-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa J. Moore, Alison C. Pridmore, Steven K. Dower, Robert C. Read, Penicillin Enhances the Toll-Like Receptor 2–Mediated Proinflammatory Activity of Streptococcus pneumoniae, The Journal of Infectious Diseases, Volume 188, Issue 7, 1 October 2003, Pages 1040–1048, https://doi.org/10.1086/378238
Close - Share Icon Share
Abstract
The Streptococcus pneumoniae cell-wall components peptidoglycan and lipoteichoic acid activate Toll-like receptor 2 (TLR2), which transduces an inflammatory response. After exposure to penicillin, type 2 S. pneumoniae strain D39, but not the isogenic autolysin-deficient mutant AL2, induced significantly enhanced interleukin-8 promoter activity in TLR2-transfected HeLa cells. Lag-phase D39 exhibited enhanced TLR2 activation after exposure to penicillin at levels below the minimum inhibitory concentration (MIC); in contrast, early log-phase S. pneumoniae were most active when exposed to the MIC. This enhancement was not ablated by heat treatment but was attenuated by autolysin inhibitors. The antimicrobial activity of moxifloxacin and erythromycin was not associated with TLR2 activation by S. pneumoniae. These data show that penicillin treatment of S. pneumoniae releases proinflammatory cell-wall components that activate TLR2 and that this activity is dependent on autolysin, the growth phase of the organism, and the antibiotic concentration
The treatment of pneumococcal infections—such as meningitis, pneumonia, and bacteremia—with β-lactam antibiotics can result in the paradoxical enhancement of inflammation as a result of the release of proinflammatory cell-wall products, such as lipoteichoic acid (LTA) and peptidoglycan (PGN) [1–4]. β-Lactam antibiotics bind to penicillin-binding proteins and thereby inhibit the formation of PGN cross-links. The resulting instability of the cell-wall activates autolytic enzymes that liberate active cell-wall components [5–7]. The major autolytic enzyme of Streptococcus pneumoniae is N-acetylmuramoyl-l-alanine amidase (LytA), which is important for daughter cell separation, stationary-phase autolysis, and penicillin-induced autolysis [5, 8–11]
Toll-like receptors (TLRs) are pattern-recognition receptors that are present on the surface of macrophages and monocytes and are important in the innate immune response to bacterial infection [12–14]. Ten TLRs have been identified, and each is activated by a finite number of microbial molecular patterns, which transduces a cascade of intracellular reactions that ultimately result in the translocation of NF-κB to the nucleus and the induction of transcription of a number of genes, including proinflammatory cytokine genes [15]. TLR4, together with 2 accessory molecules, CD14 and MD2, confers responsiveness to the lipopolysaccharide (LPS) present on gram-negative cell envelopes [12, 13, 16–18]. TLR2, along with its coreceptor CD14, is involved in the response to a number of microbial components, including the LTA and PGN present within gram-positive cell walls [19–22]. However, the signaling characteristics of TLR2 can be modified by cooperation with other TLRs, such as TLR1 and TLR6 [14]
We postulated that the proinflammatory effect of the treatment of pneumococcal infections with β-lactam antibiotics might be at least partly a consequence of the release of cell-wall products that are active via TLR2. We measured TLR2 activation by pneumococci using HeLa cells, which do not normally express TLR2 or CD14 [14], by transfecting them with plasmids that express cDNA encoding TLR2 and CD14. Proinflammatory transduction within cells challenged by bacteria was quantified using an interleukin (IL)–8 promoter reporter construct that correlates directly with the transcriptional activation by NF-κB [17]. This sensitive experimental system enabled a detailed investigation of the proinflammatory effect of exposure of S. pneumoniae to penicillin. We examined the importance of antibiotic concentration, bacterial growth phase, and the requirement for autolysin activity in eliciting this effect [8, 9, 23–25]
Materials and Methods
BacteriaD39, a type 2 pneumococcus, and its isogenic lytA-negative mutant (AL2) [26–28] (generously donated by Tim Mitchell, University of Glasgow, UK), were used in these experiments. Midlog stocks of the bacteria were prepared by culture in brain-heart infusion (BHI) broth supplemented with 10% heat-inactivated fetal calf serum (HIFCS) in 5% CO2 at 37°C. To select for lytA mutants, AL2 cells were grown in BHI medium supplemented with 0.5 μg/mL erythromycin. Bacteria were stored at −70°C in broth until required
AntibioticsBenzyl penicillin (Britannia Pharmaceuticals), moxifloxacin powder (a gift from Bayer Pharmaceuticals), and erythromycin (Sigma-Aldrich) were used as representatives of the major antibiotic classes. Each antibiotic was dissolved in an appropriate solvent, filter sterilized using a 0.22-μm filter unit (Millipore), and stored at −20°C until required. The MIC of each antimicrobial agent for each organism was determined using the macrobroth dilution method in BHI plus 10% HIFCS, according to NCCLS guidelines [29]
Exposure of pneumococci to antibioticsDuring broth culture, S. pneumoniae was exposed to penicillin by the addition of the antibiotic to the growth medium at either the MIC or one-eighth of the MIC for 30 min or 3 or 6 h. The effect of the addition of the antibiotic during the lag, early log, and stationary-phase growth stages was compared. Thus, penicillin exposure began either shortly after inoculation into broth (lag phase), 3 h after inoculation, when the OD at 620 nm was not >0.099 (early log), or 9 h after inoculation, at OD 0.999 (stationary phase). Separate S. pneumoniae cultures were used for each time treatment and growth phase, but all experiments were run in parallel. Bacterial counts were then checked spectrophotometrically and by viable count analysis [30]. Penicillin activity was arrested with Bacillus cereus penicillinase (Sigma-Aldrich), at a final concentration of 10 μg/mL
Controls included untreated bacteria, bacteria plus penicillinase, broth that contained antibiotic only, and broth only. Half of each culture volume was used as live cells, and the remaining culture volume was filtered through a 0.45-μm Millex-GV filter unit (Millipore) prior to its addition to HeLa cells
Choline and ethanolamine culture to inhibit LytA activity in the parent strainThe activity of LytA requires the presence of choline; however, culture in excess choline (e.g., 2%) inhibits the enzyme, and culture in the choline analogue ethanolamine (2%) prevents conversion of the enzyme to an active form [5, 8–11, 27]. To inhibit the activity of LytA in the D39 parent, bacteria were cultured in BHI plus 10% HIFCS that contained excess choline at 2%. Bacteria were grown to the midlog phase, washed in PBS, centrifuged at 1200 g then resuspended in fresh broth plus 2% choline or broth plus 2% ethanolamine, to ensure the inactivity of LytA. Normal viable growth and MICs were also checked and compared with untreated cells (data not shown)
Heat treatment of penicillin-exposed pneumococcal cells and filtratesTo test whether the inflammatory potential of antibiotic-treated cells or filtrates were heat stable, 50 μL of filtered and nonfiltrated antibiotic treated D39 were heat shocked at 100°C for 5 min. Heat-killed, untreated cells and filtrates of D39 were included as additional controls
Plasmid purificationExpression and reporter vectors were purified from recombinant Escherichia coli using the EndoFree Maxi purification kit (UK-Qiagen), according to the manufacturer’s instructions. The expression vectors—pSU1 (TLR2), pNM1 (MD2), pCDM8-CD14 (CD14) (a gift of B. Seed, Molecular Biology Department, Harvard Medical School, Boston), and pcDNA3 (an empty vector control)—have been described elsewhere [14, 17]. The reporter vectors were pRL-Tk (Promega), which contains the Renilla luciferase gene tagged to a constitutive reporter, and pIL8-pLuc, which contains sequences of the human IL8 gene (GeneBANK M28130), with the bases at the −174 to +45 region of the IL-8 promoter cloned into pGL3-Basic vector (Promega) to drive the expression of firefly luciferase. HeLa cells do not express CD14/TLR2 or MD2 but do inherently express TLR4. TLR4 does not function unless cells are cotransfected with MD2 [20]
HeLa cell transfection, stimulation, and dual luciferase reporter assayHeLa cells (European collection of animal cell cultures 85060701) were seeded at 1.5×105 cells/mL in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL) supplemented with 10% HIFCS in a 96-well cell culture microtiter tray (Corning Life Sciences) and incubated overnight in 5% CO2 at 37°C. After overnight incubation, the cells were inspected for adherence to the microtiter wells (80%–90% confluence). All HeLa cells were transfected with pCDM8-CD14 vector (CD14) and the reporters pRL-Tk and pIL-8-pLuc. The expression vectors pcDNA3, pSU1, and pNM1 were transfected separately, as described elsewhere [17]. Transient transfection was done using SuperFECT reagent (UK-Qiagen). After 2.5 h incubation in 5% CO2 at 37°C, the transfection/SuperFECT mixture was replaced with 100 μL/well fresh DMEM and 10% HIFCS and incubated overnight in 5% CO2 at 37°C
Prior to their addition to HeLa cells, antibiotic-treated or -untreated bacteria were declumped by vortexing with 0.3-cm glass beads (Fischer Scientific) for 5 s. Viability counts were checked, and 10 μL of each bacterial sample was instilled into 100 μL of DMEM-treated HeLa cells in 96-well plates at 37°C in 5% CO2 for 6 h. DMEM and broth preparations were included as negative controls. PGN (Staphylococcus aureus; Sigma-Aldrich) at 1 μg/mL and ultrapure LPS at 1 μg/mL [31] were included as TLR2- and MD2-positive controls, respectively. After incubation, the wells were washed twice in PBS and lysed with 1 volume of passive lysis buffer; levels of Firefly and Renilla luciferase were measured using a dual luciferase reporter assay system (Promega), according to the manufacturer’s instructions. The level of IL-8 promoter activity in each lysate was measured as the luminescence signal (in relative light units) before the addition of luciferase reagent (Promega), followed by stop and glo buffer/substrate (Promega). The illuminance signal was read and recorded using an ML3000 program. The number of relative light units is directly proportional to the level of IL-8 promoter activity in normalized data. Data were further corrected by normalization with empty vector controls
Statistical analysisAll values were checked for normality using the Anderson-Darling normality test. For the comparison of treated versus untreated cells and within-treatment comparisons, normally distributed data were analyzed by t test analysis with a Bonferrroni correction. For nonnormally distributed data, the Mann-Whitney U test was used for independent data sampling. To test the effect of bacterial dose and of growth stage (lag, early log, and stationary) on live/filtrate activation of the IL-8 promoter, the 1-way analysis of variance (ANOVA) multiple-group analysis was used. Significance was set at P⩽.005, with correction of the level where appropriate
Results
Response of TLR2-transfected cells to live S. pneumoniae. To measure signaling through TLR4/MD2 and TLR2, live D39 at final concentrations of 107, 5×106, 106, 5×105, 105, 5×104, and 104 cfu/mL were inoculated onto MD2- and TLR2-transfected HeLa cells, respectively. At each concentration of live bacteria, there was significant activation of the IL-8 promoter in TLR2-transfected HeLa cells (figure 1) (P<.005, 1-way ANOVA) after 6 h incubation. Among MD2-transfected cells, there was no significant activation of the IL-8 promoter at the same doses of bacteria (figure 1). TLR2 activation was dose responsive and was maximal when cells were exposed to 106 cfu/mL of S. pneumoniae but relatively diminished at the higher dose of 107 cfu/mL
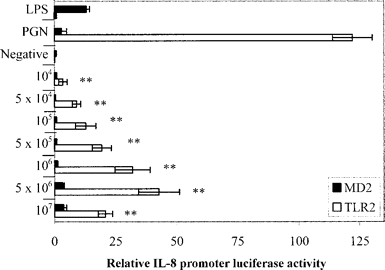
Response of Toll-like receptor 2 (TLR2)– and MD2-transfected HeLa cells to live doses of early log D39, as measured by interleukin (IL)–8 promoter activity. All values displayed for TLR2 activity were first normalized with the values for the constitutive Renilla control and then with vector control. Data are expressed as mean±SE for 2 experiments in quadruplicate. White bars signaling via TLR2 at different cfu/mL of live bacteria. Black bars signaling via MD2. Lipopolysaccharide (LPS) was used as an MD2-positive control, and peptidoglycan (PGN) from Staphylococcus aureus (1 μg/mL) was used as a TLR2-positive control. LPS (1 μg/mL) was used in an ultrapure preparation from Escherichia coli K235, and the negative control was Dulbecco’s modified Eagle’s medium plus brain-heart infusion and 10% heat-inactivated fetal calf serum. **P<.005, signaling vs. negative control (1-way analysis of variance)
Effect of growth phase of S. pneumoniae on TLR2 inductionThe magnitude of TLR2 induction by live, untreated S. pneumoniae was found to be dependent on the growth phase of the organism at the point of inoculation of HeLa cells (figure 2). Live bacteria exhibited significantly different TLR2 signaling profiles at each growth phase, with the early log phase eliciting the greatest signal (P<.005; 1-way ANOVA). The TLR2-mediated IL-8 promoter activity of filtrates was also different at each growth phase, with the stationary phase eliciting the greatest signal (P<.05; 1-way ANOVA)
![Effect of pneumocccal (D39) growth stage on the induction of Toll-like receptor 2–mediated interleukin (IL)–8 promoter activity. Transfected cells were inoculated with 5×106 live bacteria or with filtrates of these bacteria. Data are expressed as mean±SE for 2 experiments in triplicate. **P<.005, early log–phase bacteria vs. lag- and stationary-phase live bacteria (1-way analysis of variance [ANOVA]); *P<.005, stationary-phase filtrates vs. lag- and early log–phase growth filtrates (1-way ANOVA)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/188/7/10.1086/378238/2/m_188-7-1040-fig002.gif?Expires=1750423775&Signature=WD50pzKWOgpleJJPYciV6m3TN8FRVrsWpymOtmwiPq0LEOIC7mOllmB3hWIbzk8Vle~hBO6B-SJFjJOWiXRO-CIC2D~pDP9zqgVeZ1z0Wq50Ghn8u9zljRNBddI3U4LhnCKaLrf-DC0~wax0MHWlhw-Sepl82YaUcvXdTx4XDjgeS0k5JpWofvmpeTm1oZPvXHOImIO-nw4FeQxkM5B6j4~g4ebcxNWnMkPjHp0qMEMfVie6IE2B8O1JKrSusjoj0UDpVY-U-5Wvhc0ZHnAuqNhEE7quXTBlsKWsE7p9p~D5olA9zPwPnLIRoXRfwBMxG14Jmvb5fXCOWy4uy8c9MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Effect of pneumocccal (D39) growth stage on the induction of Toll-like receptor 2–mediated interleukin (IL)–8 promoter activity. Transfected cells were inoculated with 5×106 live bacteria or with filtrates of these bacteria. Data are expressed as mean±SE for 2 experiments in triplicate. **P<.005, early log–phase bacteria vs. lag- and stationary-phase live bacteria (1-way analysis of variance [ANOVA]); *P<.005, stationary-phase filtrates vs. lag- and early log–phase growth filtrates (1-way ANOVA)
Effect of penicillin on growth and TLR2-mediated proinflammatory activity of S. pneumoniae.Figure 3A–3D show the viability profiles and optical density readings for D39 and its isogenic LytA mutant AL2 when treated in penicillin at one-eighth of the MIC and at the MIC for the respective strains at the time points 30 min, 3 h, and 6 h. Both strains show similar viability characteristics when treated at one-eighth of the MIC that were almost identical to those of untreated bacteria. In contrast, D39 exposed to penicillin at the MIC displayed rapid loss in both viability and optical density over 6 h, whereas AL2 exhibited only gradual loss of both viable count and optical density
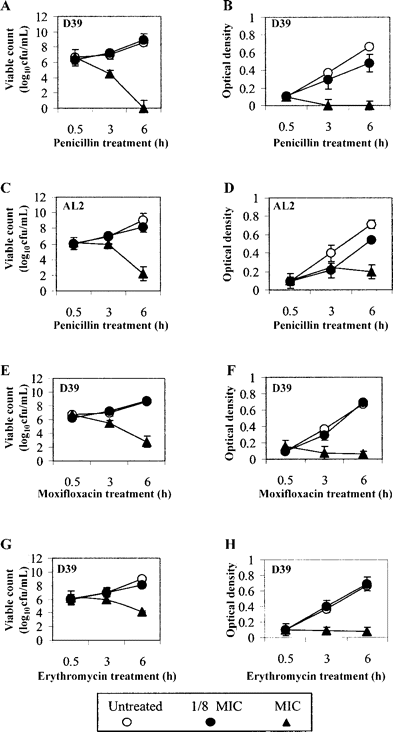
Effect of penicillin, moxifloxacin, and erythromycin on the viable growth (in log10 colony-forming units per milliliter) and OD at 620 nm of D39 Streptococcus pneumoniae (A, B, E, F and G) and the effect of penicillin on the viable growth and optical density of the LytA mutant AL2 (C and D). Data are expressed as mean±SE for 2 experiments in triplicate
Lag phaseLive D39 pneumococci exposed to one-eighth of the MIC of penicillin for 3 or 6 h beginning during the lag phase caused significant enhancements of TLR2-mediated IL-8 promoter activity, compared with untreated controls (figure 4A–4Cgray bars). Exposure for 3 h resulted in a 9-fold increase (P⩽.005, paired t test), and exposure for 6 h resulted in a 20-fold increase (P<.005, paired t test) (figure 4B4C). These enhancements at one-eighth of the MIC were not ablated by heat treatment (data not shown). The exposure of live D39 S. pnemoniae to MIC concentrations of penicillin for 6 h beginning during the lag phase produced an ablation of activity via TLR2, compared with untreated controls (P<.001, paired t test). However, this ablation coincided with the absence of viable cells, (figure 3A3B). This observation was also noted at high penicillin concentrations (10× MIC) (data not shown). Lag-phase filtrates of penicillin-exposed D39 were active via TLR2, but these were not significantly different than untreated filtrate controls (data not shown). The treatment of bacteria for 30 min had no significant effect on TLR2-mediated IL-8 promoter activity (figure 4A)
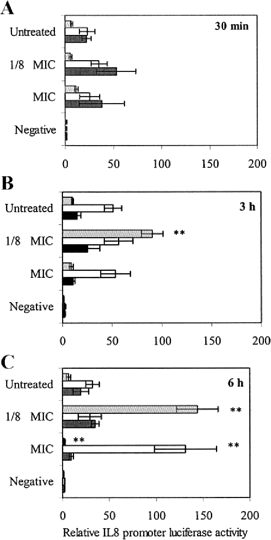
Toll-like receptor 2–mediated interleukin (IL)–8 promoter activity in response to Streptococcus pneumoniae treated with penicillin during the lag (gray bars) early log (white bars) or stationary (black bars) phase for 30 min (A) 3 h (B) or 6 h (C). The negative control was brain-heart infusion, 10% heat-inactivated fetal calf serum, Dulbecco’s modified Eagle’s medium, and penicillin at MIC penicillin concentrations. Data are expressed as mean±SE for 4 separate experiments. The paired t test was used to compare values for penicillin-treated live bacteria with untreated controls. **P⩽.005, vs. untreated controls
Early-log phaseExposure of bacteria to penicillin during the early log phase (figure 4A–4C, white bars) produced different effects from those noted for lag-phase bacteria. Live D39 exposed to the MIC or one-eighth of the MIC for penicillin for 30 min (figure 4A) or 3 h (figure 4B) displayed no significant differences in TLR2 signaling compared with untreated controls. Exposure to one-eighth of the MIC for penicillin for 6 h (figure 4C) during the early log phase also produced no significant deviation from controls. However, after 6 h treatment at the MIC, there was a 3.5-fold increase in IL-8 promoter activity elicited by whole bacteria, which occurred despite an apparent absence of viable bacteria (figure 3A) (P<.005, paired t test). In contrast, filtrates of bacteria treated with penicillin at the MIC were most active. After just 30 min of exposure to MIC concentrations of penicillin, there was a significant increase in IL-8 promoter activity (P<.05, paired t test), which also occurred at 3 and 6 h (P<.05, paired t test) (data not shown). Higher penicillin concentrations (10× MIC) exhibited TLR2 signaling characteristics that were very similar to those of the MIC (data not shown). The heat treatment of penicillin-exposed live cells and filtrates did not remove the enhancements observed at the MIC
Stationary phaseBoth live cells and filtrates (filtrate data not shown) of stationary-phase bacteria were active via TLR2 (figure 4A–4C, black bars), and treatment with penicillin at the concentrations tested had no significant additional effect, which suggests that penicillin does not modify the TLR2-mediated proinflammatory activity of stationary-phase pneumococci
Enhancement of TLR2-mediated proinflammatory activity of penicillin-treated S. pneumoniae requires autolysinTo test the role of the major autolysin, LytA, in penicillin-mediated pneumococcal inflammatory activation, a lytA isogenic D39 mutant was compared with the wild type. The MIC for this organism was found to be 2-fold lower than D39 (data not shown), and the period of exposure required for no viable recovery in MIC concentrations of penicillin was longer (figure 3C). This mutant strain had the same pattern of signaling at various growth phases and viable counts as the wild-type organism (data not shown). However, in comparison with the wild type, the lytA mutant did not display the enhancements at one-eighth of the MIC during the lag growth phase or at the MIC during early-log growth (figure 5A) (P<.005, Student’s t test)

Role of the functional LytA enzyme of Streptococcus pneumoniae during the penicillin-induced enhancement of Toll-like receptor 2–mediated interleukin (IL)–8 promoter activity. A D39 and LytA mutant treated for 6 h with penicillin added during the lag phase. **P<.005 between 2 strains (Student’s t test). B D39 treated with penicillin for 6 h added during the lag phase and also with the LytA inhibitors choline and ethanolamine. *P<.005, between treated and untreated controls (Student’s t test). The negative control was brain-heart infusion, 10% heat-inactivated fetal calf serum, Dulbecco’s modified Eagle’s medium, and penicillin at the MIC for the strain. All data are expressed as mean±SE for 4 separate experiments done in triplicate
The addition of the LytA inhibitors choline or ethanolamine before penicillin exposure during the lag phase resulted in a significant decrease in TLR2-mediated IL-8 promoter activity, compared with untreated controls (figure 5B). Ethanolamine treatment produced a decrease in 6-h enhancement at one-eighth of the MIC, by 83% (P<.05, independent t test); choline decreased this enhancement by 62% (P<.05, Student’s t test). The ablation of enhanced activity of bacteria treated with penicillin at the MIC was also removed by the addition of either choline or ethanolamine
Effect of nonbacteriolytic agents moxifloxacin and erythromycin on viable growth of S. pneumoniae.Figure 3E–3H shows the viability profile of D39 treated with moxifloxacin or erythromycin for 30 min or 3 or 6 h. At one-eighth of the MIC, the optical density and viable count for D39 treated in moxifloxacin (figure 3E 3F) or erythromycin (figure 3G 3H) show very similar growth characteristics to the untreated controls. Treatment at the MIC for each of the antibiotics caused slower killing and reduced lysis at the treatment times tested
Fluoroquinolone and macrolide antibiotics do not enhance pneumococcal activation via TLR2The fluoroquinolone moxifloxacin and the macrolide erythromycin were found to have no effect on TLR2-mediated IL-8 promoter activity compared with untreated controls. At the concentrations tested (one-eighth of the MIC and MIC), these agents did not modulate the activity of live D39 during the lag or early log phases (figure 6A6B). Compared with penicillin-treated live cells at the same MIC ratio, moxifloxacin and erythromycin were significantly less active (P<.005, Mann-Whitney U test)
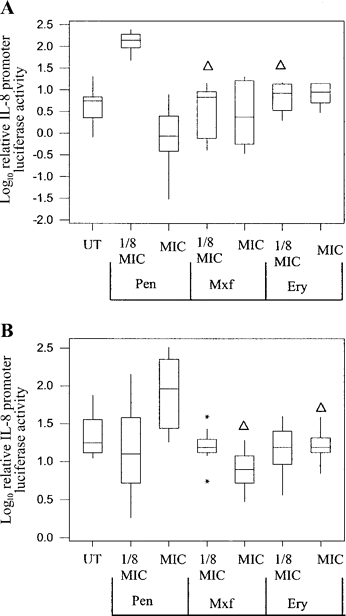
Effect of 3 different antibiotics on live D39 pneumococcal-mediated activation of the Toll-like receptor 2/interleukin (IL)–8 promoter system. The treatment of bacteria with penicillin (Pen), moxifloxacin (Mxf), or erythromycin (Ery) began during the lag (A) or early-log growth (B) stage and lasted 6 h. Data represent 3 experiments done in triplicate. Outliers are marked with asterisks.▵P<.05, when Mxf or Ery were compared with Pen at same the MIC ratio (Mann Whitney U test). UT, untreated
Normal transfection efficiencyThe viability of the HeLa cell preparations was confirmed by trypan blue staining (Stemcell Technology) before and after S. pneumoniae inoculation. Transfection efficiency was confirmed using a green fluorescent protein (GFP) plasmid construct; successful transfection of the cell was confirmed by florescent microscopy (data not shown). To control for differences between cells in each experimental condition, the Renilla luciferase reporter was used as an internal control
Discussion
We have shown that the exposure of S. pneumoniae to MIC ratios of penicillin increases the proinflammatory activity of human cells mediated by TLR2 and that this is dependent on the bacterial growth stage and requires the presence of a functional LytA enzyme. S. pneumoniae did not signal activity via the TLR4/MD2 system. Concentrations at 10 times the MIC for penicillin (data not shown) showed very similar proinflammatory activity via TLR2 to that noted at the MIC. Heat treatment had no effect on the proinflammatory potential of antibiotic-treated cells, which suggests that the active factor is a cell-wall product generated as a product of penicillin activity rather than a protein
It has been known for many years that cell-wall components of pneumococci can induce the release of proinflammatory cytokines from host cells and that treatment of the organism with β-lactam antibiotics increases this activity. In a rabbit model of pneumococcal meningitis, the liberation of cell-wall components such as PGN and LTA during β-lactam chemotherapy was associated with increased leukocytosis and inflammatory products in the subarachnoid space [1, 32, 33]. The inflammatory potential of LTA and, in particular, PGN is greatest when they are liberated from the cell wall within complex branched stem peptides [34, 35]. These inflammatory cell-wall fractions are released naturally during LytA activity and have almost equal potency against LPS [34, 35]. Both PGN and LTA have been shown to interact with TLR2, a process that can induce proinflammatory cytokine synthesis [36]. The liberation of LTA and teichoic acid (TA) increases during β-lactam therapy, and this is not seen during therapy with nonbactericidal antibiotics [37]. Modifications of LTA or TA also decrease the inflammatory potential of the fraction. Teichoication has been found to be directly associated with enhanced induction of IL-1 release [38]
The current study advances our knowledge because we have shown that the enhancement of proinflammatory activity by penicillin is mediated via TLR2, although this does not exclude a role for other pattern-recognition receptors. Furthermore, because of the sensitivity of the assay, we have been able to show that bacterial growth phase, antibiotic concentration, and functional activity of autolysin each critically influence this activity
The major pneumococcal autolysin, LytA, is an important enzyme associated with the separation of daughter cells, stationary-phase autolysis, and penicillin-mediated autolysis [5, 8–11]. The addition of penicillin to sensitive pneumococcal cells results in instability of the cell wall and the activation of LytA, which hydrolyzes the bonds between the glycan chain and stem peptides of peptidoglycan. The exogenous application of purified LytA to pneumococcal cell cultures results in the enzymatic release of branched stem peptides that are proinflammatory [35]. Similarly, the addition of penicillin to pneumococcal cultures induces the intrinsic activity of LytA and the release of proinflammatory cell components that we have shown to be active via TLR2. In the present study, the treatment of a lytA-positive strain of S. pneumoniae in penicillin resulted in the enhanced proinflammatory activation of TLR2, the absence of LytA in an isogenic mutant, or its selective inhibition by ethanolamine or excess choline treatment that significantly decreased its proinflammatory potential. The results of previous studies that used immunization challenge experiments in mice have associated directly the activity of LytA with the pathogenesis of pneumococcal disease [26]. Mice immunized with purified autolysin survive pneumococcal challenge longer than mice immunized with D39. A lytA mutant exhibited markedly reduced virulence in mice, as indicated by increased LD50 values, compared with its D39 LytA-positive parent
The bacterial growth phase is directly associated with bacterial susceptibility to β-lactam antibiotics [39, 40]. Penicillin, for example, is only bactericidal when it is applied at early growth stages, but, as the bacterial population increases, susceptibility decreases. During the penicillin treatment of experimental myositis caused by infection with Streptococcus pyogenes these events were associated with alterations in the profile of the penicillin-binding proteins (PBPs) [39]; at stationary-phase growth, a number of important PBPs are lost, decreasing the penicillin susceptibility
In the current study, bacteria at the lag and early-log phase exhibited different proinflammatory activity after exposure to penicillin, probably as a result of the cell-wall changes that occurred at different growth phases. The treatment of bacteria with β-lactam antibiotics at concentrations closer to the MIC resulted in a greater release of proinflammatory products than bacteria treated at concentrations above the MIC [3, 41, 42]. Concentrations below the MIC of β-lactam antibiotics, such as penicillin, lead to elongation, filamentation, and cell enlargement. Both of these events near and below the MIC are dependent on the distribution of PBP3 at the bacterial cell surface [3, 41–43]. The elongation of the pneumococcal cells due to the selective inhibition of PBP3 at concentrations below the MIC of penicillin may be a factor in the enhancement of proinflammatory TLR2 activation. However, the absence of these enhancements in the lytA mutant or in ethanolamine/choline-treated D39 at concentrations below the MIC suggests that autolysin still has an important role in these effects below the MIC
In contrast to β-lactam antibiotics, antimicrobial agents, such as the fluoroquinolones and the macrolides, are active at all stages of the bacterial growth cycle. These antibiotics do not induce the release of proinflammatory products from the cell wall [6]. A number of agents such as the fluoroquinolone trovafloxacin have even been shown to subdue inflammation [44]. β-lactam therapy is still extremely effective in disease treatment when it is administered at optimal concentrations to patients infected with penicillin-susceptible bacteria. However, in the era of penicillin resistance, it is possible that the administration of penicillin to some patients may result in concentrations of the drug below the MIC within some infected tissues. Our results suggest that this might augment the release of proinflammatory products from pneumococci within tissues, especially those in lag-phase growth. Indeed, Metlay et al. [45] showed that human infections due to penicillin-nonsusceptible pneumococci are associated with no increase in mortality, but patients are at a greater risk of suppurative complications of infection. Although bacteria do follow the typical lag, exponential, and stationary growth phases in the host, individual growth phases may be extended or, in some cases, absent. Factors such as host susceptibility to disease and the virulence of the invading pathogen are also key factors in bacterial proliferation in vivo [46]
To exclude the possibility that the treatment of HeLa cells with bacteria induced their apoptosis and, as a consequence, influenced the results of the HeLa cell assay, we checked cell viability at the end of each experiment by assessing trypan blue staining and GFP transfection efficiency (data not shown). To confirm that the inflammatory events noted were associated with the MIC and not simply the concentration of penicillin, D39 were treated in absolute concentrations of penicillin, and the IL-8 promoter results contrasted with those for the MIC. The events noted were confirmed to be dependent on the MIC ratio (data not shown)
TLR2 is a key signaling receptor for LTA and the branched stem peptide moieties of PGN [36, 47]. The activity of TLR2 transduces the induction of proinflammatory cytokine genes. The use of ectopic TLR2 transfection and an IL-8 promoter reporter construct has allowed us to quantify the effects of β-lactams on pneumococci at a high level of sensitivity. Our data have demonstrated that penicillin enhances the TLR2-mediated proinflammatory activity of S. pneumoniae and that this is dependent on the growth phase of the organism, can occur at concentrations below the MIC, and can only occur if autolysin activity is intact. These data highlight the importance of the LytA enzyme in the induction of the proinflammatory potential of S. pneumoniae; however, the activity and inhibition of the PBPs during β-lactam treatment may also be important factors contributing to these inflammatory events, particularly at concentrations below the MIC
Acknowledgments
We thank Glen Tillotson, Chris Dowson, Stephen Gillespie, Ian Sabroe, and Jane Ambler for their valuable advice and support
References
Presented in part: 3rd International Symposium On Pneumococci and Pneumococcal Disease, Anchorage, 5–9 May 2002 (poster 240)
Financial support: Bayer Pharmaceuticals




