-
PDF
- Split View
-
Views
-
Cite
Cite
Bernadette Biondi, Francesco S Celi, Elizabeth A McAninch, Critical Approach to Hypothyroid Patients With Persistent Symptoms, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 10, October 2023, Pages 2708–2716, https://doi.org/10.1210/clinem/dgad224
Close - Share Icon Share
Abstract
Hypothyroidism is a common condition, and numerous studies have been published over the last decade to assess the potential risks associated with this disorder when inappropriately treated. The standard of care for treatment of hypothyroidism remains levothyroxine (LT4) at doses to achieve biochemical and clinical euthyroidism. However, about 15% of hypothyroid patients experience residual hypothyroid symptoms. Some population-based studies and international population-based surveys have confirmed dissatisfaction with LT4 treatment in some hypothyroid patients. It is well established that hypothyroid patients treated with LT4 exhibit higher serum thyroxine:triiodothyronine ratios and can have a persistent increase in cardiovascular risk factors. Moreover, variants in deiodinases and thyroid hormone transporter genes have been associated with subnormal T3 concentrations, persistent symptoms in LT4-treated patients, and improvement in response to the addition of liothyronine to LT4 therapy.
The American (ATA) and European Thyroid Association (ETA) guidelines have recently evolved in their recognition of the potential limitations of LT4. This shift is reflected in prescribing patterns: Physicians’ use of combination therapy is prevalent and possibly increasing. Randomized clinical trials have recently been published and, while they have found no improvement in treating hypothyroid patients, a number of important limitations did not allow generalizability. Meta-analyses have reported a preference rate for combination therapy in 46.2% hypothyroid patients treated with LT4. To promote discussions about an optimal study design, the ATA, ETA, and British Thyroid Association have recently published a consensus document.
Our study provides a useful counterpoint on the controversial benefits of treating hypothyroid patients with combination therapy.
In endogenous euthyroidism, the thyroid produces and secretes thyroxine (T4) and triiodothyronine (T3) in levels that normalize serum thyrotropin (TSH). Levothyroxine (synthetic T4, LT4) monotherapy administered at doses to achieve a normal serum TSH concentration came to be adopted as the standard of care for treatment of overt hypothyroidism (1-6) after the landmark discovery of peripheral T4-to-T3 conversion (7). LT4 is safe, efficacious in treating the sequalae of severe hypothyroidism, convenient, and inexpensive (1-3). Most patients with hypothyroidism do well with this therapeutic strategy and experience restoration of clinical and biochemical euthyroidism. However, a minority of patients, about 15% to 20%, experience residual hypothyroid symptoms despite normalization of the serum TSH (4-6). This has led some investigators, providers, and patients to wonder whether thyroid hormone signaling is universally restored among LT4-treated patients. Indeed, there is mounting basic and clinical evidence that there is genetic diversity in the thyroid hormone signaling pathway that can lead to small, but potentially clinically significant, differences in serum T3 availability (4, 5). Likewise, it is increasingly well recognized in clinical trials that select patients benefit from the addition of liothyronine (synthetic T3, LT3) in so-called LT4 + LT3 “combination therapy” (4, 5). In the available randomized clinical trials, the superiority of combination therapy has not been consistently demonstrated (5, 8) but these initial studies likely had major limitations. Before larger and more sophisticated clinical trials can provide additional insight (5), providers are often left in the clinical scenario with how to treat a patient with hypothyroidism and residual symptoms to achieve the best possible clinical outcome.
Using LT4 + LT3 combination therapy is made more complex by the pharmacokinetics of currently available formulations of LT3. LT3 has a short half-life and its administration can result in supraphysiologic serum peaks, unlike the physiologic, stable serum T3 levels observed in endogenous euthyroidism. There are also potential safety concerns due to a relative lack of long-term outcome data from trials, but the currently available data from clinical studies are overall reassuring. All thyroid professional societies now acknowledge that clinicians can consider an n-of-1 therapeutic trial of LT4 + LT3 combination therapy in nonpregnant adults with residual symptomatology due to hypothyroidism (4-6). Indeed, prescribing patterns show that more combination therapy is being used (9), but this topic is still one of great controversy and confusion, so much so that at the 2022 Endocrine Society and American Thyroid Association (ATA) meetings, LT4 + LT3 combination therapy was the topic of the Meet the Professor Series sessions. Here, we the speakers outline various approaches to a common clinical scenario to illustrate the options in the management of hypothyroidism.
Clinical Case Vignette
A 40-year-old woman was seen for routine evaluation of postsurgical hypothyroidism. She had a personal history of Hashimoto thyroiditis and a family history of autoimmune diseases (Graves disease, vitiligo, and celiac disease), and LT4 was started at a dose of 1.2 mcg/kg/d) when hypothyroidism was diagnosed. She had a multinodular goiter, and 2 years after her first evaluation the patient underwent total thyroidectomy following the diagnosis of papillary thyroid cancer (low risk). LT4 dosage was increased after surgery (to 1.4 mcg/kg/d; in her case, 125 mcg/d) because of the well-documented necessity for higher doses of LT4 in athyreotic patients compared to those with autoimmune hypothyroidism (1). Despite the increase in LT4 dosage, the patient complained of persistent symptoms of hypothyroidism (fatigue, brain fog, depression, and weight gain of 4 kg up from her baseline of 85 kg), despite unchanged lifestyle after surgery. Suboptimal control of hypothyroidism during LT4 therapy was investigated and possible factors interfering with LT4 therapy considered (1, 2). Therefore, the possibility of poor adherence due to continuous shift to different manufacturers and/or incorrect LT4 dosage and/or administration were excluded. In addition, the patient was not taking any drugs interfering with LT4 absorption or inducing an increased turnover or excretion of LT4 for concomitant illnesses (1, 2). Associated autoimmune diseases occur in 14% of patients with Hashimoto disease and include autoimmune endocrine and nonendocrine disorders; therefore, the coexistence of celiac disease, autoimmune gastritis, adrenal insufficiency, type 1 diabetes, and other autoimmune conditions were also excluded. Our final evaluation led to the conclusion that the patient was taking appropriate doses of LT4 with good compliance; moreover, the LT4 doses did not exceed the theoretical daily dose calculated according to her body weight.
The evaluation of the thyroid function in our patient confirmed the presence of biochemical euthyroidism during the periodic follow-up, showing a normal serum TSH and the presence of thyroid hormones within the normal reference range (serum was TSH 0.98 mIU/L at the last evaluation); however, free T4 (FT4) levels were in the upper limits of the normal reference range with an increase in FT4/FT3 ratio. Moreover, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL) levels increased after surgery, and so did blood pressure with the onset of diastolic hypertension (Fig. 1).
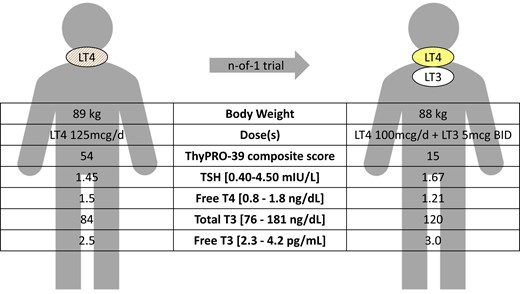
Clinical case of a 40-year-old woman with postsurgical hypothyroidism, on levothyroxine (LT4) monotherapy with normal thyroid function tests. However, she had symptoms that could be related to residual symptoms of hypothyroidism, including fatigue, brain fog, depression, and weight gain. Other etiologies that could possibly contribute to these symptoms were ruled out; she had no comorbidities that would preclude use of liothyronine (LT3) and was not planning for pregnancy. She and her provider opted for a trial of therapy with LT4 + LT3 combination therapy. Three months after starting combination therapy, her serum thyrotropin (TSH) was similar to baseline, the T4:T3 ratios had lowered, she was able to lose 1 kg, and her ThyPRO-39 composite score improved (0-100; higher scores represent worse quality of life) (62). The patient stated that she preferred the combination therapy, so she and her provider agreed to continue the regimen.
Significance of the Clinical Problem
Data from international surveys (10, 11) have shown that some patients with hypothyroidism can be dissatisfied despite adequate LT4 therapy. This dissatisfaction could be associated with the patients’ expectation that replacement therapy with LT4 would resolve all symptoms and/or to a negative relationship with their general practitioner or thyroid specialist (10, 11). The persistence of symptoms could lead unsatisfied patients to participate in surveys and share their negative experience, and this could represent a bias in the interpretation of the results of LT4 therapy. The relevant causes of dissatisfaction reported in the ATA survey were fatigue or reduced energy (77%), difficulty with weight management (69%), memory or other cognitive problems with thinking (58%), and mood (45%) (10). These symptoms of hypothyroidism can overlap with symptoms of other potential associated disorders as they are nonspecific. For instance, fatigue is a common symptom among the general population, being associated with physical, psychological, and social stress (12). Therefore, the causal relationship between patients’ symptoms, thyroid dysfunction, and its treatment remains poorly understood. In addition, whether thyroid autoimmunity per se is responsible for persistent symptoms remains unclear (13).
Hypothyroid-associated brain fog is frequently reported in unsatisfied patients receiving LT4; it is characterized by fatigue, depressed mood, and cognitive difficulties in the areas of memory and executive function, with lack of mental clarity and poor concentration leading to a considerable impairment of quality of life (14). Weight gain after surgery is also very common among patients receiving LT4. Some studies indicate a net increase in body weight following total thyroidectomy, with the greater gain occurring within the first 2 years following surgery (15) This suggests that athyreotic LT4-treated individuals can have a higher body mass index despite lower reported calorie consumption (16).
Interestingly, our patient had worsened hypercholesterolemia and developed diastolic hypertension, which are both objective signs of hypothyroidism, although not always specific in the presence of coexistent obesity. Dyslipidemia, arterial hypertension, endothelial dysfunction, and insulin resistance play a crucial role in the development of atherosclerosis in patients with hypothyroidism, leading to an increased morbidity and mortality in patients with overt disease and in those with subclinical hypothyroidism (SHypo) and serum TSH of 10 mIU/L or greater (3, 17). Even the moderate form of SHypo (defined by a serum TSH 7-9.9 mIU/L) can be considered for treatment with LT4 for the increased risk of coronary heart disease mortality and fatal stroke (17). These recommendations on SHypo are based on the results of meta-analyses and references published in high-impact journals (17). Thyroid hormone influences cholesterol metabolism, and hypothyroid patients can have an impaired lipid profile with raised levels of TC and increased oxidation of LDL, which promotes atherogenesis (18, 19). Data from meta-analyses and large randomized controlled studies suggest a trend in recovering the lipid profile during LT4 therapy with TSH normalization (18, 19). However, the efficacy of replacement doses of LT4 to completely normalize the impaired lipid profile of hypothyroid patients is under debate because of the lack of large prospective studies considering the different variables (severity of hypothyroidism, genetic background, age, lifestyle, etc) that can affect this evaluation. A systematic review and meta-analysis in adults with overt hypothyroidism assessed this intriguing issue and concluded that LT4-treated hypothyroid patients with normal serum TSH levels had 3.31 ± 1.64 mg/dL higher serum LDL levels (P = .044) and 9.60 ± 3.55 mg/dL higher serum TC levels (P = .007) compared to controls (19), confirming similar findings in rodent models of hypothyroidism during LT4 monotherapy. The American College of Cardiologists and the American Heart Association recommend normalizing LDL values with lipid-lowering drugs, especially in patients with high cardiovascular (CV) risk (20). In some population studies, hypothyroid individuals on replacement therapy used more statins (21). Muscle pain, myopathy, and elevated creatine kinase levels are commonly present in untreated hypothyroid patients, and the onset of rhabdomyolysis and severe myositis correlates with the severity of hypothyroidism. The coexistence of hypothyroidism and statin therapy may increase the risk of myopathy, and symptoms of hypothyroidism can overlap with complications of statin treatment (22). For this reason, the treatment of hypercholesterolemia is more difficult in hypothyroid patients.
Rationale for Treatment
The patient described in the vignette meets the criteria for the diagnosis of “an athyreotic patient with persistent symptoms and poor quality of life, despite the evidence of biochemical euthyroidism during replacement therapy with LT4.” The presence of symptoms and (relative to the lipid profile) biochemical signs compatible with hypothyroidism prompts the question whether the patient has a deficit in conversion of the exogenous LT4 into T3. Genetic background and functional events (weight changes, concomitant inflammation) can affect the peripheral metabolism of thyroid hormone. In healthy individuals, 20% of serum T3 is derived from thyroidal T3 secretion and 80% originates from the extrathyroidal conversion of T4 into T3 catalyzed by type 1 and type 2 deiodinases (D1 and D2) (16). Our patient had an increase in FT4/FT3 ratio during LT4 therapy, supporting the possibility that extrathyroidal T3 production might not always be adequate during LT4 monotherapy to compensate for absent thyroidal T3 secretion after total thyroidectomy (16). A large survey showed that athyreotic patients with normal TSH during LT4 treatment had a shift to the right in the distribution curve of FT4 value and a shift to the left in that of FT3 compared to a healthy population; thus, FT4 levels were above the upper normal limit in 7.2% of the population and FT3 values below the lower normal limit in 15.2% of patients (23). Low FT3/FT4 ratios occurred in 29.6% of patients despite comparable serum TSH levels (23). These data could suggest that while LT4 therapy is very effective in normalizing serum TSH levels, it could fail in restoring universal tissue euthyroidism because of the differential expression of thyroid hormone transporters and iodothyronine deiodinases in different target tissues. The effect of each deiodinase on the local T3 content is different and some tissues depend largely on serum T3 for their T3 content, whereas others can produce T3 for themselves by deiodination of local T4. In humans, the T3 content in TSH-secreting cells is greatly influenced by the local D2 activity, whose preferred substate is T4. The thyrotroph is highly enriched in D2, making this tissue highly sensitive to exogenous LT4 with a resulting “inappropriate” suppression of TSH secretion when compared with the metabolic needs of the peripheral tissues (24). As a consequence, normal serum TSH levels do not necessarily indicate a euthyroid state for all peripheral tissues. In addition, while the changes in thyroid economy during LT4 are well described in the literature, their relationship to patients’ symptoms remain unclear, including the contradictory findings on deiodinase polymorphisms.
The common Thr92Ala D2 polymorphism is associated with subtle differences in TSH (and T3) secretion following a thyrotropin-releasing hormone injection (25), and a study in athyreotic patients indicates that carriers of the Ala92 allele require a higher dose of LT4 to achieve TSH suppression (26), suggesting that this mutation may play a role in the patient's clinical manifestations (27). Moreover, data from Panicker et al (28) suggest that carriers of the Ala92 allele have a significant improvement in quality of life following LT3 and LT4 combination. Of note, these data have not been replicated (29). Moreover, other genetic polymorphisms of the deiodinases and other components of thyroid hormone signaling have been associated with small but measurable changes in circulating thyroid hormone levels (27, 30) and potentially with clinical presentation (31). On the other hand, the point estimate at the population level is low (32) and one should consider that the interaction between different genetic polymorphisms may amplify or nullify the effect of a single polymorphism. For example, a second polymorphism (−258 A/G, also reported as 258 A[D2-ORFa-Gly3Asp]) in the D2 gene, which is not in linkage disequilibrium with the Thr92Ala, was associated with lower FT4 concentrations (33). A pharmacogenomic study demonstrated that carriers of the minor allele achieved lower TSH and FT4 levels following a thyrotropin-releasing hormone injection while maintaining peak T3 levels identical to controls. These findings are consistent with an activation of D2 (34). In vitro data indeed demonstrated that the −258 A/G polymorphism results in an increase in D2 transcription due to loss of a repressor site in the D2 gene promoter region (35). Collectively, the data indicate that the regulation of the peripheral conversion of thyroid hormone is likely the result of multiple genetic (and potentially functional) factors rather than a single dominant genetic variant. Thus, several strategies could be deployed to overcome this condition.
Potential Options for Treatment
To improve symptoms in our patient, we assessed different therapeutic options.
Option 1: Increased Levothyroxine Dosage to Achieve Low-Normal Serum Thyrotropin
Our patient increased LT4 dosage on her own by taking an extra pill per week to try to improve her quality of life. Six months later, she came to us with palpitations, insomnia, and nervousness. Her serum TSH was undetectable and FT4 values were slightly increased. Around 20% of treated hypothyroid patients are overreplaced during LT4 therapy. In an old study, some patients with primary hypothyroidism reported improved well-being when LT4 dosage was titrated to obtain a serum TSH in the lower part of the reference range (36). However, two double-blind, randomized clinical trials with a crossover design in subjects with primary hypothyroidism showed that a regimen of three doses of LT4 to achieve a TSH in the upper, middle, or lower part of the reference range did not produce significant changes in hypothyroid symptoms, well-being, quality of life (37, 38) mood or cognition (38); however, patients preferred the doses they thought were higher (38). Moreover, in randomized, double-blind studies, unchanged, higher, or lower LT4 doses in patients with hypothyroidism did not have major effects on energy expenditure or body composition (39). Although similar to previous reports, LT4-treated patients preferred higher LT4 doses (39). TSH suppression during LT4 therapy did not improve body weight in our patient, whereas, interestingly, serum levels of LDL were normalized. Cholesterol levels tend to normalize in athyreotic patients only when TSH is mildly suppressed and T3 normalized during LT4 therapy (40). However, there is evidence that undetectable serum TSH and higher FT4 levels, even at the upper limit of the normal reference range, can result in a risk of atrial arrhythmias and atrial fibrillation and increase CV risk and risk of osteoporosis (41). We discussed with our patient the negative effects of TSH suppression, and she reduced the LT4 dosage again, back to her baseline that previously resulted in normal serum TSH.
Option 2: Therapeutic Trial With Desiccated Thyroid
The physiologic T4-to-T3 ratio secreted by the human thyroid is approximately 14:1, of which about 5 to 6.5 mcg of T3 is secreted daily (5). Desiccated animal thyroid has been used to treat hypothyroidism for more than a century (42), but its reputation was tarnished many decades ago by inconsistencies in manufacturing. However, modern desiccated porcine thyroid (DT) is now manufactured according to USP standards and is US Food and Drug Administration (FDA) regulated, but not yet FDA approved (43, 44). Yet, DT is still being prescribed (9, 45). The T4-to-T3 ratio in DT is approximately 4:1, thus containing potentially supratherapeutic T3 doses (during its relatively short distribution half-life) and subtherapeutic T4 doses. One grain (60 mg) of DT contains about 38 mcg of T4 and 9 mcg of T3. As the pharmacodynamic equivalence of T4 to T3 is around 3:1 (44), then one can propose a reasonable starting dose of DT knowing the LT4 dose at which the patient's serum TSH is normalized. If serum T3 levels are measured a few hours after the ingestion of the DT, it is likely that supratherapeutic serum T3 levels will be noted. In the absence of thyrotoxic symptoms, the potential risks of such transient hypertriiodothyroninemia have not been demonstrated in clinical trials (46). It is becoming increasingly better recognized that some patients exhibit a preference for DT over LT4 monotherapy and LT4 + LT34 combination therapy (45, 46), DT can normalize serum TSH (47, 48), and that several small clinical trials found no adverse effects in short-term follow-up (47, 48).
Option 3: Therapeutic Trial With Liothyronine
The substitution of LT3 for LT4 represents the most radical approach to the imputed deficit of peripheral conversion of thyroid hormone. This strategy has been used in a small study whereby individuals devoid of endogenous thyroid hormone production were treated in a double-blind, crossover intervention with LT4 or LT3, with the therapeutic goal of a TSH of 0.5 to 1.5 mcIU/mL (44). After a period of sustained (45 days) TSH within therapeutic target, study volunteers were then admitted to a clinical research unit for detailed metabolic testing before crossing over to the other treatment arm under the same regimen. The LT3 treatment resulted in a significantly higher T3 concentration when compared to LT4, demonstrating that the thyrotroph is uniquely sensitive to T4 levels (44). Compared to the LT4 (standard of care), LT3 treatment resulted in a significant weight loss and decrease in TC and LDL (44). Of interest, no significant difference was observed in depression or quality of life, and no significant adverse event was recorded in the LT3 treatment arm (one episode of anxiety was observed in a patient receiving LT4 with a TSH within normal range). This study indicated the pharmaco-equivalence of LT3 for LT4 (mcg/mcg) is 0.3:1 (44). Owing to the short distribution half-life of T3 (2.3 ± 0.11 hours) (44), even distributing the doses on a thrice-daily regimen resulted in significant peaks and troughs of T3 concentrations up to 32% of the time. It is important to note that the concern for exposure to peaks in T3 concentrations above normal range are extrapolated from the well-known toxicity resulting from sustained elevation of T3. Another potential concern derives from the rapid, nongenomic effects of T3 that have been characterized primarily in endothelial cell systems in vitro. Interestingly, a recent study demonstrated that healthy volunteers exposed to a single dose of LT3 able to acutely increase the T3 concentration by 328 ± 57 ng/dL did not experience any measurable CV effects during a 4-hour interval following administration (49). These data seem to support the notion that in vivo the metabolic effects of T3 are due to genomic (transcriptional) effects. To this end, temporary fluctuations of T3 concentration would have little to no clinically relevant effect. This is important since one of the major hurdles associated with LT3-based therapy is the perceived need to use multiple daily administration regimens, which are inherently associated with reduced adherence to treatment. One major contraindication for the use of LT3-alone therapy is the fact that our hypothetical patient is of reproductive age, and this treatment modality is not indicated during pregnancy because of the risk of causing fetal hypothyroidism.
Option 4: Therapeutic Trial With Levothyroxine Plus Liothyronine
A more common approach to the treatment of dissatisfied patients is the LT4 + LT3 combination therapy. In this case the therapeutic goal is the replacement of the thyroidal contribution to the serum T3 pool, and potentially from a relative deficit in peripheral conversion of T4-to-T3. The daily production of T3 for a 70-kg individual is estimated to be 30 mcg, of which 5 to 6 mcg is released by the thyroid, and the rest (∼ 25 mcg/d) deriving from peripheral conversion (50). Thus, a “physiologic” replacement therapy aimed to provide only the T3 lost by functional or surgical removal of the thyroid would require minimal doses of LT3. This is indeed the strategy endorsed (within the confinement of individual experimentation) by the European Thyroid Association (ETA), with a proposed LT3:LT4 ratio of 1:16, with a twice-daily administration regimen (4). In practicality, should this approach be applied to an individual taking LT4 150 mcg/d, the resulting regimen would be 92 mcg LT4 and 3.25 mcg LT3, the latter on a twice-daily regimen. Mathematical modeling indicates that this approach would result in a 17% increase in serum T3 (16 ng/dL) with serum levels consistently within normal range. A more practical approach, guided by the LT3 formulations available in the US market and by the empirical data on LT3-LT4 pharmaco-equivalence, would entail decreasing the levothyroxine dose to 125 mcg while administering 5 mcg LT3 twice daily. This strategy would result in a 44% increase in serum T3 (41 ng/dL) with serum levels consistently within normal range. Conversely, a more aggressive regimen of decreasing the levothyroxine dose to 100 mcg while administering 10 mcg LT3 twice daily would result in a 88% increase in average serum T3 (82 ng/dL). Of note, with this approach one can expect serum levels above normal range for approximately 14% of the time (47). As discussed in the “LT3 alone” section, the twice-daily administration regimen of LT3 is based on the concern for fluctuations in circulating levels of T3. As discussed, recent data seem to support the notion that no clinically relevant thyroid hormone action can be ascribed to rapid, nongenomic effects of T3 (49-51). To this end, empirical observations should be performed to assess whether a single LT3 administration is safe and effective (51). This could in turn dramatically simplify the treatment regimen, increasing patient adherence.
Clinical Trial Results of Combination Therapy
Attempts to improve residual symptomatology on LT4 monotherapy, and better approximate physiologic serum T4:T3 ratio, have been made in more than a dozen modern clinical trials of combination therapy with LT4 plus LT3. Interestingly, these have had somewhat inconsistent results, with meta-analyses failing to demonstrate the superiority of combination therapy and even having inconclusive results regarding patient preference (8, 52, 53) One substantial barrier to the interpretation of these clinical trials has been their heterogeneity in design and recruitment (52). For example, T4:T3 ratios differed greatly from 4:1 to 19:1, most were performed with the LT3 administration once daily and only a few using twice daily, and the duration of follow-up ranged from a mere 5 weeks to 1 year (5). The ATA and the ETA have acknowledged the difficulties with heterogeneity among available clinical trial data and have proposed key design elements for new trials (4, 5). Importantly, the majority of randomized controlled trials evaluating combination therapy did not specifically recruit patients with residual hypothyroid symptoms or prevalent dissatisfaction (5, 8); there was 75% consensus among experts that those individuals most likely to benefit from combination therapy may not yet have been included in trials in sufficient numbers to detect an adequately powered response (5).
Since several key studies have shown a differential psychological benefit and preference for combination therapy with the Thr92AlaD2 genotype (27, 28), and there is some clinical evidence that the prevalent Thr92AlaD2 may dampen T3 signaling (27, 54) and cause cellular stress due to aberrant localization in the Golgi apparatus (55, 56), interest in applying the concept of personalized medicine to hypothyroidism treatment has been ignited. The benefit of combination therapy among Thr92AlaD2 carriers in population studies has not been consistently reproduced (57), but interest remains given possible mechanistic implications. Thus in the ATA, ETA, and British Thyroid Association Consensus Statement, there was 100% consensus among experts that future trials of combination therapy should consider (i) including genotyping for the Thr92AlaD2 polymorphism, (ii) should be adequately powered to study the effect of this polymorphism on study outcomes, and (iii) should be adequately powered to study the effect of polymorphisms in thyroid hormone transporters (eg, MCT8, MCT10, OATP1C1) on study outcomes (5). Until new, and likely very expensive, clinical trial data can be ascertained, clinicians ultimately must consider the biochemical parameters, symptomatology, and any comorbidities when deciding whether to pursue an n-of-1 trial of therapy with their patient.
Potential Benefits and Harms of Long-term Combination Therapy
There is lack of data on the safety of long-term safety use of combination therapy with LT4 and LT3. Most of the published studies on combination therapy had a short duration and involved heterogeneous and a relatively small number of patients. An old study that used up to 60 mcg of LT3 per day reported an increased risk of palpitations among patients receiving LT3 (58). Moreover, atrial arrhythmias have been reported in overtreated patients during LT3 and LT4 treatment. A 17-year, observational, population-based study in Scotland of patients receiving thyroid hormone replacement therapy compared the results of 400 LT3 users with those of 33 955 patients taking LT4 (59). During follow-up, the median TSH was higher for patients receiving LT4 alone (2.08 vs 1.07 mU/L; P < .001) Patients using LT3 (with or without LT4) had no increased risk of CV disease (hazard ratio [HR] 1.04; 95% CI, 0.70-1.54), atrial fibrillation (HR 0.91; 95% CI, 0.47-1.75), or fractures (HR 0.79; 95% CI, 0.49-1.27) after adjusting for age (59). There was no difference in the number of prescriptions for bisphosphonates or statins although there was an increased risk of new prescriptions for antipsychotic medication (HR 2.26; 95% CI, 1.64-3.11; P < .0001), which was proportional to the number of LT3 prescriptions. In addition, the authors reported a nonsignificant trend toward an increase in breast cancer and new use of antidepressant medications. The risk for breast cancer was not increased in a Swedish registry study of 57 5461 individuals, 1147 of whom were taking LT3 (60). No increased all-cause mortality or cancer mortality, including breast cancer, was identified during a median follow-up of 8.1 years in LT3 users vs LT4 users (60). A systematic review of 18 clinical trials comparing combined therapy (LT4 + LT3) vs monotherapy (LT4) for adult patients with hypothyroidism did not report a significant difference in adverse events between the treatment groups. However, a meta-analysis was not possible because only a few heterogeneous studies have assessed the CV and bone effects of combined LT4 + LT3 therapy (8). In a retrospective multicenter trial, the risks of heart failure and stroke were higher in LT3 users with a history of thyroid cancer and those who underwent 52 weeks or less of LT3 therapy when compared to LT4-only users (61). Potential adverse events of combined therapy should be further clarified. Future large, prospective, randomized controlled trials would be necessary to establish the potential effects of combination treatment on bone and heart and the optimal LT4-to-LT3 ratio to avoid harmful effects. Treatment with LT3 is not advisable in patients with a history of arrhythmias and chronic ischemic heart disease and is not recommended in pregnant women, children, or older patients. LT3 and combined therapy are contraindicated, and LT4 monotherapy should be administered with caution in patients with abnormal conduction pathway.
We have no evidence in favor of the superiority of combination therapy compared to LT4 treatment in hypothyroid patients. However, the pooled prevalence rate for preference of combination therapy over LT4 was 46.2% (95% CI, 40.2%-52.4%) (P = .231 for the difference from chance) in a meta-analysis including data from 7 double-blind, randomized controlled trials in which patients and investigators were blinded to treatment distribution (53). In sensitivity analyses, combination treatment preference was explained in part by treatment effects on TSH concentration, mood, and symptoms, but not quality of life or body weight (53). Self-medication among unsatisfied LT4-treated patients is frequent and often leads clinicians to opt for combination therapy. Many patients are overreplaced with LT4 monotherapy or DT, and clinicians should discuss with their patient the importance of periodic follow-up to avoid overtreatment.
Returning to the Patient
After counseling on the risks and benefits of all treatment options, our patient decided to embark on a trial of combination therapy, and we agreed that she was an appropriate candidate. LT4 + LT3 doses estimates can be obtained by knowing what LT4 dose made her serum TSH normal and understanding that the pharmacodynamic equivalence of T4 to T3 is around 3:1 (39). Although the physiologic T4-to-T3 ratio in humans is approximately 14:1, LT3 is available only in 5-, 25-, and 50-mcg tablets, so multiples of 2.5 mcg (half tablet) are usually employed, and ratios approximated.
The patient’s LT4 dose was decreased from 125 mcg/day to 100 mcg/day, and LT3 at 5 mcg twice daily was added. After about 6 weeks her serum thyroid hormone levels were assessed, exactly 3 hours after ingestion of the morning LT3 dose. Her serum TSH was 1.67 mIU/L, FT4 was 1.21 ng/dL, total T3 was 120 ng/dL, and FT3 was 3.0 pg/mL (see Fig. 1). Her subjective symptoms of fatigue, brain fog, and depression improved, but she still had difficulty losing weight. She stated that she preferred the LT4 + LT3 combination therapy over LT4 monotherapy. With this therapeutic response, we agreed to continue this regimen and repeat thyroid function tests in about 3 months. Although our patient is young and healthy now, at what age LT3 should be discontinued is not known. We recommended that she has postmenopausal bone density screenings per the guidelines, and if she were to develop future osteopenia or osteoporosis, we would recommend counseling the patient to transition back to a regimen of LT4 monotherapy. Likewise, if she develops cardiac disease in the future, we would recommend transitioning back to a regimen of LT4 monotherapy, as the safety of LT3 in patients with comorbid osteopenia, osteoporosis, heart disease, and atrial fibrillation is unknown. If the patient were considering pregnancy, she should notify us when she plans to conceive and ideally transition to LT4 monotherapy before conception. If she develops vasomotor symptoms with menopause, a trial of decreasing LT3 to 2.5 mcg twice daily and adjusting the LT4 dose to achieve a normal serum TSH could be attempted.
Summary and Suggested Approach
The standard of care for the treatment of hypothyroidism remains LT4 at doses to achieve biochemical and clinical euthyroidism. However, it is well established that some hypothyroid patients can have a relative T3 deficiency within the peripheral tissues despite LT4 treatment. Moreover, variants in deiodinases and thyroid hormone transporter genes have been associated with subnormal T3 concentrations, persistent symptoms in LT4-treated patients, and improvement in response to the addition of LT3 to LT4 therapy.
The ATA and ETA guidelines have recently evolved in their recognition of the potential limitations of LT4. This shift is reflected in prescribing patterns: Physicians’ use of combination therapy is prevalent and possibly increasing. They suggest that patients who feel “unwell,” “not benefited,” or who have “persistent complaints” could be considered for a trial of combination therapy. The increased use of DT products from the thyroid glands of domesticated animals and using overreplacement therapy with LT4 to improve symptoms, weight, and quality of life can lead to important adverse effects on the heart and bone for the risk of iatrogenic subclinical thyrotoxicosis. While in the aggregate randomized clinical trials have found no improvement in treating hypothyroid patients, several important limitations did not allow generalizability.
An algorithm for the evaluation of patients with hypothyroidism with persistent symptoms during LT4 treatment is shown in Fig. 2. Medical history and physical examination may suggest possible causes of biochemical hypothyroidism that need to be excluded. If LT4 treatment fails to improve symptoms and clinical signs of hypothyroidism in patients with biochemical euthyroidism, clinicians and patients should discuss the decision to replace LT4 treatment with combination therapy. There is still plenty of empirical information missing, and carefully designed studies are absolutely necessary.
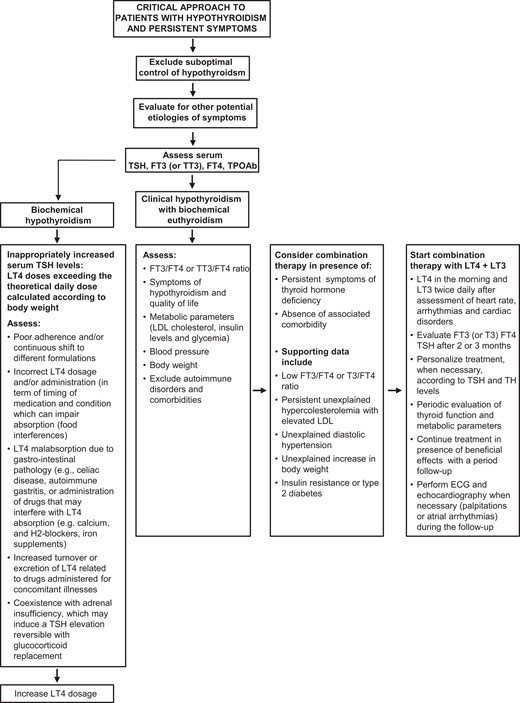
Critical approach to patients with hypothyroidism and persistent symptoms.
Funding
The work of F.S.C. is supported in part by the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (grant No. 1 R21 DK122310-01A1).
Disclosures
The authors declare that they have no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
Abbreviations
- ATA
American Thyroid Association
- CV
cardiovascular
- D2
type 2 deiodinase
- DT
desiccated porcine thyroid
- ETA
European Thyroid Association
- FT3
free triiodothyronine
- FT4
free thyroxine
- HR
hazard ratio
- LDL
low-density lipoprotein cholesterol
- LT3
liothyronine
- LT4
levothyroxine
- SHypo
subclinical hypothyroidism
- T3
triiodothyronine
- T4
thyroxine
- TC
total cholesterol
- TSH
thyrotropin



