMI subsets and all visits, the ≥70-year-old boosted group (group 1) did not. Specifically, there were no significant differences at baseline between the ≥70-year-old boosted group and the vaccinated younger group of 60–69-year-old participants with respect to any of the VZV-specific memory subsets, including effector memory, central memory, and total memory cells.
Number of Varicella Zoster Virus (VZV)–Specific Effector Cells, by Study Group
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 201 | 52 (43–64) | 199 | 38 (31–47) | 100 | 74 (60–92) | 98 | 127 (107–151) |
| Week 1 | 200 | 136 (114–161) | 197 | 100 (81–123) | 100 | 167 (135–206) | 96 | 266 (223–317) |
| Week 6 | 198 | 101 (86–119) | 195 | 82 (67–100) | 98 | 149 (124–179) | 97 | 209 (178–246) |
| Week 52 | 182 | 74 (62–88) | 171 | 47 (38–59) | 89 | 106 (85–325) | 91 | 150 (125–180) |
| GMFR | ||||||||
| Week 1 | 200 | 2.6 (2.2–3.0) | 197 | 2.6 (2.3–3.0) | 100 | 2.3 (2.0–2.6) | 96 | 2.1 (1.9–2.3) |
| Week 6 | 198 | 1.9 (1.7–2.2) | 195 | 2.2 (1.9–2.5) | 98 | 2.0 (1.8–2.3) | 97 | 1.6 (1.5–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 171 | 1.3 (1.1–1.4) | 89 | 1.4 (1.2–1.6) | 91 | 1.1 (1.0–1.3) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 201 | 52 (43–64) | 199 | 38 (31–47) | 100 | 74 (60–92) | 98 | 127 (107–151) |
| Week 1 | 200 | 136 (114–161) | 197 | 100 (81–123) | 100 | 167 (135–206) | 96 | 266 (223–317) |
| Week 6 | 198 | 101 (86–119) | 195 | 82 (67–100) | 98 | 149 (124–179) | 97 | 209 (178–246) |
| Week 52 | 182 | 74 (62–88) | 171 | 47 (38–59) | 89 | 106 (85–325) | 91 | 150 (125–180) |
| GMFR | ||||||||
| Week 1 | 200 | 2.6 (2.2–3.0) | 197 | 2.6 (2.3–3.0) | 100 | 2.3 (2.0–2.6) | 96 | 2.1 (1.9–2.3) |
| Week 6 | 198 | 1.9 (1.7–2.2) | 195 | 2.2 (1.9–2.5) | 98 | 2.0 (1.8–2.3) | 97 | 1.6 (1.5–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 171 | 1.3 (1.1–1.4) | 89 | 1.4 (1.2–1.6) | 91 | 1.1 (1.0–1.3) |
VZV-specific effector cells are defined as SFCs secreting interferon γ, with or without secretion of interleukin 2. Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMC, geometric mean count; GMFR, geometric mean fold-rise from baseline; SFC, spot-forming cell.
a Data are mean number of SFCs/106 peripheral blood mononuclear cells in VZV-stimulated wells, after subtracting the mean number of SFCs in control wells containing medium.
Number of Varicella Zoster Virus (VZV)–Specific Effector Cells, by Study Group
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 201 | 52 (43–64) | 199 | 38 (31–47) | 100 | 74 (60–92) | 98 | 127 (107–151) |
| Week 1 | 200 | 136 (114–161) | 197 | 100 (81–123) | 100 | 167 (135–206) | 96 | 266 (223–317) |
| Week 6 | 198 | 101 (86–119) | 195 | 82 (67–100) | 98 | 149 (124–179) | 97 | 209 (178–246) |
| Week 52 | 182 | 74 (62–88) | 171 | 47 (38–59) | 89 | 106 (85–325) | 91 | 150 (125–180) |
| GMFR | ||||||||
| Week 1 | 200 | 2.6 (2.2–3.0) | 197 | 2.6 (2.3–3.0) | 100 | 2.3 (2.0–2.6) | 96 | 2.1 (1.9–2.3) |
| Week 6 | 198 | 1.9 (1.7–2.2) | 195 | 2.2 (1.9–2.5) | 98 | 2.0 (1.8–2.3) | 97 | 1.6 (1.5–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 171 | 1.3 (1.1–1.4) | 89 | 1.4 (1.2–1.6) | 91 | 1.1 (1.0–1.3) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 201 | 52 (43–64) | 199 | 38 (31–47) | 100 | 74 (60–92) | 98 | 127 (107–151) |
| Week 1 | 200 | 136 (114–161) | 197 | 100 (81–123) | 100 | 167 (135–206) | 96 | 266 (223–317) |
| Week 6 | 198 | 101 (86–119) | 195 | 82 (67–100) | 98 | 149 (124–179) | 97 | 209 (178–246) |
| Week 52 | 182 | 74 (62–88) | 171 | 47 (38–59) | 89 | 106 (85–325) | 91 | 150 (125–180) |
| GMFR | ||||||||
| Week 1 | 200 | 2.6 (2.2–3.0) | 197 | 2.6 (2.3–3.0) | 100 | 2.3 (2.0–2.6) | 96 | 2.1 (1.9–2.3) |
| Week 6 | 198 | 1.9 (1.7–2.2) | 195 | 2.2 (1.9–2.5) | 98 | 2.0 (1.8–2.3) | 97 | 1.6 (1.5–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 171 | 1.3 (1.1–1.4) | 89 | 1.4 (1.2–1.6) | 91 | 1.1 (1.0–1.3) |
VZV-specific effector cells are defined as SFCs secreting interferon γ, with or without secretion of interleukin 2. Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMC, geometric mean count; GMFR, geometric mean fold-rise from baseline; SFC, spot-forming cell.
a Data are mean number of SFCs/106 peripheral blood mononuclear cells in VZV-stimulated wells, after subtracting the mean number of SFCs in control wells containing medium.
Number of Varicella Zoster Virus (VZV)–Specific Effector Memory Cells, by Study Group
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 200 | 47 (39–56) | 199 | 36 (31–47) | 100 | 56 (60–92) | 97 | 104 (81–134) |
| Week 1 | 199 | 88 (75–102) | 197 | 65 (81–123) | 100 | 99 (135–206) | 95 | 156 (125–193) |
| Week 6 | 197 | 90 (77–104) | 195 | 73 (67–100) | 98 | 114 (124–179) | 96 | 161 (130–199) |
| Week 52 | 182 | 65 (54–78) | 176 | 37 (38–59) | 88 | 82 (85–325) | 89 | 102 (78–133) |
| GMFR | ||||||||
| Week 1 | 199 | 1.9 (1.7–2.1) | 197 | 1.9 (1.7–2.1) | 100 | 1.8 (1.5–2.1) | 95 | 1.5 (1.3–1.8) |
| Week 6 | 197 | 1.9 (1.7–2.2) | 195 | 2.1 (1.9–2.4) | 98 | 2.0 (1.7–2.4) | 96 | 1.5 (1.3–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 176 | 1.1 (.9–1.3) | 89 | 1.5 (1.2–1.8) | 89 | 1.0 (.8–1.2) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 200 | 47 (39–56) | 199 | 36 (31–47) | 100 | 56 (60–92) | 97 | 104 (81–134) |
| Week 1 | 199 | 88 (75–102) | 197 | 65 (81–123) | 100 | 99 (135–206) | 95 | 156 (125–193) |
| Week 6 | 197 | 90 (77–104) | 195 | 73 (67–100) | 98 | 114 (124–179) | 96 | 161 (130–199) |
| Week 52 | 182 | 65 (54–78) | 176 | 37 (38–59) | 88 | 82 (85–325) | 89 | 102 (78–133) |
| GMFR | ||||||||
| Week 1 | 199 | 1.9 (1.7–2.1) | 197 | 1.9 (1.7–2.1) | 100 | 1.8 (1.5–2.1) | 95 | 1.5 (1.3–1.8) |
| Week 6 | 197 | 1.9 (1.7–2.2) | 195 | 2.1 (1.9–2.4) | 98 | 2.0 (1.7–2.4) | 96 | 1.5 (1.3–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 176 | 1.1 (.9–1.3) | 89 | 1.5 (1.2–1.8) | 89 | 1.0 (.8–1.2) |
VZV-specific effector memory cells are defined as SFCs secreting interferon γ and interleukin 2. Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMC, geometric mean count; GMFR, geometric mean fold-rise from baseline; SFC, spot-forming cell.
a Data are mean number of SFCs/106 peripheral blood mononuclear cells in VZV-stimulated wells, after subtracting the mean number of SFCs in control wells containing medium.
Number of Varicella Zoster Virus (VZV)–Specific Effector Memory Cells, by Study Group
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 200 | 47 (39–56) | 199 | 36 (31–47) | 100 | 56 (60–92) | 97 | 104 (81–134) |
| Week 1 | 199 | 88 (75–102) | 197 | 65 (81–123) | 100 | 99 (135–206) | 95 | 156 (125–193) |
| Week 6 | 197 | 90 (77–104) | 195 | 73 (67–100) | 98 | 114 (124–179) | 96 | 161 (130–199) |
| Week 52 | 182 | 65 (54–78) | 176 | 37 (38–59) | 88 | 82 (85–325) | 89 | 102 (78–133) |
| GMFR | ||||||||
| Week 1 | 199 | 1.9 (1.7–2.1) | 197 | 1.9 (1.7–2.1) | 100 | 1.8 (1.5–2.1) | 95 | 1.5 (1.3–1.8) |
| Week 6 | 197 | 1.9 (1.7–2.2) | 195 | 2.1 (1.9–2.4) | 98 | 2.0 (1.7–2.4) | 96 | 1.5 (1.3–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 176 | 1.1 (.9–1.3) | 89 | 1.5 (1.2–1.8) | 89 | 1.0 (.8–1.2) |
| End Point, Time . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | Participants, No. . | SFCs, No.a (95% CI) . | |
| GMC | ||||||||
| Day 1 | 200 | 47 (39–56) | 199 | 36 (31–47) | 100 | 56 (60–92) | 97 | 104 (81–134) |
| Week 1 | 199 | 88 (75–102) | 197 | 65 (81–123) | 100 | 99 (135–206) | 95 | 156 (125–193) |
| Week 6 | 197 | 90 (77–104) | 195 | 73 (67–100) | 98 | 114 (124–179) | 96 | 161 (130–199) |
| Week 52 | 182 | 65 (54–78) | 176 | 37 (38–59) | 88 | 82 (85–325) | 89 | 102 (78–133) |
| GMFR | ||||||||
| Week 1 | 199 | 1.9 (1.7–2.1) | 197 | 1.9 (1.7–2.1) | 100 | 1.8 (1.5–2.1) | 95 | 1.5 (1.3–1.8) |
| Week 6 | 197 | 1.9 (1.7–2.2) | 195 | 2.1 (1.9–2.4) | 98 | 2.0 (1.7–2.4) | 96 | 1.5 (1.3–1.8) |
| Week 52 | 182 | 1.4 (1.2–1.6) | 176 | 1.1 (.9–1.3) | 89 | 1.5 (1.2–1.8) | 89 | 1.0 (.8–1.2) |
VZV-specific effector memory cells are defined as SFCs secreting interferon γ and interleukin 2. Group 1 comprised subjects aged ≥70 years who received zoster vaccine (ZV) ≥10 years previously, group 2 comprised subjects aged ≥70 years who never received ZV and were matched to group 1 subjects by 5-year age increments, group 3 comprised subjects aged ≥60 to <70 years who never received ZV, and group 4 comprised subjects aged ≥50 to <60 years who never received ZV.
Abbreviations: CI, confidence interval; GMC, geometric mean count; GMFR, geometric mean fold-rise from baseline; SFC, spot-forming cell.
a Data are mean number of SFCs/106 peripheral blood mononuclear cells in VZV-stimulated wells, after subtracting the mean number of SFCs in control wells containing medium.
Counts of VZV-specific total effector CD4+ T-cells (Table 4) significantly increased in all groups after ZV administration. As expected for effector responses, the peak responses were at week 1. Boosted ≥70-year-old subjects had higher numbers of SFCs than their first-time-immunized age-matched controls at weeks 1 (136 vs 100 SFCs/106 PBMCs; P = .014), 6 (101 vs 82 SFCs/106 PBMCs; P = .074), and 52 (74 vs 47 SFCs/106 PBMCs; P = .0001). The younger age group (groups 3 and 4) generally had higher numbers of IFN-γ–secreting SFCs after immunization, compared with each of the older age groups (P ≤ .05), and the 50–59-year-old group generally had higher responses than the 60–69-year-old group (P ≤ .05), considering all postimmunization visits.
VZV-specific effector memory CD4+ T-cell counts, measured by dual-positive cells (ie, cells secreting IFN-γ and IL-2), increased after ZV administration in all groups, peaking at week 6, as expected for memory T cells (Table 5). The boosted ≥70-year-old subjects had higher responses than their first-time-immunized age-matched controls at weeks 1 (88 vs 65 SFCs/106 PBMCs; P = .006), 6 (90 vs 73 SFCs/106 PBMCs; P = .054) and 52 (65 vs 37 SFCs/106 PBMCs; P = <.0001). Furthermore, compared with 60–69-year-old subjects, the VZV-specific effector memory responses to ZV were similar in magnitude in the boosted ≥70-year-old subjects, while they remained significantly lower in the first-time-vaccinated ≥70-year-old subjects. The 50–59-year-old subjects had significantly higher effector memory responses after ZV receipt than older subjects at all time points except the week 52 comparison, when there was no difference from the 60–69-year-old subjects.
Analyses of VZV-specific differentiated effector cells showed kinetics over time and differences across age groups similar to those of the total effector cells, except that counts of differentiated effector cells were significantly higher only at 52 weeks after vaccination for ≥70-year-old boosted individuals, compared with the ≥70-year-old first-time-immunized group (10.9 vs 6.3 SFCs/106 PBMCs; P = .004; Supplementary Table 1). Analyses of VZV-specific central memory T-cells showed similar kinetics and differences across age groups as the effector memory T-cells (Supplementary Table 2). Analyses of the ≥70-year-old groups showed marginally higher numbers of SFCs in the boosted subjects, compared with those immunized for the first time, at weeks 1 and 52 but not week 6 after vaccination. Compared with 60–69-year-old subjects, VZV-specific central memory SFCs reached a similar magnitude after ZV receipt in the boosted ≥70-year-old subjects but remained significantly lower in the first-time-vaccinated ≥70-year-old subjects. The 50–59-year-old group had the highest numbers of SFCs secreting IL-2 but not IFN-γ after ZV receipt. Total VZV-specific memory T cells had similar kinetics as the effector memory and central memory subsets (Supplementary Table 3). Total memory responses peaked at week 6 after vaccination and were significantly higher in the ≥70-year-old boosted subjects, compared with the first-time-immunized age-matched controls at weeks 1 and 52. Both ≥70-year-old groups had lower total memory responses after vaccination than the groups aged 60–69 or 50–69 years.
Correlation of gpELISA Findings With ELISPOT Findings
Fold-rise in gpELISA titers at 6 weeks after vaccination is a nonmechanistic correlate of protection conferred by ZV, as shown in previous efficacy trials [5]. Since protection against HZ is conferred by CMI [3], we performed correlation analyses between the gpELISA and ELISPOT results at baseline and after vaccination (Table 6 and Supplementary Table 4). The data for groups 2–4, which received ZV for the first time, are presented as a pooled analysis, whereas data for group 1, which received a booster dose of ZV, are presented separately. At baseline, gpELISA titers significantly correlated with the effector memory cells in the pooled analysis (correlation coefficient = 0.14; P = .007) but not with any other T-cell subsets in the same groups or in group 1. At week 6, gpELISA titers significantly correlated with total effector, effector memory, total memory, and differentiated effector subsets in the groups 2–4 at all time points and with IL-2–secreting but not IFN-γ–secreting central memory cell subsets at entry and 6 weeks after vaccination (correlation coefficient range, 0.12–0.29; P value range, < .001 to .03). In group 1, the coefficient of correlation was generally similar but did not reach statistical significance, owing to the lower number of subjects (Table 6). In groups 2–4, the change in the gpELISA titer from baseline to week 6, which was the most predictive measure for vaccine-conferred protection in previous efficacy studies of 50–59 ZV recipients [4], positively correlated with total and differentiated effector cells at all time points and with total and effector memory responses after vaccination only (coefficients of correlation, ≥0.11; P < .05). In group 1, correlations were not significant. Individual analyses of groups 2–4 (Supplementary Tables 4) showed similar trends, compared with the pooled analysis, but the correlations did not always reach statistical significance; this was not unexpected, considering the smaller number of subjects in each group. Correlations between week 6 gpELISA concentrations and CMI were similarly distributed across groups 2–4. Correlations of the gpELISA fold-increase from baseline to week 6 with CMI were more common in group 4 than in groups 2 or 3 indicating than an effect of age could not be excluded on the relationship of gpELISA fold-increase with CMI.
Correlation Between Varicella Zoster Virus (VZV)–Specific Cell-Mediated and Humoral Immunity After Zoster Vaccine (ZV) Administration, Using Pooled Analysis
| Variable . | First-Time ZV Recipients Aged ≥50 Years Old (Groups 2–4) . | Booster ZV Recipients Aged ≥70 Years Old (Group 1) . | ||||
|---|---|---|---|---|---|---|
| Participants, No. . | Coefficient of Correlation . | P Value . | Participants, No. . | Coefficient of Correlation . | P Value . | |
| Baseline gpELISA findings | ||||||
| Total effector | 393 | 0.091 | .072 | 195 | 0.104 | .147 |
| Effector memory | 390 | 0.137 | .007 | 194 | 0.102 | .157 |
| Total memory | 393 | 0.075 | .138 | 196 | 0.074 | .304 |
| Central memory | 379 | 0.055 | .283 | 194 | 0.0414 | .566 |
| Differentiated effector | 344 | 0.057 | .293 | 161 | 0.0904 | .256 |
| Week 6 gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.218 | <.0001 | 198 | 0.222 | .002 |
| Effector memory | 385 | 0.295 | <.0001 | 196 | 0.168 | .019 |
| Total memory | 386 | 0.2165 | <.0001 | 194 | 0.224 | .002 |
| Central memory | 378 | 0.148 | .004 | 192 | 0.284 | <.0001 |
| Differentiated effector | 375 | 0.20321 | <.0001 | 195 | 0.229 | .001 |
| Week 6 gpELISA and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.233 | <.0001 | 198 | 0.166 | .020 |
| Effector memory | 385 | 0.255 | <.0001 | 197 | 0.174 | .015 |
| Total memory | 390 | 0.182 | .001 | 194 | 0.137 | .056 |
| Central memory | 382 | 0.061 | .2316 | 192 | 0.082 | .260 |
| Differentiated effector | 354 | 0.152 | .004 | 180 | 0.226 | .002 |
| Week 6 gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.167 | .002 | 182 | 0.183 | .013 |
| Effector memory | 340 | 0.12587 | .020 | 181 | 0.179 | .016 |
| Total memory | 344 | 0.107 | .048 | 180 | 0.107 | .152 |
| Central memory | 335 | 0.061 | .263 | 176 | 0.144 | .056 |
| Differentiated effector | 308 | 0.157 | .006 | 165 | 0.094 | .232 |
| Week 6/baseline gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.140 | .006 | 198 | 0.084 | .239 |
| Effector memory | 385 | 0.144 | .005 | 196 | 0.091 | .205 |
| Total memory | 386 | 0.131 | .010 | 194 | 0.124 | .085 |
| Central memory | 378 | 0.093 | .070 | 192 | 0.127 | .079 |
| Differentiated effector | 375 | 0.113 | .029 | 195 | 0.034 | .635 |
| Week 6 gpELISA/baseline and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.139 | .006 | 198 | 0.025 | .726 |
| Effector memory | 385 | 0.130 | .010 | 197 | 0.050 | .484 |
| Total memory | 390 | 0.106 | .036 | 194 | 0.061 | .402 |
| Central memory | 382 | 0.068 | .188 | 192 | 0.038 | .598 |
| Differentiated effector | 354 | 0.106 | .046 | 180 | 0.011 | .885 |
| Week 6/baseline gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.198 | <.001 | 182 | −0.007 | .922 |
| Effector memory | 340 | 0.210 | .0001 | 181 | 0.021 | .776 |
| Total memory | 344 | 0.176 | .001 | 180 | 0.016 | .830 |
| Central memory | 335 | 0.094 | .084 | 176 | 0.031 | .679 |
| Differentiated effector | 308 | 0.122 | .033 | 165 | −0.070 | .370 |
| Variable . | First-Time ZV Recipients Aged ≥50 Years Old (Groups 2–4) . | Booster ZV Recipients Aged ≥70 Years Old (Group 1) . | ||||
|---|---|---|---|---|---|---|
| Participants, No. . | Coefficient of Correlation . | P Value . | Participants, No. . | Coefficient of Correlation . | P Value . | |
| Baseline gpELISA findings | ||||||
| Total effector | 393 | 0.091 | .072 | 195 | 0.104 | .147 |
| Effector memory | 390 | 0.137 | .007 | 194 | 0.102 | .157 |
| Total memory | 393 | 0.075 | .138 | 196 | 0.074 | .304 |
| Central memory | 379 | 0.055 | .283 | 194 | 0.0414 | .566 |
| Differentiated effector | 344 | 0.057 | .293 | 161 | 0.0904 | .256 |
| Week 6 gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.218 | <.0001 | 198 | 0.222 | .002 |
| Effector memory | 385 | 0.295 | <.0001 | 196 | 0.168 | .019 |
| Total memory | 386 | 0.2165 | <.0001 | 194 | 0.224 | .002 |
| Central memory | 378 | 0.148 | .004 | 192 | 0.284 | <.0001 |
| Differentiated effector | 375 | 0.20321 | <.0001 | 195 | 0.229 | .001 |
| Week 6 gpELISA and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.233 | <.0001 | 198 | 0.166 | .020 |
| Effector memory | 385 | 0.255 | <.0001 | 197 | 0.174 | .015 |
| Total memory | 390 | 0.182 | .001 | 194 | 0.137 | .056 |
| Central memory | 382 | 0.061 | .2316 | 192 | 0.082 | .260 |
| Differentiated effector | 354 | 0.152 | .004 | 180 | 0.226 | .002 |
| Week 6 gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.167 | .002 | 182 | 0.183 | .013 |
| Effector memory | 340 | 0.12587 | .020 | 181 | 0.179 | .016 |
| Total memory | 344 | 0.107 | .048 | 180 | 0.107 | .152 |
| Central memory | 335 | 0.061 | .263 | 176 | 0.144 | .056 |
| Differentiated effector | 308 | 0.157 | .006 | 165 | 0.094 | .232 |
| Week 6/baseline gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.140 | .006 | 198 | 0.084 | .239 |
| Effector memory | 385 | 0.144 | .005 | 196 | 0.091 | .205 |
| Total memory | 386 | 0.131 | .010 | 194 | 0.124 | .085 |
| Central memory | 378 | 0.093 | .070 | 192 | 0.127 | .079 |
| Differentiated effector | 375 | 0.113 | .029 | 195 | 0.034 | .635 |
| Week 6 gpELISA/baseline and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.139 | .006 | 198 | 0.025 | .726 |
| Effector memory | 385 | 0.130 | .010 | 197 | 0.050 | .484 |
| Total memory | 390 | 0.106 | .036 | 194 | 0.061 | .402 |
| Central memory | 382 | 0.068 | .188 | 192 | 0.038 | .598 |
| Differentiated effector | 354 | 0.106 | .046 | 180 | 0.011 | .885 |
| Week 6/baseline gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.198 | <.001 | 182 | −0.007 | .922 |
| Effector memory | 340 | 0.210 | .0001 | 181 | 0.021 | .776 |
| Total memory | 344 | 0.176 | .001 | 180 | 0.016 | .830 |
| Central memory | 335 | 0.094 | .084 | 176 | 0.031 | .679 |
| Differentiated effector | 308 | 0.122 | .033 | 165 | −0.070 | .370 |
Total effector cells were measured on the basis of spot-forming cells (SFCs) secreting interferon γ (IFN-γ), with or without secretion of interleukin 2 (IL-2); effector memory cells were measured on the basis of SFCs secreting IFN-γ and IL-2; total memory cells were measured on the basis of SFCs secreting IL-2, with or without secretion of IFN-γ; central memory cells were measured on the basis of SFCs secreting IL-2 but not IFN-γ; and differentiated effector cells were measured on the basis of SFCs secreting IFN-γ but not IL-2.
Abbreviations: ELISPOT, enzyme-linked immunosorbent spot; gpELISA, VZV glycoprotein–based enzyme-linked immunosorbent assay.
Correlation Between Varicella Zoster Virus (VZV)–Specific Cell-Mediated and Humoral Immunity After Zoster Vaccine (ZV) Administration, Using Pooled Analysis
| Variable . | First-Time ZV Recipients Aged ≥50 Years Old (Groups 2–4) . | Booster ZV Recipients Aged ≥70 Years Old (Group 1) . | ||||
|---|---|---|---|---|---|---|
| Participants, No. . | Coefficient of Correlation . | P Value . | Participants, No. . | Coefficient of Correlation . | P Value . | |
| Baseline gpELISA findings | ||||||
| Total effector | 393 | 0.091 | .072 | 195 | 0.104 | .147 |
| Effector memory | 390 | 0.137 | .007 | 194 | 0.102 | .157 |
| Total memory | 393 | 0.075 | .138 | 196 | 0.074 | .304 |
| Central memory | 379 | 0.055 | .283 | 194 | 0.0414 | .566 |
| Differentiated effector | 344 | 0.057 | .293 | 161 | 0.0904 | .256 |
| Week 6 gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.218 | <.0001 | 198 | 0.222 | .002 |
| Effector memory | 385 | 0.295 | <.0001 | 196 | 0.168 | .019 |
| Total memory | 386 | 0.2165 | <.0001 | 194 | 0.224 | .002 |
| Central memory | 378 | 0.148 | .004 | 192 | 0.284 | <.0001 |
| Differentiated effector | 375 | 0.20321 | <.0001 | 195 | 0.229 | .001 |
| Week 6 gpELISA and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.233 | <.0001 | 198 | 0.166 | .020 |
| Effector memory | 385 | 0.255 | <.0001 | 197 | 0.174 | .015 |
| Total memory | 390 | 0.182 | .001 | 194 | 0.137 | .056 |
| Central memory | 382 | 0.061 | .2316 | 192 | 0.082 | .260 |
| Differentiated effector | 354 | 0.152 | .004 | 180 | 0.226 | .002 |
| Week 6 gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.167 | .002 | 182 | 0.183 | .013 |
| Effector memory | 340 | 0.12587 | .020 | 181 | 0.179 | .016 |
| Total memory | 344 | 0.107 | .048 | 180 | 0.107 | .152 |
| Central memory | 335 | 0.061 | .263 | 176 | 0.144 | .056 |
| Differentiated effector | 308 | 0.157 | .006 | 165 | 0.094 | .232 |
| Week 6/baseline gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.140 | .006 | 198 | 0.084 | .239 |
| Effector memory | 385 | 0.144 | .005 | 196 | 0.091 | .205 |
| Total memory | 386 | 0.131 | .010 | 194 | 0.124 | .085 |
| Central memory | 378 | 0.093 | .070 | 192 | 0.127 | .079 |
| Differentiated effector | 375 | 0.113 | .029 | 195 | 0.034 | .635 |
| Week 6 gpELISA/baseline and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.139 | .006 | 198 | 0.025 | .726 |
| Effector memory | 385 | 0.130 | .010 | 197 | 0.050 | .484 |
| Total memory | 390 | 0.106 | .036 | 194 | 0.061 | .402 |
| Central memory | 382 | 0.068 | .188 | 192 | 0.038 | .598 |
| Differentiated effector | 354 | 0.106 | .046 | 180 | 0.011 | .885 |
| Week 6/baseline gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.198 | <.001 | 182 | −0.007 | .922 |
| Effector memory | 340 | 0.210 | .0001 | 181 | 0.021 | .776 |
| Total memory | 344 | 0.176 | .001 | 180 | 0.016 | .830 |
| Central memory | 335 | 0.094 | .084 | 176 | 0.031 | .679 |
| Differentiated effector | 308 | 0.122 | .033 | 165 | −0.070 | .370 |
| Variable . | First-Time ZV Recipients Aged ≥50 Years Old (Groups 2–4) . | Booster ZV Recipients Aged ≥70 Years Old (Group 1) . | ||||
|---|---|---|---|---|---|---|
| Participants, No. . | Coefficient of Correlation . | P Value . | Participants, No. . | Coefficient of Correlation . | P Value . | |
| Baseline gpELISA findings | ||||||
| Total effector | 393 | 0.091 | .072 | 195 | 0.104 | .147 |
| Effector memory | 390 | 0.137 | .007 | 194 | 0.102 | .157 |
| Total memory | 393 | 0.075 | .138 | 196 | 0.074 | .304 |
| Central memory | 379 | 0.055 | .283 | 194 | 0.0414 | .566 |
| Differentiated effector | 344 | 0.057 | .293 | 161 | 0.0904 | .256 |
| Week 6 gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.218 | <.0001 | 198 | 0.222 | .002 |
| Effector memory | 385 | 0.295 | <.0001 | 196 | 0.168 | .019 |
| Total memory | 386 | 0.2165 | <.0001 | 194 | 0.224 | .002 |
| Central memory | 378 | 0.148 | .004 | 192 | 0.284 | <.0001 |
| Differentiated effector | 375 | 0.20321 | <.0001 | 195 | 0.229 | .001 |
| Week 6 gpELISA and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.233 | <.0001 | 198 | 0.166 | .020 |
| Effector memory | 385 | 0.255 | <.0001 | 197 | 0.174 | .015 |
| Total memory | 390 | 0.182 | .001 | 194 | 0.137 | .056 |
| Central memory | 382 | 0.061 | .2316 | 192 | 0.082 | .260 |
| Differentiated effector | 354 | 0.152 | .004 | 180 | 0.226 | .002 |
| Week 6 gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.167 | .002 | 182 | 0.183 | .013 |
| Effector memory | 340 | 0.12587 | .020 | 181 | 0.179 | .016 |
| Total memory | 344 | 0.107 | .048 | 180 | 0.107 | .152 |
| Central memory | 335 | 0.061 | .263 | 176 | 0.144 | .056 |
| Differentiated effector | 308 | 0.157 | .006 | 165 | 0.094 | .232 |
| Week 6/baseline gpELISA and week 1 ELISPOT findings | ||||||
| Total effector | 386 | 0.140 | .006 | 198 | 0.084 | .239 |
| Effector memory | 385 | 0.144 | .005 | 196 | 0.091 | .205 |
| Total memory | 386 | 0.131 | .010 | 194 | 0.124 | .085 |
| Central memory | 378 | 0.093 | .070 | 192 | 0.127 | .079 |
| Differentiated effector | 375 | 0.113 | .029 | 195 | 0.034 | .635 |
| Week 6 gpELISA/baseline and week 6 ELISPOT findings | ||||||
| Total effector | 387 | 0.139 | .006 | 198 | 0.025 | .726 |
| Effector memory | 385 | 0.130 | .010 | 197 | 0.050 | .484 |
| Total memory | 390 | 0.106 | .036 | 194 | 0.061 | .402 |
| Central memory | 382 | 0.068 | .188 | 192 | 0.038 | .598 |
| Differentiated effector | 354 | 0.106 | .046 | 180 | 0.011 | .885 |
| Week 6/baseline gpELISA and week 52 ELISPOT findings | ||||||
| Total effector | 342 | 0.198 | <.001 | 182 | −0.007 | .922 |
| Effector memory | 340 | 0.210 | .0001 | 181 | 0.021 | .776 |
| Total memory | 344 | 0.176 | .001 | 180 | 0.016 | .830 |
| Central memory | 335 | 0.094 | .084 | 176 | 0.031 | .679 |
| Differentiated effector | 308 | 0.122 | .033 | 165 | −0.070 | .370 |
Total effector cells were measured on the basis of spot-forming cells (SFCs) secreting interferon γ (IFN-γ), with or without secretion of interleukin 2 (IL-2); effector memory cells were measured on the basis of SFCs secreting IFN-γ and IL-2; total memory cells were measured on the basis of SFCs secreting IL-2, with or without secretion of IFN-γ; central memory cells were measured on the basis of SFCs secreting IL-2 but not IFN-γ; and differentiated effector cells were measured on the basis of SFCs secreting IFN-γ but not IL-2.
Abbreviations: ELISPOT, enzyme-linked immunosorbent spot; gpELISA, VZV glycoprotein–based enzyme-linked immunosorbent assay.
DISCUSSION
This study examined the safety and immunogenicity of a booster dose of ZV in subjects ≥70 years of age who received a dose of ZV ≥10 years previously and compared these responses to those in cohorts of age-matched and younger vaccinees who recently received their first dose of ZV. The GMTs of VZV antibody were similar in all age groups at baseline, confirming that antibody levels are similar over a wide age spectrum and do not decline with increasing age [3, 24]. All age groups developed an increase in GMT at week 1 after ZV receipt that peaked at week 6. The booster dose of ZV administered to adults ≥70 years old after ≥10 years elicited a GMT and geometric mean fold-rise in VZV antibody titer that was noninferior to that of ZV administered as a first dose to subjects ≥70 years old.
At baseline, the boosted-dose group maintained a small but statistically significant higher number of VZV-specific total effector, effector memory, and total memory T cells. Effector memory T cells are characterized by a high capacity to multiply and to rapidly increase cytokine production after they encounter their cognate antigen. These cells may contribute to the early response to VZV reactivation, and their increased number may be advantageous to the host [13, 14]. The extent to which circulating effector memory CD4+ T cells represent their tissue counterparts is unknown.
gpELISA titers at 6 weeks after ZV administration and their increase from baseline have been shown to correlate with protection conferred by ZV [3, 4]. However, the fold-rise in antibody titer is a nonmechanistic correlate of protection against HZ [5]. Since this study demonstrates a significant correlation between gpELISA titers at 6 weeks after vaccination and the CMI response to ZV, this may explain the predictive value of the antibody response to ZV [3–5]. Correlation analyses of gpELISA with VZV-specific CMI in previous studies have yielded dissimilar results. However, the older studies used mostly proliferation-based assays, which have higher variability than ELISPOT, while we used a highly sensitive and reproducible fluorescence-based ELISPOT assay to measure SFCs secreting IFN-γ and/or IL-2. We also used strict quality control criteria for accepting assay results, which increased the reproducibility of our assay, compared with ELISPOT in older studies.
The magnitude of the VZV IFN-γ ELISPOT response to ZV has been demonstrated to correlate with protection against HZ [3]. The GMCs in this study at baseline were higher in the younger age groups, as predicted by the previously demonstrated age-related decline in VZV-specific CMI [3, 6]. The IFN-γ ELISPOT-based GMCs increased significantly at all postvaccination time points in each study group, with the peak GMC occurring at week 1, which is consistent with previous studies of this VZV-specific CMI response [12, 25]. However, GMCs at all times were significantly higher in the younger groups, confirming previous reports that ZV administration at a younger age results in a greater VZV-specific CMI responses and greater efficacy of ZV [2, 3].
Similar to their baseline comparison, the IFN-γ GMCs in ≥70-year-old subjects receiving a second dose of ZV were significantly greater at week 1 and week 52 than in those receiving a first dose. The comparisons between groups were very similar whether the VZV-specific CMI readout in the ELISPOT assay was the number of total effector, effector memory, or total memory cells. Thus, recipients of a booster dose of ZV ≥10 years after a first dose developed robust measures of VZV-specific CMI that demonstrated significant residual effects of the first dose. This is not unexpected, given that protection persists long after ZV administration, albeit with a time-dependent decline, in such individuals [21].
Our results revealed not only persistence of increased VZV-specific CMI ≥10 years after vaccination in individuals ≥70 years of age, but also that these individuals predominantly had responses to a booster dose of ZV of the same magnitude as 60–69-year-old individuals vaccinated for the first time. This observation raises the question of the long-term persistence of ZV-boosted CMI in adults who receive the vaccine at the age of 50–59 years, when VZV-specific CMI after ZV is significantly higher than that of older individuals. Furthermore, we hypothesize that a second dose of ZV administered ≥10 years after the first ZV in this age group would increase VZV-specific CMI even further, compared with findings for individuals immunized for the first time at ≥60 years of age. Studies are needed to test this hypothesis, to establish the duration of protection conferred by ZV to 50–59-year-old vaccinees.
The local and systemic reactions to ZV in vaccinees receiving a second dose were similar to those in an age-matched control group receiving a first dose and to that observed in the pivotal trial for ZV [26]. The increased frequency of local adverse events in younger vaccinees was previously observed with ZV. The absence of increased reactogenicity for subjects receiving a second dose was expected, given that ZV administration to people with a prior episode of HZ or prior ZV is typically uneventful [25, 27, 28].
This study was limited by the choice of a single interval for administering a second dose of ZV and the absence of clinical correlations. Although the practical implications of the current findings are not fully understood, the similarity of ELISPOT responses to those observed in the successful efficacy trial of ZV in vaccinees ≥60 years of age [3] supports further investigation of administration of ZV at an early age versus at a later age and further investigation of a booster dose for elderly individuals at an appropriate interval after initial immunization against HZ.
Notes
Financial support. Funding for this research was provided by Merck, Sharp, & Dohme Corp., a subsidiary of Merck & Co., Inc. (sponsor). Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authors and may not necessarily reflect those of the sponsor.
Potential conflicts of interest. M. J. L. has received grants from Merck and personal fees from Merck and GSK and receives royalties for a patent with Merck. K. E. S. has received grants from Merck. A. W.-D.'s institution received support for this study through a contract with Merck, and her spouse receives royalties from Merck for the Zostavax patent. N. L. has received grants from Merck. G. Z. has equity interest in Abbott, J&J, Merck, Medtronics, and Pfizer and had a contract with Merck as statistician. J. P., L. P., and Z. P. are current or former employees of Merck and may or may not own stock or stock options in the company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Merck employee at the time of this research.




