-
PDF
- Split View
-
Views
-
Cite
Cite
Tonia Woodberry, Todd J. Suscovich, Leah M. Henry, Jennifer K. Davis, Nicole Frahm, Bruce D. Walker, David T. Scadden, Frederick Wang, Christian Brander, Differential Targeting and Shifts in the Immunodominance of Epstein‐Barr Virus–Specific CD8 and CD4 T Cell Responses during Acute and Persistent Infection, The Journal of Infectious Diseases, Volume 192, Issue 9, 1 November 2005, Pages 1513–1524, https://doi.org/10.1086/491741
Close - Share Icon Share
Abstract
The evolution of Epstein‐Barr virus (EBV)–specific T cell responses that occurs during the acute and persistent stages of infection remains poorly characterized despite its importance for developing immune interventions for EBV‐associated disorders. This study assessed T cell responses to 113 EBV‐derived epitopes in 40 subjects with acute or persistent EBV infection. Although no significant differences were seen in the breadth of CD8 and CD4 T cell responses, their magnitude differed significantly over time; acutely infected subjects generated especially strong responses to lytic viral antigens. The cross‐sectional shift in immunodominance was also confirmed in subjects followed longitudinally from acute to persistent infection. In addition, human leukocyte antigen–matched siblings with discordant histories of symptomatic EBV infection showed no significant differences in their response patterns, suggesting that symptomatic EBV infection does not lead to unique persistent‐stage responses. These data provide an assessment of immunodominance patterns and guidance for developing immunotherapeutic interventions for EBV‐associated disorders.
Epstein‐Barr virus (EBV) is an endemic and persistent human pathogen that, if acquired during childhood, typically results in a clinically asymptomatic infection [1]. In contrast, infection in adulthood often causes a symptomatic acute viral syndrome termed “acute infectious mononucleosis” (AIM). The increased occurrence of EBV‐associated disorders in immunocompromised individuals [2] and the clinical improvements observed after the adoptive transfer of EBV‐specific T cells strongly suggest that EBV‐specific cellular immunity is important for effective viral control [3, 4]. Previous studies have shown that healthy adults with persistent EBV infection maintain strong EBV‐specific cytotoxic T lymphocyte (CTL) responses directed against HLA class I–restricted viral epitopes for years after the initial infection [2, 5]; these responses often target epitopes derived from latently expressed viral proteins or, on occasion, lytic antigen–derived epitopes [6–8]. In contrast, during the acute phase of EBV infection, CTL responses have been found to frequently target lytic gene products, and responses to latently expressed viral proteins have often been reported to be weak or absent [9–12]. In comparison to that on CTL responses, less information is available on virus‐specific CD4 T cell responses [5, 13]. Nevertheless, a number of EBV‐encoded proteins that induce CD4 T cell responses have been identified, and, to date, ∼30 HLA class II–restricted epitopes have been described. However, data on their restricting HLA alleles are often incomplete, and their relative contribution to the total EBV‐specific immunity at different stages of infection has not been determined [14, 15].
EBV‐specific T cell responses have been reported to differ considerably in breadth, magnitude, and immunodominance when assessed during either the acute or the persistent phase of infection [9–11, 13, 16–18]. However, published studies have often assessed only a limited number of epitopes at a time and have not investigated T cell responses to HLA class I– and HLA class II–restricted epitopes simultaneously. Therefore, to better understand the evolution of T cell responses that occurs during the acute and persistent stages of EBV infection and to extend the results of previous studies to a broader representation of HLA alleles, the present study assessed EBV‐specific T cell responses in 40 individuals at different stages of infection by use of a panel of 113 previously described EBV‐derived CD8 T cell– and CD4 T cell–specific epitopes. Longitudinal as well as cross‐sectional analyses show significant shifts in the immunodominance of both the CD8 and the CD4 T cell responses over time and suggest that responses during the persistent stage of infection are comparable in individuals who acquired EBV symptomatically or asymptomatically.
Subjects, Materials, and Methods
Study subjects. A total of 32 healthy subjects with persistent EBV infection and 8 subjects with acute, symptomatic EBV infection (recruited within 14 days after the onset of symptoms) were enrolled from hospitals in the Boston area. For 3 of the 8 subjects with acute infection, hospitalization was required at the time of diagnosis. Of the 32 subjects with persistent infection, 5 reported a history of symptomatic acute infection 3–12 years before enrollment in the study. Persistent EBV infection was confirmed serologically, and the clinical diagnosis of AIM was confirmed by the Quidel Quickview+ infectious mononucleosis test. Intermediate‐ to high‐resolution HLA class I typing was performed as described elsewhere [19]. HLA diversity in the 32 subjects with persistent EBV infection (39 different HLA class I alleles were present) was comparable to that in the 8 subjects with AIM (28 different HLA class I alleles were present, with 24 alleles common to both groups).
EBV‐derived T cell–specific epitopes and enzyme‐linked immunospot (ELISPOT) assays. A set of 113 EBV‐derived T cell–specific epitopes (80 HLA class I–restricted and 33 HLA class II–restricted epitopes) was used to screen peripheral blood mononuclear cells (PBMCs) by ELISPOT assay. Epitope sequences and their reported restricting HLA alleles are included in tables 1 and 2. For ELISPOT assays, individual epitopes were added at a final concentration of 10 μg/mL, and no epitope was added to 5 negative control wells. After overnight incubation at 37°C in 5% CO2, plates were developed as described elsewhere [39], and spots were counted using the AID EliSpot Reader System (Autoimmun Diagnostika). The threshold for positive responses was a minimum of 5 sfc/well or a response that exceeded the mean number of spot‐forming cells per well in negative wells plus 3 SDs, whichever gave the higher value. For 2 subjects, an insufficient number of PBMCs was available, and those samples were therefore tested against the HLA class I–restricted epitopes only.
Frequency of recognition of HLA class I–restricted Epstein‐Barr virus (EBV)–derived epitopes.
Frequency of recognition of HLA class II–restricted Epstein‐Barr virus (EBV)–derived CD4 T cell–specific epitopes.
Statistical analysis. Statistical analyses included the Student’s t test (2‐tailed) for comparison of breadth and magnitude and Pearson’s correlation (2‐tailed) for correlations.
Results
Wide recognition of EBV‐derived CD8 T cell– and CD4 T cell–specific epitopes in subjects with AIM and persistent EBV infection. Although EBV‐specific immunity has been assessed in an extensive number of studies, these analyses have often been restricted to a few selected epitopes and HLA alleles, which makes it unclear whether the conclusions drawn from these studies are fully applicable to a genetically heterogeneous population [2, 5]. Additionally, only limited information exists regarding CD4 T cell responses at either stage of infection, their evolution over time, and their relations to EBV‐specific CD8 cells. Thus, the present study tested a total of 32 subjects with persistent EBV infection and 8 subjects with AIM against a panel of 113 EBV‐derived epitopes, which included 80 previously described HLA class I–restricted epitopes derived from both lytic and latent EBV antigens and 33 previously described HLA class II–restricted epitopes derived predominantly from latent EBV antigens (tables 1 and 2). Because freshly isolated cells were used for this study and, in general, the HLA types were not known at the time of the assay, all samples were tested with the panel of 113 epitopes, irrespective of the subjects’ individual HLA types. Although some epitope‐specific responses were detected in subjects not expressing the restricting HLA allele, the data analysis included only positive responses found in subjects expressing the originally described restricting HLA allele.
When all 40 samples were tested against the panel of epitopes, 54 (70%) of 77 HLA class I–restricted epitopes were recognized by samples from at least 1 subject expressing the appropriate restricting HLA class I allele (table 1). Of the HLA class II–restricted epitopes, 31 (94%) of 33 were recognized at least once. The detailed description of HLA class I restriction for the tested CD8 T cell–specific epitopes allowed for a cohort‐wide immunodominance analysis (table 1). These data revealed a wide range of frequencies at which each epitope was recognized in subjects expressing the restricting HLA class I allele: for instance, 100% of the subjects expressing HLA‐B8 recognized 2 EBNA‐derived epitopes (EBNA‐3a‐B8‐1 and EBNA‐3a‐B8‐3), and 87% recognized a third epitope (BZLF1‐B8‐2). However, none of the responses restricted by the most common HLA class I allele in this cohort, HLA‐A2, reached >67% frequency of recognition, and all but 3 HLA‐A2–restricted epitopes were recognized by <20% of the subjects expressing HLA‐A2. However, it is possible that some responses were missed, because they may have been under the detection limit of the ELISPOT assay used in this study, and, thus, the epitope’s true cohort‐wide immunodominance may have been underestimated. More importantly, however, the data clearly show that a number of lytic antigen– and latent antigen–derived epitopes can be frequently recognized in subjects with AIM and subjects with persistent EBV infection, indicating that responses to lytic antigen–derived epitopes can be maintained during persistent EBV infection and that responses to latent antigen–derived epitopes can be detected during AIM.
Similar breadth but not magnitude of CD8 and CD4 T cell responses during AIM and persistent EBV infection. Previous studies have described sometimes dramatic shifts in epitope‐specific CD8 T cell responses, from those toward lytic antigens during AIM to those toward latent antigens during persistent EBV infection [2, 5]. To confirm these findings in a genetically diverse cohort and on the basis of multiple epitopes, we assessed the breadth and magnitude of lytic antigen– and latent antigen–specific CD8 T cell responses in the 2 groups. The analyses showed a similar number of targeted HLA class I–restricted epitopes (breadth of response) in subjects with AIM and subjects with persistent EBV infection (figure 1A). There was also no significant difference in the breadth of responses to EBV lytic antigen–derived or latent antigen–derived epitopes in subjects with persistent EBV infection and subjects with AIM (figure 1B), confirming the previous observation that responses to latent antigens can be detected during early EBV infection and that responses to lytic antigens can be maintained during persistent EBV infection. The data obtained with the HLA class II–restricted epitopes revealed a similar breadth in the CD4 T cell responses in subjects with persistent EBV infection and subjects with AIM (figure 1A) and indicate that latent antigen–specific CD4 T cell responses are induced during early infection, because subjects with AIM were frequently found to mount such responses.
![Responses to Epstein‐Barr virus (EBV) lytic antigen–derived epitopes in subjects with acute infectious mononucleosis (AIM) and persistent EBV infection. A, Breadth of the ex vivo T cell responses. Responses to CD8 T cell– and CD4 T cell–specific epitopes were compared in subjects with AIM and subjects with persistent EBV infection. B, CD8 T cell responses to HLA class I–restricted epitopes. Responses were further stratified into those against lytic antigens and those against latent antigens and were compared between subjects with AIM and subjects with persistent EBV infection. C and D, Magnitude of response (spot‐forming cells [sfc]/1×106 peripheral blood mononuclear cells [PBMCs]). Medians are indicated by horizontal lines.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/192/9/10.1086/491741/2/m_192-9-1513-fig001.gif?Expires=1750157966&Signature=QKWB1sOiWyyHpvb2lBcUyvni42XFZhgGD0id-4ZoEVtfl9-FGzrQQyJYVtwC4RWL8ZEEhbsJwFtR9eFB15QbuQRxuXJ3RLO2RHQ8LkSJWbCM4l0-eDR8V1T~aIR-RsOlBrnxXKtWyDj50wmJBrU8ar7HTS8Ar6fOWtVzuZln8M8-JpkAFSVxgITpAXImZPypRlx5KM6kmedzNT4B4C9nMruEd6m-VB5BR~zjvW2XrCr60mSHE23YK3g6MU0WzGRI9a9sGUOcz~t~Hh98ueMQia0HJ1zw91nMdh3k8ck7c7MxZSWE-NatWfO~R5LQi7W8wB9FQ-~PF3pvr9pyM~cvkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Responses to Epstein‐Barr virus (EBV) lytic antigen–derived epitopes in subjects with acute infectious mononucleosis (AIM) and persistent EBV infection. A, Breadth of the ex vivo T cell responses. Responses to CD8 T cell– and CD4 T cell–specific epitopes were compared in subjects with AIM and subjects with persistent EBV infection. B, CD8 T cell responses to HLA class I–restricted epitopes. Responses were further stratified into those against lytic antigens and those against latent antigens and were compared between subjects with AIM and subjects with persistent EBV infection. C and D, Magnitude of response (spot‐forming cells [sfc]/1×106 peripheral blood mononuclear cells [PBMCs]). Medians are indicated by horizontal lines.
Although no significant differences in the breadth of responses were observed, the magnitude of CD8 T cell responses differed significantly between subjects with AIM and subjects with persistent EBV infection (P=.04) (figure 1C). Stratification of these responses into lytic antigen–derived and latent antigen–derived HLA class I–restricted epitopes revealed that the increased overall magnitude in subjects with AIM was due to significantly stronger responses to lytic antigen–derived epitopes (P=.006) but not to latent antigen–derived epitopes (figure 1D). In contrast, the total magnitude of responses to CD4 T cell targets did not differ between subjects with persistent EBV infection and subjects with AIM (figure 1C), likely because primarily latent antigen–derived epitopes were tested (table 2). Notably, the number of epitopes recognized and the total magnitude of the response in a given subject were directly related (P<.0001 for both CD8 and CD4 T cell responses; data not shown), indicating that broader responses did not diminish the average strength of these responses. However, when the breadth or the magnitude of the CD8 and CD4 T cell responses were compared, no significant association emerged (data not shown). Together, these data indicate that broad CD8 and CD4 T cell responses can be detected during early EBV infection and show that, although strong responses to latent and lytic antigens can be observed in both stages of infection, the responses to lytic antigens are significantly stronger during AIM than during persistent EBV infection.
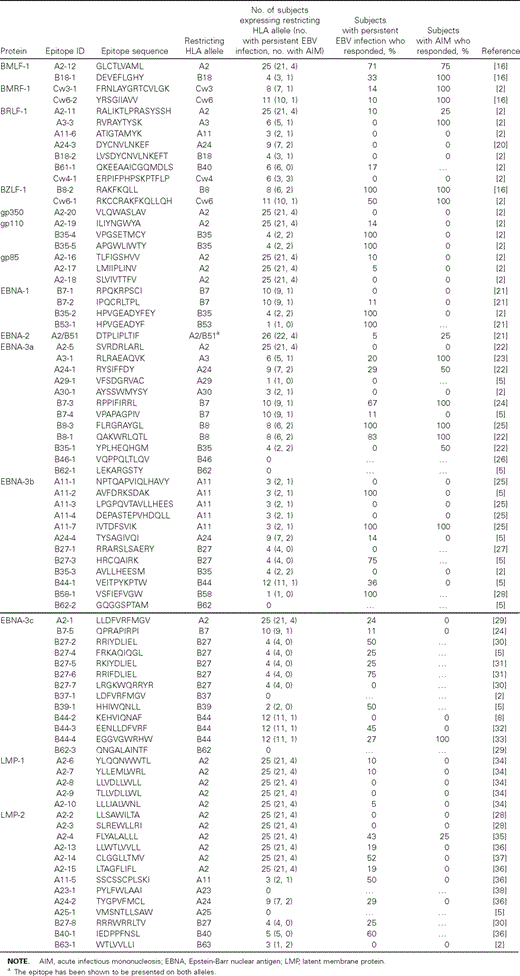
Dominant responses during AIM and persistent EBV infection. The above data were further analyzed to determine whether particular EBV‐derived epitopes were predominantly recognized during AIM or persistent EBV infection. Indeed, the individual immunodominant responses of subjects with AIM were more frequently directed against lytic antigen–derived epitopes, and only 2 subjects with AIM had an immunodominant response to a latent antigen–derived epitope (figure 2A). In contrast, latent antigens were more frequently targeted by the immunodominant response in subjects with persistent EBV infection than in subjects with AIM. These data are in accordance with previously published data, which were based on fewer selected epitopes and subjects with a narrower distribution of HLA alleles [2, 5]. For HLA class II–restricted epitopes, most subjects with persistent EBV infection mounted their dominant CD4 T cell response to any of the EBNA‐1–derived epitopes. In contrast, the majority of subjects with AIM mounted their immunodominant response to EBNA‐3c–derived epitopes (figure 2B).

Shift in the target of dominant responses during acute infectious mononucleosis (AIM) and persistent Epstein‐Barr virus (EBV) infection. The protein encoding the immunodominantly targeted epitope was recorded for each subject and compared between subjects with AIM and subjects with persistent EBV infection. A, Frequency of subjects in which the immunodominant HLA class I–restricted epitope was derived from each protein. Shown are results for subjects with AIM and subjects with persistent EBV infection. B, Frequency of subjects in which the immunodominant HLA class II–restricted epitope was derived from each protein. Shown are results for subjects with AIM and subjects with persistent EBV infection. EBNA, Epstein‐Barr nuclear antigen; LMP, latent membrane protein.
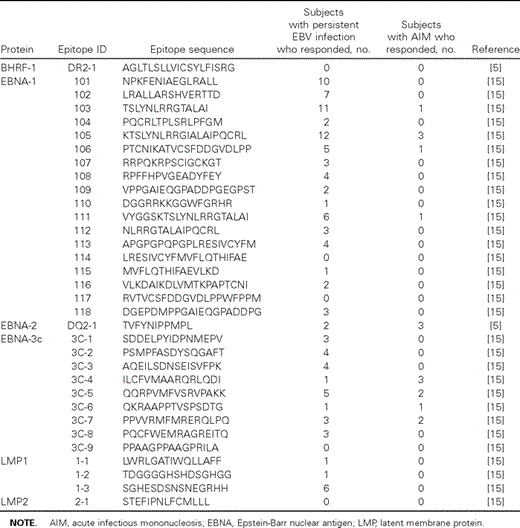
These data are consistent with a gradual shift from targeting lytic antigen–derived epitopes during AIM to targeting latent antigen–derived epitopes during persistent EBV infection and a shift in the CD4 T cell response from EBNA‐3c to EBNA‐1 during the duration of the infection. These shifts was further supported by the data from 2 subjects tested during both AIM and persistent EBV infection: subject K48 responded to 5 CD8 T cell–specific epitopes (1 lytic antigen–derived and 4 latent antigen–derived epitopes) during AIM, with the immunodominant response directed against a BZLF‐1–derived epitope (figure 3A). Five months later, 7 responses, mostly directed against latent antigens, were detected. In addition, 5 new CD4 T cell responses, all directed against EBNA‐1–derived epitopes, became detectable. Similarly, subject K50 showed 2 CD8 T cell responses during AIM, and, 7 months later, a total of 5 responses, mostly to latent antigen–derived epitopes, were detected (figure 3B), whereas no CD4 T cell responses were detected at either time point. Similarly, 2 subjects with persistent EBV infection, who were initially tested 6 and 15 months after AIM, respectively, were tested again 24 months later. In both subjects, 1–3 new latent antigen–specific CD8 T cell responses were detected at the later time point (data not shown). In addition, both subjects developed strong CD4 T cell responses over time, with 1 subject showing 5 new CD4 T cell responses—4 of which were directed against EBNA‐1—and 1 subject showing 3 new CD4 T cell responses—2 of which were directed against EBNA‐1–derived epitopes (data not shown). Together, the longitudinal analyses confirmed the trends observed in the cross‐sectional cohort, with lytic antigen–specific CD8 T cell responses of significant magnitude detectable in subjects with AIM and increasing numbers of latent antigen–specific CD8 T cell responses emerging over time and with a strong focus on EBNA‐1–specific CD4 T cell responses in subjects with persistent EBV infection.
Epstein‐Barr virus–specific T cell responses during acute infectious mononucleosis (AIM) and 5 or 7 months later. A, Responses to CD8 T cell– and CD4 T cell–specific epitopes during AIM (black bars) and 5 months later (white bars) for subject K48. B, Responses to CD8 T cell– and CD4 T cell–specific epitopes during AIM (black bars) and 7 months later (white bars) for subject K50. Results are expressed as spot‐forming cells (sfc) per 1×106 peripheral blood mononuclear cells (PBMCs). Epitope sequences are shown in table 1. EBNA, Epstein‐Barr nuclear antigen; LMP, latent membrane protein.
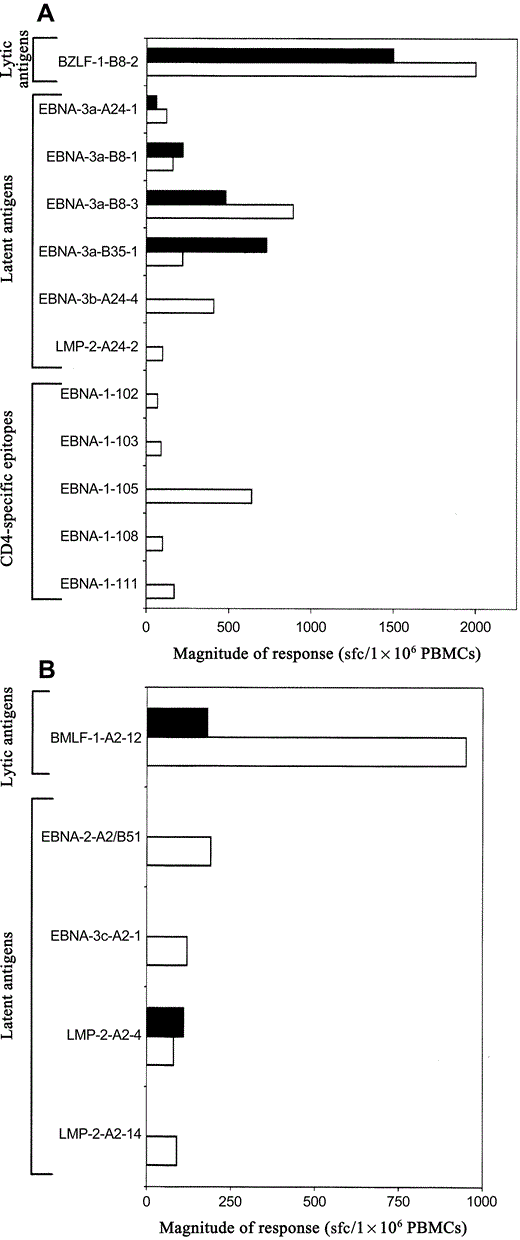
Largely overlapping response patterns in HLA‐matched subjects, regardless of history of AIM. To investigate whether response patterns during persistent EBV infection were affected by clinical manifestations at the time of EBV infection, partly or fully HLA‐matched subjects with a discordant history of AIM were identified and tested with the panel of epitopes. This group included 2 siblings (K67 and K68) who had the same 3 HLA class I alleles, only 1 of whom (K67) had a history of symptomatic EBV infection (12 years before enrollment). Samples from both siblings had remarkably similar responses to the same 5 HLA class I–restricted epitopes after testing all 77 HLA class I–restricted epitopes, and only 1 additional response was observed in sibling K67, who had an unmatched HLA class I allele (HLA‐A2) targeting a subdominant epitope among the 23 potential HLA‐A2–restricted epitopes (figure 4A). In addition, 2 of these epitopes have not been described as being restricted by the alleles expressed by these subjects, so they likely are promiscuously binding epitopes. Such promiscuous antigen presentation has been described for at least 2 EBV‐encoded CD8 T cell‐specific epitopes, shared by HLA‐A2 and HLA‐B51 and by HLA‐A23 and HLA‐A24, respectively [40, 41]. Of note, by testing all CD8 T cell–specific epitopes against samples from all subjects enrolled in this study, responses in subjects without the originally described restricting allele were observed in >72% of all tested subjects. However, the in vivo relevance of these responses is unclear, and the data analysis therefore focused on responses in subjects expressing the correct HLA restricting alleles.
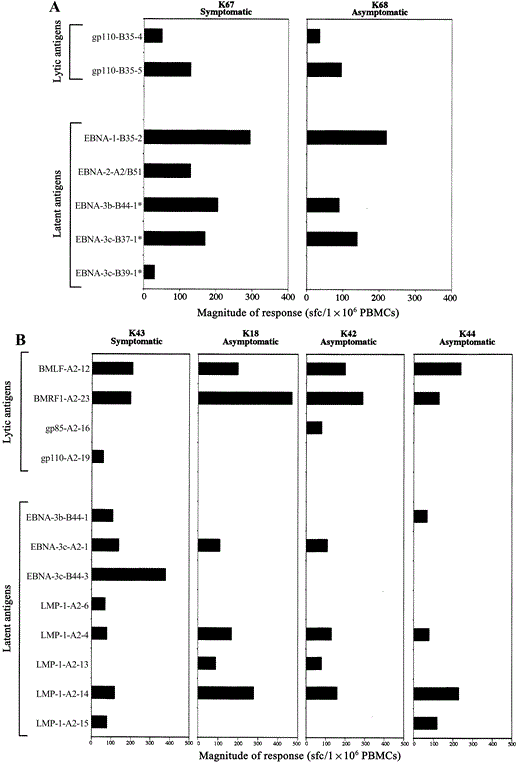
Epstein‐Barr virus (EBV)–specific immune responses in genetically related subjects. A, CD8 T cell responses in 2 siblings, K67 and K68. The siblings have A3, B35, and C4 alleles in common, and only K67 reported a history of acute infectious mononucleosis (AIM). Epitopes marked with an asterisk are responses to epitopes not previously described to be presented by the subjects’ HLA allele(s). B, Responses to CD8 T cell–specific epitopes in HLA class I–identical siblings K18, K42, K43, and K44. The siblings have A2, B13, B44, Cw5, and Cw66 alleles in common. K43 reported a history of AIM and required hospitalization; the other siblings acquired EBV asymptomatically. Results are expressed as spot‐forming cells (sfc) per 1×106 peripheral blood mononuclear cells (PBMCs). Epitope sequences are shown in table 1. EBNA, Epstein‐Barr nuclear antigen; LMP, latent membrane protein.
In addition to the above 2 siblings, 4 siblings (K18, K42, K43, and K44) with identical HLA class I alleles were identified, and only 1 sibling (K43) had a history of severe symptomatic EBV infection (11 years before enrollment). The siblings recognized between 6 and 10 CD8 T cell–specific epitopes, and all siblings responded to the same 4 epitopes (figure 4B). An additional 4 responses were shared between 2 or 3 siblings. Overall, there was no difference in the breadth or magnitude of responses in the sibling with a history of AIM, compared with that in the siblings who acquired EBV asymptomatically. This indicates that symptomatic EBV infection likely does not lead to a distinct response pattern during persistent EBV infection, compared with that seen in subjects infected asymptomatically.
Discussion
A number of studies have investigated the specificity, magnitude, cytolytic activity, and phenotypic characteristics of EBV‐specific T cells [5, 6, 9–11, 17]. However, these studies have often focused on a limited number of viral epitopes and HLA alleles and did not assess the association between virus‐specific CD8 and CD4 T cell responses. The present study was designed to overcome these limitations by using a large set of EBV‐derived HLA class I– and HLA class II–restricted epitopes and testing a genetically diverse cohort consisting of subjects with AIM and subjects with persistent EBV infection. Overall, no statistically significant differences were found in the total breadth of EBV‐specific CD8 and CD4 T cell responses between subjects with AIM and subjects with persistent EBV infection (figures 1A and 1B). This finding contrasts with those from other studies that found few or absent responses to latent antigen–derived epitopes during AIM and decreased responses to lytic antigen–derived epitopes during persistent EBV infection [9, 10, 17], and this difference in findings may be because the present study was based on a larger number of tested epitopes and a more genetically heterogeneous population. The detection of responses to latent antigen–derived epitopes during AIM and responses to lytic antigen–derived epitopes during persistent EBV infection suggests that sporadic, asymptomatic EBV reactivation may support long‐term maintenance of responses to lytic antigen–derived epitopes during persistent EBV infection [42, 43] and that the presence of latently infected cells with a type III gene expression profile (EBNA‐3 and latent membrane proteins 1 and 2) during AIM may induce early responses to latent antigen–derived epitopes [44, 45]. In addition, sporadic exposure to infectious virus may further contribute to the maintenance of responses to lytic antigen–derived epitopes during persistent EBV infection. Together, the data thus demonstrate that the induction of strong responses to lytic antigen–derived epitopes during AIM does not preclude the generation of responses to latent antigen–derived epitopes, although the latter may not reach immunodominance until later in the course of infection. Importantly, responses to lytic antigen–derived epitopes were found to dominate during AIM, even though some studies have suggested that the T cells that mount these responses may be subject to rapid cell death in vitro and thus may be underestimated in the assays used in the present study [9–11, 46, 47]. However, an analysis of ELISPOT assays in which shorter incubation times (<4 h) were used demonstrated that no additional responses were detected, compared with the number detected in overnight incubations, suggesting that, even if the T cells were dying in vitro, all responses were still detected with the traditional ELISPOT approach (data not shown).
The paucity of described lytic antigen–derived, HLA class II–restricted T cell–specific epitopes prevented a comparison of CD4 T cell responses to lytic antigen–derived epitopes and latent antigen–derived epitopes during AIM and persistent EBV infection. Despite this limitation, the data revealed a striking shift in CD4 T cell responses, from those directed toward EBNA‐3c during AIM to those directed toward EBNA‐1 during persistent EBV infection. This late induction of EBNA‐1–specific CD4 T cells is in accordance with the findings of other studies that have shown that EBNA‐1–specific CD4 T cells were present during persistent EBV infection [14, 15] and is supported by the predominate type I gene expression profile (EBNA‐1) during persistent EBV infection [2]. The late emergence of EBNA‐1–specific CD4 T cell responses is also in accordance with the late development of an EBNA‐1–specific antibody response relative to other specific responses [48]. Whether, and how, the delayed induction of CD4 T cellular and humoral responses to EBNA‐1 are linked is unclear, but specific antigen availability during the later, but not the early, stages of infection could potentially explain this observation.
Although they provide an extensive immunodominance analysis of EBV‐derived epitope–specific responses, the present analyses failed to reveal a direct correlation between either the breadth or the magnitude of EBV‐specific CD8 and CD4 T cell responses. However, it is important to note that, despite testing a relatively large number of epitopes, the present analyses are by no means comprehensive. Although CD8 T cell responses to other viruses, such as HIV and hepatitis C virus, can be determined by use of panels of epitopes that span the entire viral genome [49, 50], for EBV and other herpesviruses, their large genome prohibits such an approach. Expanding studies beyond the 10 viral proteins for which CD8 T cell– and CD4 T cell–specific epitopes have been described would be very valuable and may reveal novel responses and/or possibly an association between CD8 and CD4 T cell activity. Similarly, identifying more subjects with AIM and testing them against such extended panels of epitopes will be needed to confirm the observations made in the present study and may reveal a closer interdependence of virus‐specific CD8 and CD4 T cell activity.
Finally, events surrounding AIM, especially the manifestation of clinical symptoms, have been proposed to influence long‐term EBV immunity and the risk of developing EBV‐associated neoplasms [51, 52]. Longitudinal analyses of 2 subjects during and after AIM showed that they had a response pattern that was not different from that in subjects with persistent EBV infection who did not have a history of AIM (data not shown). In addition, data from largely HLA‐matched or HLA‐identical siblings with a discordant history of AIM showed no differences in the breadth or magnitude of the responses during persistent EBV infection. Although we cannot rule out the possibility that some functional differences in the respective CD8 T cell populations between the siblings may have been retained during persistent EBV infection, it appears, at least in these healthy adults, that EBV‐specific responses were not significantly impacted by having a symptomatic or an asymptomatic initial infection. These findings and the identification of dominant responses to lytic antigen–derived epitopes and latent antigen–derived epitopes during AIM and persistent EBV infection may have significant implications for EBV vaccine development and immune‐based treatment strategies for EBV‐associated disorders.
Acknowledgments
We thank Alan B. Rickinson (CRC Institute for Cancer Studies, University of Birmingham, Birmingham, UK), for helpful comments and unpublished epitope sequences; Caitlyn Linde and Hannah Hewitt, for review of the manuscript; and all subjects, especially the members of the 2 families included in this study, for their blood donations.
References
Potential conflicts of interest: none reported.
Financial support: National Institutes of Health/National Institute of Dental and Craniofacial Research program (grant PO1 DE01438‐01).




