-
PDF
- Split View
-
Views
-
Cite
Cite
Anne Karin Brigtsen, Dennis L. Kasper, Carol J. Baker, Harold J. Jennings, Hilde-Kari Guttormsen, Induction of Cross-Reactive Antibodies by Immunization of Healthy Adults with Types Ia and Ib Group B Streptococcal Polysaccharide-Tetanus Toxoid Conjugate Vaccines, The Journal of Infectious Diseases, Volume 185, Issue 9, 1 May 2002, Pages 1277–1284, https://doi.org/10.1086/340324
Close - Share Icon Share
Abstract
Types Ia and Ib group B streptococcal (GBS) capsular polysaccharides (PSs) are structural isomers but are antigenically distinct. Immunization of healthy adults with GBS type Ia PS-tetanus toxoid (Ia-TT) or Ib-TT glycoconjugate vaccines induced ⩾4-fold increases in specific immunoglobulin G to the heterologous PS in more than two-thirds of subjects. Ib-TT vaccine-induced IgG bound with substantially higher affinity to homologous (Ib) than to heterologous (Ia) PS and promoted opsonophagocytic killing of GBS type Ib but not type Ia organisms. The failure of the Ib-TT- and Ia-TT-induced human antibodies to kill bacteria of the cross-reactive serotype contrasts with the results of previous studies in animals. Inhibition enzyme-linked immunosorbent assays demonstrated that Ib-TT-induced IgG to the homologous PS bound mainly to native Ib PS, whereas the cross-reactive antibodies recognized both native and derivative PSs. These results indicate that GBS Ia and Ib PSs should be included in a multivalent conjugate vaccine to prevent GBS disease.
Group B streptococci (GBS) are a leading cause of invasive infections in neonates and young infants in the United States [1]. The widespread implementation of intrapartum antibiotic prophylaxis in this country has coincided with a decrease in the incidence of early-onset GBS disease in neonates <7 days old from 1.7 (1993) to 0.6 (1998) per 1000 live births; in contrast, the incidence of late-onset disease (7–89 days) has remained unchanged at 0.4 per 1000 live births [1]. Nine serotypes of GBS have been identified on the basis of capsular polysaccharides (PSs) [2–4]. Together, serotypes Ia and Ib account for ∼40% of early-onset infections [5]. The capsular PSs of serotypes Ia and Ib are structural isomers [6] that differ only in the glycosidic linkage between the side-chain galactose and N-acetyl-glucosamine residues (figure 1).
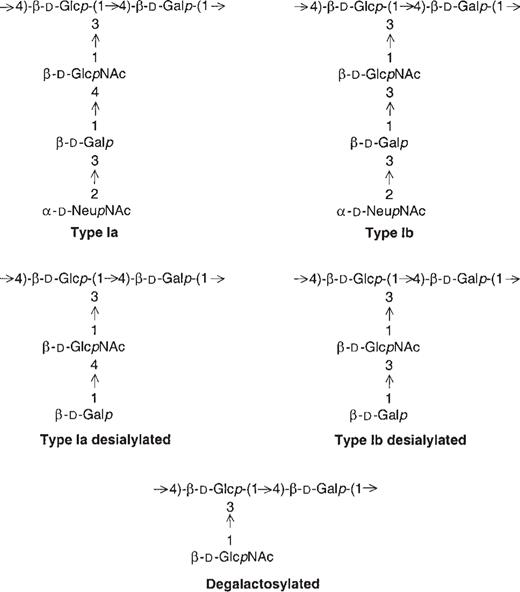
Structure of repeating units of group B streptococcus types Ia and Ib capsular polysaccharides. Native, desialylated types Ia and Ib and the degalactosylated common structure of types Ia and Ib are shown.
Lancefield [7] demonstrated in 1938 that rabbit antiserum raised to formalin-killed types Ia and IbGBS reactedwith the heterologous serotype in precipitin reactions. Passive immunization with these cross-reacting antibodies protected adult mice against homologous and heterologous lethal infection. However, immunogenicity studies demonstrated the antigenic uniqueness of the Ia and Ib organisms; homologous bacteria removed from the antiserum antibodies that protected against either serotype, whereas heterologous bacteria removed only antibodies that protected against self [7]. Lancefield named this protective cross-reactive epitope common to types Ia and Ib GBS the “Iabc cross-reactive antigen” [8]. Subsequent immunochemical studies suggested that the cross-reactive epitope was the backbone of the repeating unit common to the types Ia and Ib PS [9].
The PS nature of the cross-reactive epitope was confirmed by studies in which rabbits were immunized with a glycoconjugate vaccine consisting of purified Ia PS from GBS conjugated to tetanus toxoid (Ia-TT) [10]. The Ia-TT glycoconjugate vaccine induced high-titer Ia PS-specific antibodies that opsonized GBS of not only type Ia but also type Ib for killing by human peripheral blood polymorphonuclear leukocytes (PMNL). Even though the relative binding affinity of these cross-reactive antibodies was substantially lower for the heterologous than for the homologous PS, passive immunization of dams with rabbit antiserum raised to Ia-TT protected their pups against lethal challenge with organisms of either serotype [10]. However, pups born to dams actively immunizedwith a trivalent glycoconjugate vaccine composed of types Ia, II, and III GBS conjugated separately to TT were not protected against infection when challenged with GBS type Ib [11]. To our knowledge, no information exists on the functional activity of these cross-reactive antibodies in humans.
GBS type III-specific antibodies are protective in humans, and low levels of maternal type III-specific IgG correlate with susceptibility to neonatal invasive GBS disease [12] (unpublished data). The administration to women of a vaccine that induces typespecific IgG [5, 13, 14] would be likely to protect infants during the first 3 months of life, the period when nearly all neonatal GBS disease occurs [15]. It is, therefore, an important goal to develop a multivalent vaccine containing PS from the serotypes responsible for the majority of cases of GBS disease. Unconjugated GBS PSs are poorly immunogenic in humans [5, 13, 14, 16, 17], but coupling of these PSs to an immunogenic carrier substantially improves the immunogenicity of the resulting vaccines in a dose-dependentmanner [5, 13, 14].We have used TT as a carrier in our model vaccines in humans [5, 13, 14], and the maximum PS doses are, therefore, limited by the total amount of TT that can be given [18]. The production of a multivalent PS-based vaccine would be simplified if the number of serotypes included could be limited through the induction of functional antibodies against cross-reactive antigens.
Our aims in this study were to investigate whether immunization with GBS Ia-TT or GBS Ib-TT conjugate vaccine induces cross-reactive functional antibodies in healthy adults and to define the cross-reactive epitope(s).
Materials and Methods
Bacterial strains. GBS type Ia strain 515 and GBS type Ib strain H36B (isolated from neonates with invasive infection [7, 19]) were used in the opsonophagocytic killing assays. Both strains are typical clinical isolates with respect to their degree of encapsulation and their susceptibility to opsonophagocytic killing [10, 11, 19, 20]. The strains were stored at −80°C in 0.5 mL of Todd Hewitt broth (Difco).
Preparation of GBS types Ia and Ib glycoconjugates. GBS types Ia and Ib PSs from the highly encapsulated strain 090 and from strain H36B, respectively, were purified, as described earlier for GBS type III PS [5, 21]. Oxidation of the sialic acid residues by sodium metaperiodate yielded reactive aldehyde groups available for controlled covalent coupling to e-amino groups on proteins. For use as the ELISA coating antigens, type Ia or Ib PS with 50% of the sialic acid residues oxidized was conjugated to human serum albumin (HSA; Sigma), as described elsewhere [22]. For use in conjugate vaccine, each of the oxidized PSs was conjugated to monomeric TT (Amvax) [5]; in this case, 25%and 9%of the sialic acid residues were oxidized [5], as determined by gas chromatography-mass spectrometry [21], in the Ia and Ib PS, respectively.
Chemically modified PSs. The following chemical modifications of the native PSs of types Ia and Ib were obtained, as described elsewhere [23, 24] (figure 1): desialylated or core PS with a terminal side-chain galactose, PS with the carboxyl group of the sialic acid residues reduced to a hydroxymethyl group (not shown), and degalactosylated PS with a common structure for both types Ia and Ib.
Human serum samples. Serum samples from 2 separate phase 1 trialswere studied [5]. In brief, paired serum samples from 70 healthy, nonpregnant women (18–40 years of age) were sampled before and 8 weeks after vaccination with unconjugated GBS type Ia PS (Ia PS), GBS type Ib PS (Ib PS), the Ia-TT or Ib-TT conjugate vaccine, or saline placebo. Vaccine groups consisted of 15 women each, and 10 women received placebo.
Quantitative ELISA. Specific IgG to Ia or Ib PS was quantitated in ELISAs, essentially as described for the quantitative determination of type III PS-specific IgG [5, 22]. In brief, microtiter plates were coated with 2.5 µ/mL HSA covalently linked to either Ia PS (Ia-HSA) or Ib PS (Ib-HSA), incubated at 30°C for 6 h, and washed, and serum was added by serial 2-fold dilution. The plates were incubated at 4°C overnight, washed, and reincubated at 30°C for 2 h with goat anti-human IgG conjugated to alkaline phosphatase (Biosource International). After washing, the plates were developed with p-nitrophenyl phosphate (Sigma) at 37°C for 1 h, and the absorbance at 405 nm (A405) was determined with a kinetic reader (model EL312E; Bio-Tek Instruments). Optimal concentrations of coating antigens and conjugate were determined by checkerboard ELISA [25]. Ia- or Ib-specific IgG was quantitated in various serum samples by comparison of the A405 of the test serum with a standard curve obtained with pooled streptococcal human reference serum (SHRS Ia and SHRS Ib). The amounts of type-specific antibodies in SHRS Ia and SHRS Ib were determined by quantitative precipitation and radioactive antigen-binding assay of nonprecipitating antibodies, as described elsewhere for type III SHRS [22]. More than 90% of the type-specific antibodies in the reference serum were of the IgG isotype, as evaluated by ELISA [22]. Internal positive and negative standard serum samples were included in each assay. Results were reported as micrograms of PS-specific IgG per milliliter. The lower limit of quantitation was 0.05 µg/mL for the Ia-specific IgG and 0.1 µg/mL for the Ib-specific IgG assays.
Competitive inhibition ELISA. To identify the epitope(s) on the PS to which the vaccine-induced cross-reactive antibodies bind, we tested serum samples from 4 Ia-TT and 5 Ib-TT recipients who had low concentration of cross-reactive antibodies in preimmunization serum (0.09–0.98 µg/mL) and a ⩾4 fold increase in cross-reactive antibodies 8 weeks after immunization (to 2.95–132.72 µg/mL). Specific inhibition of binding of the serum to plates coated with Ia- HSA or Ib-HSAwas assessed, as described above for the ELISA, except that the serum was incubated with the native Ia and Ib PSs and derivatives of the native PSs at concentrations of 1.5 ng/mL−30 µg/mL. The test serum was used at a dilution yielding an A405 of 1 after 1 h of incubation, a value near the middle linear portion of the titration [25]. The results were expressed as the percentage of inhibition of binding of the test serum in the presence of inhibitors, compared with binding without inhibitors.
Opsonophagocytic killing assay. A modification of the opsonophagocytic killing assay described by Baltimore et al. [20] was applied to test the ability of vaccine-induced serum to opsonize GBS for killing by PMNL. We tested serum from 2 Ia-TT and 5 Ib-TT recipients who had low concentrations of cross-reactive antibodies in preimmunization serum (0.09–0.98 µg/mL) and a ⩾20-fold increase in cross-reactive antibodies 8 weeks after immunization (to 2.95–132.72 mg/mL). For these studies, we used serum from irradiated rabbits as our complement source, because pilot studies revealed that complement from human serum absorbed with GBS types Ia and Ib organisms resulted in reduction in colony-forming units in the GBS type Ia killing assay in the absence of added type-specific antibodies, whereas rabbit complement did not. Furthermore, complement from irradiated rabbits and absorbed human serum gave comparable killing of GBS when type-specific antibodies were added (data not shown). Reaction mixtures (250 µL) consisted of heat-inactivated (56°C for 30 min) test serum diluted to yield various concentrations of PS-specific IgG, PMNL isolated from healthy adult volunteers [26], bacteria grown to mid-logarithmic phase, and 10% serum from irradiated rabbits (as the complement source). The ratio of PMNL to bacteria was 2-4:1. A positive internal standard, the SHRS for the homologous PS at a concentration of 2 µg/mL, was included in each assay, as were negative controls lacking serum and/or complement or PMNL. All serumsamples and controls were tested in duplicate. Results are expressed as the mean percentage of log reduction in colony-forming units per milliliter in the tested serum, compared with the positive internal standard after incubation at 37°C for 1 h.
Statistics. Continuous data are presented as means ± SDs. IC50 values of the native PSs and their derivatives were compared in unpaired t tests. Proportions were compared by χ2 test. P < .05 was considered to be significant.
Results
Type-specific IgG response. The PS-TT conjugate vaccines were more immunogenic than their unconjugated PS vaccine counterparts with respect to the induction of cross-reactive PS-specific IgG. Eleven (73%) of 15 Ia-TT recipients (figure 2A) and 5 (33%) of 15 unconjugated Ia PS recipients (figure 2B) developed ⩾4-fold increases in specific IgG to the heterologous PS, Ib PS, as shown by the dashed lines (P = .028; figure 2). Ten (67%) of 15 Ib-TT recipients (figure 2C) and 7 (47%) of 15 unconjugated Ib PS recipients (figure 2D) had ⩾4-fold increases of specific IgG to the heterologous PS, Ia PS, as shown by the dashed lines (P = .269). The median concentrations of crossreactive IgG in serum from women immunized with Ia vaccines were 6.6 µg/mL (25th–75th quartiles, 0.84–11.56 µg/mL) and 0.2 µg/mL (25th–75th quartiles, 0.1–2.1 µg/mL) for recipients of Ia-TT conjugate vaccine and Ia PS vaccine, respectively (P < .02). Similarly, the median concentrations of cross-reactive IgG after immunization with Ib-TT and Ib PS vaccines were 7.5 µg/mL (25th-75th quartiles, 1.1–43.3 µg/mL) and 4.8 µg/mL (25th–75th quartiles, 0.5–18.7 µg/mL), respectively. No change in PS-specific IgG levels was noted in placebo recipients (data not shown).
![Reverse cumulative distributions of fold increase in polysaccharide (PS)-specific antibodies 8 weeks after immunization with group B streptococcus type Ia PS-tetanus toxoid conjugate vaccine (Ia-TT [n = 15]; A), type Ib PS-TT conjugate (Ib-TT [n = 15]; C), unconjugated type Ia PS (n = 15; B), or unconjugated type Ib PS (n = 15; D). Data are proportion of vaccine-induced antibodies recognizing homologous PS (solid lines) and heterologous PS (dashed line). Lower limits of quantitation were 0.05 and 0.1 µg/mL, respectively, for Ia PS- and Ib PS-specific IgG ELISAs.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/185/9/10.1086_340324/2/m_185-9-1277-fig002.jpeg?Expires=1750157984&Signature=gjak81~Zto~qpTX7B4WRlS6RbQyFr8iZP7KePYUubNzFc5tP~002iwAqLNy8WHQrzLJvpJirmNjA4JtAa8lNcIMhSIaUeTQ0fcWvmeeegOXP9WwAjVrshjSWgRBpEmvrTgBCQUUOef7qoxUnrqjh4vT9kkFSYFQlbrEeZIYKLMYW~H4dixxVKkC~U~8UkMBnoW3j4l75resU-3pKRWMQnn5dGDj8EZZSGYvqVMy4vmt~J1mVmY3dTuR2SFKRu-zGAsSMlyXZVawloGLdVdW3igGmuDuFMVJeevmrKpve6unouwPqI4s3DR5WWImI~eUSCmbQxoM0DDsO7cbGT3wVMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Reverse cumulative distributions of fold increase in polysaccharide (PS)-specific antibodies 8 weeks after immunization with group B streptococcus type Ia PS-tetanus toxoid conjugate vaccine (Ia-TT [n = 15]; A), type Ib PS-TT conjugate (Ib-TT [n = 15]; C), unconjugated type Ia PS (n = 15; B), or unconjugated type Ib PS (n = 15; D). Data are proportion of vaccine-induced antibodies recognizing homologous PS (solid lines) and heterologous PS (dashed line). Lower limits of quantitation were 0.05 and 0.1 µg/mL, respectively, for Ia PS- and Ib PS-specific IgG ELISAs.
Nine of the 15 Ia-TT-immunized and 8 of the 15 Ib-TT-immunized women had low levels of cross-reactive antibodies (<1 µg/mL) before immunization and had developed ⩾4-fold increases in cross-reactive antibodies at 8 weeks after immunization. Of these, 5 serum samples from Ib-TT vaccine recipients and 2 serumsamples from Ia-TT vaccine recipients with low preimmunization PS-specific IgG to both the homologous and heterologous PSs (0.09–0.98 µg/mL) had developed ⩾20-fold increases in Ia PS-specific and Ib PS-specific IgG, respectively, at 8 weeks (2.95–132.97 µg/mL). Serum samples from these 7 subjects were selected for studies of their ability to promote opsonophagocytosis of type Ia GBS for killing by PMNL, relative affinity for the homologous versus the heterologous PS, and epitope specificity (Ib-TT recipients only).
In vitro functional activity of vaccine-induced antibodies. The pooled reference sera, SHRS Ib and SHRS Ia, were included in each experiment at a concentration of 2 µg/mL (i.e., 100 ng of PS-specific IgG in the final reaction mixture) as an internal standard to permit adjustment for variations in the ability of the adult PMNL to kill bacteria. The opsonic activity of test serum was expressed as the percentage of reduction in colony-forming units per milliliter obtained for SHRS Ib and SHRS Ia. Serum from Ib-TT recipients containing 13.1–202.2 µg/mL of Ib PS-specific IgG killed homologous type Ib strain H36B in a concentration-dependent manner (figure 3). A mean of 25 ng of type Ib PS-specific IgG in the reaction mixture (0.5 µg/mL) promoted statistically significant killing of the homologous strain (figure 3A). In contrast, serum from the Ib-TT vaccine recipients containing 2.95–132.72 µg/mL of Ia PS-specific IgG did not promote killing of the heterologous type Ia strain 515 at any concentration tested (up to 6600 ng of type Ia PS-specific IgG in the reaction mixture; figure 3B [and data not shown]). Serum from 2 Ia-TT recipients containing 12.6 and 42.6 µg/mL of Ia PS-specific IgG, respectively, killed homologous Ia strain 515 in a concentration-dependent manner (data not shown). In contrast, serum from the 2 Ia-TT vaccine recipients, containing 11.4 and 12.6 µg/mL of Ib PS-specific IgG, did not promote killing of the heterologous type Ib strain H36B at any concentration tested (data not shown). Neither the preimmunization serum samples nor the controls included in the assay promoted opsonophagocytosis and killing of either GBS strain.
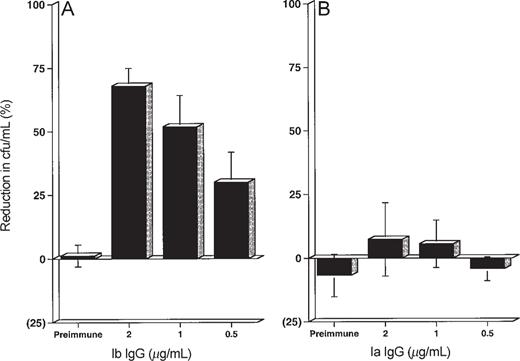
In vitro opsonophagocytosis and killing of group B streptococci (GBS) type Ia strain 515 and type Ib strain H36B by GBS Ib polysaccharide (PS)-tetanus toxoid conjugate vaccine (Ib-TT)-induced antibodies. Killing is expressed as percentage of log reduction (compared with positive internal standard) in colony-forming units of GBS type Ia (B) and type Ib (A) after 60 min of incubation. Concentrations of type Ib PS-specific IgG (A) and type Ia PS-specific IgG (B) are given in micrograms per milliliter. Each bar represents mean ± SD for duplicate determinations with 5 serum samples.
Relative affinity of Ib-TT conjugate vaccine-induced antibodies. The relative affinity of the type Ib PS-specific IgG induced by Ib-TT was substantially higher for the homologous Ib PS than for the heterologous PS (figures 4 and 5). In a competitive inhibition ELISA with Ib-HSA as the coating antigen, the mean IC50 for Ib PS was >100-fold lower (figure 4, top left) than that for Ia PS (figure 4, top right; mean ± SD, 90 ± 50 vs. 1269 ± 1085 ng/mL; P = :0318). The cross-reactive type Ia PS-specific IgG elicited after immunization with Ib-TT conjugate recognized both Ia and Ib PS. Like the Ib-TT conjugate vaccine-induced antibodies recognizing the homologous Ib PS, the Ib-TT-induced cross-reactive antibodies to heterologous type Ia PS bound with substantially higher relative affinity to Ib than to Ia PS (figure 5). With the exception of 1 serumsample, the IC50 of Ib PS was nearly 100-fold lower than that of Ia PS (3 ± 2 vs. 200 ± 85 ng/mL; P50 = .006). When the excluded serum sample was included in the analysis, the difference was still significant, with the mean IC50 of Ib PS increasing to 50 ± 110 ng/mL (P = .04). Similarly, the relative affinity of the type Ia PS-specific IgG induced by Ia-TT was 1–3 logarithmic orders of magnitude higher for the homologous Ia PS than for the heterologous Ib PS (data not shown).
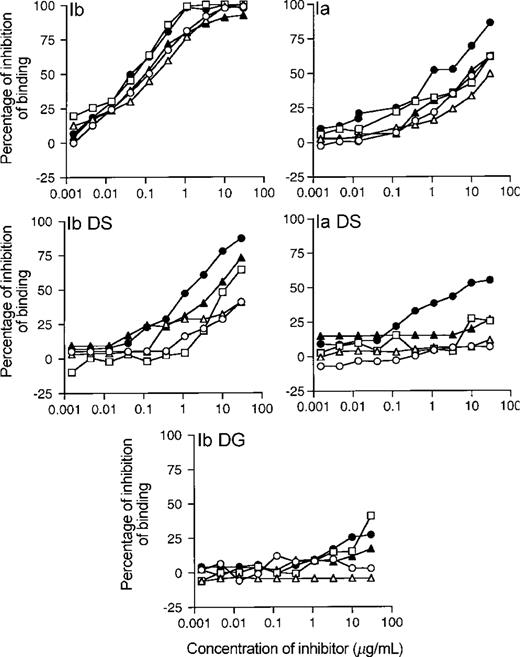
Epitope specificity of antibodies induced by group B streptococcus (GBS) type Ib polysaccharide (PS)-tetanus toxoid conjugate vaccine (Ib-TT) for Ib PS. Each of 5 serum samples is represented by a separate symbol; symbols indicate mean percentage of inhibition of IgG binding to ELISA plates coated with Ib PS conjugated to human serum albumin by 5 serum samples obtained 8 weeks after immunization, tested in duplicate. Inhibitors used are shown in upper left corner of each panel: Ib, native Ib PS; Ia, native Ia PS; Ib DS, desialylated Ib PS; Ia DS, desialylated Ia PS; Ib DG, degalactosylated GBS type Ib PS. Concentrations of inhibitors are expressed in micrograms per milliliter.
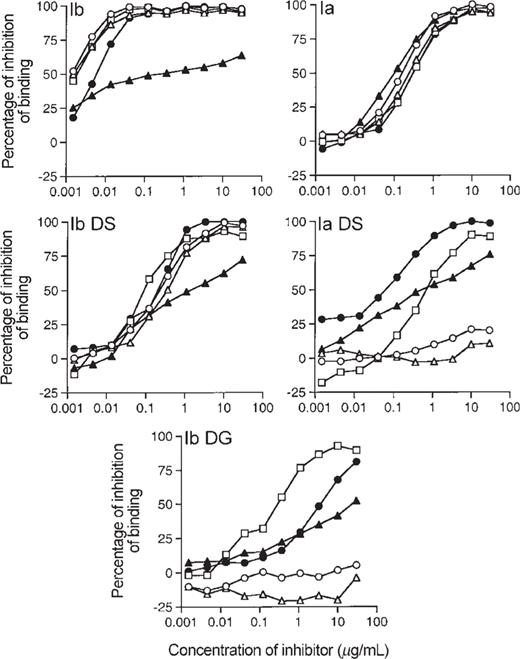
Epitope specificity of antibodies elicited by group B streptococcus (GBS) Ib polysaccharide (PS)-tetanus toxoid conjugate vaccine (Ib-TT) for Ia PS. Each of 5 serum samples is represented by a separate symbol; symbols indicate mean percentage of inhibition of IgG binding to ELISA plates coated with Ia PS conjugated to human serum albumin by 5 serum samples obtained 8 weeks after immunization, tested in duplicate. Inhibitors used are shown in upper left corner of each panel: Ib, native Ib PS; Ia, native Ia PS; Ib DS, desialylated Ib PS; Ia DS, desialylated Ia PS; Ib DG, degalactosylated GBS type Ib PS. Concentrations of inhibitors are expressed in micrograms per milliliter.
Epitope specificity of Ib-TT conjugate vaccine-induced antibodies. To further examine which epitopes bind Ib-TT conjugate-induced antibodies, derivatives of the native PS were used as inhibitors in competitive ELISAs. In the homologous ELISA (Ib-HSA), the antibodies demonstrated a homogeneous binding pattern, with all antibodies recognizing the native homologous and heterologous PS (figure 4). The Ib IgG in serum from the 5 Ib-TT vaccine recipients recognized desialylated Ib PS (i.e., the core Ib), albeit with a substantially lower IC50 than that for native PS. For 3 of these serum samples, the majority of the Ib PS-specific IgG was inhibited by the core Ib (IC50 <60-fold lower than that for native Ib PS); for the 2 remaining serum samples from Ib-TT recipients, the core inhibited, 50% of binding at the highest concentration tested. The inhibition pattern for the Ib derivative with reduced carboxyl groups on the sialic acid residues was identical to that of the core Ib PS (data not shown). No inhibition of binding was detected with the degalactosylated PS (the common backbone); this structure has been hypothesized to be the common immunodeterminant for type Ia and type Ib [9].
In stark contrast, the cross-reactive Ib-TT-induced antibodies demonstrated a heterogeneous binding pattern to the derivative PS in the heterologous ELISA (Ia-HSA; F). In general, the number of serum samples containing antibodies that bound to the native PS and their derivatives increased with the degree of antigenic completeness of the PS. Only 2 of 5 serum samples were completely inhibited by the common backbone—that is, the degalactosylated PS. Two of the serum samples that were completely inhibited by the native heterologous PS were inhibited to the same degree by the desialylated homologous PS. One of the 5 serum samples required the α(1⊔4) conformation in the native Ia PS for optimal inhibition.
Discussion
Induction of cross-reactive antibodies to GBS type Ia or Ib was first demonstrated in 1938, when Lancefield [7] passively immunized mice with hyperimmune rabbit antiserum derived after immunization with either type Ia or type Ib organisms and demonstrated protection against lethal challenge with homologous and heterologous organisms. The antigenic uniqueness of these PSs was established by absorption studies in which the type Ib bacteria removed from the GBS type Ib-hyperimmune rabbit antiserum antibodies that protected against either type Ia or Ib, whereas the type Ia strain removed only the antibodies that protected against type Ia organisms [7]. In 1993, Wessels et al. [10] corroborated and extended these studies by demonstrating that cross-protective antibodies could be elicited in animals by type Ia PS conjugated to TT. In this report, we describe the induction of cross-reactive antibodies by GBS type Ia-TT and Ib-TT conjugate vaccines in humans. Although there is considerable interindividual variability in the magnitude of this response, cross-reactive antibodies were induced in the majority of conjugate vaccine recipients. Indeed, more than two-thirds of the recipients of either Ia-TT or Ib-TT responded with a ⩾4-fold increase in antibodies to the heterologous PS. Fewer recipients of the corresponding unconjugated Ia or Ib PS vaccines developed cross-reactive antibodies, probably because of their significantly poorer immunogenicity [5].
The distribution of GBS serotypes has varied over time, by geographic region and by site of invasion [27–30]. In particular, surveillance for invasiveGBS disease has demonstrated a changing distribution of serotypes,with serotypeVemerging as a prominent cause of such cases [31] and serotypes Ia, Ib, II, III, and V accounting for 98% of cases of invasive disease. In recent years, serotype Ia has emerged as a more dominant serotype in colonized women and neonates [32] and in neonates with early-onset GBS disease (i.e., disease occurring in the first 6 days of life) [28, 30]. Accordingly, type Ia accounted for nearly 40% and type Ib for 5%–10% of GBS isolates from neonates with early-onset disease in several recent studies [28–30]. Thus, both type Ia and type Ib represent important serotypes to include in a multivalent GBS vaccine to prevent perinatal GBS disease. However, the total dose of TT that can be administered in amultivalent vaccine is limited [18], and this limitation, in turn, restricts the dose of PS that can be used in a conjugate vaccine. The implication is that a higher dose of a given type of PS may be used if fewer serotypes are included. Because there is a clear doseresponse relationship between the amount of PS administered and the levels of PS-specific antibodies produced [5, 13, 14], a higher dose of PS is advantageous. Thus, if either Ia-TT or Ib-TT conjugate vaccine elicits cross-reactive antibodies that opsonize the strains of the heterologous serotype for phagocytosis and killing by PMNL, then only 1 of these monovalent vaccines may be needed for a multivalent GBS conjugate vaccine.
Our study demonstrates that antibodies elicited by the Ib-TT and Ia-TT conjugate vaccines in healthy adults promote in vitro opsonophagocytosis and killing of homologous, but not heterologous, organisms. At a concentration as high as 133 µg/mL, Ib-TT vaccine-induced type Ia PS-specific IgG did not promote in vitro opsonophagocytosis and killing of type Ia organisms, whereas type Ib PS-specific IgG induced by the same vaccine at concentrations as low as 0.5–1 µg/mL promoted in vitro opsonophagocytosis and killing of the homologous type Ib organism. The failure of the Ib-TT- and Ia-TT-induced antibodies to kill bacteria of the cross-reactive serotype (Ia) contrasts with the results of a previous study, in which rabbit serum raised to Ia-TT opsonized type Ib organisms for in vitro opsonophagocytic killing [10]. It also contrasts with the reported direct correlation observed between the level of PS-specific IgG to the homologous serotype induced by both type Ia and type Ib PS-TT and unconjugated PS vaccines and the ability of these sera to promote in vitro opsonophagocytosis and killing of homologous bacteria [5]. The lower level of opsonophagocytic activity displayed by the cross-reacting antibodies is similar to the findings of Nahm et al. [33] in studies of vaccinees receiving multivalent pneumococcal PS vaccines. This discrepancy may be explained by lower relative affinities for heterologous than for homologous PS and/or by the binding of antibodies to different epitopes on the homologous than on the heterologous capsular PS.
The cross-reactive type Ia PS-specific IgG and type Ib PS-specific IgG induced by Ib-TT and Ia-TT conjugate vaccines, respectively, bound with greater relative affinity to native PS than to heterologous PS in inhibition ELISAs. Indeed, the relative binding affinity of Ib-TT vaccine-induced type Ia PS-specific IgG was nearly 100-fold lower. This result is similar to that of Wessels et al. [10], who found that type Ia PS-specific IgG elicited by Ia-TT vaccine in rabbits bound with substantially greater affinity to Ia than to Ib PS. The complete inhibition of antibody binding to Ia PS by Ib PS proves that the same antibodies are bound to these 2 PSs, indicating that the type Ib PS-specific and the cross-reactive Ia PS-specific IgG do bind to the same epitope, albeit with different affinities. Accordingly, the difference in 1 glycosidic bond in the side chain of the repeating-unit structure of isomeric types Ia and Ib appears to be important for the relative binding affinity of antibodies elicited by Ib-TT conjugate vaccine.
revious investigations have suggested that degalactosylated PS is a common immunodeterminant to which both type Ia and type Ib rabbit antisera react [9]. Our experiments using human serum indicate that this degalactosylated PS is not the common immunodeterminant. None of 5 serum samples fromadults given Ib-TT conjugate vaccine recognized degalactosylated Ib PS in the homologous Ib-HSA ELISA, and only 3 of 5 did in the heterologous Ia-HSA ELISA. The lower relative binding affinity of the derivatives than of the native PSs indicates that native PS is required to elicit optimal binding between antigen and antibody. An increase in the affinity of antigen-antibody binding as oligosaccharide size increases has been shown for capsular oligosaccharides of both gram-negative and gram-positive bacteria, including GBS type III, Streptococcus pneumoniae type 14, Neisseria meningitidis group B, Escherichia coli K1, Haemophilus influenzae type b, and Salmonella serogroup B [34–38]. Several investigators have suggested that antibodies to PSs recognize a particular conformation of the antibody-binding epitope that is fully expressed only in the high-molecular-weight forms of the antigen [35, 38]. The same may be true for the Ib-TT-induced antibodies, although in this instance the repeating unit itself has been reduced in size, whereas the number of repeating units remains unchanged. Previous reports have indicated that the terminal sialic acid residues appear to be critical to the immunodeterminant on Ia PS and type III PS, and these residues are also critical to the immunodeterminant on Ib PS.
In summary, we have documented that cross-reactive GBS antibodies can be induced in humans by types Ia and Ib glycoconjugate vaccines. Moreover, we have shown that the presence of these cross-reactive antibodies (detected by ELISA) does not predict function. In spite of the strong structural similarities between types Ia and Ib GBS, Ib-TT and Ia-TT conjugate vaccines elicit antibodies, in some persons, that bind to the heterologous capsular PS with substantially lower affinity and do not promote opsonophagocytosis and killing of heterologous strains in vitro. These results support that, for optimal protection, both type Ia and type Ib should be included in a multivalent conjugate vaccine.
Acknowledgements
Invaluable technical assistance in the production of vaccines and antigens was provided by April Blodgett, Julianne Pinel, Ken D. Johnson, and Barbara G. Reinap at the Channing Laboratory. Marcia A. Rench, Melissa E. Hickman, Morven S. Edwards, and Judith R. Campbell, at Baylor, and Angela C. Tramontano, at Channing, applied their expertise to the recruitment of subjects, collection of specimens, performance of assays, and analysis of antibody responses.
References
Presented in part: 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 28 September 1999 (abstract 1395).
A statement of informed consent was obtained from vaccinees, and human subject research experimentation guidelines of the US Department of Health and Human Services were followed during the conduct of the clinical trial.
Financial support: National Institutes of Health (contracts AI-75326 and AI-65316 and merit award AI-23339 [to D.L.K.]).
The contents of this publication do not necessarily reflect the views or politics of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Author notes
Present affiliation: Department of Pediatrics, Ullevål University Hospital, University of Oslo, Oslo, Norway.




