-
PDF
- Split View
-
Views
-
Cite
Cite
Akihiko Saitoh, Karen Hsia, Terence Fenton, Christine A. Powell, Cindy Christopherson, Courtney V. Fletcher, Stuart E. Starr, Stephen A. Spector, Persistence of Human Immunodeficiency Virus (HIV) Type 1 DNA in Peripheral Blood Despite Prolonged Suppression of Plasma HIV-1 RNA in Children, The Journal of Infectious Diseases, Volume 185, Issue 10, 15 May 2002, Pages 1409–1416, https://doi.org/10.1086/340614
Close - Share Icon Share
Abstract
Human immunodeficiency virus (HIV) type 1 DNA in peripheral blood mononuclear cells (PBMC) was quantified in 31 children who received efavirenz, nelfinavir, and 1 or 2 nucleoside reverse-transcriptase inhibitors for ⩾2 years and in whom undetectable plasma HIV-1 RNA levels (<50 copies/mL) were sustained, to determine the usefulness of HIV-1 DNA as a marker of virus suppression. The median baseline HIV-1 DNA level was 750 copies/106 PBMC. After initiation of highly active antiretroviral therapy (HAART), HIV-1 DNA levels decreased gradually, reaching a plateau from week 80 through week 104 (median HIV-1 DNA level, 263 copies/ 106 PBMC). Children who had plasma HIV-1 RNA levels <50 copies/mL after receiving HAART for 8 weeks (n = 16) had persistently lower quantities of intracellular HIV-1 DNA than children whose HIV-1 RNA levels reached <50 copies/mL after 8 weeks of HAART (n = 15). The median half-life for intracellular HIV-1 DNA was 60 weeks. Thus, despite prolonged maintenance of undetectable levels of plasma HIV-1 RNA, HIV-1 DNA remains detectable in PBMC of children and may be a useful marker of further virus suppression.
The increased availability of antiretroviral agents that are active against human immunodeficiency virus (HIV) type 1 has made it possible to maintain prolonged virus suppression with sustained increases in CD4+ lymphocyte counts [1, 2]. Associated with this prolonged reduction in HIV-1 replication have been delayed disease progression and significant decreases in opportunistic complications and mortality [3]. The benefits of highly active antiretroviral therapy (HAART) have been observed in children, as well as in adults; an annual mortality <1% was recently found among HIV-infected children followed up within the Pediatric AIDS Clinical Trials Group (PACTG) [4, 5].
Currently, optimal management of HIV-1 infection in a patient who is receiving antiretroviral therapy includes monitoring of plasma HIV-1 RNA levels and CD4+ lymphocyte counts [6–9]. After the initiation of HAART, most patients who are adherent to treatment regimens will have undetectable levels (<50 copies/ mL) of plasma HIV-1RNA [5, 10]. There currently is no broadly used marker assay for further monitoring the decrease in HIV-1 in peripheral blood once HIV-1 RNA has reached an undetectable level. Such markers may be helpful in understanding HIV-1 pathogenesis and identifying subgroups of patients who are more or less likely to have sustained virus suppression and increases in CD4+ lymphocyte counts.
During potent and prolonged therapy, HIV-1DNAcan remain detectable in peripheral blood mononuclear cells (PBMC) [11, 12] and lymphoid tissues [13], even when virus suppression has been sustained with long-term treatment. Additionally, replication- competent virus can be recovered from resting memory CD4+ lymphocytes in adults [14, 15] and children [13, 16, 17]. The level of HIV-1 DNA has been found to decrease after long-term antiretroviral treatment and appears to reach a plateau even when potent HAART is continued [11, 12]. The dynamics of plasma HIV-1 RNA in children after initiation of HAART are similar to what is observed in adults [18]. However, immune responses to HAART that occur in children may differ from those described for adults. The thymus, which has an important role in T cell maturation and proliferation, is more functional in children. During HAART, children reconstitute CD4+ lymphocyte populations with naive CD4+ cells earlier than do adults, who tend to have an initial predominant restoration of memory CD4+ lymphocytes [19–21]. Thus, the quantity of residual HIV-1 remaining in the CD4+ lymphocytes in children may differ from that in adults, reflecting the different populations of cells found in the peripheral circulation when HAART is successful.
Reduction of total HIV-1 DNA in peripheral blood has been reported during administration of several combination antiretroviral regimens in adults [22–27]. However, no data are available that identify the dynamics of HIV-1 DNA in children treated with HAART who have sustained virus suppression and increased CD4+ lymphocyte counts. The present study was undertaken to determine the dynamics of HIV-1 DNA in the peripheral blood of HIV-1-infected children in whom undetectable plasma HIV- 1 RNA levels were sustained for >1 year after the initiation of HAART. The relationships between HIV-1 DNA levels and plasma HIV-1 RNA levels or CD4+ lymphocyte counts before and after initiation of treatment were also assessed.
Subjects, Materials, and Methods
Subjects. Subjects evaluated in this study were participants in PACTG 382, which was designed to evaluate the pharmacokinetics, tolerance, and potential efficacy of a therapeutic regimen including efavirenz, nelfinavir, and 1 or 2 nucleoside reverse-transcriptase inhibitors (NRTIs) in children. Patients enrolled in the PACTG study were nonnucleoside reverse-transcriptase inhibitor and protease inhibitor naive but could have received prior NRTIs. Children received either 1 NRTI or 2 NRTIs in addition to efavirenz and nelfinavir. Under this open-label protocol, 57HIV-1-infected children aged 3–17 years were enrolled at some point from October 1997 through February 1998 as part of the PACTG study. The combination therapy used as part of the PACTG study was well tolerated and had a potent and sustained antiviral effect in children [5].
A subgroup of 41 children (median age, 5.6 years; range, 1.7–16.8 years) were selected for the HIV-1 DNA study described here on the basis of having persistently undetectable plasma HIV-1 RNA levels while receiving study HAART for >2 years (table 1). Children who received <2 years of study treatment, in whom HIV-1 RNA levels <50 copies/mL were not attained, or who had ⩾2 consecutive detectable plasma HIV-1 RNA measurements (⩾50 copies/ mL) after HIV-1 RNA levels had decreased to <50 copies/mL were excluded from the study. Thirty of 31 children included in the final study group were documented to have received previous treatment with ⩾1 NRTI.
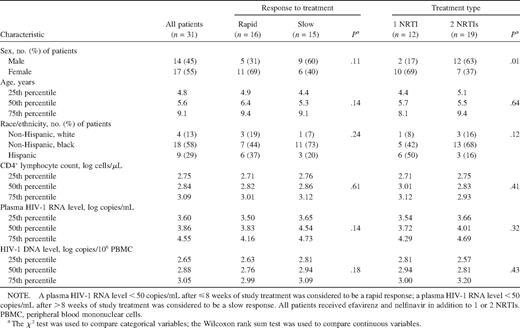
Baseline characteristics of human immunodeficiency virus (HIV) type 1 infection for all study participants and comparison of data for patients who had a rapid response to treatment versus those who had a slow response and for patients who received 1 nucleoside reverse-transcriptase inhibitor (NRTI) versus those who received 2 NRTIs.
Quantification of HIV-1 DNA. PBMC were isolated from whole blood by Ficoll-Paque gradient separation (Amersham Pharmacia). Cells were frozen and stored immediately at −70°C in freezing medium that contained 10% dimethyl sulfoxide and 90% fetal calf serum. HIV-1 DNA levels were quantified by the Amplicor HIV-1 Monitor assay (Roche Molecular Systems) [28]. Measurement of total DNA (cellular plus viral) was performed with Hoechst dye (bisbenzimide; Pharmacia) by use of a fluorometer. The level of HIV-1 DNA is expressed as copies per microgram of cellular DNA. The HIV-1 DNA copy number was calculated using the following formula: HIV-1 copies = dtotal HIV-1 optical density/total quantitation standard optical density) × input quantitation standard copy number. The HIV-1 copies were normalized to total genomic DNA and expressed as HIV-1 copies per microgram of extracted DNA, using the conversion 1 μg of DNA = 150,000 PBMC.
DNA levels were calculated and expressed in 3 ways: (1) copies per 106 PBMC, (2) copies per 106 CD4+ lymphocytes, using CD4+ lymphocyte counts from the same day that the PBMC were obtained, and (3) copies per milliliter of whole blood. HIV-1 DNA levels in PBMC were measured at baseline (prior to initiation of study treatment) and at weeks 2, 4, 8, 20, 48, 80, and 104.
Quantitation of plasma HIV-1 RNA. The level of plasma HIV-1 RNA was measured by a commercially available polymerase chain reaction assay, the Amplicor HIV-1 Monitor assay (Roche Molecular Systems). Plasma samples with <400 copies/mL of HIV-1 RNA, which is the limit of quantitation of this assay, were tested by use of the Ultrasensitive HIV-1 Monitor assay (version 1.0; Roche Molecular Systems), which has a limit of quantitation of 50 copies/mL of HIV-1 RNA. The values of the ultrasensitive assay were used for data analysis when samples were retested.
Statistical analysis. For the purposes of statistical analysis, data from continuous measures, including DNA and RNA levels and CD4+ lymphocyte count data, were transformed to log10. Correlations among these variables were assessed by means of Spearman's rank order methods. Group comparisons between these continuous variables consisted of Wilcoxon rank sum tests, when 2 groups were involved, and Kruskal-Wallis tests, when >2 groups were being compared. Within-subject assessments of change over time in continuous variables consisted of Wilcoxon matched-pairs signed rank tests. χ2 tests were used to make pairwise comparisons between categorical variables. Covariance analyses and half-life estimation were done using regression models. P ⩽ .05 was considered to be significant.
Results
All children in the present study had undetectable plasma HIV- 1 RNA levels by week 48 and sustained undetectable plasma HIV RNA levels (<50 copies/mL) throughout the rest of the study period. Twenty-seven patients (87%) were black or Hispanic. At baseline, the median CD4+ lymphocyte count was 2.8 log10 cells/μL, and the median plasma HIV-1 RNA level was 3.9 log10 copies/μL. Thirty-nine percent of patients (12 of 31 patients) received 1 NRTI; the remaining 61% (19 of 31 patients) received 2 NRTIs. The number of NRTIs administered was decided by each child's primary HIV care provider. All patients received efavirenz and nelfinavir. The 31 study patients were divided into 2 groups, on the basis of response to HAART: patients with a rapid response (“rapid responders”; n = 16), whose plasma HIV-1 RNA levels decreased to <50 copies/mL after ⩽8 weeks of HAART, and patients with a slow response (“slow responders”; n = 15), whose HIV-1 RNA levels decreased to <50 copies/mL after >weeks of HAART. Nostatistically significant differences were seen at baseline between the rapid responders and slow responders in background characteristics, including sex, age, race, CD4+ lymphocyte count, and plasma HIV-1 RNA level (table 1).
Association between continuous suppression of plasma HIV-1 RNA and sustained increases in CD4 + lymphocyte counts. All 31 patients enrolled in this study had detectable levels of plasma HIV-1 RNA (range, 2.9–5.2 log10 copies/mL) before initiation of antiretroviral therapy. Fourteen patients (45%) had HIV-1 RNA levels >4 log10 copies/mL at baseline. Sixteen (52%) of the 31 patients had an undetectable plasma HIV-1 RNA level at week 8, and all patients had an undetectable level at week 48. Plasma HIV-1 RNA remained undetectable in all patients after week 48. CD4+ lymphocyte counts increased from baseline (median, 693 cells/μL; range, 248–2616 cells/μL) through week 20 (median, 928 cells/μL; range, 242–2705 cells/μL) and week 104 (median, 960 cells/μL; range, 260–1921 cells/μL).
Correlation between HIV-1 DNA copies per 106 PBMC and HIV-1 DNA copies per 106 CD4 + lymphocytes. Data from HIV-1 DNA quantification were expressed as HIV-1 DNA copies per 106 PBMC and copies per 106 CD4+ lymphocytes. At baseline, HIV-1 DNA values expressed as copies per 106 CD4+ lymphocytes was strongly correlated with values expressed as copies per 106 PBMC (r = 0.82; P<.0001), and this correlation remained strong throughout the study period (r > 0.85; P < .0001). The change in HIV-1 DNA levels from baseline through week 104 observed in data expressed as copies per 106 CD4+ lymphocytes also correlated highly with the decrease observed when data were expressed as copies per 106 PBMC (r = 0.91; P < .0001). Thus, unless otherwise specified, HIV-1 DNA levels are expressed as copies per 106 PBMC in the analyses that follow. The change in CD4+ lymphocyte counts from baseline through week 104 was not correlated with the change in HIV-1 DNA during this time period (r = −0.19; P = .32), which indicates that the decrease in intracellular HIV-1 DNA represented a decrease in the absolute quantity of HIV-1 DNA present within cells and did not simply reflect an increase in the absolute number of CD4+ lymphocytes.
Decrease in intracellular HIV-1DNA during HAART. Baseline HIV-1 DNA levels were not significantly correlated with age (r = −0.24; P = .20); moreover, baseline HIV-1 DNA values did not vary as a function of race/ethnicity (P = .70). The median quantities of HIV-1 DNA were compared at each visit during administration of HAART. Before the initiation of HAART, the median level of intracellular HIV-1 DNA was 750 copies/ 106 PBMC (range, 204–4464 copies/106 PBMC; n = 31). After the initiation of HAART, levels of intracellular HIV-1 DNA dropped gradually, reaching median levels of 519 copies/106 PBMC at week 8 (range, 180–2505 copies/106 PBMC; n = 30) and 433 copies/106 PBMC at week 20 (range, 60–2028 copies/ 106 PBMC; n = 30) (figure 1). Statistically significant differences were seen between the median quantity of intracellular HIV-1 DNA at baseline and that at week 8 (P = .003) and between the median quantity at baseline and that at weeks 20, 48, 80, and 104 (P < .001). HIV-1 DNA quantities decreased further from week 20 through week 80 (P = .003), with a marginally significant decrease from week 48 through week 80 (P = .07), and reached a plateau from week 80 through week 104 (P = .7). However, levels of HIV-1 DNA remained detectable in all patients through week 104 (median, 263 copies/106 PBMC; range, 51–1321 copies/106 PBMC). The estimated half-life for intracellular HIV-1 DNA, calculated by regression coefficient analysis, was 60 weeks.
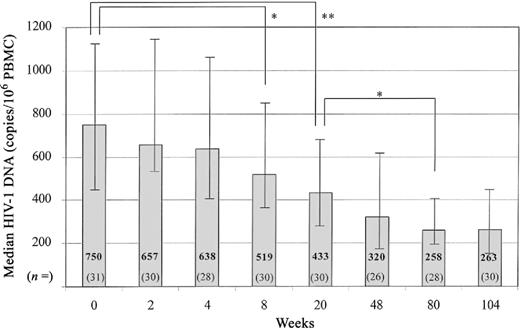
Median level of human immunodeficiency virus (HIV) type 1 DNA in copies per 106 peripheral blood mononuclear cells (PBMC). Median HIV-1 DNA levels decreased throughout treatment. Bars indicate the range of HIV-1 DNA between the 25th and 75th percentiles. There was a significant difference between intracellular HIV-1 DNA levels at baseline and weeks 8 (P = .003) and 20, 48, 80, and 104 (P < .001). HIV-1 DNA significantly decreased from week 20 through weeks 80 (P = .005) and 104 (P = .002). n, No. of patients in each group for each week. Variation in sample size was due to no. of patients available for study at each time point. *P < .05. **P < .001.
The baseline intracellular HIV-1 DNA level was a predictor of HIV-1 DNA levels at each subsequent week. There were high correlations between baseline HIV-1 DNA and the HIV-1 DNA levels at weeks 2–20 (r < 0.74; P < .0001). This correlation diminished but remained statistically significant from week 48 through week 104 (r > 0:39; P < .05).
Decrease in intracellular HIV-1 DNA levels in children who received 1 NRTI and those who received 2 NRTIs. Because children who received efavirenz, nelfinavir, and 2 NRTIs may have had a more rapid decrease in intracellular HIV-1 DNA than those who received only 1NRTI, we examined the absolute intracellular HIV-1 DNA load and rate of decrease in both groups (table 2). Children who received 2 NRTIs had a median baseline intracellular HIV-1 DNA load of 2.81 log10 copies/106 PBMC, versus 2.94 log10 copies/106 PBMC for children who received a single NRTI (P = .43). At week 48, intracellular HIV-1 DNA loads had decreased to 2.51 and 2.52 log10 copies/106 PBMC for the 2- and 1-NRTI groups, respectively (P = .93). By week 104, intracellular HIV-1 DNA loads were 2.4 and 2.61 log10 copies/106 PBMC, respectively (P = .08). The rates of intracellular HIV-1 DNA decrease for the 2 groups did not differ at any of the intervals examined
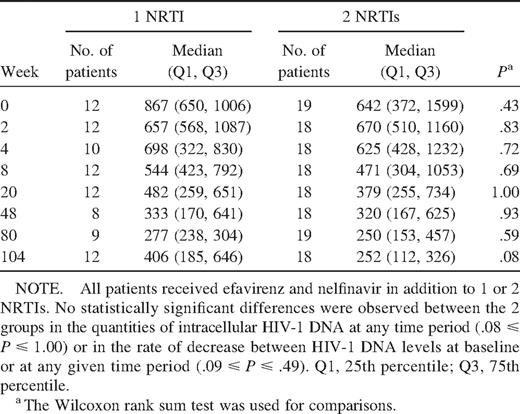
Comparison of median intracellular quantities of HIV-1 DNA in patients who received 1 nucleoside reverse-transcriptase inhibitor (NRTI) and patients who received 2 NRTIs.
Correlation between intracellular HIV-1 DNA and plasma HIV-1 RNA. To estimate the ratio of replicating virus to nonreplicating virus and to approximate the number of cells producing virus, the association between intracellular HIV-1 DNA and plasma HIV-1 RNA was examined. Figure 2 shows the correlation between intracellular HIV-1 DNA and plasma HIV-1 RNA at baseline and at weeks 2, 8, and 20. At baseline (n=31), a weak correlation was seen between HIV-1 DNA and HIV-1 RNA levels (n =31; r=0.39; P=.03), and stronger correlations were observed at weeks 2 (n=30; r =0.52; P=.005), 4 (n=28; r =0.51; P=.006), and 8 (n=30; r =0.53; P=.003), at which times the majority of patients continued to have detectable plasma HIV-1 RNA levels. However, by week 20, at which time most patients (89%) had undetectable HIV-1 RNA levels, little correlation was seen between plasma HIV-1 RNA values and intracellular HIV-1 DNA levels (n = 27; r = 0.11; P = .58). The baseline plasma HIV-1 RNA level was predictive of change in HIV-1 DNA levels from baseline through weeks 4 (P = .04; β =0.156) and 8 (P=.0001; β =0.254), when the analysis was controlled for baseline HIV-1 DNA level.
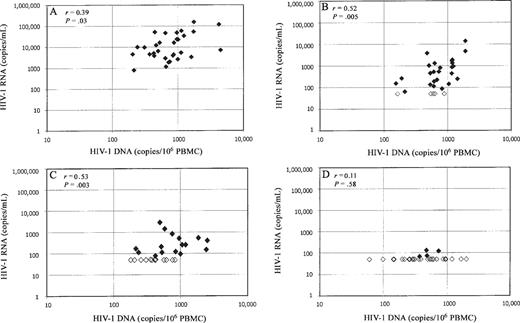
Correlation between human immunodeficiency virus (HIV) type 1 DNA and HIV-1 RNA at baseline (A) and weeks 2 (B), 8 (C), and 20 (D). Statistically significant, moderate correlations are observed between intracellular HIV-1 DNA and plasma HIV-1 RNA levels during the first 8 weeks of highly active antiretroviral therapy. The correlation is no longer present by week 20, at which time 27 (87%) of 31 patients had undetectable HIV-1 RNA levels (<50 copies/mL). Open diamonds, undetectable HIV-1 RNA levels; filled diamonds, detectable HIV-1 RNA levels. PBMC, peripheral blood mononuclear cells.
To examine the ratio of HIV-1 RNA to HIV-1DNA, the HIV-1 DNA concentration in each patient was converted from copies per 106 PBMC to copies per milliliter of whole blood, so that HIV-1 DNA and HIV-1 RNA concentrations were expressed in the same units. At baseline, the ratio of HIV-1 RNA to HIV-1 DNA was 10.8:1. This ratio decreased rapidly with treatment; median ratios of 0.46:1, 0.14:1, and 0.12:1 were observed at weeks 2, 4, and 8, respectively.
Intracellular HIV-1 DNA levels in rapid versus slow responders. Study patients were divided into 2 groups, rapid responders (n=16), whose plasma HIV-1 RNA levels decreased to <50 copies/mL after ⩽8 weeks of HAART, and slow responders (n = 15), whose plasma HIV-1 RNA levels decreased to <50 copies/mL after >8 weeks of HAART. Median intracellular HIV-1 DNA levels were lower among rapid responders than among slow responders at baseline and throughout follow-up. Statistically significant differences were seen at weeks 2 (P=.01), 4 (P=.01), 8 (P=.02), 48 (P=.02), and 80 (P=.009) (figure 3). These differences were not significant at baseline (P=.18), week 20 (P=.14), or week 104 (P=.31).
![Comparison of median intracellular quantities of human immunodeficiency virus (HIV) type 1 DNA in patients who had a rapid response to therapy (“rapid responders”; children in whom the plasma HIV-1 RNA levels decreased to <50 copies/mL after ⩽8 weeks of highly active antiretroviral therapy [HAART]) and patients who had a slow response to therapy (“slow responders”; children in whom the plasma HIV-1 RNA levels decreased to <50 copies/mL after >8 weeks of HAART). The median intracellular quantity of HIV-1 DNA in rapid responders is consistently lower than that observed in slow responders, at weeks 2 (P = .01), 4 (P = .01), 8 (P = .02), 48 (P = .02), and 104 (P = .31). n, No. of patients in each group for each week; PBMC, peripheral blood mononuclear cells. Variation in sample size was due to no. of patients available for study at each time point. *P < .05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/185/10/10.1086_340614/2/m_185-10-1409-fig003.jpeg?Expires=1750096876&Signature=fdEnU3bIOSgREga4YuikTpwXvQD5V1yWXs~-4sT44P~5CT6yk97moPfVdh-9aj7INbbGMFbPhNX2h0FdzFD3aXyhV-~dzZ6WNlskvwQ-0NBSxlwrkVl22g~BPNM9fwugFp6S1NYw3GL3rF71dKpk8WqXx5bQRCOdFvgf5hG57DFIk-gcAmc0Z-jPBopSNui-mEz9Uw4dNsR8FUcdKBN-lR9uRGlPyj5xukK1iSSNbCuhwL5D7pf2WzeVb2y7vefApUwViEs8auFhSMFK9nOwqNci2IA0Roxi1jyruPLLe21s1DaXR1WzonVbd7wHnOEgzW7c8qoncjd2Z6IqWIueOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comparison of median intracellular quantities of human immunodeficiency virus (HIV) type 1 DNA in patients who had a rapid response to therapy (“rapid responders”; children in whom the plasma HIV-1 RNA levels decreased to <50 copies/mL after ⩽8 weeks of highly active antiretroviral therapy [HAART]) and patients who had a slow response to therapy (“slow responders”; children in whom the plasma HIV-1 RNA levels decreased to <50 copies/mL after >8 weeks of HAART). The median intracellular quantity of HIV-1 DNA in rapid responders is consistently lower than that observed in slow responders, at weeks 2 (P = .01), 4 (P = .01), 8 (P = .02), 48 (P = .02), and 104 (P = .31). n, No. of patients in each group for each week; PBMC, peripheral blood mononuclear cells. Variation in sample size was due to no. of patients available for study at each time point. *P < .05.
Sixty-three percent of rapid responders had HIV-1DNAlevels lower than the median at week 8, whereas 73% of slow responders had levels higher than the median (P=.045) (table 3), which suggests that rapid responders had lower intracellular HIV-1 DNA levels than did slow responders at week 8. At weeks 2, 4, 8, 48, and 80, rapid responders had levels of HIV-1 DNA that were significantly lower than levels in slow responders (P < .05). After week 8, both rapid and slow responders exhibited a continued decrease in intracellular HIV-1 DNA from week 8 through week 104 of 0.34 log10 copies/106 PBMC (P = .011) and 0.39 log10 copies/106 PBMC (P = .001), respectively. In addition, 27 (87%) of 31 patients had undetectable levels of HIV-1 RNA by week 20. These patients exhibited a decrease in HIV-1 DNA of 0.13 log10 copies/106 PBMC from week 20 through week 104 (P = .015), which indicates that a decrease in intracellular HIV-1 DNA levels could be demonstrated during the period in which HIV-1 RNA levels were undetectable.
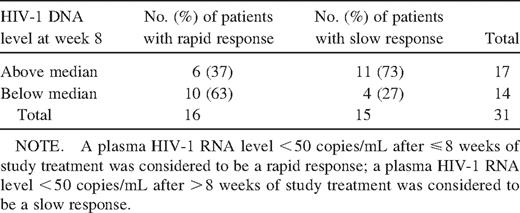
Association between median human immunodeficiency virus (HIV) type 1 DNA level at week 8 and response to treatment.
Correlation between intracellular HIV-1 DNA levels and CD4+ lymphocyte counts. Although a gradual increase in CD4+ lymphocyte counts was seen in the children who participated in this study, there was no correlation between a patient's intracellular HIV-1 DNA level and the CD4+ lymphocyte count at baseline or at weeks 2, 4, 8, 20, or 48 (r =−0:09 to 0.06; P> .63). Additionally, baseline CD4+ lymphocyte counts were not predictive of change in intracellular HIV-1 DNA over time (P>.05 for change in intracellular HIV-1 DNA from baseline to all follow-up weeks). Finally, an examination of HIV-1 DNA levels by CD4+ lymphocyte count plots failed to reveal a threshold level of HIV-1 DNA at which patients with substantial increases in CD4+ lymphocyte counts could be distinguished from those with little or no increase in CD4+ lymphocyte counts. However, the ratio of CD4+ lymphocyte count to HIV-1 DNA copies progressively increased from baseline through week 80. When HIV-1 DNA was expressed in copies per milliliter and CD4+ lymphocyte counts were expressed in cells per milliliter, the baseline ratio of HIV-1 DNA to CD4+ lymphocytes was 1:371. This decreased to 1:407, 1:477, and 1:569 at weeks 2, 4, and 8, respectively, and reached a plateau of <1:1100 at weeks 80 and 104
Discussion
Our research findings demonstrate that HIV-1 DNA remains detectable in the PBMC of infected children who have received HAART for ⩾2 years, despite maintenance of sustained plasma HIV-1 RNA levels <50 copies/mL. HIV-1 DNA levels gradually decreased from baseline through week 48, reaching a plateau from week 80 through week 104. Thus, even when the level of viral RNA in plasma was undetectable, PBMC continued for at least 2 years to harbor HIV-1 DNA that could be quantified, and HIV-1 DNA in PBMC may be a useful marker of continued virus suppression.
The median quantity of intracellularHIV-1DNAhad decreased by 31% at week 8, by 42% at week 20, and by 57% at week 48, compared with the baseline level. The estimated half-life of HIV- 1 DNA for this patient population, calculated by regression coefficient analysis, was 60 weeks (∼14 months), which is longer than the viral half-lives reported elsewhere for infected adults receiving effective antiretroviral therapy, which were estimated to be between 5 and 10 months [29–31].
Several hypotheses could explain the prolonged half-life of intracellular HIV-1 DNA in children, compared with that in adults. Children have higher numbers of CD4+ lymphocytes, which can serve as targets for HIV-1 infection and may be capable of harboring low levels of HIV-1 DNA and nonreplicating virus, than do adults. Alternatively, a differential susceptibility to the antiviral activity of the antiretroviral therapies used could explain the observed differences in the HIV-1 DNA half-lives observed in children. In addition, because 97% of the children in the present study had received ⩾1 NRTI before the initiation of study treatment, diminished susceptibility to the antiretroviral agents used may have affected the quantity of HIV-1 DNA and the rate of decrease that we observed.
It is important to note that the present study investigated only the pool of virus present in peripheral blood. Other reservoirs of HIV-1-infected cells exist throughout the body, including the lymph nodes, the central nervous system, and some solid organs, in which replication-competent virus likely will persist despite sustained virus suppression as determined by plasma HIV-1 RNA. Therefore, the complete eradication of HIV-1 from an infected individual by use of currently available antiretroviral agents likely will not be possible.
Intracellular levels of HIV-1DNA at baseline were predictive of future quantities of HIV-1 DNA in the children evaluated in the present study. Patients who had high HIV-1 DNA levels at baseline continued to have higher quantities of HIV-1 DNA throughout the 104 weeks of follow-up. These higher baseline levels of HIV-1 DNA may indicate a relative increase in the number of cells susceptible to productive HIV-1 infection. The persistence of higher levels of intracellular HIV-1 DNA in patients who had the highest baseline levels of HIV-1 DNA during sustained antiretroviral-induced virus suppression suggests that the virus set point may be predetermined by the pool of susceptible long-lived cells that are capable of maintaining HIV-1 DNA without virus replication or cell death. Similar findings have been reported for HIV-1-infected adults receiving HAART, independent of baseline CD4+ lymphocyte counts [30, 32]. At baseline and while expression of HIV-1 continued to be detectable during the first 8 weeks of therapy, quantities of intracellular HIV-1 DNA correlated with quantities of plasma HIV-1 RNA. By week 20, correlations between HIV-1 DNA and HIV-1 RNA were no longer meaningful, because HIV-1 RNA levels had become undetectable (<50 copies/mL) in almost all members of the subgroup studied.
The rate of decrease in plasma HIV-1 RNA levels in the early phase of treatment is useful for the evaluation of the potential response to HAART in children [33]. In the larger cohort from which the subgroup for this study was derived, 75% of rapid responders maintained virus suppression (<50 copies/mL) for ⩾24 weeks, compared with 48% of slow responders. Additionally, baseline plasma HIV-1 RNA levels are predictive of the nadir achieved and of sustained HIV-1 RNA suppression after initiation of HAART. The correlation between HIV-1 DNA and therapeutic efficacy has been reported for adults [34]. In that study, mean HIV-1 DNA copy numbers for patients who did not respond to treatment were 4.54-fold higher than those for patients who did respond to treatment and for untreated patients. In the present study, rapid responders were found to have median intracellular HIV-1 DNA levels at baseline that were lower than those for slow responders.Of interest, even when antiretroviral therapy was effective (defined as achievement of plasma HIV-1 RNA levels <50 copies/mL), slow responders continued to have greater quantities of intracellular HIV-1 DNA than did rapid responders, throughout 104 weeks of HAART.
The assay used for our studies measured total HIV-1 DNA, both integrated and unintegrated. Integrated HIV-1 DNA is stable and is located mainly in resting memory CD4+ T cells, which are the major reservoirs of HIV-1 virus [13]. Unintegrated HIV-1 DNA is the direct product of acute replicating virus, is unstable, and is the major form of HIV-1 DNA that decreases during the initial period of effective antiretroviral therapy [35]. It is possible, therefore, that the HIV-1 DNA found in rapid responders after 8 weeks of therapy predominantly is integrated HIV-1 DNA, whereas slow responders harbor a greater ratio of unintegrated HIV-1 DNA to integrated HIV-1 DNA; since unintegrated HIV- 1 DNA accumulates during the early phase of infection, this would suggest that therapy has been less active. Targeting and eliminating the latent reservoirs containing integrated HIV-1 DNA will be required if eradication of HIV-1 is to be achieved.
In conclusion, our research has shown that levels of intracellular HIV-1 DNA in PBMC remain detectable despite prolonged suppression of HIV-1 RNA to <50 copies/mL in children who have received HAART for ⩾2 years. HIV-1 DNA levels gradually decrease and appear to reach a plateau after 80 weeks of therapy. The quantitation of intracellular HIV-1 DNA in PBMC may be a useful marker of further virus suppression when plasma HIV-1 RNA levels have become undetectable by highly sensitive assays. The nature and pathogenetic impact of sustained levels of HIV-1 DNA in children receiving HAART requires further investigation.
References
Presented in part: 8th Conference on Retroviruses and Opportunistic Infections, Chicago, February 2001 (abstract 685B).
Informed consent was obtained from study participants. This study followed the human experimentation guidelines of the US Department of Health and Human Services and the University of California, San Diego, review board.
Financial support: Pediatric AIDS Clinical Trials Group and National Institute of Allergy and Infectious Diseases (grant nos. AI-39004, AI-27563, AI-33835, and AI-41110; AI-36214 to the University of California Center for AIDS Research); Dupont Pharmaceuticals Company; Agouron Pharmaceuticals, Inc.




