-
PDF
- Split View
-
Views
-
Cite
Cite
Toru Takahashi, Ken Maeda, Tadaki Suzuki, Aki Ishido, Toru Shigeoka, Takayuki Tominaga, Toshiaki Kamei, Masahiro Honda, Daisuke Ninomiya, Takenori Sakai, Takanori Senba, Shozo Kaneyuki, Shota Sakaguchi, Akira Satoh, Takanori Hosokawa, Yojiro Kawabe, Shintaro Kurihara, Koichi Izumikawa, Shigeru Kohno, Taichi Azuma, Koichiro Suemori, Masaki Yasukawa, Tetsuya Mizutani, Tsutomu Omatsu, Yukie Katayama, Masaharu Miyahara, Masahito Ijuin, Kazuko Doi, Masaru Okuda, Kazunori Umeki, Tomoya Saito, Kazuko Fukushima, Kensuke Nakajima, Tomoki Yoshikawa, Hideki Tani, Shuetsu Fukushi, Aiko Fukuma, Momoko Ogata, Masayuki Shimojima, Noriko Nakajima, Noriyo Nagata, Harutaka Katano, Hitomi Fukumoto, Yuko Sato, Hideki Hasegawa, Takuya Yamagishi, Kazunori Oishi, Ichiro Kurane, Shigeru Morikawa, Masayuki Saijo, The First Identification and Retrospective Study of Severe Fever With Thrombocytopenia Syndrome in Japan, The Journal of Infectious Diseases, Volume 209, Issue 6, 15 March 2014, Pages 816–827, https://doi.org/10.1093/infdis/jit603
Close - Share Icon Share
Abstract
Background. Severe fever with thrombocytopenia syndrome (SFTS) is caused by SFTS virus (SFTSV), a novel bunyavirus reported to be endemic in central and northeastern China. This article describes the first identified patient with SFTS and a retrospective study on SFTS in Japan.
Methods. Virologic and pathologic examinations were performed on the patient's samples. Laboratory diagnosis of SFTS was made by isolation/genome amplification and/or the detection of anti-SFTSV immunoglobulin G antibody in sera. Physicians were alerted to the initial diagnosis and asked whether they had previously treated patients with symptoms similar to those of SFTS.
Results. A female patient who died in 2012 received a diagnosis of SFTS. Ten additional patients with SFTS were then retrospectively identified. All patients were aged ≥50 years and lived in western Japan. Six cases were fatal. The ratio of males to females was 8:3. SFTSV was isolated from 8 patients. Phylogenetic analyses indicated that all of the Japanese SFTSV isolates formed a genotype independent to those from China. Most patients showed symptoms due to hemorrhage, possibly because of disseminated intravascular coagulation and/or hemophagocytosis.
Conclusions. SFTS has been endemic to Japan, and SFTSV has been circulating naturally within the country.
(See the editorial commentary by Qiu and Kobinger on pages 811–2.)
Severe fever with thrombocytopenia syndrome (SFTS), an infectious disease with a high case-fatality rate, is caused by SFTS virus (SFTSV), a novel bunyavirus reported to be endemic to central and northeastern parts of China [1, 2]. SFTSV, which is classified into the genus Phlebovirus and the family Bunyaviridae, is suspected to be a tick-borne virus owing to evidence of its presence in 2 species of ticks: Haemaphysalis longicornis and Rhipicephalus microplus [2, 3]. Approximately 2% of H. longicornis organisms collected from sheep, cattle, and dogs in Shandong Province tested positive for SFTSV in virus genome amplification assays [4]. A similar disease, which was named fever, thrombocytopenia and leukopenia syndrome (FTLS), was independently reported to be caused by a novel virus, Henan fever virus (HNFV) [1]. Despite the different names, “SFTS” and “FTLS” represent the same condition and “SFTSV” and “HNFV” represent the same virus. The case-fatality rate of SFTS is reported to be approximately 12% [1, 2]. Human-to-human transmission of SFTSV was reported to occur through close contact with the blood and/or body secretions of infected patients [5–9]. To our knowledge, there were no published reports of SFTS outside of China before we performed the study described here. Another tick-borne phlebovirus, the Heartland virus, which was detected in Missouri, is phylogenetically associated with SFTSV, caused severe febrile illness with thrombocytopenia, leukopenia in the total blood cell count, and elevated levels of liver enzymes [10].
We report the first identification of SFTS in Japan, which was detected in a previously healthy woman aged 50–59 years who died of multiple-organ failure in the autumn of 2012, and findings from a subsequent retrospective study of SFTS in Japan.
MATERIALS AND METHODS
Next-Generation Sequencing
Culture supernatants were subjected to viral RNA extraction using High Pure Viral Nucleic Acid Kit (Roche Diagnostics). Complementary DNA (cDNA) was synthesized using SuperScript III (Invitrogen) with random primers and then randomly amplified using the illustra GenomiPhi V2 Kit (GE Healthcare Life Sciences). A cDNA library was prepared using the Nextera DNA Sample Prep Kit (Illumina). A sequencing run for 50 nucleotides was performed with MiSeq (Illumina), using the MiSeq Reagent sequencing kit (Illumina). The assembled nucleotide sequences were used to determine homologous sequences by tBlast at the National Center for Biotechnology Information Web site (available at: http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Viral Genome Detection, Virus Isolation, and Antibody Detection in Sera
The SFTSV genome was detected by reverse-transcription polymerase chain reaction (RT-PCR) in total RNA extracted from patient sera, using a High Pure Viral RNA kit (Roche Applied Science). Reverse transcription was performed with Ready-to-Go RT-PCR beads (GE Healthcare), using random nucleotide hexamers. PCR was performed to amplify SFTSV-specific cDNA fragments. PCR primer sets were designed to amplify the nucleoprotein (NP) region of the SFTSV genome (Supplementary Table 1). The PCR conditions were as follows: 1 cycle at 55°C for 30 minutes followed by 95°C for 2 minutes; 45 cycles at 94°C for 30 seconds, 52°C for 30 seconds, and 68°C for 30 seconds; followed by 1 cycle at 68°C for 5 minutes.
Vero cells were inoculated with RT-PCR–positive patient sera for virus isolation, cultured for 4–7 days, and examined for SFTSV antigen detection by indirect immunofluorescence assay (IFA) with a polyclonal antibody raised against SFTSV recombinant NP (rNP; rabbit anti-SFTSV rNP serum), which was produced as follows. SFTSV rNP tagged with histidine-tag on the C-terminus was expressed in a baculovirus expression system, as previously described [11, 12]. The NP gene of SFTSV strain HB29 (GenBank accession no. NC_018137) was artificially synthesized. Anti-SFTSV rNP serum was raised in rabbits by immunization with the purified SFTSV rNP, as previously described [13–15].
The neutralizing antibody to SFTSV Chinese isolate HB29 strain [2, 16] and Japanese isolate YG1 (this study) was detected as reported previously, except for the target virus [15]. With the exception of the antigen preparation, immunoglobulin G (IgG) antibody titers to SFTSV were determined by indirect IFA, using SFTSV HB29–infecting Vero cells as previously described [14].
Pathologic Studies With Histopathologic, Immunohistochemical, and In Situ Hybridization AT-Tailing (ISH-AT) Methods
Histopathologic studies of formalin-fixed and paraffin-embedded specimens were performed using hematoxylin-eosin stain.
Immunohistochemical analysis was performed as previously described, with some modifications [17]. The rabbit anti-SFTSV rNP serum and the peroxidase-labeled, polymer-conjugated anti-rabbit immunoglobulin (EnVision/HRP, Dako) were used in the immunohistochemical analysis as the primary and the secondary antibodies, respectively. Normal rabbit serum and lymph nodes of necrotizing lymphadenitis without SFTSV infection were used as negative controls for the antibody and tissue specimens, respectively.
ISH-AT was used for detection of SFTSV genomic RNA (negative-strand RNA) as reported previously, with the exception of the strand-specific oligonucleotide probes [18–20], which were designed for the S and L segments of the SFTSV genome (Supplementary Table 1).
Quantitative Amplification of the SFTSV Genome in the Autopsied Tissues and Organs
The SFTSV copy number was determined by performing quantitative real-time RT-PCR analysis of RNA samples extracted from paraffin-embedded sections as previously described, with some modifications [17]. The amount of human β-actin messenger RNA (mRNA) in the DNase-treated RNA extracted from each section was also determined and used as an internal reference for normalization [21]. The primers and labeled probes are shown (Supplementary Table 1).
Electron Microscopic Analysis
The culture supernatant from Vero cells inoculated with SFTSV isolated from the first patient was used for electron microscopic analysis. The samples were fixed with 4% glutaraldehyde. The fixed samples were negatively stained with 2% phosphotungstic acid and then observed using a JEM-1400 transmission electron microscope (JEOL, Tokyo, Japan).
Recruitment of Patients With Suspected SFTS
The first diagnosis of SFTS in Japan was made public through an announcement from the Ministry of Health, Labor, and Welfare of Japan on 30 January 2013. Physicians were asked to volunteer information if they had treated patients who satisfied the following case definition: (1) fever of >38°C; (2) gastrointestinal tract symptoms, such as nausea, vomiting, abdominal pain, diarrhea, and melena; (3) thrombocytopenia, with <100 × 109 platelets/L; (4) leukopenia, with <4 × 109 white blood cells/L; (5) elevated levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase; (6) absence of other causes; and (7) death or admission to an intensive care unit because of the severity symptoms. Information about patients was collected from their physicians. The retrospective recruitment of patients with suspected SFTS was conducted from 30 January to 31 March 2013. Written informed consent was obtained from patients or responsible relatives.
Serum samples, which had been collected from the patients for future analyses to clarify the etiology and had been stored in each hospital by the respective physicians, used for the present study were sent to the Department of Virology 1 at the National Institute of Infectious Diseases (NIID; Tokyo, Japan) for virologic analyses. Clinical and laboratory data of the patients with SFTS were also sent to the corresponding author (M. Saijo) without information that made it possible to identify individuals.
Phylogenetic Analysis
cDNAs prepared from the patients' sera samples were used to determine SFTSV genome sequences. The terminal sequences of SFTSV genomes were determined by rapid amplification of cDNA ends (RACE) at the 3′ and 5′ ends, performed by RT-PCR after 3′ and 5′ linker ligation using a DynaExpress miRNA Cloning Kit, High Efficient (BioDynamics Laboratory, Tokyo, Japan). Purified RNA from the culture supernatant of SFTSV (YG1)–infected Vero cells was subjected to the 3′ linker ligation according to the manufacturer's protocol. Next, the 5′ linker was ligated to the 5′-phosphate end of the purified 3′ linker ligated RNA, according to the manufacturer's protocol, following RNA purification by NucleoSpin RNA Clean-up XS (Takara Bio, Shiga, Japan). The 3′ and 5′ linker ligated RNA was then reverse transcribed according to the manufacturer's protocol by SuperScript III (Invitrogen, Carlsbad, CA), using either the 3′ forward RT primer, which is the antiparallel sequence of the 3′ linker sequence, or the pd(N)6 primer (random hexamer). RT-PCR was performed using Q5 Hot Start High-Fidelity DNA Polymerase (NEB, Ipswich, MA) and SFTSV gene-specific primers (Supplementary Table 1) with the 3′ RT primer or the 5′ primer, which is the antiparallel sequence of the 5′ linker sequence.
Nucleotide sequences of each full segment of SFTSV in patients' sera were aligned using MUSCLE, an en suite program in the Molecular Evolutionary Genetics Analysis 5.1 software (MEGA Team, Japan). Evolutionary distances were estimated using Kimura's 2-parameter method, and phylogenetic trees were constructed using the neighbor-joining method. The robustness of the trees was tested using 1000 bootstrap replications. Accession numbers of the nucleotide sequences of SFTSV L-, M-, and S-segments are described in Supplementary Table 2.
Ethics Statement
Serum samples were used for virologic analysis after obtaining written informed consent from the patients themselves (for those who survived) or their responsible family members (for those who died). The clinical and laboratory data of the patients with SFTS were sent to the corresponding author without personally identifying information. All of the protocols and procedures were approved by the research and ethics committees of the NIID.
The polyclonal antibody to SFTSV rNP was produced by immunizing rabbits with purified SFTSV rNP, with approval from the Institutional Animal Care and Use Committee of the NIID (no. 111 124).
RESULTS
The First Patient in Japan Who Received a Diagnosis of SFTS
A previously healthy woman aged 50–59 years who lived in the Yamaguchi prefecture of Japan was hospitalized with high fever, fatigue, vomiting, and melena in the autumn of 2012. Her body temperature was 39.2°C. Tick bite wounds were not observed anywhere on her skin. Laboratory tests revealed a low platelet count of 89 × 109 platelets/L (normal range, 150–250 × 109 platelets/L) and a low white blood cell count of 0.4 × 109 cells/L (normal range, 4.0–8.0 × 109 cells/L). Serum levels of alanine aminotransferase, aspartate aminotransferase, and creatine kinase were abnormally high, while the C-reactive protein level was normal. A coagulation study revealed a prolonged activated partial thromboplastin time and a high D-dimer level. The patient's serum ferritin level of >40 000 µg/L was extremely elevated (normal range, 3–166 µg/L). Urinary analysis showed proteinuria and microhematuria. The blood culture was sterile. Computed tomography of the chest and abdomen showed right axillary lymphadenopathy and bilateral renal swelling; however, there was no evidence of hepatosplenomegaly. Bone marrow aspiration revealed mildly hypocellular marrow with an increase in levels of activated histiocytes and hemophagocytes. The patient's condition deteriorated rapidly on the day following admission with the appearance of macrohematuria and a massive amount of tarry stool. Death occurred on the third day of hospitalization.
Serum was used for virus isolation using Vero and Felis catus whole fetus (Fcwf-4) cells. Cytopathic effect appeared in both cells within 5 days. Many DNA fragments that were homologous to those of SFTSV were detected in the culture supernatant of the cells inoculated in next-generation sequencing.
DNA amplified by conventional RT-PCR using either of 2 primer pairs showed the expected sizes of 458 or 461 bp in agarose-electrophoresis (data not shown). The inoculated Vero cells were tested for the presence of SFTSV antigen in indirect IFA with rabbit anti-SFTSV rNP serum and showed a similar positive reactivity (Figure 1A). Enveloped and spherical virions with approximate diameters of 100 nm were detected by electron microscopy (Figure 1B). The morphology of the virion is compatible with that of a bunyavirus. The virus isolated from the patient was named SFTSV YG1.
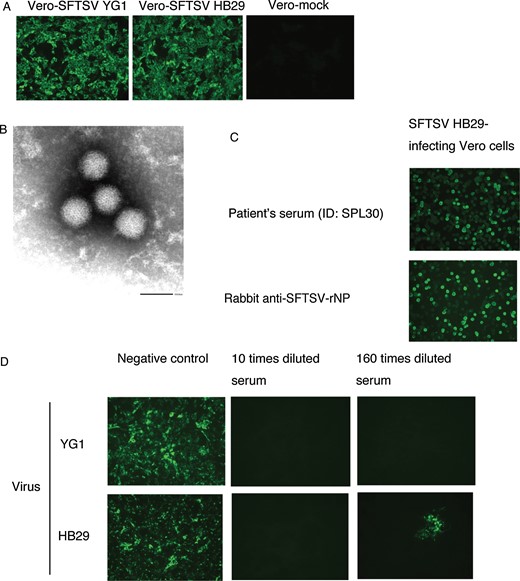
Reactivity of patient sera samples to severe fever with thrombocytopenia syndrome virus (SFTSV). A, Detection of SFTSV antigen in Vero cells by indirect immunofluorescence assay (IFA) with rabbit anti-SFTSV recombinant nucleoprotein (rNP) serum. B, Virions in the culture supernatant detected by electron microscopy (bar in the image indicates the length of 100 nm, ID: SPL004). C, Positive indirect IFA results of the serum collected from a surviving patient (ID: SPL030) in the convalescent phase of SFTSV HB29. D, Neutralizing antibody activity was induced in the serum collected from a patient (ID: SPL032) in the convalescent phase of SFTSV YG1 and SFTSV HB29.
Autopsy findings included right axillary lymphadenopathy (3.5 × 2.0 cm; Figure 2A), bilateral renal swelling, mild retention of pericardial fluid (140 mL), and hepatic steatosis. Gastric ulceration was also observed in the pyloric region.
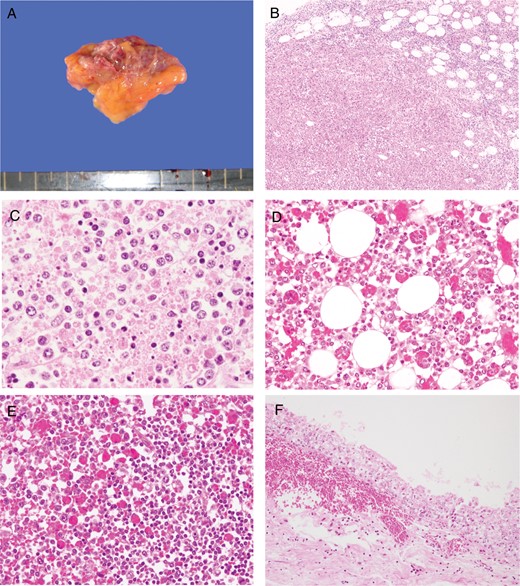
Macroscopic and microscopic findings under hematoxylin-eosin staining. A, Gross appearance of the swollen right axillary lymph node (3.5 × 2.0 cm). B and C, Microscopic findings in the right axillary lymph node. The basic architecture of the lymph node has been replaced by massive necrosis. The necrotic regions contain histiocytes, immunoblasts, nuclear debris, and eosinophilic ghosts but no neutrophils. D and E, Marked erythrophagocytosis in the bone marrow (D) and spleen (E). F, Acute subepithelial hemorrhage in the renal pelvis.
Severe necrotizing lymphadenitis with massive necrosis, the depletion of small lymphocytes, and severe infiltration of the swollen right axillary and right cervical lymph nodes by histiocytes and immunoblasts were observed (Figure 2B and 2C). Necrosis, which comprised nuclear debris and eosinophilic ghosts but not granulocytes, was distributed throughout the cortical area, the sinuses, and the capsule of the lymph node and had spread to the perinodal adipose tissue. No clusters of epithelioid histiocytes, stellate microabscesses, or granulomas were observed. There were no obvious intranuclear or intracytoplasmic viral inclusions. Prominent hemophagocytosis was observed in these lymph nodes, the bone marrow, and the spleen (Figure 2D and 2E). The bone marrow was relatively hypocellular, with no reduction in the number of megakaryocytes. The liver showed mild microvesicular fatty changes in zone 3 and mild inflammation, comprising lymphocytes and macrophages, around the portal tracts. The kidney showed subepithelial hemorrhage within the renal pelvis (Figure 2F). Significant hemophagocytosis were also observed in the mediastinal, hilar, and abdominal lymph nodes with no evidence of necrosis (Figure 3A, 3C, and 3E). The findings in the remaining visceral organs were unremarkable.
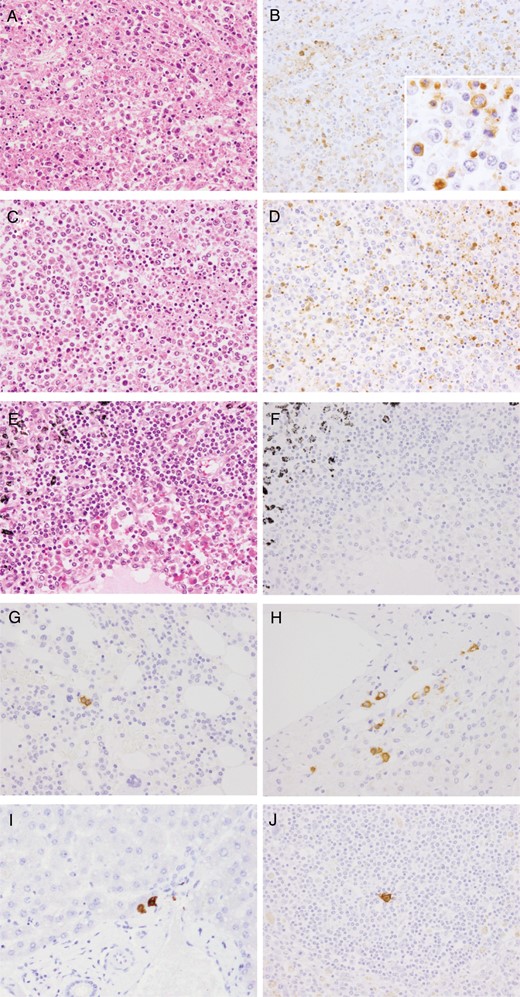
Microscopic and immunohistochemical tissue images. Hematoxylin-eosin staining (A, C, and E) and subsequent immunohistochemical analysis reveal the presence of severe fever with thrombocytopenia syndrome virus (SFTSV) NP (B, D, F, G, H, I, and J) in the right axillary (A and B), right cervical (C and D), and the mediastinal (E and F) lymph nodes. A, C, and E, Infiltration by immunoblasts and prominent hemophagocytosis was generally observed in the right axillary, right cervical, mediastinal, hilar, and abdominal lymph nodes; however, necrosis was only observed in the right axillary and cervical lymph nodes. B, D, and F, Viral antigen–positive cells were detected in the right axillary and cervical lymph nodes. Positive signals for SFTSV NP antigen were detected in the cytoplasm of blastic cells (B; inset). Inset shows a higher magnification (40×). In contrast, no signals were detected in the mediastinal lymph nodes, regardless of immunoblast infiltration and hemophagocytosis. G, H, I, and J, Immunohistochemical analysis of SFTSV NP was performed in the bone marrow (G), adrenal glands (H), liver (I), and spleen (J). A few SFTSV antigen–positive cells were observed in these tissues, with no notable cytopathic effects or necrosis (magnification, 20×; inset, 40×).
Positive signals for SFTSV NP antigen were detected in the cytoplasm of blastic cells and necrotic regions in the cortical area of the right axillary lymph node (Figure 3B). Viral antigen-positive cells were also detected in the right cervical lymph nodes (Figure 3D) but not in the mediastinal lymph nodes (Figure 3F), regardless of the level of immunoblast infiltration and hemophagocytosis (Figure 3E). Relatively few SFTSV antigen–positive cells were detected in the bone marrow, adrenal glands, the liver, and the spleen (Figure 3G–J), with no notable cytopathic effects or necrosis. No antigen-positive cells were detected in the heart, lungs, kidneys, gastrointestinal tract, aorta, or iliopsoas muscle.
The SFTSV genome, SFTSV genomic RNA (negative-strand RNA; Figure 4A), and mRNA (positive-strand RNA; Figure 4B) were detected in the blastic cells in the right cervical lymph node by ISH-AT analysis [18–20], while no signals were detected using an irrelevant probe as a negative control (Figure 4C). No signals showing necrotizing lymphadenitis without SFTSV infection were detected in the lymph nodes (negative control; Figure 4D).
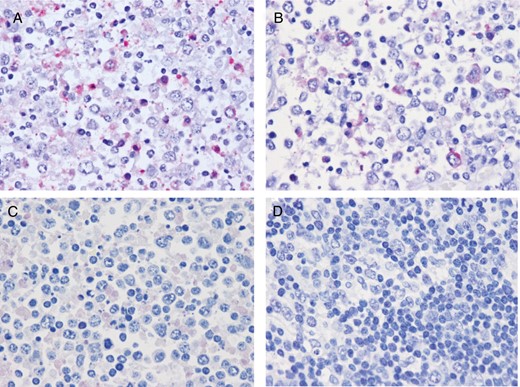
Detection of severe fever with thrombocytopenia syndrome virus (SFTSV) RNA in the right cervical lymph node by the in situ hybridization AT-tailing method. A, SFTSV genomic RNA was detected in the right cervical lymph node by the in situ hybridization AT-tailing method and a sense probe. SFTSV genomic RNA was detected in the cytoplasm of the blastic cells. B, The in situ hybridization AT-tailing method with an anti-sense probe detected a few cells in the right cervical lymph node that were positive for SFTSV messenger RNA (mRNA). SFTSV mRNA was also detected in the cytoplasm of blastic cells. C, No signals were detected in the right axillary lymph node by the in situ hybridization AT-tailing method with an irrelevant probe (negative control). D, A SFTSV sense probe detected no signals in lymph node sections showing necrotizing lymphadenitis without SFTSV infection.
SFTSV RNA was present, with high copy numbers, in the right axillary and cervical lymph node sections (Supplementary Table 3). Consistent with immunohistochemical analysis results, low copy numbers (100–1000 copies) of SFTSV RNA were also detected in the bone marrow, the spleen, the liver, and the adrenal glands. A few copies (<100) were detected in other tissue sections that did not contain antigen-positive cells (as assessed by immunohistochemical analysis), suggesting that quantitative real-time RT-PCR detected cell-free circulating SFTSV; a high viral load in the serum is characteristic of SFTS infection [22]. The number of SFTSV RNA copies/cell was calculated using the β-actin mRNA copy number, estimated at 1500 copies/cell [17]. The SFTSV RNA copy numbers in the right axillary and cervical lymph node sections were the highest among the tissues tested, while the bone marrow, spleen, and liver showed relatively lower copy numbers per cell (Supplementary Table 3).
The Retrospective Study
Serum samples collected from 23 patients who were retrospectively suspected of having SFTS were sent to the Department of Virology 1, NIID (Figure 5). The male-to-female ratio was 18:5. The earliest infection dated back to 2005 (Figure 5B). Serum samples collected from 21 patients (for 2 patients, acute phase serum samples were not available for virologic analysis) were subjected to virus isolation and RT-PCR for amplification of the SFTSV genome. Serum samples collected from all 23 patients were tested for IgG and immunoglobulin M (IgM) antibodies to SFTSV in the indirect IFA. Of the 23 patients, 8 men and 2 women received a diagnosis of SFTS: 7 had positive results of virus isolation and genome amplification tests; 1 had positive results of virus genome amplification testing and IgM antibody to SFTSV but had negative results of virus isolation analysis; and 2 were positive for IgG antibody to SFTSV. In the present study, the 2 patients who tested positive for SFTSV on the basis of detection of IgG to SFVSV were regarded as SFTS positive because their symptoms were reminiscent of SFTS and because reports of asymptomatic cases in China are quite rare [23, 24]. For the purposes of the present study, the first patient was included with the 10 retrospective cases, for a total of 11 diagnosed cases.
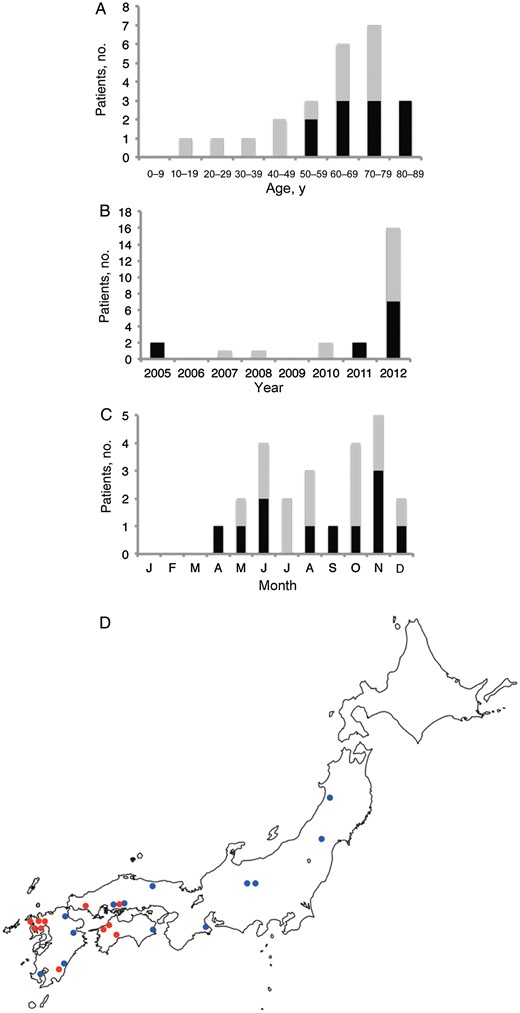
Chronological (A), age-based (B), geographic (C), and seasonal (D) distributions of patients with retrospectively diagnosed severe fever with thrombocytopenia syndrome (SFTS) in Japan. Black and gray bars in panels A–C indicate patients with and those without SFTS, respectively. The red and blue circles in panel D indicate the areas where patients with and those without SFTS were located.
All of the 11 patients in whom SFTS was diagnosed were aged ≥50 years (Figure 5A) and came from western Japan (Figure 5D). Disease onset occurred in all patients between the months of April and December (Figure 5C). Six of the 11 cases were fatal. There was clear evidence of tick bite in 2 cases.
Clinical Manifestation of 11 SFTS Patients
The clinical manifestations observed in SFTS patients are summarized in Table 1. All patients showed nonspecific febrile symptoms with gastrointestinal tract symptoms in the early phase of the disease. Deterioration in consciousness, characterized by dysarthria, disorientation, and alteration in consciousness, was commonly observed. Generalized convulsions were seen in the late stages of the disease in 5 of the 6 fatal cases. Respiratory symptoms were rarely observed. Superficial lymphadenopathy was detected in 5 patients.
Summary of Clinical Manifestation of Retrospectively Diagnosed Japanese Severe Fever With Thrombocytopenia Syndrome Cases
| Symptom or Laboratory Parameter . | Variable (n = 11) . |
|---|---|
| Clinical manifestation, positive/negative/unknown | |
| Fever | 11/0/0 |
| General symptoms | |
| General fatigue | 11/0/0 |
| Myalgia | 2/5/4 |
| Arthralgia | 1/6/4 |
| Headache | 6/4/1 |
| Gastrointestinal tract symptoms | |
| Overall | 11/0/0 |
| Nausea | 9/2/0 |
| Vomiting | 6/5/0 |
| Abdominal pain | 6/5/0 |
| Diarrhea | 7/4/0 |
| Anorexia | 11/0/0 |
| Respiratory symptoms | |
| Overall | 3/8/0 |
| Throat pain | 2/9/0 |
| Cough | 1/10/0 |
| Neurologic symptoms | |
| Overall | 10/1/0 |
| Dysarthria | 3/8/0 |
| Consciousness disturbance | 8/3/0 |
| Seizure | 6/5/0 |
| Hemorrhage | |
| Overall | 9/2/0 |
| Hemoptysis | 1/10/0 |
| Purpura | 3/8/0 |
| Bloody diarrhea | 4/7/0 |
| Gingival bleeding | 5/6/0 |
| Nasal hemorrhage | 0/11/0 |
| Genitourinary tract hemorrhage | 0/11/0 |
| Others | |
| Lymphadenopathy | 5/6/0 |
| Laboratory finding, no. (%) | |
| Total blood cell count | |
| Leukopenia | 11 (100) |
| Thrombocytopenia | 11 (100) |
| Serum chemistry | |
| Total protein level < 6.0 mg/dL (hypoproteinemia) | 3 (27) |
| Albumin level < 3.0 mg/dL (hypoalbuminemia) | 1 (9) |
| Aspartate aminotransferase level >30 IU/L | 11 (100) |
| Alanine aminotransferase level >30 IU/L | 11 (100) |
| Lactate dehydrogenase level >250 IU/L | 11 (100) |
| Creatine kinase level >200 IU/L | 11 (100) |
| Blood urea nitrogen level >20 mg/dL | 7 (64) |
| Creatinine level >1 mg/dL | 7 (64) |
| Inflammatory parameter | |
| C-reactive protein level >1 mg/dL | 3 (27) |
| Urinalysis | |
| Hematuria | 9 (90)a |
| Proteinuria | 10 (100)a |
| Coagulopathy, no. (%) | |
| Abnormality in either DIC parameterb | 11 (100) |
| Hemophagocytosis, no. (%) | |
| Bone marrow examination | |
| Hemophagocytosis | 5 (100)c |
| Positive increased ferritin level | 8 (100)d |
| Tick bite within 2 weeks of onset, no. (%) | 2 (18) |
| Symptom or Laboratory Parameter . | Variable (n = 11) . |
|---|---|
| Clinical manifestation, positive/negative/unknown | |
| Fever | 11/0/0 |
| General symptoms | |
| General fatigue | 11/0/0 |
| Myalgia | 2/5/4 |
| Arthralgia | 1/6/4 |
| Headache | 6/4/1 |
| Gastrointestinal tract symptoms | |
| Overall | 11/0/0 |
| Nausea | 9/2/0 |
| Vomiting | 6/5/0 |
| Abdominal pain | 6/5/0 |
| Diarrhea | 7/4/0 |
| Anorexia | 11/0/0 |
| Respiratory symptoms | |
| Overall | 3/8/0 |
| Throat pain | 2/9/0 |
| Cough | 1/10/0 |
| Neurologic symptoms | |
| Overall | 10/1/0 |
| Dysarthria | 3/8/0 |
| Consciousness disturbance | 8/3/0 |
| Seizure | 6/5/0 |
| Hemorrhage | |
| Overall | 9/2/0 |
| Hemoptysis | 1/10/0 |
| Purpura | 3/8/0 |
| Bloody diarrhea | 4/7/0 |
| Gingival bleeding | 5/6/0 |
| Nasal hemorrhage | 0/11/0 |
| Genitourinary tract hemorrhage | 0/11/0 |
| Others | |
| Lymphadenopathy | 5/6/0 |
| Laboratory finding, no. (%) | |
| Total blood cell count | |
| Leukopenia | 11 (100) |
| Thrombocytopenia | 11 (100) |
| Serum chemistry | |
| Total protein level < 6.0 mg/dL (hypoproteinemia) | 3 (27) |
| Albumin level < 3.0 mg/dL (hypoalbuminemia) | 1 (9) |
| Aspartate aminotransferase level >30 IU/L | 11 (100) |
| Alanine aminotransferase level >30 IU/L | 11 (100) |
| Lactate dehydrogenase level >250 IU/L | 11 (100) |
| Creatine kinase level >200 IU/L | 11 (100) |
| Blood urea nitrogen level >20 mg/dL | 7 (64) |
| Creatinine level >1 mg/dL | 7 (64) |
| Inflammatory parameter | |
| C-reactive protein level >1 mg/dL | 3 (27) |
| Urinalysis | |
| Hematuria | 9 (90)a |
| Proteinuria | 10 (100)a |
| Coagulopathy, no. (%) | |
| Abnormality in either DIC parameterb | 11 (100) |
| Hemophagocytosis, no. (%) | |
| Bone marrow examination | |
| Hemophagocytosis | 5 (100)c |
| Positive increased ferritin level | 8 (100)d |
| Tick bite within 2 weeks of onset, no. (%) | 2 (18) |
a Basic number is 10.
b Disseminated intravascular coagulation (DIC) parameters include prothrombin time, activated partial thromboplastin time, and levels of antithrombin 3, fibrinogen, D-dimer, and fibrinogen degradation products.
c Basic number is 5.
d Basic number is 8.
Summary of Clinical Manifestation of Retrospectively Diagnosed Japanese Severe Fever With Thrombocytopenia Syndrome Cases
| Symptom or Laboratory Parameter . | Variable (n = 11) . |
|---|---|
| Clinical manifestation, positive/negative/unknown | |
| Fever | 11/0/0 |
| General symptoms | |
| General fatigue | 11/0/0 |
| Myalgia | 2/5/4 |
| Arthralgia | 1/6/4 |
| Headache | 6/4/1 |
| Gastrointestinal tract symptoms | |
| Overall | 11/0/0 |
| Nausea | 9/2/0 |
| Vomiting | 6/5/0 |
| Abdominal pain | 6/5/0 |
| Diarrhea | 7/4/0 |
| Anorexia | 11/0/0 |
| Respiratory symptoms | |
| Overall | 3/8/0 |
| Throat pain | 2/9/0 |
| Cough | 1/10/0 |
| Neurologic symptoms | |
| Overall | 10/1/0 |
| Dysarthria | 3/8/0 |
| Consciousness disturbance | 8/3/0 |
| Seizure | 6/5/0 |
| Hemorrhage | |
| Overall | 9/2/0 |
| Hemoptysis | 1/10/0 |
| Purpura | 3/8/0 |
| Bloody diarrhea | 4/7/0 |
| Gingival bleeding | 5/6/0 |
| Nasal hemorrhage | 0/11/0 |
| Genitourinary tract hemorrhage | 0/11/0 |
| Others | |
| Lymphadenopathy | 5/6/0 |
| Laboratory finding, no. (%) | |
| Total blood cell count | |
| Leukopenia | 11 (100) |
| Thrombocytopenia | 11 (100) |
| Serum chemistry | |
| Total protein level < 6.0 mg/dL (hypoproteinemia) | 3 (27) |
| Albumin level < 3.0 mg/dL (hypoalbuminemia) | 1 (9) |
| Aspartate aminotransferase level >30 IU/L | 11 (100) |
| Alanine aminotransferase level >30 IU/L | 11 (100) |
| Lactate dehydrogenase level >250 IU/L | 11 (100) |
| Creatine kinase level >200 IU/L | 11 (100) |
| Blood urea nitrogen level >20 mg/dL | 7 (64) |
| Creatinine level >1 mg/dL | 7 (64) |
| Inflammatory parameter | |
| C-reactive protein level >1 mg/dL | 3 (27) |
| Urinalysis | |
| Hematuria | 9 (90)a |
| Proteinuria | 10 (100)a |
| Coagulopathy, no. (%) | |
| Abnormality in either DIC parameterb | 11 (100) |
| Hemophagocytosis, no. (%) | |
| Bone marrow examination | |
| Hemophagocytosis | 5 (100)c |
| Positive increased ferritin level | 8 (100)d |
| Tick bite within 2 weeks of onset, no. (%) | 2 (18) |
| Symptom or Laboratory Parameter . | Variable (n = 11) . |
|---|---|
| Clinical manifestation, positive/negative/unknown | |
| Fever | 11/0/0 |
| General symptoms | |
| General fatigue | 11/0/0 |
| Myalgia | 2/5/4 |
| Arthralgia | 1/6/4 |
| Headache | 6/4/1 |
| Gastrointestinal tract symptoms | |
| Overall | 11/0/0 |
| Nausea | 9/2/0 |
| Vomiting | 6/5/0 |
| Abdominal pain | 6/5/0 |
| Diarrhea | 7/4/0 |
| Anorexia | 11/0/0 |
| Respiratory symptoms | |
| Overall | 3/8/0 |
| Throat pain | 2/9/0 |
| Cough | 1/10/0 |
| Neurologic symptoms | |
| Overall | 10/1/0 |
| Dysarthria | 3/8/0 |
| Consciousness disturbance | 8/3/0 |
| Seizure | 6/5/0 |
| Hemorrhage | |
| Overall | 9/2/0 |
| Hemoptysis | 1/10/0 |
| Purpura | 3/8/0 |
| Bloody diarrhea | 4/7/0 |
| Gingival bleeding | 5/6/0 |
| Nasal hemorrhage | 0/11/0 |
| Genitourinary tract hemorrhage | 0/11/0 |
| Others | |
| Lymphadenopathy | 5/6/0 |
| Laboratory finding, no. (%) | |
| Total blood cell count | |
| Leukopenia | 11 (100) |
| Thrombocytopenia | 11 (100) |
| Serum chemistry | |
| Total protein level < 6.0 mg/dL (hypoproteinemia) | 3 (27) |
| Albumin level < 3.0 mg/dL (hypoalbuminemia) | 1 (9) |
| Aspartate aminotransferase level >30 IU/L | 11 (100) |
| Alanine aminotransferase level >30 IU/L | 11 (100) |
| Lactate dehydrogenase level >250 IU/L | 11 (100) |
| Creatine kinase level >200 IU/L | 11 (100) |
| Blood urea nitrogen level >20 mg/dL | 7 (64) |
| Creatinine level >1 mg/dL | 7 (64) |
| Inflammatory parameter | |
| C-reactive protein level >1 mg/dL | 3 (27) |
| Urinalysis | |
| Hematuria | 9 (90)a |
| Proteinuria | 10 (100)a |
| Coagulopathy, no. (%) | |
| Abnormality in either DIC parameterb | 11 (100) |
| Hemophagocytosis, no. (%) | |
| Bone marrow examination | |
| Hemophagocytosis | 5 (100)c |
| Positive increased ferritin level | 8 (100)d |
| Tick bite within 2 weeks of onset, no. (%) | 2 (18) |
a Basic number is 10.
b Disseminated intravascular coagulation (DIC) parameters include prothrombin time, activated partial thromboplastin time, and levels of antithrombin 3, fibrinogen, D-dimer, and fibrinogen degradation products.
c Basic number is 5.
d Basic number is 8.
Hemorrhagic symptoms such as petechiae, purpura, melena, bloody vomit, gingival bleeding as a form of discharge, and excessive bleeding at the site of skin biopsy were observed in 9 of the 11 patients.
Blood urea nitrogen and creatinine levels were elevated in 8 of 11 patients. Hematuria and proteinuria were observed in most patients.
In all 5 patients for whom bone marrow observation was performed, hemophagocytosis, with or without bone marrow cell dysplasia, was observed. The ferritin level was elevated in the blood of 8 patients, including the 5 in whom bone marrow examination was performed. Abnormalities were observed in tests in all of the patients for coagulopathy, prothrombin time, activated partial thromboplastin time, fibrin/fibrinogen degradation products, fibrinogen, and/or D-dimer.
Phylogenetic Analysis
The Japanese SFTSV strains were closely related to the SFTSV Chinese isolates but formed an independent cluster for each segment (Figure 6). No geographic or chronological relationship was found between the Japanese and Chinese strains.

Phylogenetic trees showing the phylogenetic positions of severe fever with thrombocytopenia syndrome virus (SFTSV) strains in Japan, compared with other known strains. Trees are based on the S segment (A; left panel), M segment (B; middle panel), and L segment (C; right panel). Heartland virus, Uukuniemi virus, Toscana virus, and Rift Valley fever virus are included in the phylogenetic analyses.
Neutralizing Antibody Response to SFTSV
Sera collected from the 5 surviving patients with SFTS in the convalescent phase showed neutralizing activities to SFTSV Japanese isolate YG1 (Figure 1D). The sera of these 5 patients also showed similar degrees of neutralizing activities to SFTSV HB29.
DISCUSSION
The clinical manifestations of Japanese SFTS were very similar to those of severe cases of Chinese SFTS [1, 2], but the case-fatality rate (6 of 11 patients [55%]) was apparently higher than that in China, where an average of 12% of cases were fatal. It is worth noting that the characteristics of Japanese SFTS presented in this study were based on a limited number of patients and a strict case definition.
To our knowledge, this may be the first report describing the pathologic findings of a patient with SFTS. The local lymph nodes, which were the primary target organ in the first patient, served as the site of virus replication and showed marked pathologic changes, including massive necrosis. No neutrophil infiltration was observed in the patient. The spleen, liver, adrenal glands, and bone marrow contained a few SFTSV-infected cells. However, no viral antigens were detected in hepatocytes or in the parenchymal cells of the adrenal glands. Viruses belonging to the Bunyaviridae family, which includes the Rift valley fever virus, Hantaan virus, and Heartland virus [10], infect monocytes/macrophages [25, 26]. These cells may control the spread (or containment) of viruses to cells within other organs. The pathologic and virologic observations suggest that SFTSV replicated in the blastic cells within the lymph nodes and that replication took place predominantly in the local lymph nodes, not in the major organs.
Macrophages with phagocytosis of bone marrow cells were observed in all of the patients in whom bone marrow examination was performed. Furthermore, pathologic examination of the lymph nodes, bone marrow, and the spleen of the initial patient revealed marked hemophagocytosis, regardless of the presence of SFTSV-infected cells (Figure 2D and 2E and Figure 3A, 3C, and 3E). Ferritin level was extremely elevated in the sera of all patients who were tested. Abnormality in coagulopathy-associated indices was also observed in all patients. SFTSV infections induced a cytokine storm, the level of which was associated with the severity of SFTS [27]. These results indicate that in addition to multiorgan dysfunction, hemophagocytosis and disseminated intravascular coagulation are major factors for poor prognosis.
The mode of the natural SFTSV lifecycle in Japan should be clarified to enable better identification of risk factors for SFTSV infection and to address strategies for reducing the risk of infections. Further study is necessary to clarify the circulation of SFTSV in nature in Japan, in terms of tick species, percentages of SFTSV positivity for each tick species, and the prevalence of SFTSV-positive ticks, to better determine and evaluate the risk factors for SFTSV infection in Japan.
Japanese isolates formed a cluster that was independent from Chinese isolates (Figure 6), indicating that SFTSV has been circulating in Japan naturally for some time. The earliest year for which we have evidence of SFTS in patient sera samples was 2005, and to our knowledge, the article by Liu et al, which concerns SFTS occurrence in China during 2006, reports the oldest cases from China [5]. It is also noteworthy that patients with SFTS were reported in South Korea in 2013 (ProMed mail: archive numbers 20130521.1729124 and 20130529.174441). The prevalence of SFTS in and around East Asia should be studied to clarify the nature of SFTS in the region.
In conclusion, SFTSV is prevalent in Japan. Japanese SFTSV strains have characteristics similar to those of Chinese isolates but an independent genotype, which indicates that SFTSV has been present in Japan for some time.
Notes
Acknowledgments. We thank Dr De-Xin Li and Dr Mi-Fang Liang, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, PRC, for providing us with the SFTSV HB29 strain; Dr Roger Hewson, Virology, Pathogenesis, and Emerging Disease, Public Health England–Microbiology Services, Porton Down, Salisbury, United Kingdom, for his critical comments; and the municipal officials who supported this work, in the prefectures in which patients with SFTS were reported.
Financial support. This work was supported by the Ministry of Health, Labor and Welfare Science Research (grants in aid H24-Shinko-Ippan-013, H22-Shinko-Ippan-006, and H25-Shinko-Shitei-009).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
T. T., K. M., and T. S. contributed equally to this work.
Presented in part: 15th International Negative Strand Virus Meeting, Granada, Spain, 16–21 June 2013; Abstract 324.




