-
PDF
- Split View
-
Views
-
Cite
Cite
Sedigheh Zakeri, J. Pedro Gil, Sandor Bereckzy, Navid D. Djadid, Anders Bjorkman, High Prevalence of Double Plasmodium falciparum dhfr Mutations at Codons 108 and 59 in the Sistan-Baluchistan Province, Iran, The Journal of Infectious Diseases, Volume 187, Issue 11, 1 June 2003, Pages 1828–1829, https://doi.org/10.1086/375250
Close - Share Icon Share
To the Editor— In Iran, transmission of Plasmodium falciparum malaria mainly occurs in the Sistan-Baluchistan province, where >90% of the annual cases are observed [1]. Chloroquine is recommended as the first-line antimalarial treatment, and sulfadoxine-pyrimethamine (SP) is recommended as the second-line treatment. SP appears to be effective in the region, although findings of in vivo and in vitro resistance in some small-scale, local drug resistance studies have already been documented [2]. However, SP resistance has been consistently reported among Afghan refugee settlements along the western border of Pakistan [3], a region from which human migration recently has increased substantially. This raises the concern that SP-resistant malaria parasites are now invading the nearby regions of Iran
Mutations in the P. falciparum dhfr and dhps genes represent a valuable tool for epidemiological surveillance of SP resistance in the region, as has been suggested elsewhere for another setting in which malaria is endemic [4]. This is supported by a positive correlation between mutations in the dhfr and dhps genes and in vivo SP resistance observed in a preliminary study in the Iranian coastal province of Hormozgan [5]
To evaluate the present status of these genes in the Sistan-Baluchistan province, we have studied the dhfr (Ser108Asn, Asn51Ile, and Cys59Arg) and dhps (Ala437Gly and Lys540Glu) single-nucleotide polymorphism (SNP) frequencies in 101 symptomatic patients with microscopically confirmed P. falciparum malaria attending the Chahbahar Health Center. The patients were all permanent residents in the province; a large fraction of them were foreigners, mostly of Afghan origin. Blood samples were obtained after written consent was given. DNA was extracted through phenol-chloroform-based protocols. Established polymerase chain reaction-restriction fragment-length polymorphism methods [6, 7] were performed for the analysis of the selected SNPs
Most patients (97%) were found to simultaneously carry the Ser108Asn and Cys59Arg pyrimethamine resistance-associated mutations, mainly in “pure form” [4], while retaining a wild-type mutation at position 51 (table 1). The Asn51Ile mutation was present in only 6 samples (6%). A similar pattern was also found in another small study of blood samples from Pakistan [8], which suggests that a regional mutation selection profile exists, with Cys59Arg mutations preceding alterations in codon 51 toward the triple mutant
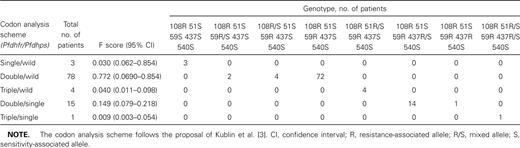
Frequencies of Plasmodium falciparum dhfr and dhps genotypes of malaria patients in the Sistan-Baluchistan Province, Iran
P. falciparum dhps Ala437Gly-carrying alleles were found in 17% of the patients, mostly in mixed form. This result was in contrast to previous observations of no mutations in P. falciparum dhps in a region of Pakistan [8], which suggests that these are recent mutation selection events, possibly specific to Iranian malaria settings. Of note, the 437Gly mutation was significantly more frequent among Iranians (13/42 patients [30.9%]), compared with patients of Afghan origin (3/54 samples [5.5%]; χ2=9.219; P<.01). The absence of mutations at the 540 position on P. falciparum dhps reinforces the view that these mutations represent, in most cases, a last step toward the quintuple mutant, recently considered to be a landmark of SP resistance [4]
In conclusion, these molecular data suggest that the high prevalence of mutations in the dhfr and dhps genes might lead toward a development of SP resistance in the southeastern Iranian provinces. The remoteness of these regions, which is associated with the potential for rapid establishment of SP resistance, stresses the need for continuing epidemiological surveillance, including the use of molecular methods, as proposed elsewhere [4]
References
Financial support: Pasteur Institute of Iran; Swedish International Development Cooperation Agency Department for Research Cooperation; Swedish International Development Cooperation Agency, Department for Research Cooperation




