-
PDF
- Split View
-
Views
-
Cite
Cite
Seun O Oladipupo, Younes Laidoudi, John F Beckmann, Xing Ping Hu, Arthur G Appel, The prevalence of Wolbachia in multiple cockroach species and its implication for urban insect management, Journal of Economic Entomology, Volume 116, Issue 4, August 2023, Pages 1307–1316, https://doi.org/10.1093/jee/toad098
Close - Share Icon Share
Abstract
Cockroach management relies heavily on the use of conventional insecticides in urban settings, which no longer provide the anticipated level of control. Knowledge of cockroach endosymbionts, like Wolbachia, might provide novel avenues for control. Therefore, we screened 16 cockroach species belonging to 3 families (Ectobiidae, Blattidae, and Blaberidae) for the presence of Wolbachia. We mapped the evolution of Wolbachia-cockroach relationships based on maximum likelihood phylogeny and phylogenetic species clustering on a multi-loci sequence dataset (i.e., coxA, virD4, hcpA, and gatB) of Wolbachia genes. We confirmed the previous report of Wolbachia in 1 Ectobiid species; Supella longipalpa (Fab.), and detected the presence of Wolbachia in 2 Ectobiid species; Balta notulata (Stål) and Pseudomops septentrionalis Hebard, and 1 Blaberid species; Gromphadorhina portentosa (Schaum). All cockroach-associated Wolbachia herein detected were clustered with the ancestor of F clade Wolbachia of Cimex lectularius L. (bed bugs). Since Wolbachia provision C. lectularius with biotin vitamins that confer reproductive fitness, we screened the cockroach-associated Wolbachia for the presence of biotin genes. In toto, our results reveal 2 important findings: (i) Wolbachia is relatively uncommon among cockroach species infecting about 25% of species investigated, and (ii) cockroach-associated Wolbachia have biotin genes that likely provide nutritional benefits to their hosts. Thus, we discuss the potential of exploring Wolbachia as a tool for urban insect management.
Introduction
Wolbachia is a genus of gram-negative intracellular maternally transmitted bacteria. It belongs to the family Ehrlichiaceae (recently emended from Anaplasmataceae) (Hertig and Wolbach 1924, Hördt et al. 2020). Wolbachia infects a diverse range of arthropods and parasitic nematodes (Hilgenboecker et al. 2008, Werren et al. 2008). Taxonomical consensus for Wolbachia strains relies on the monophyletic lineage or groups, also known as supergroups or clades, which run from A to T, and the recently identified supergroup U from bat mites (Casiraghi et al. 2005, Kaur et al. 2021, Olanratmanee et al. 2021).
Wolbachia encompasses a broad spectrum of relationships with its host. Phenotypes range from reproductive modifications like feminization (Bouchon et al. 1998, Badawi et al. 2015), male-killing (Hurst and Majerus 1993, Perlmutter et al. 2019), parthenogenesis (Stouthamer et al. 1990, Hagimori et al. 2006), and cytoplasmic incompatibility (Laven 1957, Yen and Barr 1971, Beckmann and Fallon 2013). Wolbachia can also induce other phenotypic effects in their hosts such as pathogen blocking (Teixeira et al. 2008, Hughes et al. 2011, Fraser et al. 2020), alteration of host immune genes (Zhang et al. 2020), and provisioning of essential nutrients (Nikoh et al. 2014). As a result, Wolbachia represents a possible tool for manipulating host biology and a sustainable approach for insect management in certain situations (Laven 1967, O’Neill et al. 2019, Zheng et al. 2019). Wolbachia has been used to achieve population suppression and reduce vector competence of medical and agricultural insect pests. This is probably because Wolbachia substrate targets are conserved across insect species (Oladipupo et al. 2023). For example, Wolbachia-infected mosquitoes were released in Australia (Hoffmann et al. 2011). Likewise, in the United States (Mains et al. 2019, Crawford et al. 2020). Following the release, wild mosquito populations were transformed and their ability to transmit dengue was reduced (Hoffmann et al. 2011, Ross et al. 2022). Similarly, Wolbachia-mediated pathogen-blocking effects have been recorded under experimental conditions (Walker et al. 2011).
Urban entomology traditionally deals with the study of insect pests, such as cockroaches, that affect people and their property. Three cockroach families particularly stand out: Ectobiidae, Blattidae, and Blaberidae. The field of urban entomology has witnessed a paradigm shift from the use of conventional insecticides to safer and more sustainable options (Gondhalekar 2019, Oladipupo et al. 2022), especially for cockroach control. For example, RNA interference was used to silence the expression of the precopulatory protein presented by the male German cockroach, Blattella germanica (L.) (Myers et al. 2018). Such a tool in that study could distort orientation and prevent successful mating between a male and female German cockroach. Essential oils were incorporated in super absorbent polymer gels to compromise the reproductive fitness of female German cockroaches (Oladipupo et al. 2020a, 2020b). Deltaproteobacteria and Clostridia were used to induce pathogenicity in bed bugs (Pietri and Liang 2018). Injury among cockroach species was exploited to increase their susceptibility to parasitoids (Tee and Lee 2017). These studies highlight a shift from conventional insecticide applications to sustainable and eco-friendly alternatives.
As existing cockroach control strategies are not always sufficient (Pai et al. 2005, Fardisi et al. 2019) and should either be bolstered by other techniques (Perry and Choe 2020, Hamilton et al. 2021) or replaced by new methods (Oladipupo et al. 2020a, 2020b), Wolbachia represents a potential new, safe (Popovici et al. 2010) tool, yet untested in cockroach management. Two cockroach species, Supella longipalpa (Fab.) and B. germanica, are known to harbor Wolbachia (Vaishampayan et al. 2007, Jin et al. 2008). Wolbachia associated with S. longipalpa belongs to the Wolbachia F supergroup (Vaishampayan et al. 2007). Most Wolbachia strains from the F supergroup encode biotin and riboflavin synthesis pathways that provide planthoppers with nutrients necessary for fecundity (Ju et al. 2020, Pan et al. 2020). Bed bugs also rely on the F supergroup Wolbachia’s biotin synthesis operon of obligate intracellular microbes (BOOM) to provide B vitamins essential for their fitness and survival (Nikoh et al. 2014, Driscoll et al. 2020). Both bed bugs and cockroach co-infestation are common in homes. Consequently, an approach to control these populations might be to take advantage of this specific interdependence between Wolbachia supergroup F and these hosts by eliminating the endosymbiont. Such a system could inhibit host development. To exploit Wolbachia as a cockroach control strategy in the future, the first step is to map the phylogenetic relationship of the endosymbiont to predict possible phenotypic effects. The impact of this technique depends on 2 components: (i) whether the cockroaches are naturally infected with Wolbachia, and (ii) whether Wolbachia is essential for cockroach survival—which has not yet been proven.
As a classification tool, the multilocus sequence typing (MLST) method can elucidate Wolbachia’s phylogenetic lineages. It involves using a range (typically 4–7) of housekeeping genes (Baldo et al. 2006). For Wolbachia, we used the hcpA (hypothetical conserved protein in bacteria), coxA (encodes a subunit of the respiratory chain), gatB (synthesizes charged tRNAs), wsp (encodes outer surface protein), and virD4 (Type IV secretion system) (see Table 1) (Baldo et al. 2006). Understandably, the adequacy of MLST at strain differentiation has been challenged and whole-genome typing approaches have been suggested instead (Bleidorn and Gerth 2018). However, due to the difficulties in bacterial isolation, the whole-genome typing approach might only sometimes be feasible for Wolbachia at present. Consequently, the MLST system is optimized to reliably identify closely related Wolbachia strains and their host associations (Wang et al. 2020, Ramalho et al. 2021). The delineation of Wolbachia to the supergroup level suggests important ecological and functional characteristics that could be exploited for insect and disease biocontrol (Kaur et al. 2021). For example, Wolbachia supergroups A and B are reproductive manipulators in arthropods (Vandekerckhove et al. 1999) C and D enhance fertility and development in Onchocercidae nematodes (Bandi et al. 1998). Wolbachia supergroup E found in mites plays a role in thelytokous parthenogenesis (Konecka and Olszanowski 2021), while those of F coinfect insect arthropods and Onchocercidae nematodes (Mansonella spp.) and play a role in nutritional mutualism (Casiraghi et al. 2005, Ju et al. 2020). Supergroup M infects aphids inducing different phenotypic effects (Wang et al. 2014). In short, the supergroup-host association can initially reveal important implications for biotechnology development. To this end, Wolbachia’s infection status herein was explored in 3 cockroach families (i.e., Ectobiidae, Blattidae, and Blaberidae) using molecular tools. We mapped the phylogenetic relationship of cockroach-associated Wolbachia and discuss our findings’ implications for urban insect management.
| Gene (amplicon length) . | Function . | Primer sequence (5’–3’) . | Annealing temperature (°C) . | Extension time (s) . |
|---|---|---|---|---|
| Biotin C (700 bp) | Biotin synthesis | *BioC F2742- CCAACGTCGGTATCTGTTCCT BioC R3700- CAGCTACCACACATACCACT | 57.6 | 45 |
| Biotin H (1,000 bp) | Biotin synthesis | *BioH F3348- TGTAGCCTGTACCTGTAC CCA BioH R4123- GTGTTTTGTCACGGTTGGGG | 57.6 | 45 |
| coxA (460 bp) | Encodes a subunit of the respiratory chain | EF013 F- CCATATTAGCTGGAATTATTGGTGG EF014 R- GCGCACAAAAGGAATGTCATTAAC | 50 | 30 |
| gatB (210 bp) | Synthesizes charged tRNAs | EF010 F- GTGATGGYGATATRGARAARGG EF012 R- GTCAAGATACCTTATTRTTYG | 47.8 | 25 |
| hcp (400 bp) | Conserved protein in bacteria | EF015 F- CGYTCTGCTATMTTTGCYGC EF016 R- CYTGTGAARTAAARGATTTTGG | 53.6 | 33 |
| Histone (380 bp) | Provides structural support to the chromosome | *H3 AF - ATGGCTCGTACCAAGCAGACVGC H3 AR- ATATCCTTRGGCATRATRGTGAC | 58 | 40 |
| virD4 (257 bp) | Type IV secretion system | LM68 F- CCTACAGGYTCKGGYAARGGTG LM69 R- GCCAAAARTCYTGYTCAGGC | 60 | 30 |
| wsp (550 bp) | Outer surface protein | *WSP F- GTCCAATARSTGATGARGAAAC WSP R- CYGCACCAAYAGYRCTRTAAA | 55 | 60 |
| Universal cox1 | Component of the respiratory chain | LCO1490- GGTCAACAAATCATAAAGATATTGG HCO2198- TAAACTTCAGGGTGACCAAAAAATCA | 57.6 | 45 |
| Gene (amplicon length) . | Function . | Primer sequence (5’–3’) . | Annealing temperature (°C) . | Extension time (s) . |
|---|---|---|---|---|
| Biotin C (700 bp) | Biotin synthesis | *BioC F2742- CCAACGTCGGTATCTGTTCCT BioC R3700- CAGCTACCACACATACCACT | 57.6 | 45 |
| Biotin H (1,000 bp) | Biotin synthesis | *BioH F3348- TGTAGCCTGTACCTGTAC CCA BioH R4123- GTGTTTTGTCACGGTTGGGG | 57.6 | 45 |
| coxA (460 bp) | Encodes a subunit of the respiratory chain | EF013 F- CCATATTAGCTGGAATTATTGGTGG EF014 R- GCGCACAAAAGGAATGTCATTAAC | 50 | 30 |
| gatB (210 bp) | Synthesizes charged tRNAs | EF010 F- GTGATGGYGATATRGARAARGG EF012 R- GTCAAGATACCTTATTRTTYG | 47.8 | 25 |
| hcp (400 bp) | Conserved protein in bacteria | EF015 F- CGYTCTGCTATMTTTGCYGC EF016 R- CYTGTGAARTAAARGATTTTGG | 53.6 | 33 |
| Histone (380 bp) | Provides structural support to the chromosome | *H3 AF - ATGGCTCGTACCAAGCAGACVGC H3 AR- ATATCCTTRGGCATRATRGTGAC | 58 | 40 |
| virD4 (257 bp) | Type IV secretion system | LM68 F- CCTACAGGYTCKGGYAARGGTG LM69 R- GCCAAAARTCYTGYTCAGGC | 60 | 30 |
| wsp (550 bp) | Outer surface protein | *WSP F- GTCCAATARSTGATGARGAAAC WSP R- CYGCACCAAYAGYRCTRTAAA | 55 | 60 |
| Universal cox1 | Component of the respiratory chain | LCO1490- GGTCAACAAATCATAAAGATATTGG HCO2198- TAAACTTCAGGGTGACCAAAAAATCA | 57.6 | 45 |
F = forward; R = reverse; *biotin primers were retrieved from Balvín et al. (2018), histone primers from Baldo et al. (2006), and universal cox1 primers from Folmer et al. (1994). The rest of the primers were designed by SOO (see author contributions for more).
| Gene (amplicon length) . | Function . | Primer sequence (5’–3’) . | Annealing temperature (°C) . | Extension time (s) . |
|---|---|---|---|---|
| Biotin C (700 bp) | Biotin synthesis | *BioC F2742- CCAACGTCGGTATCTGTTCCT BioC R3700- CAGCTACCACACATACCACT | 57.6 | 45 |
| Biotin H (1,000 bp) | Biotin synthesis | *BioH F3348- TGTAGCCTGTACCTGTAC CCA BioH R4123- GTGTTTTGTCACGGTTGGGG | 57.6 | 45 |
| coxA (460 bp) | Encodes a subunit of the respiratory chain | EF013 F- CCATATTAGCTGGAATTATTGGTGG EF014 R- GCGCACAAAAGGAATGTCATTAAC | 50 | 30 |
| gatB (210 bp) | Synthesizes charged tRNAs | EF010 F- GTGATGGYGATATRGARAARGG EF012 R- GTCAAGATACCTTATTRTTYG | 47.8 | 25 |
| hcp (400 bp) | Conserved protein in bacteria | EF015 F- CGYTCTGCTATMTTTGCYGC EF016 R- CYTGTGAARTAAARGATTTTGG | 53.6 | 33 |
| Histone (380 bp) | Provides structural support to the chromosome | *H3 AF - ATGGCTCGTACCAAGCAGACVGC H3 AR- ATATCCTTRGGCATRATRGTGAC | 58 | 40 |
| virD4 (257 bp) | Type IV secretion system | LM68 F- CCTACAGGYTCKGGYAARGGTG LM69 R- GCCAAAARTCYTGYTCAGGC | 60 | 30 |
| wsp (550 bp) | Outer surface protein | *WSP F- GTCCAATARSTGATGARGAAAC WSP R- CYGCACCAAYAGYRCTRTAAA | 55 | 60 |
| Universal cox1 | Component of the respiratory chain | LCO1490- GGTCAACAAATCATAAAGATATTGG HCO2198- TAAACTTCAGGGTGACCAAAAAATCA | 57.6 | 45 |
| Gene (amplicon length) . | Function . | Primer sequence (5’–3’) . | Annealing temperature (°C) . | Extension time (s) . |
|---|---|---|---|---|
| Biotin C (700 bp) | Biotin synthesis | *BioC F2742- CCAACGTCGGTATCTGTTCCT BioC R3700- CAGCTACCACACATACCACT | 57.6 | 45 |
| Biotin H (1,000 bp) | Biotin synthesis | *BioH F3348- TGTAGCCTGTACCTGTAC CCA BioH R4123- GTGTTTTGTCACGGTTGGGG | 57.6 | 45 |
| coxA (460 bp) | Encodes a subunit of the respiratory chain | EF013 F- CCATATTAGCTGGAATTATTGGTGG EF014 R- GCGCACAAAAGGAATGTCATTAAC | 50 | 30 |
| gatB (210 bp) | Synthesizes charged tRNAs | EF010 F- GTGATGGYGATATRGARAARGG EF012 R- GTCAAGATACCTTATTRTTYG | 47.8 | 25 |
| hcp (400 bp) | Conserved protein in bacteria | EF015 F- CGYTCTGCTATMTTTGCYGC EF016 R- CYTGTGAARTAAARGATTTTGG | 53.6 | 33 |
| Histone (380 bp) | Provides structural support to the chromosome | *H3 AF - ATGGCTCGTACCAAGCAGACVGC H3 AR- ATATCCTTRGGCATRATRGTGAC | 58 | 40 |
| virD4 (257 bp) | Type IV secretion system | LM68 F- CCTACAGGYTCKGGYAARGGTG LM69 R- GCCAAAARTCYTGYTCAGGC | 60 | 30 |
| wsp (550 bp) | Outer surface protein | *WSP F- GTCCAATARSTGATGARGAAAC WSP R- CYGCACCAAYAGYRCTRTAAA | 55 | 60 |
| Universal cox1 | Component of the respiratory chain | LCO1490- GGTCAACAAATCATAAAGATATTGG HCO2198- TAAACTTCAGGGTGACCAAAAAATCA | 57.6 | 45 |
F = forward; R = reverse; *biotin primers were retrieved from Balvín et al. (2018), histone primers from Baldo et al. (2006), and universal cox1 primers from Folmer et al. (1994). The rest of the primers were designed by SOO (see author contributions for more).
Materials and Methods
Cockroach Collection and DNA Extraction
From March to July 2020, specimens (n = 100) representing 16 cockroach species belonging to 3 families: Ectobiidae, Blattidae, and Blaberidae, were collected either from the field around the Auburn University campus or retrieved from laboratory-maintained specimens in the Urban Entomology laboratory at Auburn University, Auburn, Alabama, USA (Table 2). As Wolbachia is concentrated in the reproductive tissues of insects (Hertig and Wolbach 1924), we dissected female medium-sized and large cockroaches to isolate reproductive tissues before DNA extraction according to Hoofman and Winston (1987) with slight modifications (Supplementary Appendix 1). For small-sized cockroaches, DNA was extracted and pooled from groups of 3–5 females, and 3–5 pooled samples were analyzed independently. DNA was extracted from the ovaries and analyzed from 5 to 8 individual females for medium-sized and large cockroaches.
| Family . | Speciesa . | Common name (US) . | Collection reference . | Number screened . |
|---|---|---|---|---|
| Ectobiidae (Oviparous) | Balta notulata (Stål) | Small-spotted cockroach | Field-collected | DNA was extracted and pooled from groups of 3–5 females for small cockroach species and 3–5 pooled samples were analyzed independently |
| Supella longipalpa (Fabricius) | Brown-banded cockroach | Field-collected | ||
| Blattella germanica (L.) | German cockroach | Field-collected and laboratory-maintained | ||
| Blattella asinahi Mizukubo | Asian cockroach | Field-collected and laboratory-maintained | ||
| Blattella vaga Hebard | Field cockroach | Laboratory-maintained | ||
| Pseudomops septentrionalis Hebard | Pale bordered field cockroach | Field-collected | ||
| Blattidae (Oviparous) | Eurycotis floridana (Walker) | Palmetto bug, Florida wood roach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Blatta lateralis (Walker) | Turkestan cockroach | Laboratory-maintained | ||
| Periplaneta fuliginosa (Serville) | Smokybrown cockroach | Laboratory-maintained | ||
| Periplaneta americana (L.) | American cockroach | Field-collected and laboratory-maintained | ||
| Blaberidae (ovoviviparous) | Nauphoeta cinerea Burmeister | Speckled cockroach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Gromphadorhina Portentosa (Schaum) | Madagascar hissing cockroach | Laboratory-maintained | ||
| Diploptera punctata (Eschscholtz) | Pacific beetle cockroach | Laboratory-maintained | ||
| Schultesia lampyridiformis Roth | Firefly mimic cockroach | Laboratory-maintained | ||
| Blaptica dubia Serville | Dubia cockroach | Laboratory-maintained | ||
| Leucophaea maderae (Fabricius) | Maderae cockroach | Laboratory-maintained |
| Family . | Speciesa . | Common name (US) . | Collection reference . | Number screened . |
|---|---|---|---|---|
| Ectobiidae (Oviparous) | Balta notulata (Stål) | Small-spotted cockroach | Field-collected | DNA was extracted and pooled from groups of 3–5 females for small cockroach species and 3–5 pooled samples were analyzed independently |
| Supella longipalpa (Fabricius) | Brown-banded cockroach | Field-collected | ||
| Blattella germanica (L.) | German cockroach | Field-collected and laboratory-maintained | ||
| Blattella asinahi Mizukubo | Asian cockroach | Field-collected and laboratory-maintained | ||
| Blattella vaga Hebard | Field cockroach | Laboratory-maintained | ||
| Pseudomops septentrionalis Hebard | Pale bordered field cockroach | Field-collected | ||
| Blattidae (Oviparous) | Eurycotis floridana (Walker) | Palmetto bug, Florida wood roach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Blatta lateralis (Walker) | Turkestan cockroach | Laboratory-maintained | ||
| Periplaneta fuliginosa (Serville) | Smokybrown cockroach | Laboratory-maintained | ||
| Periplaneta americana (L.) | American cockroach | Field-collected and laboratory-maintained | ||
| Blaberidae (ovoviviparous) | Nauphoeta cinerea Burmeister | Speckled cockroach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Gromphadorhina Portentosa (Schaum) | Madagascar hissing cockroach | Laboratory-maintained | ||
| Diploptera punctata (Eschscholtz) | Pacific beetle cockroach | Laboratory-maintained | ||
| Schultesia lampyridiformis Roth | Firefly mimic cockroach | Laboratory-maintained | ||
| Blaptica dubia Serville | Dubia cockroach | Laboratory-maintained | ||
| Leucophaea maderae (Fabricius) | Maderae cockroach | Laboratory-maintained |
aSpecies in bold are PCR-positive for the presence of Wolbachia.
| Family . | Speciesa . | Common name (US) . | Collection reference . | Number screened . |
|---|---|---|---|---|
| Ectobiidae (Oviparous) | Balta notulata (Stål) | Small-spotted cockroach | Field-collected | DNA was extracted and pooled from groups of 3–5 females for small cockroach species and 3–5 pooled samples were analyzed independently |
| Supella longipalpa (Fabricius) | Brown-banded cockroach | Field-collected | ||
| Blattella germanica (L.) | German cockroach | Field-collected and laboratory-maintained | ||
| Blattella asinahi Mizukubo | Asian cockroach | Field-collected and laboratory-maintained | ||
| Blattella vaga Hebard | Field cockroach | Laboratory-maintained | ||
| Pseudomops septentrionalis Hebard | Pale bordered field cockroach | Field-collected | ||
| Blattidae (Oviparous) | Eurycotis floridana (Walker) | Palmetto bug, Florida wood roach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Blatta lateralis (Walker) | Turkestan cockroach | Laboratory-maintained | ||
| Periplaneta fuliginosa (Serville) | Smokybrown cockroach | Laboratory-maintained | ||
| Periplaneta americana (L.) | American cockroach | Field-collected and laboratory-maintained | ||
| Blaberidae (ovoviviparous) | Nauphoeta cinerea Burmeister | Speckled cockroach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Gromphadorhina Portentosa (Schaum) | Madagascar hissing cockroach | Laboratory-maintained | ||
| Diploptera punctata (Eschscholtz) | Pacific beetle cockroach | Laboratory-maintained | ||
| Schultesia lampyridiformis Roth | Firefly mimic cockroach | Laboratory-maintained | ||
| Blaptica dubia Serville | Dubia cockroach | Laboratory-maintained | ||
| Leucophaea maderae (Fabricius) | Maderae cockroach | Laboratory-maintained |
| Family . | Speciesa . | Common name (US) . | Collection reference . | Number screened . |
|---|---|---|---|---|
| Ectobiidae (Oviparous) | Balta notulata (Stål) | Small-spotted cockroach | Field-collected | DNA was extracted and pooled from groups of 3–5 females for small cockroach species and 3–5 pooled samples were analyzed independently |
| Supella longipalpa (Fabricius) | Brown-banded cockroach | Field-collected | ||
| Blattella germanica (L.) | German cockroach | Field-collected and laboratory-maintained | ||
| Blattella asinahi Mizukubo | Asian cockroach | Field-collected and laboratory-maintained | ||
| Blattella vaga Hebard | Field cockroach | Laboratory-maintained | ||
| Pseudomops septentrionalis Hebard | Pale bordered field cockroach | Field-collected | ||
| Blattidae (Oviparous) | Eurycotis floridana (Walker) | Palmetto bug, Florida wood roach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Blatta lateralis (Walker) | Turkestan cockroach | Laboratory-maintained | ||
| Periplaneta fuliginosa (Serville) | Smokybrown cockroach | Laboratory-maintained | ||
| Periplaneta americana (L.) | American cockroach | Field-collected and laboratory-maintained | ||
| Blaberidae (ovoviviparous) | Nauphoeta cinerea Burmeister | Speckled cockroach | Laboratory-maintained | DNA was extracted and analyzed from 5–8 individual females |
| Gromphadorhina Portentosa (Schaum) | Madagascar hissing cockroach | Laboratory-maintained | ||
| Diploptera punctata (Eschscholtz) | Pacific beetle cockroach | Laboratory-maintained | ||
| Schultesia lampyridiformis Roth | Firefly mimic cockroach | Laboratory-maintained | ||
| Blaptica dubia Serville | Dubia cockroach | Laboratory-maintained | ||
| Leucophaea maderae (Fabricius) | Maderae cockroach | Laboratory-maintained |
aSpecies in bold are PCR-positive for the presence of Wolbachia.
PCR screening, cloning, and sequencing
Cockroach genomic DNA samples were PCR amplified, cloned, and Sanger sequenced using primers sets (Table 1) targeting 5 housekeeping genes: coxA, gatB, hcpA, wsp, and virD4 genes. All PCR reactions were conducted as described (Supplementary Appendix 1). All sequences were obtained in triplicates; the corresponding amplicon sizes are provided in Table 1. The Wolbachia-infected Drosophila simulans Sturtevant and D. melanogaster Meigen DNA, as well as DNA-free water and Wolbachia-free D. simulans and D. melanogaster DNA, were included as control templates at each PCR reaction. DNA samples were amplified for the target genes listed above in a total volume of 100 µl using high-fidelity polymerase Phusion (New England Biolabs). PCR cycling conditions were performed as reported (Supplementary Appendix 2). Amplified DNA was eluted by E.Z.N.A Gel Extraction Kit (Omega Bio-Tek).
NotI and BamHI restriction sites were added to forward and reverse primers, respectively, downstream cloning into pBluescript II SK+. PCR cycling conditions were performed as reported (Supplementary Appendix 2), and purified DNA amplicons were subcloned into pBluescript II SK+ (coxA, gatB, and hcpA) or pJET1.2/blunt (virD4 and wsp) vectors and transformed into competent Escherichia coli Top10F cells. Clones were screened for the presence of recombinant plasmids with the desired insert by gene-specific PCR. Mini-prepped plasmids were sent to Molecular Cloning Laboratories (San Francisco, CA) for forward and reverse Sanger sequencing.
In addition, both Biotin C (700 bp) and Biotin H (1,000 bp), and the cockroach cox1 gene (708 bp) were PCR amplified and sequenced using specific primer sets (Table 1) as described previously (Balvín et al. 2018) (see Supplementary Appendix 2 for more details). For each amplicon (i.e., wsp, VirD4, or histone), the expected bands were resolved through gel electrophoresis. Corresponding gels derived from the same PCR reaction were placed side by side (Fig. 1). All gels were processed in parallel using Adobe Photoshop 2020. The original full-length gels are provided (see Supplementary Appendix 3).
Phylogenetics
Gene dataset preparation and maximum likelihood phylogeny.
All available homologous DNA sequences (n = 942) of the searched Wolbachia housekeeping genes (i.e., coxA, virD4, hcpA, and gatB) were retrieved from the GenBank database using the Multiple Sequence Alignment Viewer (MSAV) 1.21.0 – NCBI (implemented as a function within Blastn). Reference sequences derived from draft/complete Wolbachia genomes were identified and aligned using MAFFT v7.490 software (Nakamura et al. 2018) before sequence concatenation with SeaView 5.0.5 software (Gouy et al. 2010). The concatenated reference sequences were then subjected to trimming using TrimAL v1.4. rev15 software (Capella-Gutierrez et al. 2009). The multisequence alignment file was split into 2 partitions: (i) the reference multilocus-sequence alignment (Ref-MLSA) containing the concatenated Wolbachia sequences from the cockroach species we sequenced and 34 other sequences from the reference entries described above, which encompasses Wolbachia supergroup A, B, D, F, J, and T, and (ii) query sequence alignment containing the remaining sequences derived from all gene datasets (n = 908). The maximum likelihood (ML) phylogeny was performed on the Ref-MLSA using IQTREE software (IQ-TREE multicore version 1.6.12 for Mac OS X 64-bit) (Nguyen et al. 2015) to generate the reference tree (Ref-Tree) under 1000 Ultra-Fast bootstrap (Hoang et al. 2018). The K3Pu (+F+G4) model was selected by ModelFinder (implemented as a function in IQTREE) (Kalyaanamoorthy et al. 2017) before tree computation.
Phylogeny postanalysis.
To evaluate Wolbachia supergroup diversity, analysis based either on the phenetic (assemble species by automatic partitioning [ASAP]) (Puillandre et al. 2021) and the automatic barcode gap discovery (ABGD) for primary species delimitation (Puillandre et al. 2012) or on the ML phylogeny (Poisson tree processes, i.e., PTP) (Zhang et al. 2013) and its Bayesian version (bPTP) (Toussaint et al. 2016). The general mixed Yule-coalescent (GMYC) (Fujisawa and Barraclough 2013) were performed on the Ref-MLSA and the Ref-Tree, respectively. Species delimitation analysis was conducted within the iTaxoTools 0.1 software (Vences et al. 2021). The partitioned results of all delimitation algorithms were then analyzed simultaneously under the Limes software (Ducasse et al. 2020).
Biotin and cockroach–Wolbachia cospeciation.
To test whether the biotin synthesis genes (Fig. 2) coevolved within the cockroach Wolbachia compared to their host phylogeny, comparative event-based analysis and cophylogenetic reconciliations were investigated on the Wolbachia hcpA against the concatenated biotin C and H genes. Briefly, each sequence dataset (i.e., hcpA, biotins C and H) of cockroach-Wolbachia strains obtained were aligned against those from the F clade Wolbachia infecting bed bugs. We chose to align with the bed bug Wolbachia because it contains a functional biotin gene with an indispensable role in bed bug development (Nikoh et al. 2014, Balvín et al. 2018, Hickin et al. 2022). The cox1 sequences of their bed bug hosts (Balvín et al. 2018) were aligned using MAFFT v7.490 software (Nakamura et al. 2018). Biotin genes were concatenated using SeaView 5.0.5 software (Gouy et al. 2010). Neighbor-joining phylogenesis was performed on each sequence alignment (i.e., hcpA, concatenated Biotin C/H, and host cox1) using MEGA6 software (Tamura et al. 2013). The cophylogenetic reconciliation between each combination of Wolbachia/host trees was performed using the maximum parsimony reconciliations (MPRs) based on the duplication-transfer-loss model within eMPRess v1 software (Santichaivekin et al. 2021). The co-speciation (host and endosymbiont speciate simultaneously) represents the null hypothesis with 0 cost event, duplication (intra-host speciation), loss (host speciates but endosymbiont fails to establish in one of the new lineages), and transfer (representing an inheritance of a Wolbachia from a kind species reflecting a crucial event in the evolution of the symbiotic relationships between species). For each cophylogenetic reconciliation, the cost of 4.63, 5.89, and 1 were selected for the duplication, transfer, and loss, respectively, using the eMPRess v1 software (Santichaivekin et al. 2021).

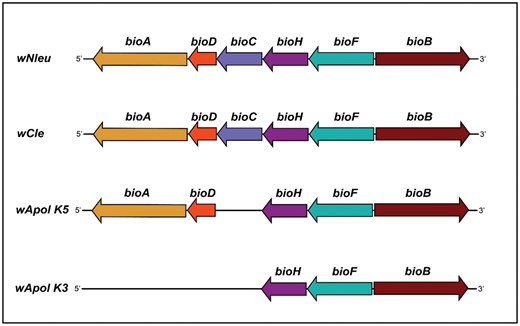
Organization and molecular analysis of the biotin operons of Wolbachia. The complete pathway for biotin is present in Wolbachia from Nomada eucophthalma (wNlue) supergroup A, and Wolbachia from bedbug Cimex lectularius (wCle) supergroup F only. Wolbachia from Atemnus politus, (wApol) lacks the bioC, bioA, and bioD genes. The presence of bioC and bioH genes in the biotin operon indicates the presence of a functional biotin operon (Lefoulon et al. 2020).
Results
PCR Screening and Phylogenetics Reveal Novel Cockroach Wolbachia Strains
Using PCR, we screened 16 cockroach species belonging to 3 families: Ectobiidae, Blattidae, and Blaberidae (Table 1). Only 4 of the cockroach species screened harbored Wolbachia (Fig. 1). The 4 Wolbachia-infected cockroach species were Gromphadorhina portentosa from the Blaberidae (ovoviviparous), along with Supella longipalpa, Pseudomops septentrionalis, and Balta notulata from the Ectobiidae (oviparous). After infections were detected, we sought to classify the Wolbachia from these 4 cockroach hosts in cladogram of Wolbachia. We cloned and Sanger sequenced 5 MLST markers (i.e., coxA, virD4, hcpA, wsp, and gatB) and concatenated consensus sequences from these isolates. Hereafter we refer to these strains as Wolbachia of G. portentosa (wGpo), S. longipalpa (wSlo), P. septentrionalis (wPse), and B. notulata (wBno). These strains clustered within the F clade that include Wolbachia from Cimex lectularius (wCle), the common bed bug (Fig. 3). Accordingly, all delimitation algorithms performed either on the ML tree (Ref-Tree) or the concatenated sequence alignment (Ref-MLSA) files classified these strains as an integral part of F clade Wolbachia. The pairwise homoplasy index (PHI) test detected significant recombination events throughout the Wolbachia strains (P-value = 3 × 10−15) (Fig. 4). While the recombination events of wCle were detected within the virD4 gene fragment, the recombination events were located in the wsp gene fragment of wBno and wGpo strains, followed by the hcpA gene of wPse and the gatB gene fragment of wSlo strains (Fig. 3).
(A) Cockroach families and species PCR screened for the presence of Wolbachia. Species in red fonts are strongly positive for Wolbachia (B) Cockroach PCR gels show amplicons from 16 cockroach species. wsp (top), virD4 (middle) primers, and histone (bottom—control for DNA quality). Water, Wolbachia-cured Drosophila simulans, and D. melanogaster are negative controls. Wolbachia-infected D. simulans and D. melanogaster are positive controls. Four cockroach species are strongly positive for Wolbachia: Balta notulata, Supella longipalpa, Pseudomorphs septentrionalis, and Gromophorina portentosa. Others are negative. For each amplicon (i.e., wsp, virD4, or histone), corresponding cropped gels (i.e., gels separated by white space in B) were derived from the same PCR reaction. All gels were processed in parallel using Adobe Photoshop 2020. The full-length original gels are provided (see Supplementary Appendix 3).
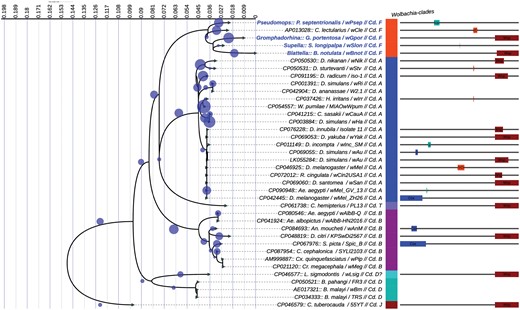
Maximum likelihood (ML) phylogeny showing the distribution of Wolbachia clades. The tree corresponds to the IQTREE inferred from 38 concatenated genes. A total of 1,544 positions with 28.7% of informative sites. Accession numbers and species names are indicated at the tip of each branch. The bold blue label indicates the sequence obtained in the study. The tree includes 908 query sequences (blue circle) representing all GenBank entries of Wolbachia gene sequences. The color-coded bare indicated the result of delimitation algorithms. The size and position of recombination events throughout the alignment are shown.

Least-cost evolutionary reconstruction between F clade Wolbachia (blue) and their arthropod hosts (black) based on the concatenated biotin C and H genes (right figure) and the hcpA gene (left tree) with the coxA host tree at each time. The best maximum parsimony reconciliation (MPR) was selected for the biotin tree while a unique MPR presentation was found for the hcpA tree. A total of 9 co-speciation, 1 duplication, 6 transfer, and 8 loss events were mapped out for both reconciliation trees using preselected cost-event spaces of 4.63, 5.89, and 1 selected for duplication, transfer, and loss, respectively.
Biotin and Cockroach–Wolbachia Cospeciation
Based on available Wolbachia genome data, the presence of biotin C and H genes suggests that a biotin operon is functional (Lefoulon et al. 2020). Thus we sought to PCR amplify and sanger sequence these 2 genes from the Wolbachia-infected cockroaches. The result of event-based analysis on the concatenated biotin C and H from F clade Wolbachia strains (i.e., cockroach and Cimicidae-associated Wolbachia) replicated the same output as the housekeeping gene hcpA from the same Wolbachia strains regarding the cox1 phylogeny of their hosts. A total of 9 co-speciation events, 1 duplication, 6 counts of transfer, and 8 counts of losses were identified throughout both tree reconciliations. As a result, a strong time consistency was found between both tree reconciliations, thereby refuting the null hypothesis, that is, Wolbachia and host trees are similar due to chance (P-value = 0.0099) (Fig. 4). Both biotin C/H and hcpA reconciliations showed a transfer event between cockroach-associated Wolbachia lineage and Cimicidae-associated Wolbachia with functional biotin (Balvín et al. 2018) (Fig. 4).
Discussion
The types of relationships between Wolbachia and its host imply relevant insights that could be exploited for host control (Nikoh et al. 2014, Ju et al. 2020, Pan et al. 2020). Therefore, we investigated the distribution of Wolbachia within 3 cockroach families: Ectobiidae, Blattidae, and Blaberidae, to understand the relationship between cockroach-associated Wolbachia and their hosts. Our results reveal 3 significant findings: (i) Wolbachia is relatively uncommon among cockroach species, (ii) cockroach-associated Wolbachia are clustered within the ancestor F clade Wolbachia, and (iii) cockroach-associated Wolbachia have biotin genes that likely provide nutritional benefits to their cockroach hosts. Thus, we discuss the potentiality of exploring Wolbachia as a tool for urban insect management.
The present study confirmed the previous reports on the occurrence of F clade Wolbachia within cockroach species like S. longipalpa (Vaishampayan et al. 2007, Gibson and Hunter 2009) and extended knowledge on B. notulata, G. portentosa, and P septentrionalis as new hosts for Wolbachia (Floate et al. 2006). We were unable to detect the presence of Wolbachia in B. germanica as previously reported (Jin et al. 2008). This suggests that geographical structure likely plays a role in Wolbachia infection frequency in host. In Lepidoptera, Wolbachia infection frequency is influenced by abiotic factors such as latitudinal gradient and geographical location (Ahmed et al. 2015). Since US and China share a relatively close latitudinal gradient, this appears not to be the case here. In short, about 25% of the cockroach species screened harbor Wolbachia. The absence of Wolbachia DNA from all other Blattella spp. (i.e., B. vaga, B. germanica, and B. asinahi) holds 2 important implications: (i) Wolbachia infections are not common in cockroach species and (ii) Wolbachia can form a symbiotic association with cockroaches.
The bioinformatics approach combining maximum likelihood (ML) phylogeny and automated species delimitation algorithms conducted herein on a multilocus sequence typing (MLST) of coxA, virD4, hcpA, and gatB genes identified all cockroach-associated Wolbachia as a monophyletic lineage within the reference F clade strain of the common bed bug C. lectularius. The F supergroup contains Wolbachia infecting termites (e.g., genus Coptotermes, Odontotermes, Kalotermes), crickets (Hapithus agitator), and scorpions (Opistophthalmus). MLST is a valid way to estimate phylogenetic relationships between closely related Wolbachia and the identification of their supergroups (Baldo and Werren 2007, Wang et al. 2020). This is because the number of Wolbachia whole-genome data sets available to date is too small, and only the MLST system is optimized enough to reliably identify closely related Wolbachia strains and their host associations (Wang et al. 2020).
We were able to PCR amplify and sequence the biotin C and H genes in the Wolbachia-associated cockroaches. The presence of both genes, which is a rarity in many sequenced Wolbachia genomes (Nikoh et al. 2014, Ju et al. 2020, Lefoulon et al. 2020), was reported only from Wolbachia strains with complete biotin operon. Thus, the presence of biotin C and H genes in these cockroach species suggests the likely presence of a biotin operon. A functional BOOM in Wolbachia provisions biotin that plays a substantial role in the adaptation, evolution, and diversification of their insect hosts (Nikoh et al. 2014, Driscoll et al. 2020). For example, Wolbachia-cured C. lectularius suffered about a 50% reduction in the number of eggs laid (Hosokawa et al. 2010). Subsequently, only about 20% of the 1st instar nymphs from these hatched eggs developed to adulthood (Hosokawa et al. 2010). This suggests that Wolbachia BOOM contributes significantly to bed bug fitness. It is now known that the relationship between these biotin-producing Wolbachia and their insect hosts is obligate nutritional mutualism (Nikoh et al. 2014). Like wCle, the Wolbachia-associated cockroaches belong to the F supergroup. Wolbachia’s nutritional and obligate benefits to their hosts are a vulnerability that could be exploited. From a control standpoint, a parsimonious approach would be to use antibiotic baits to cure Wolbachia in natural populations of cockroaches such as S. longipalpa and P. septentrionalis, known to cluster around or in homes. Understandably, before this can be sought, 2 crucial experiments are necessary. First, the nutritional benefits of Wolbachia in their cockroach hosts must be ascertained. Second, cockroaches are omnivorous. If the nutritional benefits are confirmed, then how the broad diets of cockroaches could be supplemented by biotin should be clarified.
The report of Wolbachia in these cockroach species, and previous reports of the ubiquitous cockroach obligate endosymbiont, Blattabacterium, suggests may be the time is right to extend the definition of sustainable and eco-friendly alternatives to include endosymbionts as currently done with RNAI and other interventions (Gondhalekar 2019). For example, Blattabacterium plays a substantial role in nitrogen recycling and essential amino acid provisioning for cockroaches (López-Sánchez et al. 2009). Wolbachia-mediated control as currently implemented with mosquitoes (Crawford et al. 2020, Ross et al. 2022) is an effective population suppression tool. Specifically, Wolbachia-infected males crossed with uninfected females produce no viable offspring (Beckmann et al. 2017). Thus, endosymbionts central to urban insect survival could be explored and exploited for control.
In conclusion, we report the presence of Wolbachia in 3 previously unreported cockroach species; B. notulata, P. septentrionalis, and G. portentosa. This cockroach-associated Wolbachia is nested in the Wolbachia supergroup F. Our findings suggest 2 potential applications. (i) The likely presence of a biotin operon in this Wolbachia suggests nutritional symbiosis. Future studies might exploit cockroach nutritional dependence on Wolbachia for the control of these nuisance insects. (ii) Wolbachia was present only in 25% of cockroach species investigated. Furthermore, the absence of Wolbachia in most cockroaches could allow for embryonic microinjection of cytoplasmic incompatibility inducing Wolbachia, into ootheca of species that do not naturally harbor Wolbachia to generate a transinfected line. For example, a population of transinfected Aedes albopictus lines was generated using embryonic microinjection (Xi et al. 2006). On Wolbachia stabilization, males can then be released with uninfected or incompatible females to achieve population suppression. Taken together, from an economic standpoint, the exploration of endosymbionts as a tool to suppress urban insect pests, such as cockroaches, could potentially become viable in the future.
Acknowledgments
The authors are grateful to Chelsea Smith (Auburn University) for help with cockroach collection, Samia Bedjaoui (Higher National Veterinary School, Issad Abbes, Oued Smar, Algiers) for help with data analysis, and Luis Mendez (Auburn University) for molecular consultation.
Funding
This research was funded in part by #BlackinEnto (S. O. O.), the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA), the Alabama Agricultural Experiment Station (AAES), and AAES Hatch/Multistate Grants ALA015-1-18039 (A. G. A), ALA015-1-19126 (X. P. H), ALA015-1-05005 (X. P. H. and A. G. A.), Auburn University’s Department of Entomology and Plant Pathology Startup Funds (J. F. B.), and USDA-NIFA Hatch Grant 1015922 (J. F. B.).
Conflict of Interest
The authors have no conflict of interest to declare.
Author Contributions
Seun Oladipupo (Conceptualization-Equal, Data curation-Lead, Formal analysis-Lead, Funding acquisition-Supporting, Investigation-Lead, Methodology-Lead, Software-Equal, Visualization-Equal, Writing – original draft-Lead, Writing – review & editing-Lead), Younes Laidoudi(Formal analysis-Supporting, Investigation-Supporting, Methodology-Supporting, Software-Equal, Visualization-Supporting, Writing – original draft-Supporting, Writing – review & editing-Supporting), John Beckmann (Conceptualization-Equal, Data curation-Supporting, Funding acquisition-Equal, Investigation-Supporting, Methodology-Supporting, Project administration-Equal, Resources-Equal, Software-Equal, Supervision-Equal, Validation-Equal, Visualization-Lead, Writing – original draft-Supporting, Writing – review & editing-Supporting), Xing Ping Hu (Conceptualization-Equal, Funding acquisition-Equal, Investigation-Supporting, Methodology-Supporting, Project administration-Equal, Resources-Equal, Supervision-Equal, Validation-Equal, Writing – original draft-Supporting, Writing – review & editing-Supporting), Arthur Appel (Conceptualization-Equal, Funding acquisition-Equal, Investigation-Supporting, Methodology-Supporting, Project administration-Equal, Resources-Equal, Supervision-Equal, Validation-Equal, Writing – original draft-Supporting, Writing – review & editing-Supporting)
Data Availability
All DNA sequences generated in the present study are available from the GenBank database under the accession numbers: ON261168-ON261171 for cox1 gene for cockroach hosts, ON406439-ON406442 for the gatB,ON406443-ON406446 for the hcpA,ON406447-ON406450 for the virD4, ON406451-ON406454 for biotin C, ON406455-ON406458 for the biotin H, and ON419495-ON419498 for coxA genes of Wolbachia. The descriptive pipeline and dataset used in the present study are available as a Git-hub repository: https://github.com/YLdz-SM/Cockroach-associated-Wolbachia.git.



