-
PDF
- Split View
-
Views
-
Cite
Cite
Holger Hebart, Jürgen Löffler, Christof Meisner, François Serey, Diethard Schmidt, Angelika Böhme, Hans Martin, Andreas Engel, Donald Bunjes, Winfried V. Kern, Ulrike Schumacher, Lothar Kanz, Hermann Einsele, Early Detection of Aspergillus Infection after Allogeneic Stem Cell Transplantation by Polymerase Chain Reaction Screening, The Journal of Infectious Diseases, Volume 181, Issue 5, May 2000, Pages 1713–1719, https://doi.org/10.1086/315435
Close - Share Icon Share
Abstract
Invasive aspergillosis (IA) has become a major cause of mortality in patients after allogeneic stem cell transplantation. To assess the potential of prospective polymerase chain reaction (PCR) screening for early diagnosis of IA, 84 recipients of an allogeneic stem cell transplant were analyzed with the investigators blinded to clinical and microbiologic data. Of 1193 blood samples analyzed, 169 (14.2%) were positive by PCR. In patients with newly diagnosed IA (n = 7), PCR positivity preceded the first clinical signs by a median of 2 days (range, 1–23 days) and preceded clinical diagnosis of IA by a median of 9 days (range, 2–34 days). Pretransplantation IA (relative risk [RR], 2.37), acute graft-versus-host disease (RR, 2.75), and corticosteroid treatment (RR, 6.5) were associated with PCR positivity. The PCR assay revealed a sensitivity of 100% (95% confidence interval [CI], 48%–100%) and a specificity of 65% (95% CI, 53%–75%). None of the PCR-negative patients developed IA during the study period. Thus, prospective PCR screening allows for identification of patients at high risk for subsequent onset of IA.
Invasive aspergillosis (IA) has become a major cause of infection-related mortality in recipients of an allogeneic stem cell transplant (SCT) [1–3]. Early initiation of antifungal therapy is thought to be crucial to reduce the high mortality rate of IA in patients at risk [3]. Thus, the lack of sensitive and specific diagnostic tests remains a major limiting factor for effective antifungal therapy.
Clinical signs of IA are usually nonspecific, as are radiologic findings [4, 5]. Bronchoscopy and biopsy procedures are often contraindicated because of thrombocytopenia, and cultures from bronchoalveolar lavage and blood samples often are negative, even in the presence of later-proven IA [1, 6]. Sputum isolates lack sensitivity and do not allow one to distinguish colonization from invasive disease [1, 7]. More recently, more sensitive diagnostic tests based on the detection of fungal cell wall components [8–10] and Aspergillus-specific DNA sequences have been reported [11, 12]. Preliminary data for selected patients indicated a potential value of polymerase chain reaction (PCR) for early diagnosis of IA [11, 12].
To evaluate the value of detection of Aspergillus-specific DNA sequences in blood samples for the early diagnosis of IA in patients after allogeneic SCT, a prospective study was conducted.
Patients and Methods
Patient enrollment
During 1996–1997, 84 patients who had undergone allogeneic SCT were enrolled in a multicenter study. The clinical characteristics of the study patients, including underlying disease, donor type, source of transplant, conditioning therapy, and degree of acute graft-versus-host disease, are shown in table 1. Prophylaxis of graft-versus-host disease was done with cyclosporm A in combination with other immunosuppressive drugs for 79 patients, and 5 patients received a T cell-depleted allograft.
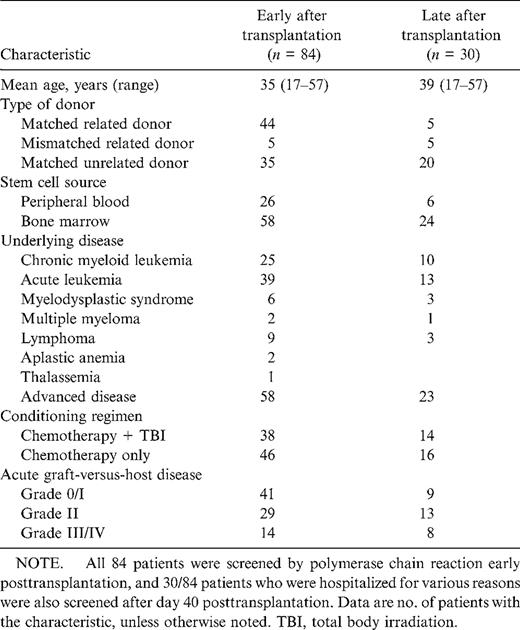
Characterization of allogeneic stem cell transplant recipients included in the study.
A history of IA before transplantation was documented for 15 patients: 4 patients presented with proven IA a mean of 11 months (range, 5–18 months) before transplantation, 6 patients with probable IA a mean of 3 months (range, 2–5 months) before transplantation, and 5 patients with possible IA a mean of 3 months (range, 2–5 months) before transplantation.
The mean number of days patients had a neutrophil count <500/µL was 15.7 (range, 5–67 days), and the mean number of days patients had a neutrophil count <100/µL was 12.2 (range, 3–59 days).
During follow-up, 35 patients died of non-Aspergillus-related causes: 6 patients of acute graft-versus-host disease, 6 of nonfungal infections, 8 of regimen-related toxicity, and 15 of relapse.
Patient management
All patients were treated in laminar airflow rooms until recovery of neutrophils to >1000/µL was documented. The mean number of days in laminar air-flow rooms was 22.4 days (range, 11–69 days). Particulate matters were removed from the air by high-efficiency filters, achieving a complete elimination of particulates >5 mm and a reduction to <100/28 L per min of particulates >0.5 mm. Staff and visitors entered the room downwind from the patient after a careful hand wash and wearing face masks. Food was not sterilized. Most routine procedures were done in the room. If a medical procedure (e.g., computed tomography) was done outside the room, patients were gowned and masked, removed from the room, and returned after the procedure.
For antifungal prophylaxis, fluconazole (400 mg/day; n = 66) and itraconazole (400 mg/day, dose adaptation according to serum levels; n = 18) were used. Fourteen of 15 patients with a pretransplantation history of IA received itraconazole for antifungal prophylaxis. For patients with severe mucositis, itraconazole was stopped, and amphotericin B, at a dose of 0.7–1.0 mg/kg of body weight, was given only to patients developing febrile neutropenia thereafter. After resolution of mucositis, itraconazole was given if possible.
Systemic antibacterial therapy was started at the onset of fever, when neutrophil counts were below 1.0 × 109/L, after a careful clinical examination was completed and a chest radiograph had been made. Blood was cultured from peripheral veins and the central venous catheter. Oropharyngeal, perianal, vaginal, and skin swabs as well as stool and urine specimens were analyzed for bacterial and fungal growth. High-resolution computed tomography was done for patients with persisting febrile neutropenia after 96 h of empirical antibiotic therapy and for all patients with pulmonary infiltrates, followed by a guided bronchoalveolar lavage if feasible.
Empirical systemic antifungal therapy with amphotericin B at a dose of 0.7–1.0 mg/kg of body weight was started after 96 h of febrile neutropenia not responding to broad-spectrum antibiotics and immediately at the onset of fever for neutropenic patients with a previous history of IA. Neutropenic patients with pulmonary infiltrates compatible with invasive pulmonary aspergillosis received intravenous amphotericin B at a dose of 1–1.25 mg/kg of body weight.
Liposomal amphotericin B (AmBisome; Nexstar, Munich) was administered only to patients developing severe amphotericin B-related side effects; doses were 3 mg/kg of body weight for patients treated for proven or probable IA and 1 mg/kg body weight for patients treated for febrile neutropenia only.
Patients after autologous SCT
A cohort of 34 patients receiving high-dose therapy followed by the infusion of autologous stem cells, who were known to be at low risk to develop IA, were included as negative controls. Blood samples were collected once weekly during neutropenia until discharge from hospital. Twentyeight patients had hematologic malignancies (lymphoma, 17; acute leukemia, 9; chronic myeloid leukemia, 2), and 6 had solid tumors. Twelve patients received a conditioning regimen with total body irradiation. None of the patients had evidence of pretransplantation IA. The mean duration of neutropenia <500 neutrophils/µL was 13.1 days (range, 7–36 days). Antifungal prophylaxis consisted of oral amphotericin B for 20 patients, itraconazole (400 mg/day, dose adaptation according to serum levels) for 8, and fluconazole (100 mg/day) for 6.
Definitions
Proven IA was defined as the recovery of any Aspergillus species from normally sterile sites or demonstration of a positive culture or septate branched hyphae from deep-tissue biopsy and autopsy specimens and compatible clinical symptoms. Probable IA was defined as the presence of clinical signs and symptoms, together with a cavitary lesion with a halo sign on computed tomography, or radiographic evidence compatible with Aspergillus infection and isolation of Aspergillus species from sputum, bronchoalveolar lavage, or throat swab samples.
Collection and handling of blood specimens
Five milliliters of EDTA-anticoagulated whole blood was collected prospectively 2–4 times weekly, starting at admission to the transplant unit until death or discharge from hospital. DNA extraction of samples was done as described elsewhere [11]. The PCR assay was run twice weekly by an investigator blinded to clinical and microbiologie data. PCR results were not known to the physicians and thus were not used in the management of the patients. Fungal DNA extraction, PCR amplification, and Aspergillus-specific hybridization were done at a single center (Tübingen). After it was demonstrated that the sensitivity of the PCR assay on EDTA-anticoagulated blood samples spiked with fungal cells and DNA and stored for up to 72 h was not affected (data not shown), samples from Ulm and Frankfurt were shipped overnight at room temperature to Tübingen and were extracted immediately after arrival. Thus, DNA extraction from all clinical samples was done within 24 h after collection of blood samples.
PCR assay
The oligonucleotide primer pair (5′-ATTGGAGGGCAAGTCTGGTG and 5′-CCGATCCCTAGTCGGCATAG) and the DNA probe specific for A. fumigatus, A. flavus, and A. versicolor (5′-CATGGCCTTCACTGGCTGTGGGGGGAACCA) have been described elsewhere [11]. Thirty-five cycles of repeated denaturation, primer annealing, and enzymatic chain extension (30 s at 94°C, 1 min at 62°C, and 2 min at 72°C) were done in a thermocycler (Trioblock; Biometra, Göttingen, Germany). A maximum of 24 samples, including controls, were run in each PCR assay. To monitor for contamination, aliquots of saline and human fibroblast DNA were prepared concurrently as negative extraction and amplification controls by the same procedure. For each 10 blood samples extracted or amplified, 1 negative control was added, according to the recommendations of the German Society for Microbiology. Blood samples spiked with different amounts of Aspergillus fumigatus cells were extracted concurrently to control the DNA extraction step. To control the sensitivity of the assay, fungal DNA was amplified in each run in concentrations with a range of 100 fg to 100 pg. Coamplification of the HLA-DR gene was done in each test tube to exclude the presence of Taq inhibitors. The amplicons were analyzed by a slot-blot test with the 5′-digoxigeninlabeled internal oligonucleotide probe. Potentially, this assay can be completed within 30 h after collection of blood samples.
Statistical analysis
Continuous variables were described by use of statistical characteristics (means and ranges). Discrete variables were described as counts and percentages. To evaluate the value of PCR as a screening test for IA, sensitivities, specificities, and positive and negative predictive values were presented, along with 95% confidence intervals (CIs). The outcome was defined as proven or probable IA. IA early after transplantation (until day 40) was analyzed as the study end point for all 84 patients, and IA after day 40 was analyzed as the study end point for 30 of these 84 patients who were hospitalized for clinical reasons and continuously screened by PCR until discharge or death. The prognostic value of clinical characteristics for positive PCR testing was statistically assessed by use of Mantel-Haenszel χ2 tests. Results are presented as relative risks (RRs) with nominal 2-tailed P values (unadjusted for multiple comparisons). Statistical comparisons were made with SAS 6.11 for Windows (SAS Institute, Cary, NC).
Results
PCR screening
Of a total of 1193 samples analyzed, 169 (14.2%) were positive and 1024 (85.8%) were negative. Thirty (35.7%) of 84 patients before day 40 and 12 (40%) of 30 patients after day 40 after transplantation had ⩾1 positive PCR result (figure 1). At the time of SCT, 6 (40%) of 15 patients with a pretransplantation history of IA were PCR positive, compared with only 2 (2.9%) of 69 patients without IA before transplantation. All 7 patients (100%) developing de novo IA were correctly identified by the PCR assay (true-positive results). Of the 69 patients without clinical evidence of IA before transplantation, 14 (21%) out of 65 patients were found to be PCR positive before day 40 and 3 (15%) out of 19 patients after day 40 after transplantation without clinical evidence of IA during the study period (false-positive results).
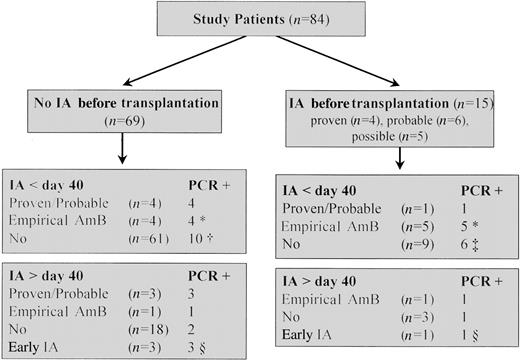
Polymerase chain reaction (PCR) results and clinical evidence of invasive aspergillosis (IA) after allogeneic stem cell transplantation. * Eighteen patients received empirical amphotericin B (AmB) for febrile neutropenia not responding to broad-spectrum antibiotic therapy; in 9 of them, fever was retrospectively explained by non-Aspergillus infections (n = 7) or graft-versus-host disease (n = 2). † One PCR-positive patient developed probable IA on day 100 after transplantation. ‡After PCR screening was stopped, 2 patients developed proven or probable IA on days 90 and 156 after transplantation (1 each PCR positive and PCR negative). § Continuously screened patients with early-onset IA.
Patients having no evidence of IA before transplantation
Of 69 patients without evidence of IA before transplantation, 27 were found to be PCR positive, 18 before day 40 and 9 after day 40 after transplantation. Seven of these 27 patients developed de novo IA during the screening period (see below).
Fourteen patients were found to be PCR positive before day 40 without evidence of proven or probable IA; of these, 4 patients received empirical amphotericin for febrile neutropenia not responding to broad-spectrum antibiotic therapy, and 1 patient developed probable IA on day 100 after the screening program was stopped. Thus, 9 patients were found to be PCR positive early after transplantation without any clinical evidence of IA (5 had 1 positive test, 3 had 2 positive tests, and one had 3 positive tests).
After day 40 after transplantation, 3 patients were found to be PCR positive without clinical evidence of proven or probable IA, 2 once and one twice.
Patients with proven or probable IA
During the study period, proven or probable IA was documented in 5 of 84 patients before day 40 and in 3 of 30 after day 40 after transplantation (figure 1), 7 of whom died. All 8 patients with proven or probable IA were PCR positive.
PCR was found to be the earliest indicator for 7 patients with de novo IA, preceding the first clinical signs of IA (taking into account nonspecific radiographic findings) by a median of 2 days (range, 1–23 days) and preceding clinical diagnosis of IA based on typical radiographic findings by a median of 9 days (range, 2–34 days; figure 2; table 2). The median time from the second positive PCR result to clinical diagnosis of IA was 3 days (range, 0–19 days).
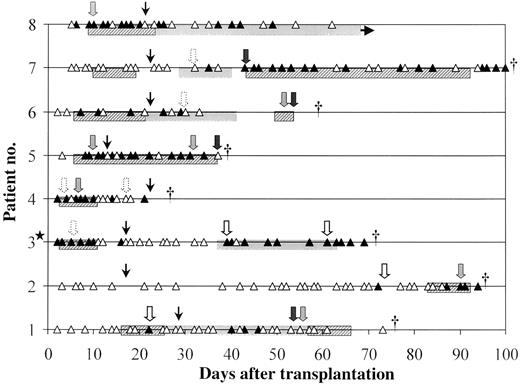
Correlation of polymerase chain reaction (PCR) results and clinical evidence among 8 continuously screened patients with proven or probable invasive aspergillosis (IA). ▲. Positive PCR result (days 1–100 after transplantation); △, negative PCR result (days 1–100 after transplantation); ★, patient with proven IA before transplantation; dotted arrows, pulmonary infiltrate (chest radiograph); open arrows, nonspecific infiltrates (computed tomography); shaded arrows, nodular infiltrates (computed tomography); solid arrows, cerebral lesions; narrow arrows, date laminar air flow was stopped; hatched bars, intravenous amphotericin B; and shaded bars, itraconazole. † Time of death.
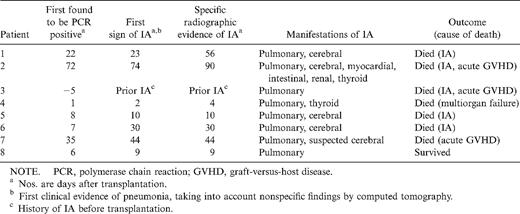
Clinical course of patients with proven or probable invasive aspergillosis (IA) after allogeneic stem cell transplantation.
A mean of 12.4 samples (range, 4–22 samples) were found to be PCR positive for patients with proven or probable IA, a mean of 3.0 samples (range, 1–6 samples) for PCR-positive patients receiving empirical antifungal therapy, and a mean of 0.52 samples (range, 0–3 samples) for patients without any clinical evidence of IA.
For early-onset IA, the sensitivity was 100% (95% CI, 48%–100%), the specificity was 65% (95% CI, 53%–75%), the negative predictive value was 100% (95% CI, 93%–100%), and the positive predictive value was 15.2% (95% CI, 5%–32%). The positive predictive value and the specificity of the assay were improved if calculations were based on 2 positive PCR tests without a loss of sensitivity (positive predictive value, 27.8% [95% CI, 10%–54%]; specificity, 84% [95% CI, 74%–91%]). For patients without a history of IA (n = 69) who tested PCR positive twice, a sensitivity of 100% (95% CI, 40%–100%), a specificity of 92.3% (95% CI, 83%–98%), a positive predictive value of 44.4% (95% CI, 14%–79%), and a negative predictive value of 100% (95% CI, 94%–100%) were documented. The value of the PCR assay as a screening assay for late-onset IA was similar (data not shown).
Patients with a history of IA before transplantation
Twelve of 15 patients with a history of IA were found to be PCR positive before day 40 after transplantation.
Two of 4 patients with a proven history of IA before transplantation developed progressive IA at days 40 and 156 after transplantation. PCR positivity was documented in both patients early after transplantation (figure 2, patient 3; figure 3, patient 2). One additional patient received empirical amphotericin B at the onset of febrile neutropenia.
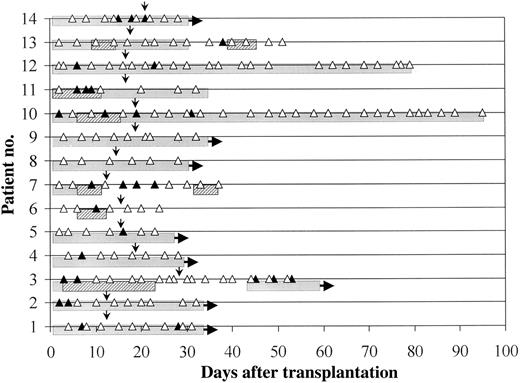
Polymerase chain reaction (PCR) results for patients with a history of invasive aspergillosis (IA) before transplantation (proven in patients 1–3, possible in patients 4–8, and probable in patients 9–14). One patient developed progressive IA during the study period (patient 3, figure 2). Patients 2 and 9 developed IA at days 156 and 90, respectively, after transplantation. Patients 5 and 10 died from relapse, patient 7 from acute graft-versus-host disease, patients 11 and 13 from regimen-related toxicity, and patient 12 from cytomegalovirus-induced interstitial pneumonia. ▲, Positive PCR result; △, negative PCR result; narrow arrows, date laminar air flow was stopped; hatched bars, intravenous amphotericin B; and shaded bars, itraconazole.
One of 6 patients with a probable history of IA before transplantation developed probable IA at day 90 after transplantation. This patient was screened only before day 40 after transplantation, and all blood samples analyzed during this time period tested negative (figure 3, patient 9). Another 2 patients (figure 3, patients 10 and 11), both PCR positive, developed new pulmonary infiltrates and were treated empirically with intravenous amphotericin B. For 1 additional patient treated empirically with amphotericin B, fever was retrospectively explained by a culture-proven viral infection.
Two of 5 patients with possible IA before transplantation received empirical antifungal therapy for febrile neutropenia not responding to broad-spectrum antibiotic treatment, and both patients were PCR positive.
Patients receiving empirical antifungal therapy
Early after transplantation, another 18 patients received systemic antifungal therapy, 9 patients with febrile neutropenia not responding to broad-spectrum antibiotic therapy after 96 h, 3 patients with pulmonary infiltrates at onset of febrile neutropenia, and 6 febrile neutropenic patients with a previous history of IA, 2 of whom developed new pulmonary infiltrates. In 7 PCR-negative patients, febrile neutropenia was retrospectively explained by culture-proven infections: Candida tropicalis in 1 patient and bacterial and viral infections in 4 and 2, respectively. In 2 of 18 patients with only 1 positive PCR result, the febrile episode was associated with severe acute graft-versus-host disease and was resolved on initiation of steroid treatment. In the remaining 9 patients, all found to be PCR positive in a mean of 3 blood samples (range, 1–6 blood samples), no clinical or microbiologic cause of febrile neutropenia could be documented. Five of these 9 patients had a history of IA before transplantation, and 4 developed pulmonary infiltrates during the febrile episode. All 9 patients showed clearance of the fungal DNA from the blood on antifungal therapy, and none died because of IA at a later stage after transplantation.
None of the PCR-negative patients receiving empirical antifungal therapy for febrile neutropenia developed IA during the study period.
Two PCR-positive patients received antifungal treatment after day 40 after transplantation.
Analysis of risk factors
The RR to become PCR positive early after transplantation was found to be increased in patients with a history of proven or probable IA before transplantation (RR, 2.37; P = .005). In the later period after transplantation, PCR positivity was found to be associated with acute graftversus-host disease grade III or IV (RR, 2.75; P = .008) and corticosteroid treatment with >1 mg/kg of body weight (RR, 6.5; P = .005).
Patients after autologous transplant
One hundred nine blood samples collected from 34 patients after autologous SCT were analyzed as negative controls. None of the patients developed proven or probable IA, and only 5 patients received empirical antifungal therapy for febrile neutropenia not responding to broad-spectrum antibiotic therapy.
Nine samples (8.3%) taken from 5 patients (14%) were found to be PCR positive. Four of 5 PCR-positive patients had hematologic malignancies. Seven PCR-positive samples were taken from 3 patients receiving empirical antifungal therapy. The other 2 PCR-positive patients without evidence of IA had only 1 single positive test result. None of the PCR-negative patients developed IA during the study period.
Discussion
This is the first study demonstrating prospective PCR screening for fungus-specific DNA sequences in blood samples to be feasible and to allow early diagnosis of IA in high-risk patients. All 8 patients with proven or probable IA were PCR positive. In patients with de novo proven or probable IA (n = 7), PCR preceded the first signs of IA by a median of 2 days (range, 1–23 days) and preceded clinical diagnosis of IA based on typical radiographic findings by a median of 9 days (range, 2–34 days). The sensitivity and the negative predictive value were found to be 100%, suggesting the potential application of the assay as a screening test. The positive predictive value was found to be rather low but could be significantly improved if calculations were based on 2 positive PCR results. When patients with a history of IA were excluded from analysis, a positive predictive value of 44% was demonstrated. Fourteen (21%) of 65 patients were found to be PCR positive before day 40 and 3 (15%) of 19 patients after day 40 without clinical evidence of IA before transplantation or during the study period. In a cohort of 34 patients without proven or probable IA after autologous SCT, 8.3% of blood samples taken from 5 patients (14%) were PCR positive. False-positive test results have been reported also for other sensitive assays [9], and, because the PCR test was not used for therapeutic decision making, empirical antifungal therapy most likely reduced the incidence of IA, the end point of the study.
This study clearly demonstrates that PCR may help to establish an early diagnosis of invasive aspergillosis after allogeneic SCT. The DNA extraction protocol applied to clinical samples has been shown to have a major impact on detection sensitivity [13, 14]. Therefore, for the detection of Aspergillus DNA, we are still relying on a time-consuming DNA-extraction protocol incorporating enzymatic lysis with µ1,3-glucanases, whereas this step is not needed for the sensitive detection of Candida DNA in blood samples [15]. Moreover, the clinical material under study is likely to affect the sensitivity of the assay [16]. A prospective comparison of PCR on plasma and whole blood samples from patients with proven or probable IA indicates a higher sensitivity for PCR on whole blood (authors' unpublished data). Moreover, a reliable number of negative and positive controls need to be added to control each step of the procedure, including DNA extraction, PCR amplification, and amplicon detection, to detect any systematic errors [17].
Three additional patients, screened only before day 40 after transplantation, developed IA late after transplantation, and 2 of these 3 were found to be PCR positive during the screening episode. Because prospective screening was stopped in about two-thirds of the patients after discharge from the hospital, the number of patients analyzed late after transplantation in this study is small. However, PCR preceded clinical symptoms of IA in 3 prospectively screened patients. A larger cohort of patients developing IA late after transplantation needs to be analyzed, to establish the role of prospective PCR screening for early diagnosis of IA in this patient group.
Patients with a history of IA, who were shown to be at high risk for Aspergillus infection reactivation after transplantation [18], were more likely to have a positive PCR result. These data support the hypothesis of reactivation of the pathogen during intense immunosuppressive therapy, possibly from proliferation of residual colonizing Aspergillus spores [19, 20]. Thus, it is reasonable to assume that, as a result of severe mucositis and subsequent disturbance of natural antimicrobial barriers, Aspergillus-specific DNA sequences might be detected in the blood of patients with a history of IA. Intensified antifungal prophylaxis and early systemic antifungal treatment at onset of febrile neutropenia in these patients are clearly indicated.
Severe acute graft-versus-host disease and corticosteroid use were found to be associated with an increased risk to become PCR positive. These results are in accordance with a large retrospective analysis demonstrating severe acute graft-versus-host disease and use of high-dose corticosteroids to be important risk factors after allogeneic SCT [1].
We conclude that prospective examination of blood samples by PCR following allogeneic SCT allows identification of patients at very high risk for IA. Future trials will have to address the question of whether PCR-based preemptive antifungal therapy will help to reduce Aspergillus-related mortality in highrisk patients.
References
Presented in part: International Society of Human and Animal Mycology meeting, Parma, Italy, 8–13 June 1997 (abstract O60); American Society of Hematology 39th annual meeting, San Diego, December 1997 (abstract 1864).
Written informed consent was obtained from all patients under the guidelines of the institutional review board at the University Hospital of Tübingen.
Financial support: Deutsche Krebshilfe (project 70-2199-Ka 1).




