-
PDF
- Split View
-
Views
-
Cite
Cite
Louise McMenemy, Fearghal P. Behan, Josh Kaufmann, David Cain, Alexander N. Bennett, Christopher J. Boos, Nicola T. Fear, Paul Cullinan, Anthony M.J. Bull, Andrew T.M. Phillips, Alison H. McGregor, Association Between Combat‐Related Traumatic Injury and Skeletal Health: Bone Mineral Density Loss Is Localized and Correlates With Altered Loading in Amputees: the Armed Services Trauma Rehabilitation Outcome (ADVANCE) Study, Journal of Bone and Mineral Research, Volume 38, Issue 9, 1 September 2023, Pages 1227–1233, https://doi.org/10.1002/jbmr.4794
Close - Share Icon Share
ABSTRACT
The association between combat‐related traumatic injury (CRTI) and bone health is uncertain. A disproportionate number of lower limb amputees from the Iraq and Afghanistan conflicts are diagnosed with osteopenia/osteoporosis, increasing lifetime risk of fragility fracture and challenging traditional osteoporosis treatment paradigms. The aim of this study is to test the hypotheses that CRTI results in a systemic reduction in bone mineral density (BMD) and that active traumatic lower limb amputees have localized BMD reduction, which is more prominent with higher level amputations. This is a cross‐sectional analysis of the first phase of a cohort study comprising 575 male adult UK military personnel with CRTI (UK‐Afghanistan War 2003 to 2014; including 153 lower limb amputees) who were frequency‐matched to 562 uninjured men by age, service, rank, regiment, deployment period, and role‐in‐theatre. BMD was assessed using dual‐energy X‐ray absorptiometry (DXA) scanning of the hips and lumbar spine. Femoral neck BMD was lower in the CRTI than the uninjured group (T‐score −0.08 versus −0.42 p = .000). Subgroup analysis revealed this reduction was significant only at the femoral neck of the amputated limb of amputees (p = 0.000), where the reduction was greater for above knee amputees than below knee amputees (p < 0.001). There were no differences in spine BMD or activity levels between amputees and controls. Changes in bone health in CRTI appear to be mechanically driven rather than systemic and are only evident in those with lower limb amputation. This may arise from altered joint and muscle loading creating a reduced mechanical stimulus to the femur resulting in localized unloading osteopenia. This suggests that interventions to stimulate bone may provide an effective management strategy. © 2023 Crown copyright and The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR). This article is published with the permission of the Controller of HMSO and the King's Printer for Scotland.
Introduction
Recent conflicts in Iraq and Afghanistan have resulted in large numbers of personnel exposed to combat related traumatic injury (CRTI) including 265 UK military personnel sustaining a total 416 amputations, most frequently of the lower limb.(1) The mean age at time of amputation was 22 years, an age at which osteoporosis is uncommon.(2) In studies of amputees over 5 years following amputation disproportionate numbers (more than 50%) of amputees are diagnosed with osteoporosis and osteopenia.(3‐5) CRTI can induce systemic inflammation(6) and hormonal changes,(7) both of which have been shown to lead to osteoporosis. CRTI with traumatic amputation has the potential to lead to osteoporosis through not only systemic inflammation and hormonal changes but also altered loading. Although a documented long‐term complication of lower limb amputation is osteoporosis, this is often observed in older less active subjects with comorbidities, thus it is unknown whether this is secondary to systemic changes or changes to the loading environment.(3,8,9) A comparison of CRTI and traumatic amputation enables the differences between systemic effects and localized effects to be investigated due to the change in loading on the amputated limb.
The long‐term consequences of osteopenia and osteoporosis include stress fractures, femoral neck and vertebral wedge fractures, with serious implications on loss of mobility, physical dependency, and morbidity, including compromising future prosthetic use. Although there is currently no direct evidence that amputation influences fracture risk, indirectly lower bone mineral density (BMD) would imply a greater risk. Current systemic treatments include hormone replacement therapy and use of bone‐preserving medication such as bisphosphonates, but such treatments have been shown to have adverse effects elsewhere in the body.(10,11) It is therefore important to understand whether amputees suffer from systemically driven osteopenia/osteoporosis or whether it is a localized phenomenon similar to disuse osteopenia.
There is some evidence in the literature from studies in older groups, using X‐ray data as a surrogate of BMD, few numbers of amputees, without control groups, and without controlling or accounting for differences in activity levels, smoking or body mass index (BMI), that amputee bone loss is commonly localized to the amputated limb.(12‐15) Thus it is postulated that BMD loss in amputees is a mechanical phenomenon, similar to disuse osteopenia, where altered, nonphysiological loading, post‐amputation, drives progressive bone loss over the course of many remodeling cycles. Literature has suggested this might come from: offloading by the predominantly ischial tuberosity weight bearing prosthetic socket(13) that would be worse for above knee amputees than below knee amputees, bedrest immediately postsurgery,(12) reduced activity as ambulation becomes more challenging,(16,17) and significantly lowered muscle forces from extreme atrophy.(18) Diagnosis discordance in measures of BMD can be used to investigate local versus systemic phenomena, where minor discordance is found when the scores at two different measurement sites are separated by one diagnostic class. Major discordance is when one site is considered osteoporotic and the other normal.(19) The presence of discordance means that the BMD loss is localized and not systemic.
The aim of this study was to test the following hypotheses in a prospective military cohort:
BMD in the CRTI group is lower than the uninjured group;
BMD in the femoral neck of the amputated limb of lower limb amputees is lower than in the CRTI non‐amputee or uninjured groups;
BMD loss is greater in above knee amputees than below knee amputees; and
BMD changes in the amputee population exhibit higher levels of diagnosis discordance than in the populations of CRTI non‐amputee and uninjured groups.
Subjects and Methods
Study design and participants
The Armed Services Trauma Rehabilitation Outcome (ADVANCE) study(20) is a prospective cohort study comprising 575 male adult UK military personnel (UK‐Afghanistan War 2003 to 2014; 153 lower limb amputees of varying amputation type and level) with CRTI (mean of 8 years since injury) who were frequency‐matched to 562 uninjured men by age, service, rank, regiment, deployment period, and role‐in‐theater. The adjusted recruitment response rates (excluding those who had died, had no known contact details or for whom no contact was attempted) were equivalent between groups at 58.0%. CRTI was defined as injury requiring aeromedical evacuation.(21) Ethical approval was received from the Ministry of Defence Research Ethics Committee and all participants provided written consent prior to inclusion. All lower limb amputee subjects were below or above‐knee amputees in at least one limb.
Variables
Dual‐energy X‐ray absorptiometry (DXA) scanning was used to measure BMD and T‐score at both the lumbar spine and femoral neck. T‐score was selected (as opposed to Z‐score) because the patient population is young therefore comparison to a healthy 30‐year‐old is appropriate to age. Measurements presented from the femoral neck of CRTI non‐amputee, bilateral amputees, and uninjured control participants represent an average of both right and left legs. In unilateral amputee participants, the data of one limb was used for analysis separately as opposed to either the amputated or non‐amputated limb.
The following data were also collected to control for any factors that could account for differences in amputee and uninjured BMD:
Age;
Height, or preinjury height for bilateral amputees;
Adjusted body mass, to include the lost mass of the amputated limb(s)(22);
Adjusted BMI;
Smoking status (pack years), to account for the known adverse effects of smoking on bone turnover(23,24); and
Activity levels (using the International Physical Activity Questionnaire [IPAQ],(25) a validated patient‐reported outcome measure where moderate activity is defined as “activities.… [that] make you breathe somewhat harder than normal” and vigorous activity as “activities that take hard physical effort and make you breathe much harder than normal”).
Statistical methods and sample size
Forty‐three amputees are required to detect a clinically significant difference between T‐score at the spine and femoral neck with 80% power and 1% level of significance(16,19); unilateral (as opposed to bilateral) amputees are required to detect a clinically significant difference between T‐score in the femur of amputated and contralateral limbs.(15) A clinically significant difference here is defined as a change from one diagnostic class to another.
Statistical analysis was carried out using Stata Version 16 (StataCorp, College Station, TX, USA). For comparisons between two groups (uninjured versus CRTI), independent t tests were used for normally distributed data, and Mann‐Whitney U tests for non‐normally distributed data. For comparisons between three or more groups (non‐injured versus CRTI non‐amputees versus amputees, subcategories of amputees), one‐way ANOVAs were used for normally distributed data, whereas the Kruskal Wallis test was used for non‐normally distributed data. Where significant differences were found, post‐hoc pairwise comparisons with Bonferroni corrections were conducted.
Logistic binary regression with femoral neck pathology (T‐score less than −1) as the outcome measure was performed to investigate the contribution of the following variables on femoral neck BMD pathology: amputee status, smoking history, total IPAQ walking minutes, total IPAQ activity minutes, and adjusted BMI. To assess discordance, Fisher's exact test tests were performed for differences in magnitude and type (spine lower than hip or hip lower than spine) of discordance between controls, injured non‐amputees, and amputees. A conservative threshold of p < 0.01 was set for statistical significance of all analyses due to the large sample size to reduce the chances of significant results for very small differences.
Results
The injured and uninjured groups are summarized in Table 1. Adjusted body mass and BMI were significantly higher in the injured compared to uninjured participants. There was no statistical difference in the T‐score at the spine between the whole injured and non‐injured groups (p = 0.959, Table 1). However, the whole injured group demonstrated a reduced femoral neck T‐score compared to the uninjured group (p < 0.001, Table 1).
Participant Details of Uninjured, Injured, and Injured Subgroups (Non‐amputees, Unilateral, and Bilateral Amputees)
| Uninjured | Injured (whole cohort) | p | Injured (non‐amputee) | Injured (unilateral amputee) | Injured (bilateral amputee) | |
| Participant details | n = 562 | n = 575 | n = 419 | n = 81 | n = 75 | |
| Age (years) | 34.3 (30.7 to 37.8) | 33.6 (30.7 to 37.8) | 0.368 | 33.8 (30.6 to 38.4) | 32.9 (30.2 to 35.9) | 33.0 (30.9 to 36.2) |
| Height (m) | 1.79 (1.75 to 1.83) | 1.79 (1.75 to 1.84) | 0.108 | 1.79 (1.74 to 1.83) | 1.80 (1.76 to 1.85) | 1.80 (1.75 to 1.86) |
| Adjusted body mass (kg) | 86.8 (80.0 to 95.2) | 88.8 (80.4 to 99.4) | 0.008 | 87.4 (79.9 to 97.8) | 93.7 (82.0 to 102.7) | 93.1 (78.8 to 102) |
| Adjusted BMI (kg/m2) | 27.2 (25.0 to 29.3) | 27.8 (25.5 to 30.5) | 0.002 | 27.5 (25.4 to 30.1) | 28.1 (26.2 to 31.1) | 29.2 (25.1 to 32.1) |
| Smoke pack years | 0 (0 to 5.5) | 0 (0 to 6.0) | 0.581 | 0 (0 to 5.0) | 0 (0 to 11.1) | 0 (0 to 6.5) |
| T‐scores | n = 562 | n = 567 | n = 414 | n = 79 | n = 74 | |
| Lumbar spine (L1–L4) | −0.19 (−0.89 to +0.51) | −0.28 (−0.91 to +0.60) | 0.959 | −0.26 (−0.86 to +0.66) | −0.31 (−0.90 to +0.58) | −0.40 (−1.35 to +0.39) |
| n = 562 | n = 560 | n = 414 | n = 79 | n = 67 | ||
| Femoral neck | −0.08 (−0.65 to +0.47) | −0.42 (−1.13 to +0.25) | 0.000 | −0.19 (0.78 to +0.44) | −1.13 (−1.69 to −0.44) | −1.67 (−2.22 to −0.76) |
| Uninjured | Injured (whole cohort) | p | Injured (non‐amputee) | Injured (unilateral amputee) | Injured (bilateral amputee) | |
| Participant details | n = 562 | n = 575 | n = 419 | n = 81 | n = 75 | |
| Age (years) | 34.3 (30.7 to 37.8) | 33.6 (30.7 to 37.8) | 0.368 | 33.8 (30.6 to 38.4) | 32.9 (30.2 to 35.9) | 33.0 (30.9 to 36.2) |
| Height (m) | 1.79 (1.75 to 1.83) | 1.79 (1.75 to 1.84) | 0.108 | 1.79 (1.74 to 1.83) | 1.80 (1.76 to 1.85) | 1.80 (1.75 to 1.86) |
| Adjusted body mass (kg) | 86.8 (80.0 to 95.2) | 88.8 (80.4 to 99.4) | 0.008 | 87.4 (79.9 to 97.8) | 93.7 (82.0 to 102.7) | 93.1 (78.8 to 102) |
| Adjusted BMI (kg/m2) | 27.2 (25.0 to 29.3) | 27.8 (25.5 to 30.5) | 0.002 | 27.5 (25.4 to 30.1) | 28.1 (26.2 to 31.1) | 29.2 (25.1 to 32.1) |
| Smoke pack years | 0 (0 to 5.5) | 0 (0 to 6.0) | 0.581 | 0 (0 to 5.0) | 0 (0 to 11.1) | 0 (0 to 6.5) |
| T‐scores | n = 562 | n = 567 | n = 414 | n = 79 | n = 74 | |
| Lumbar spine (L1–L4) | −0.19 (−0.89 to +0.51) | −0.28 (−0.91 to +0.60) | 0.959 | −0.26 (−0.86 to +0.66) | −0.31 (−0.90 to +0.58) | −0.40 (−1.35 to +0.39) |
| n = 562 | n = 560 | n = 414 | n = 79 | n = 67 | ||
| Femoral neck | −0.08 (−0.65 to +0.47) | −0.42 (−1.13 to +0.25) | 0.000 | −0.19 (0.78 to +0.44) | −1.13 (−1.69 to −0.44) | −1.67 (−2.22 to −0.76) |
Note: Values are median (interquartile range). Values of p represent univariable comparisons between uninjured and injured participants (whole injured cohort). Bold values represent significant results, p < 0.01, Bonferroni corrected.
Participant Details of Uninjured, Injured, and Injured Subgroups (Non‐amputees, Unilateral, and Bilateral Amputees)
| Uninjured | Injured (whole cohort) | p | Injured (non‐amputee) | Injured (unilateral amputee) | Injured (bilateral amputee) | |
| Participant details | n = 562 | n = 575 | n = 419 | n = 81 | n = 75 | |
| Age (years) | 34.3 (30.7 to 37.8) | 33.6 (30.7 to 37.8) | 0.368 | 33.8 (30.6 to 38.4) | 32.9 (30.2 to 35.9) | 33.0 (30.9 to 36.2) |
| Height (m) | 1.79 (1.75 to 1.83) | 1.79 (1.75 to 1.84) | 0.108 | 1.79 (1.74 to 1.83) | 1.80 (1.76 to 1.85) | 1.80 (1.75 to 1.86) |
| Adjusted body mass (kg) | 86.8 (80.0 to 95.2) | 88.8 (80.4 to 99.4) | 0.008 | 87.4 (79.9 to 97.8) | 93.7 (82.0 to 102.7) | 93.1 (78.8 to 102) |
| Adjusted BMI (kg/m2) | 27.2 (25.0 to 29.3) | 27.8 (25.5 to 30.5) | 0.002 | 27.5 (25.4 to 30.1) | 28.1 (26.2 to 31.1) | 29.2 (25.1 to 32.1) |
| Smoke pack years | 0 (0 to 5.5) | 0 (0 to 6.0) | 0.581 | 0 (0 to 5.0) | 0 (0 to 11.1) | 0 (0 to 6.5) |
| T‐scores | n = 562 | n = 567 | n = 414 | n = 79 | n = 74 | |
| Lumbar spine (L1–L4) | −0.19 (−0.89 to +0.51) | −0.28 (−0.91 to +0.60) | 0.959 | −0.26 (−0.86 to +0.66) | −0.31 (−0.90 to +0.58) | −0.40 (−1.35 to +0.39) |
| n = 562 | n = 560 | n = 414 | n = 79 | n = 67 | ||
| Femoral neck | −0.08 (−0.65 to +0.47) | −0.42 (−1.13 to +0.25) | 0.000 | −0.19 (0.78 to +0.44) | −1.13 (−1.69 to −0.44) | −1.67 (−2.22 to −0.76) |
| Uninjured | Injured (whole cohort) | p | Injured (non‐amputee) | Injured (unilateral amputee) | Injured (bilateral amputee) | |
| Participant details | n = 562 | n = 575 | n = 419 | n = 81 | n = 75 | |
| Age (years) | 34.3 (30.7 to 37.8) | 33.6 (30.7 to 37.8) | 0.368 | 33.8 (30.6 to 38.4) | 32.9 (30.2 to 35.9) | 33.0 (30.9 to 36.2) |
| Height (m) | 1.79 (1.75 to 1.83) | 1.79 (1.75 to 1.84) | 0.108 | 1.79 (1.74 to 1.83) | 1.80 (1.76 to 1.85) | 1.80 (1.75 to 1.86) |
| Adjusted body mass (kg) | 86.8 (80.0 to 95.2) | 88.8 (80.4 to 99.4) | 0.008 | 87.4 (79.9 to 97.8) | 93.7 (82.0 to 102.7) | 93.1 (78.8 to 102) |
| Adjusted BMI (kg/m2) | 27.2 (25.0 to 29.3) | 27.8 (25.5 to 30.5) | 0.002 | 27.5 (25.4 to 30.1) | 28.1 (26.2 to 31.1) | 29.2 (25.1 to 32.1) |
| Smoke pack years | 0 (0 to 5.5) | 0 (0 to 6.0) | 0.581 | 0 (0 to 5.0) | 0 (0 to 11.1) | 0 (0 to 6.5) |
| T‐scores | n = 562 | n = 567 | n = 414 | n = 79 | n = 74 | |
| Lumbar spine (L1–L4) | −0.19 (−0.89 to +0.51) | −0.28 (−0.91 to +0.60) | 0.959 | −0.26 (−0.86 to +0.66) | −0.31 (−0.90 to +0.58) | −0.40 (−1.35 to +0.39) |
| n = 562 | n = 560 | n = 414 | n = 79 | n = 67 | ||
| Femoral neck | −0.08 (−0.65 to +0.47) | −0.42 (−1.13 to +0.25) | 0.000 | −0.19 (0.78 to +0.44) | −1.13 (−1.69 to −0.44) | −1.67 (−2.22 to −0.76) |
Note: Values are median (interquartile range). Values of p represent univariable comparisons between uninjured and injured participants (whole injured cohort). Bold values represent significant results, p < 0.01, Bonferroni corrected.
To test the mechanical loading hypothesis, a subgroup analysis on the femoral neck BMD was conducted in which the injured group was divided into non amputees (expected normal loading), the unaffected limb of unilateral amputees (expected normal or elevated loading), and the amputated limbs of both unilateral and bilateral amputees separated into below knee amputee (BKA; expected reduced loading) or above knee amputee (AKA; expected greater reduced loading). There was no difference in the femoral neck BMD between any of the groups with intact limbs. The amputated limb groups had significantly lower femoral neck BMD than all other groups (Fig. 1). This difference was greater for the above knee amputated limbs than the below knee amputated limbs (p = 0.000).
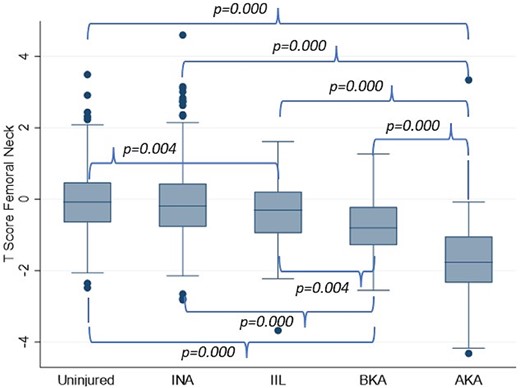
BMD T‐scores at the femoral neck in uninjured (mean value of both sides), injured non‐amputees (INA, mean value of both sides), the intact limb of unilateral amputees (injured intact limb, IIL), and the amputated limbs of unilateral and bilateral amputees separated into below knee amputation (BKA) and above knee amputation (AKA).
Activity levels cannot be separated per limb; therefore, activity was analyzed for uninjured, injured non‐amputee, injured unilateral amputee, and injured bilateral amputee groups. There was no difference in the activity levels between the groups as measured by the IPAQ, except for lower vigorous activity (p = 0.002) and fewer walking minutes (p = 0.001) for the injured non‐amputees compared to the uninjured group (Fig. 2).
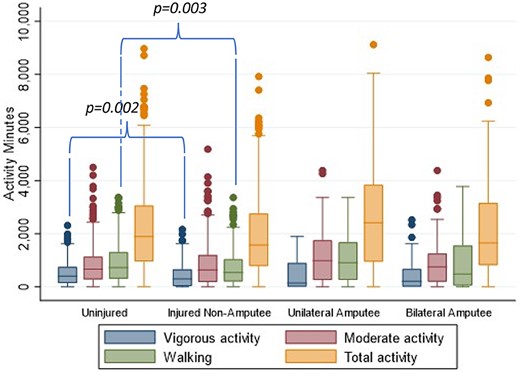
Self‐reported activity levels in minutes of walking, moderate activity and vigorous activity over the last 7 days using the International Physical Activity Questionnaire (IPAQ) for non‐injured, injured non‐amputee, injured unilateral amputee, and injured bilateral amputee groups.
Lower limb amputee status, total IPAQ walking minutes, total IPAQ activity minutes, adjusted BMI, and smoking pack‐years were entered into a binary logistic regression analysis with the outcome variable of femoral neck pathology (defined by normal T‐scores or T‐scores diagnostic of osteopenia or osteoporosis). Amputee status and adjusted BMI were the only variables that significantly contributed to the model. Adjusted BMI had an odds ratio of 0.90, while having a lower limb amputation had an odds ratio of 12.02; ie, having a lower limb amputation increases the odds of femoral neck BMD pathology by more than 12 times, while having a higher BMI reduced the odds of femoral neck pathology.
There was a significant difference in discordance between amputees, injured non‐amputees, and uninjured groups (Table 2), where the injured amputees exhibited greater major and minor discordance than the other two groups and the discordance was focused on the femoral neck having a lower BMD than spine BMD.
Discordance Level and Type Between Uninjured, Injured Non‐amputees, and Injured Amputees
| Discordance level | Discordance type | |||||||
| Parameter, n (%) | No diagnostic discordance | Minor discordance | Major discordance | p | No diagnostic discordance | Femoral neck > spine | Spine > femoral neck | p |
| Uninjured | 433 (77) | 126 (22) | 3 (1) | <0.001 | 433 (77) | 93 (17) | 36 (6) | <0.001 |
| Injured non‐amputees | 316 (76) | 101 (24) | 1 (0) | 316 (76) | 51 (12) | 51 (12) | ||
| Injured amputees | 65 (43) | 75 (50) | 11 (7) | 65 (43) | 13 (9) | 73 (48) | ||
| Discordance level | Discordance type | |||||||
| Parameter, n (%) | No diagnostic discordance | Minor discordance | Major discordance | p | No diagnostic discordance | Femoral neck > spine | Spine > femoral neck | p |
| Uninjured | 433 (77) | 126 (22) | 3 (1) | <0.001 | 433 (77) | 93 (17) | 36 (6) | <0.001 |
| Injured non‐amputees | 316 (76) | 101 (24) | 1 (0) | 316 (76) | 51 (12) | 51 (12) | ||
| Injured amputees | 65 (43) | 75 (50) | 11 (7) | 65 (43) | 13 (9) | 73 (48) | ||
Discordance Level and Type Between Uninjured, Injured Non‐amputees, and Injured Amputees
| Discordance level | Discordance type | |||||||
| Parameter, n (%) | No diagnostic discordance | Minor discordance | Major discordance | p | No diagnostic discordance | Femoral neck > spine | Spine > femoral neck | p |
| Uninjured | 433 (77) | 126 (22) | 3 (1) | <0.001 | 433 (77) | 93 (17) | 36 (6) | <0.001 |
| Injured non‐amputees | 316 (76) | 101 (24) | 1 (0) | 316 (76) | 51 (12) | 51 (12) | ||
| Injured amputees | 65 (43) | 75 (50) | 11 (7) | 65 (43) | 13 (9) | 73 (48) | ||
| Discordance level | Discordance type | |||||||
| Parameter, n (%) | No diagnostic discordance | Minor discordance | Major discordance | p | No diagnostic discordance | Femoral neck > spine | Spine > femoral neck | p |
| Uninjured | 433 (77) | 126 (22) | 3 (1) | <0.001 | 433 (77) | 93 (17) | 36 (6) | <0.001 |
| Injured non‐amputees | 316 (76) | 101 (24) | 1 (0) | 316 (76) | 51 (12) | 51 (12) | ||
| Injured amputees | 65 (43) | 75 (50) | 11 (7) | 65 (43) | 13 (9) | 73 (48) | ||
Discussion
This analysis has shown that CRTI in UK service personnel is associated with BMD loss when compared to a matched non‐injured comparison group, but that the association is limited to loss in the femoral neck of the amputated limbs in amputees. Additionally, the loss was greater for above knee amputees. We found no evidence of systemic BMD loss, which has important implications on future research and treatment strategies. The higher BMI in the injured group could be considered as protective against generalized osteopenia, enhancing the localized phenomenon. A recent study on the same cohort found an increased prevalence of metabolic syndrome and arterial stiffness, which may explain the higher BMI, but this requires further investigation.(21)
Unlike the results found in most existing studies of amputee activity level,(26,27) uninjured and amputee groups demonstrated no statistical difference in terms of weekly activity level. This suggests that global activity cannot explain reduced BMD in this amputee population but that differences could be because of localized loading changes. Furthermore, this challenges the view that BMD loss in this young traumatic active amputee group is due to disuse osteopenia and presents more localized unloading osteopenia (due to bone stress shielding). Activity results show that amputees perform, on average, 440 minutes of vigorous exercise per week. Therefore, adequate load bearing exercise is being undertaken by the amputee group on a weekly basis. It is evident that for the unilateral amputee group activity levels are sufficient to maintain BMD in the unamputated side; however, not in the amputated side neck of femur. This warrants further investigation and suggests that the load transfer to the amputated side is disrupted through, for example, the socket, thus causing stress shielding at the femoral neck. Treatment options that provide localized loading at the proximal femur could be considered, such as local heavy resistance training or high loading activities including hopping or jumping.
Our findings lend evidence to the argument that BMD loss in the amputee population is localized and is driven by abnormal or reduced loading in the femur on the amputated side, possibly due to intentional offloading through the design of the prosthetic socket and prosthetic limb.(28) Additionally, there is no difference in spine BMD in unilateral and bilateral amputees. Abnormal loading occurring at the femur seemingly does not affect loading at the spine to the same degree, further implicating the prosthetic socket as a key contributor. Evidence that the BMD loss is due to abnormal loading is found in newer prosthetic options such as direct skeletal fixation (DSF) which has been used in UK military cohorts.(29) Long‐term follow up of DXA data from DSF patients demonstrates a reversal in the reduced BMD at the femoral neck with time when loading through the osseointegrated fixation.(30)
The true burden of femoral neck fractures in amputees is not currently understood. With an aging population and increasing numbers of diabetics and vasculopaths, an increase in global incidence of amputation has been seen.(31) Although outcomes for amputees sustaining hip fractures have not been shown to be different from an age‐matched population, the only studies in the literature looking at outcomes are for patients over the age of 50 years.(32) The traumatic amputee population seen in both the UK and allied nations following recent conflicts are much younger than the traditional diabetic or vascular amputee and the results here show that they are highly active. Therefore, at a much younger age there is a potential increased risk of femoral neck fracture in the presence of BMD loss resulting in reduced activity levels, prosthetic fit difficulties, and generalized deconditioning. The young traumatic amputee population in the UK may be relatively small, but globally over 35 million people in 2017 were living with a traumatic lower limb amputation representing a potential burden for femoral neck fractures due to BMD loss.(33)
Using clinical diagnostic criteria, it is possible to compare these results to some of the larger, population‐based studies investigating diagnosis discordance in the aging population. By analyzing subjects on an individual case‐by‐case basis, major diagnosis discordance was found in this study in 7% of amputees and minor discordance in a further 50%. Unlike in the general population,(19,34) however, this diagnosis discordance overwhelmingly reflects a lower T‐score in the femoral neck than in the lumbar spine. Of all the amputee cases where discordance is present, 85% had a lower T‐score in the femur than in the lumbar spine. By comparison, Moaayeri and colleagues(19) and Mounach and colleagues(34) found this in just 40% and 17% of male non‐amputee populations, respectively, where discordance occurred much more commonly in the reverse direction. These results suggest that the rate of BMD loss in the femur of young amputees is higher than in the lumbar spine and, possibly, the rest of the body. If the causes of bone loss were systemic then a lower BMD in the spine would be expected or there would be no discordance. Clinically, the presence of discordance should alert a clinician to the likelihood of localized BMD, as opposed to systemic osteoporosis. Over 40% (43%) of the amputees had no discordance; this may be reflective of the difference in unloading between below knee, through knee, and above knee amputees.
Although reduced BMD levels in lower‐limb amputees is used to diagnose osteoporosis or osteopenia,(3,4,34) more recently, researchers have begun to make subtle distinctions between BMD loss in amputees and BMD loss in the general population. Sherk and colleagues(12) first highlighted the possible similarities between BMD loss post‐amputation and BMD loss because of periods of prolonged unloading from scuba diving,(35) bed rest,(36) and space travel studies.(37) Later studies by Bemben and colleagues,(13) Ramirez and colleagues,(14) and Flint and colleagues(15) made similar comparisons and do not equate amputee BMD loss to osteoporosis or osteopenia but rather to physical phenomena such as bone stress shielding by the socket resulting in reduced load transmission through the skeleton. Although the concept of disuse osteopenia is well understood, the activity levels seen in this cohort suggest that the reduced BMD seen is more likely secondary to bone stress shielding through socket design.
Osteoporosis is typically associated with endocrine changes in the older population. As a result, it is treated with hormone replacement therapy or bone‐preserving medication, such as bisphosphonates. The long‐term use of both would be inappropriate for a young population given the well‐documented, adverse side‐effects in older patients,(10,11) although proper management with treatment holidays is known to reduce these side effects.(38) Safety in much younger patients represented by this cohort is not known. Moreover, the use of systemic treatments for a localized issue risks introducing additional problematic side effects. We therefore advocate that young amputees with BMD loss limited to the amputated side femoral neck not be diagnosed with osteoporosis, instead the BMD loss is to be classified as localized unloading osteopenia. A diagnosis of localized unloading osteopenia creates a clear diagnostic distinction between systemic osteoporosis and BMD loss following amputation. This may prevent inappropriate use of systemic treatment and will, we hope, drive research into the ideal loading environment to potentially reverse BMD changes, and further research into prosthetic socket design.
Study limitations
Bone biomarkers are now commonly used in clinical diagnosis of systemic osteoporosis.(39) As such, their use may have been valuable in evaluating the cause of BMD loss in the young amputee population. Determining whether BMD loss is attributed to reduction in bone formation or increase in bone resorption through biomarkers would represent a further step toward determining the exact etiology of the problem in young amputees. Conversely, given the localization of deterioration in amputees, it is also possible that global bone biomarkers would fail to capture local differences.
CRTI can induce systemic inflammation and hormonal changes that can lead to a complex multisystemic effect that was not assessed in this study.
Additionally, all subjects evaluated in this study come from an active military background. As such, it could be suggested that results do not fully transfer to nonmilitary amputees because serving military personnel are known to be more active than their civilian counterparts.(40)
Bemben and colleagues(13) tracked the progression of BMD loss in amputees over the first year postamputation whereas this study provides only data at one time point. In the future, ADVANCE will be capable of completing a similar study with longer‐term follow‐up of 10 to 15 years postamputation.
Conclusion
CRTI on its own does not induce changes in BMD. Amputation, however, results in a lower BMD at the proximal femur that is worse for above knee amputees and can occur at a young age relatively soon after injury. These findings support the proposal that these changes are localized unloading osteopenia and should not be associated with a diagnosis of systemic osteoporosis that could have adverse effects on patient's rehabilitation outlook and medical treatment strategies.
Author Contributions
LM: Conceptualization; formal analysis; methodology; writing – original draft and revisions. FPB: Formal analysis; methodology; visualization; writing – review & editing. JK, DC: Formal analysis; methodology; writing – original draft. ANB, CB, NTF, PC: Data curation; formal analysis; funding acquisition; investigation; project administration; writing – review & editing. AMJB: Data curation; formal analysis; funding acquisition; investigation; project administration; visualization; writing – review & editing. ATMP: Conceptualization; project administration; supervision; writing – review & editing. AHM: Conceptualization; project administration; supervision; writing – review & editing.
Acknowledgments
This work is part of the ADVANCE study which is supported by the ADVANCE Charity (https://www.advancestudydmrc.org.uk/advance-charity/). Key contributors to this charity are the Headley Court Charity (principal funder), HM Treasury (LIBOR Grant), Help for Heroes, Nuffield Trust for the Forces of the Crown, Forces in Mind Trust, National Lottery Community Fund, Blesma ‐ The Limbless Veterans and the UK Ministry of Defence. Other financial support was provided by the Royal British Legion as part of the Royal British Legion Centre for Blast Injury Studies at Imperial College London.
Disclosures
NF receives funding from the UK Ministry of Defence. LM, FPB, JK, DC, ANB, CB, PC, AMJB, ATMP, and AHM all have no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4794.
Data Availability Statement
Data are available upon reasonable request. Given the sensitive nature of the participants, the data have not been widely available. Requests for data will be considered on a case‐by‐case basis and subject to UK Ministry of Defence clearance.



