-
PDF
- Split View
-
Views
-
Cite
Cite
Mike Flint, Christin H. Goodman, Scott Bearden, Dianna M. Blau, Brian R. Amman, Alison J. Basile, Jessica A. Belser, Éric Bergeron, Michael D. Bowen, Aaron C. Brault, Shelley Campbell, Ayan K. Chakrabarti, Kimberly A. Dodd, Bobbie R. Erickson, Molly M. Freeman, Aridth Gibbons, Lisa W. Guerrero, John D. Klena, R. Ryan Lash, Michael K. Lo, Laura K. McMullan, Gbetuwa Momoh, James L. Massally, Augustine Goba, Christopher D. Paddock, Rachael A. Priestley, Meredith Pyle, Mark Rayfield, Brandy J. Russell, Johanna S. Salzer, Angela J. Sanchez, Amy J. Schuh, Tara K. Sealy, Martin Steinau, Robyn A. Stoddard, Céline Taboy, Maryann Turnsek, David Wang, Galina E. Zemtsova, Marko Zivcec, Christina F. Spiropoulou, Ute Ströher, Jonathan S. Towner, Stuart T. Nichol, Brian H. Bird, Ebola Virus Diagnostics: The US Centers for Disease Control and Prevention Laboratory in Sierra Leone, August 2014 to March 2015, The Journal of Infectious Diseases, Volume 212, Issue suppl_2, October 2015, Pages S350–S358, https://doi.org/10.1093/infdis/jiv361
Close - Share Icon Share
Abstract
In August 2014, the Viral Special Pathogens Branch of the US Centers for Disease Control and Prevention established a field laboratory in Sierra Leone in response to the ongoing Ebola virus outbreak. Through March 2015, this laboratory tested >12 000 specimens from throughout Sierra Leone. We describe the organization and procedures of the laboratory located in Bo, Sierra Leone.
Since the discovery of Ebola virus (EBOV) in 1976, there have been >20 outbreaks, but the West African epidemic of 2013–2015 is the largest recorded, with >10 000 fatalities and 25 000 persons infected as of 29 March 2015, surpassing all the other outbreaks combined. EBOV is classified in the family Filoviridae, genus Ebolavirus, with the Zaire ebolavirus species being responsible for the 2014 West African outbreak [1]. The EBOV genome is negative-strand RNA approximately 19 kb long that encodes 7 genes: nucleoprotein (NP), viral protein (VP) 35, VP40, glycoprotein, VP30, VP24 and the viral polymerase L [2]. Several tests can be used to diagnose EBOV infection, including reverse-transcription polymerase chain reaction (RT-PCR) to detect the virus genome [3–6], enzyme-linked immunosorbent assay to measure virus-specific immunoglobulin (Ig) M or IgG [7, 8], or assays for viral antigen, either in blood (with enzyme-linked immunosorbent assay) [7] or in skin or liver biopsy specimens (with immunohistochemistry) [9, 10]. RT-PCR has been the most common assay used in the current outbreak, detecting EBOV RNA in whole blood samples obtained from living patients or oral swab samples collected from dead bodies.
Laboratories have been established in many parts of the outbreak region, often associated with an EBOV treatment unit (ETU), to provide testing for diagnostic purposes, aiding admittance, treatment, and discharge decisions. For living patients, RT-PCR may give a negative result if performed <72 hours after onset of clinical symptoms, apparently owing to low viremia during the early stages of infection [11, 12]. Thus, a negative test result may be valid only if the blood sample is obtained ≥72 hours after onset. Because reverse-transcriptase inhibitors in the sample can cause false-negative results, the laboratory will usually include a control for this, either through amplification of a human transcript such as β2-microglobulin (B2M; which also acts as a check for sample integrity), or by addition of an exogenous RNA, which is then also detected with RT-PCR.
The Viral Special Pathogens Branch (VSPB) of the US Centers for Disease Control and Prevention (CDC) has many decades of experience in establishing and operating diagnostic laboratories in low-resource settings, in support of public health efforts to control EBOV and Marburg virus disease outbreaks. In August 2014, in response to an ongoing EBOV outbreak in West Africa, the Sierra Leone Ministry of Health and Sanitation invited the CDC to establish an EBOV diagnostic laboratory in Sierra Leone. The laboratory was initially situated at Kenema Government Hospital, but owing to westward movement of cases, it was moved in late September to the Bandajuma region of Bo within the Médicins Sans Frontières (MSF) case management center (Figure 1). When it opened, the CDC laboratory was one of only 3 EBOV diagnostic laboratories operating in Sierra Leone, and during the next 7 months it received samples from 12 of Sierra Leone's 14 districts. Here we describe the organization and work flow of the Bo laboratory. We hope this information will facilitate the establishment and aid the rapid deployment of EBOV diagnostic field laboratories for this and future outbreaks.
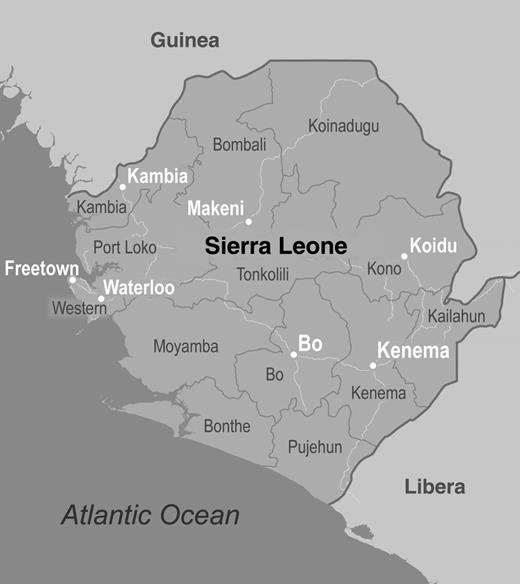
Map of Sierra Leone, with the different districts shown. District names are in black text, city names in white. The Centers for Disease Control and Prevention laboratory was initially established in Kenema but was relocated to Bo in September 2014.
MATERIALS AND METHODS
Biosafety
It was not possible to establish a biosafety level 4 (BSL-4) containment laboratory in Sierra Leone. To allow CDC scientists to work safely, a “hot laboratory” was set up. This was the only area where patient samples were handled. All persons entering the hot laboratory were trained by VSPB scientists experienced in working with EBOV under BSL-4 conditions. The personal protective equipment (PPE) worn in the hot laboratory included scrubs and Croc clog shoes (Uniform Advantage), level 3 surgical gowns (Proxima Sirus; Medline Industries), gloves (High Five Products), extended cuff gloves (Purple Nitrile-Xtra; Kimberly-Clark), and shoe covers (Proshield 3; DuPont). Powered air purifying respirators (PAPRs) and accessories were manufactured by 3M (Air-Mate). Potentially infected materials were decontaminated using 5% (vol/vol) Micro-Chem Plus disinfectant: a mixture of dimethyl benzyl ammonium chloride, dimethyl ethyl benzyl ammonium chloride, and polyethylene mono-ether glycols (National Chemical Laboratories). Extensive safety testing confirmed that this concentration of disinfectant was virucidal for EBOV (P. Jahrling, personal communication). Specimen transport containers were Air Sea BioJar (code 500; Biopack 2; 1.5-L UN combination packaging 4G/class 6.2) from Air Sea Containers, and absorbent paper pads were from Saf-T-Pak.
RNA Extraction and RT-PCR Assays
RNA was extracted from patient specimens using the MagMAX magnetic bead system (Life Technologies), according to the manufacturer's instructions. In this process, 100 µL of each specimen was mixed with 400 µL of lysis buffer supplemented with 2 µL of carrier RNA solution. RNA was extracted, using either a MagMAX Express-96 Deep-Well magnetic particle extractor or a BeadRetriever automated magnetic bead separation system, and then eluted in 90 µL of elution buffer. Full details of the RT-PCR assays will be described elsewhere, but are available on request. Briefly, 3 tests were performed concurrently: 2 specific for EBOV NP and VP40 genes and 1 for the human B2M gene as a control for sample integrity. RT-PCR assays were performed on a CFX96 Real-Time System (BioRad). Samples for use as positive controls were generated in the BSL-4 laboratory at the CDC in Atlanta, Georgia; Z. ebolavirus (Mayinga variant from the 1976 outbreak) from the VSPB collection was grown in Vero E6 cells, and viral RNA was extracted from the culture medium.
Results from the quantitative RT-PCR (qRT-PCR) assays were evaluated comprehensively with attention paid to each of the 3 targets qRT-PCR test results, the date of onset of clinical illness, the date of collection of the specimen, and the case history of the patient (if known). Specimens were considered negative if 3 criteria were met: (1) both EBOV NP and VP40 were undetected, (2) the internal extraction control B2M was detected in an acceptable range of cycle threshold values (blood specimens, approximately 18–30; oral swab samples, approximately 28–39), and (3) the specimen was collected >72 hours after onset of clinical illness. Specimens were considered positive regardless of the timing of collection relative to onset of clinical illness if (1) either of the NP or VP40 assays were detected (cycle threshold, <39) and (2) the B2M internal control was detected in the acceptable ranges. In instances where results were discordant between the NP and VP40 assays (typically in the first 1–3 days after onset of illness when viremia levels were lower) a follow-up specimen was requested for final definitive confirmation.
Results were considered pending if (1) the NP and VP40 assays were not detected and the B2M internal control also failed detection or (2) the specimen was collected <72 hours after onset of clinical illness. In these pending cases, a subsequent (>72 hours) follow-up specimen was requested for definitive diagnosis of EBOV status. A small minority of specimens were rejected as nondiagnostic if the B2M internal control failed to be detected after 2 separate RNA extractions and qRT-PCR test runs. The overwhelming majority of the rejected specimens were surveillance oral swab samples obtained from dead bodies whose poor quality was probably due either to improper collection technique or to excessive transit time to the laboratory.
RESULTS
Establishment of the CDC Field Laboratory in Sierra Leone for EBOV Diagnostics
MSF provided a building on the grounds of their Bo case management center for CDC use; this space was converted into a field laboratory. Running water was provided by the MSF water and sanitation engineers, and electric power was supplied by medium capacity generators on the MSF compound and with small portable high quality backup generators (HondaEU2000i) for sensitive electronic equipment. Three rooms within the house were designated as work areas: a clean room to set up PCR and RNA extraction reagents, a room for extracting RNA and adding it to PCR plates, and a room for performing the PCR assays (Figure 2A). The third room also served as an office area for laboratory workers during their deployment.
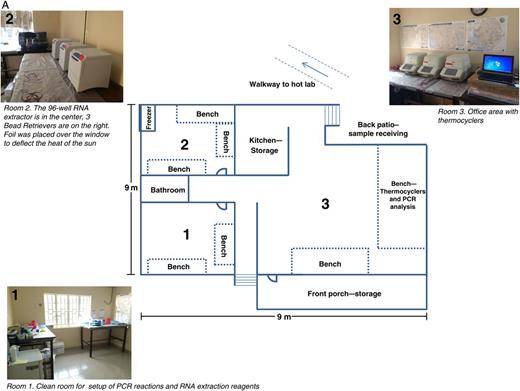
A, Floor plan of the Centers for Disease Control and Prevention (CDC) laboratory at Bo. Room 1 is the clean room, used for preparation of polymerase chain reaction (PCR) master mix and reagents for RNA extraction, room 2 is used for RNA extraction and the addition of RNA to PCR plates, and room 3 is an office area with thermocyclers, used for performing the PCR reactions and for data analysis. Work is undertaken in a unidirectional flow, from room 1 to room 2 to room 3.
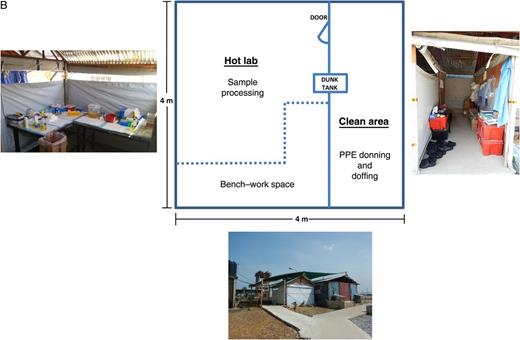
B, “Hot laboratory” at the CDC Bo laboratory. Constructed by Médicins Sans Frontières, this is a temporary wooden structure with corrugated metal roofing and plastic sheeting walls. An anteroom serves as a changing area, separated from the exterior by a plastic sheet that can be raised or lowered for privacy. Abbreviation: PPE, personal protective equipment.
Although many field laboratories opt to use class III biological safety cabinets (glove boxes), we established a separate hot laboratory. Glove boxes require electrical power, can be uncomfortable to work in for extended periods, and the glove ports can block the scientist's field of view while manipulating samples. The gloves may be corroded and weakened by repeated exposure to the decontaminating solution. However, they can be used with a more minimal PPE and in the presence of air conditioning. In contrast, a hot laboratory, with a larger working area and less restricted movement, allows multiple scientists to work on the same specimen, in a production line with different scientists performing individual steps of the processing sequence.
For the hot laboratory, MSF constructed a wood-framed scaffold with corrugated iron roofing and plastic sheeting walls about 20 yards from the main laboratory building (Figure 2B). An anteroom was used as a changing area where scrubs, PPE, and PAPRs were donned and doffed. To allow transfer of materials out of the hot laboratory, a storage trunk was filled with disinfectant (approximately 40 L) and situated with one end accessible from the hot area and the other end in the changing room. This was used as a “dunk tank,” in which items from the hot area could be submerged and the exterior surfaces disinfected before retrieval in the changing room. The disinfectant solution in the dunk tank was changed once every 3–4 weeks.
Only a single area (room 1 inside the house) was air conditioned, and temperatures in the other rooms, especially the hot lab, frequently exceeded 32°C. Consequently, when workers were inside the hot laboratory, those outside would check on their status every 5–10 minutes. In addition, a wireless doorbell system was arranged to allow signaling from inside the hot laboratory to the outside, to indicate that assistance was required. The hot laboratory was not a closed structure, having an opening between the walls and the roofing all around its perimeter (Figure 2B), so it was possible to communicate with those inside from the anteroom area. For routine communications with scientists working in the hot laboratory no additional PPE was required for those in the anteroom.
The CDC laboratory teams routinely consisted of 4 members: 3 scientists to perform sample testing and a team leader responsible for data entry, quality control, and reporting of results. On days with high sample loads, 2 scientists processed samples in the hot laboratory, while the other scientist performed RNA extraction and PCR in the main laboratory building. On days with fewer samples, a single person was required for the hot laboratory, and the other 2 implemented RNA extraction and PCR. On most days, a morning and an afternoon extraction and RT-PCR assay were performed. Generally, the laboratory teams were deployed for 28 days, with 1–3 days of overlap with the previous team. Each team worked 10–14 hours a day, 7 days a week, with no days off.
Daily Work Flow and Specimen Processing
Samples were received from referring facilities, most frequently delivered by a motorcycle courier, but sometimes delivered by ambulance or by a United Nations–coordinated helicopter. Most deliveries, but not all, were accompanied by the appropriate case investigation forms. On receipt of the sample, the transport boxes, the outside of the triple-package Air Sea shipping containers, and each sheet of paperwork were decontaminated by misting with disinfectant. The sheets of paper were allowed to air dry before examination. The containers were stored, unopened, at 4°C before processing and were never opened outside the hot laboratory. For each sample listed on the case investigation form, the time of delivery, the patient's name, and the referring facility's identification number was recorded, and a unique laboratory identification number was assigned. A 2-mL Wheaton cryogenic tube and a screw-cap Sarstedt tube were labeled with the same unique laboratory identifier, the former for an aliquot of the sample material (for freezing, in case a repeat of the assay was required), the latter tube with 400 µL of lysis buffer supplemented with carrier RNA for sample inactivation before RNA extraction.
Owing to differences in work requirements, the PPE used in the hot laboratory differed significantly from that used by MSF clinicians for patient care in treatment centers (Figure 3). The hot laboratory workers used lightweight level 3 laboratory gowns, which are much cooler than a full-Tyvek suit, an important consideration given the ambient environmental conditions. For face and respiratory protection instead of N-95 respirators and goggles, the hot laboratory workers wore a full-face shield and hooded PAPR, which, in addition to respiratory protection, provided some air flow and relief from the heat. The filter unit of the PAPR was worn underneath the gowns and was fully protected from contamination, thus allowing for easier surface decontamination without harming the internal high-efficiency particulate air filter and electronics.
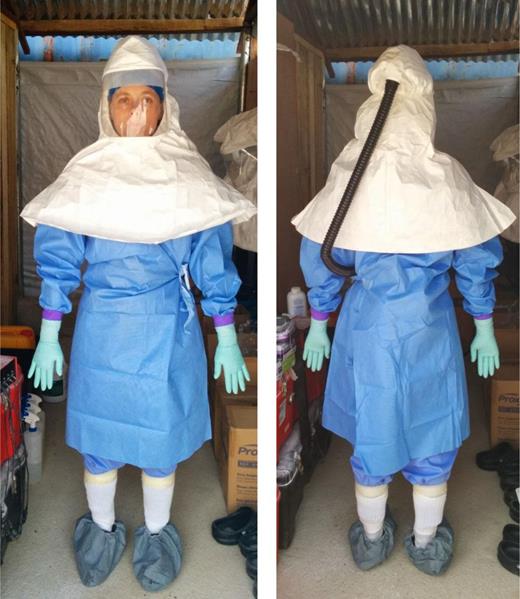
Personal protective equipment used within the “hot laboratory” at the Centers for Disease Control and Prevention laboratory in Sierra Leone.
In the changing room of the hot laboratory structure, scientists removed all jewelry and clothing, including underwear, and put on scrubs, socks, and lightweight plastic footwear (Crocs or equivalent) and shoe covers. The scrub pants were tucked into the socks and taped in place to prevent them from falling and revealing the skin. A PAPR with full hood was donned next, with a level 3 closed-front gown over the scrubs with the upper portion of the gown fitting between the inner and outer folds of the PAPR hood. An inner pair of gloves was taped to the sleeves of the gown; a middle pair, with extended cuffs, went over the first pair and was taped also. A third pair of gloves went over the second pair, the third pair being light colored to make any splashes on the gloves more visible. Once donned, the PPE was checked by another scientist, with special attention to the rear of the gown to ensure that the PAPR and the scientist's back were not exposed.
The objective of the hot laboratory work was to ensure that the received samples were correctly documented, to transfer an aliquot of the specimen to a cryogenic tube for freezing, and to place 100 µL of the specimen into lysis buffer for RNA extraction. Scientists therefore entered the hot laboratory with the shipping containers, a list of the samples that were documented as being inside the containers, cryovials for specimen storage, and tubes containing lysis buffer (each tube labeled with a unique VSPB identifying number). The first task inside the hot laboratory was to open the shipping containers and to sort the specimens for processing, identify any that might be missing or unlisted, and record any information present on the specimen containers that differed from that on the case investigation form. A scientist outside the hot laboratory took notes of discrepancies, passed in additional tubes, and corrected the log books if necessary.
Once sample identification and documentation was complete, the specimen tubes were opened one at a time at arms length over a bucket of disinfectant. When possible, a disposable transfer pipette was used to transfer up to 1.5 mL of the sample to the 2-mL cryogenic tube. Specimens that were inaccessible by transfer pipette were retrieved using a regular micropipette with an aerosol barrier tip. Then, from the cryogenic tube, 100 µL of specimen was transferred to a screw-cap tube containing lysis buffer and carrier RNA. The latter tube was shaken to ensure complete mixing, and each tube was transferred to a plastic, lidded box with holes in the bottom (so that the box would flood with disinfectant when submerged in the dunk tank, without the tubes floating out). After use, each pipette tip or transfer pipette was used to aspirate disinfectant to decontaminate its interior, and then it was discarded into a disinfectant waste bucket, along with any excess specimen and the now empty specimen tubes. After processing of each sample, the scientist's gloves were sprayed with disinfectant. If pipettors were used, they were wiped down with a paper towel soaked in disinfectant after each sample.
Once all samples were processed, the plastic boxes were immersed in the dunk tank, and weighed down with a rock, for at least a 3-minute contact time. The reusable shipping containers were “dunked out” in the same fashion. To leave the hot laboratory, the scientist opened the door back to the changing room and, while standing in the doorway, was sprayed from head to toe with disinfectant by another scientist wearing a face shield, gown, and gloves. Once sprayed, the hot laboratory scientist removed and discarded his or her shoe covers and outer gloves. The shoes, middle gloves, and tape attaching the gloves to the gown were also then sprayed with disinfectant, and the middle gloves removed. The inner gloves were then sprayed with disinfectant, and the scientists stepped out of the hot area. The tape, the gown, and the inner gloves were removed and safely discarded. The PAPR hood was removed, with only the interior surface touched. Tubes were retrieved from the dunk tank, specimen aliquots were frozen at −20°C, and RNA was extracted from the samples in lysis buffer.
Discarded specimen tubes, transfer pipettes, and tips were soaked in disinfectant overnight, before disposal the next morning. A colander set over a bucket was used to filter them from the liquid, and they were then disposed along with other solid waste, double bagged in biohazard autoclave bags. The exterior of the double-bagged trash was sprayed with disinfectant, transferred out of the hot laboratory, and placed in a third biohazard bag, which was in turn decontaminated. For liquid waste, the lid, handle, surfaces and sides of the waste bucket were sprayed with disinfectant, and the bottom of the bucket was dipped into the dunk tank. The bucket could then be passed out of the hot laboratory. Solid waste was incinerated by MSF workers, and liquid waste was disposed in the compound septic tank.
Challenges and Solutions
The packaging of samples delivered to the laboratory was frequently challenging. Samples were generally either whole blood obtained from living patients, into purple-top tubes containing anticoagulant ethylenediaminetetraacetic acid or red-top tubes with no anticoagulant, or oral swab samples obtained from dead bodies (in a transport medium). The supply chain to the districts was inconsistent for several months; initially, samples were received in a variety of containers, including coffee pots (possibly intended to insulate the samples), bloodied urine containers wrapped in gloves, filled syringes with needles attached, and sample tubes in plastic shopping bags. Occasionally samples were received many days after collection, with the clotted blood stuck to the stopper lids. The referring facilities, when it was possible to contact them, were responsive to feedback and were generally able to work toward fixing these biosafety issues. To improve sample transport safety, materials for a triple-layer packing system were purchased by CDC and provided to couriers on each sample delivery for return to the referring facilities. The packing system consisted of a watertight, leak-proof, shatter-resistant shipping container filled with soft-paper tissues for padding and reclosable bags containing absorbent pads into which specimen tubes could be placed. Each container was inserted in a corrugated cardboard box to protect it during transport.
The triple packing system became widely adopted for sample transportation, but the quality of the samples themselves remained inconsistent. For example, no standardized swabbing system was in use, and persistent problems were associated with these specimens. Often the swab samples were dry and required rehydration with lysis buffer. The wooden shafts of swabs were sometimes broken off, leaving the sharp, splintered ends exposed when the specimen tube was opened; sometimes the shafts were too long for the lid to be fitted back on to the tube, causing the lid to be loose and the tube contents to leak inside the shipping container. One district repeatedly sent swab samples in tubes wedged inside Vacutainer tubes, which could not be retrieved. Two districts consistently sent swab samples in bacterial agar transport medium. The CDC attempted to provide swabs and tubes of virus transport medium to various districts, but these did not always make it to the intended recipients, and specimens prepared with these materials were only rarely received by the laboratory.
It was sometimes difficult to link patients with their results; multiple patients with identical names could be present in an ETU simultaneously. On occasion, one identifier was assigned at a holding facility and a different identifier was given at the ETU. The use of unique patient identifiers eventually solved these issues, but this did not become standard practice until many months into the outbreak.
Another challenge was the supply of electricity to the laboratory. The Bo laboratory received electrical power from a generator operated by MSF, which was reliable, for the most part. Power failures did occasionally occur, and the ability of the BioRad real-time system to restart an assay at the point that it had stopped, once the power supply resumed, proved extremely useful; brief power outages did not affect the data quality. Another useful feature of this instrument was that it does not require an internal control fluorophore, such as Rox, which simplified reaction setup. To avoid the loss of power during RNA extractions, the instruments were generally powered by a portable gasoline-powered generator (HondaEU2000i), though overheating or possibly the quality of the locally sourced fuel was sometimes responsible for problems with these generators.
Laboratory Throughput
The laboratory received and processed specimens daily from 22 August 2014 through 22 March 2015, including the day of relocation from Kenema to Bo, Thanksgiving, Christmas Day, and New Year's Day. More than 12 000 specimens were tested in total (Figure 4A). (Note added in proof: at the time of manuscript acceptance, more than 20 000 specimens had been tested.) The mean number of samples tested per day was 63, with the highest number tested being 178 (Figure 4B). Samples transported from far afield sometimes arrived too late in the day for processing and were tested the next morning, but on average, 71% of samples were processed on the day they arrived at the laboratory, with the results distributed that evening. Samples were held over for next-day processing for several reasons: to reduce fatigue on the team, for safety concerns due to low light in the hot laboratory after dark, and, most importantly, if patients would not be moved after dark and the laboratory results would not be acted on until the next day. In all, 99.9% of samples were tested either on the day of receipt or the next day. Full details of the assay performance, the laboratory results, and their impact on the outbreak response will be published elsewhere.
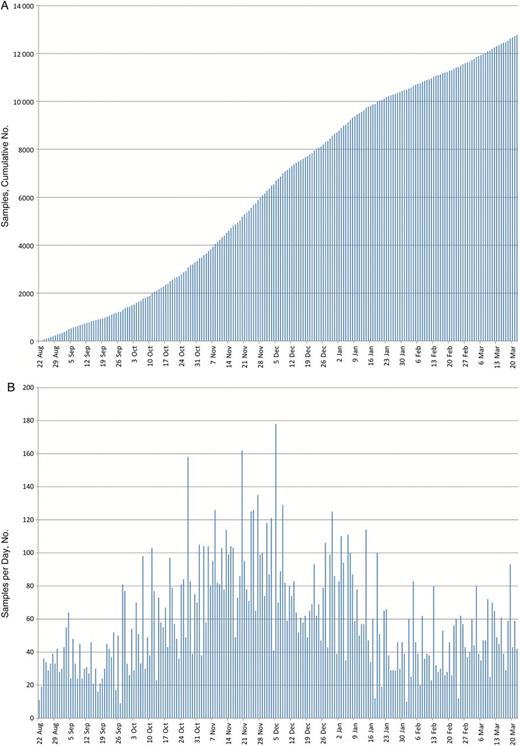
A, Cumulative number of samples tested for Ebola virus (EBOV) RNA by the Centers for Disease Control and Prevention laboratory in Sierra Leone, 22 August 2014 to 22 March 2015. B, Number of samples tested per day.
DISCUSSION
The role of the US CDC laboratory in Bo was to provide EBOV diagnostics for 3 purposes: (1) testing blood drawn from patients in holding centers to guide their transfer to an ETU or otherwise, (2) testing patients in the ETU to help with decisions regarding their discharge, and (3) testing oral swab samples collected from dead bodies to facilitate contact tracing and the implementation of safe burial protocols. Although we believe that the CDC laboratory was an important resource for the clinicians caring for EBOV patients across much of Sierra Leone, improvements to the laboratory services were possible. Owing to the excessive workloads, assays other than the EBOV RT-PCR tests were not performed until early February 2015, when daily sample numbers had dropped significantly. At this stage it proved possible to implement blood chemistry tests and a rapid diagnostic assay for malaria.
Other tests that could have helped guide patient care include testing for other infectious diseases, such as Lassa fever, shigellosis, and typhoid fever. Serological assays for anti-EBOV IgG and IgM were occasionally requested by ETU clinicians. The CDC laboratory did not have the capacity to perform these assays in Sierra Leone. The IgM assay would have been useful to inform contact tracing efforts. The IgG assay could have been used to assess titers in the blood of EBOV disease survivors, before its transfusion as an emergency experimental treatment. In these cases, testing for human immunodeficiency, hepatitis B, and hepatitis C viruses would also have been useful. Such an application was not a common occurrence, however.
Although the assays described here have been successfully used under low-resource conditions, they are relatively expensive and require some laboratory infrastructure and trained staff. A simple, rapid, point-of-care diagnostic test that could detect EBOV in blood from a finger prick would be invaluable for testing in community settings. Such a test would need to be sensitive, with a clear algorithm for retesting negative patients. Several platforms are currently under evaluation, and will probably be deployed as a presumptive diagnostic test designed to complement the existing RT-PCR assays.
In summary, we have described the organization and procedures of the US CDC EBOV diagnostic laboratory located in Bo, Sierra Leone. Visitors to this facility have commented on the simplicity of the laboratory setup, and expressed surprise at its throughput, diagnostic accuracy, and rapid turn-around in support of the EBOV outbreak response in Sierra Leone. We hope that this article aids the deployment and establishment of other laboratories, to serve the patients of this EBOV outbreak and any future ones.
Notes
Acknowledgments. This article is dedicated to all those working to combat the EBOV outbreak, especially the medical technicians and phlebotomists who have risked their lives drawing blood and delivering it to the laboratory. We thank Issah French, Will Pooley, Mambu Momoh, Ian Crozier, Kaci Hickox, Frederique Jacquerioz, Suzanne Donovan, Lewis Rubinson, Darrio Gramuglia, Henry Kyobe, and Monia Sayah; your courage and devotion to helping those afflicted has awed and inspired us. We thank Tanya Klimova for assistance with editing this manuscript. We thank the Sierra Leone Ministry of Health and Sanitation, the World Health Organization and the Global Outbreak Alert and Response Network for organizing the deployment of Centers for Disease Control and Prevention (CDC) scientists to Sierra Leone. We thank the nurses, physicians, and other employees of Kenema Government Hospital and MSF Bo. We also thank the International Rescue Committee for their support of Bo Government Hospital. The laboratory scientist pictured in Figure 3 provided explicit written consent for use of the image.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




