-
PDF
- Split View
-
Views
-
Cite
Cite
Emil Kääntä, Roope Parviainen, Marjaana Tikanmäki, Suvi Alenius, Juha‐Jaakko Sinikumpu, Eero Kajantie, Maternal Smoking During Pregnancy and Offspring's Risk for Bone Fracture in Childhood and Adolescence, Journal of Bone and Mineral Research, Volume 38, Issue 12, 1 December 2023, Pages 1791–1799, https://doi.org/10.1002/jbmr.4923
Close - Share Icon Share
ABSTRACT
Conditions during gestation, such as maternal smoking, may affect offspring's bone structure. This could increase the offspring's risk of bone fractures during childhood. In this study, we aimed to assess the association between prenatal exposure to maternal smoking and childhood bone fracture risk. We used a register‐based birth cohort that included all children born in Finland between January 1987 and September 1990. After exclusions, the final study population consisted of 220,699 persons. Using a unique national identification number, we linked the cohort data to the fracture diagnosis in specialty care and covariate data using the Medical Birth Register (MBR), Statistics Finland and Care Register for Health Care (CRHC). The fractures were analyzed in three groups: all fractures, non‐high‐energy fractures, and high‐energy fractures. The analyses were adjusted for sex, parity, child's year of birth, mother's age at childbirth, mother's and father's educational level, and mother's fracture status. We tested the association in three age groups: <1 year, 1–<5 years, and 5–<15 years using Cox and (recurrent fractures) Poisson regression. A total of 18,857 (8.5%) persons had at least one bone fracture diagnosis before the age of 15 years. In the age group 5–<15 years, maternal smoking during pregnancy was associated with higher fracture risk in all of the studied fracture groups: hazard ratio (HR) = 1.12 (95% confidence interval [CI] 1.06–1.17) in all fractures, 1.13 (95% CI 1.07–1.19) in non‐high‐energy, and 1.15 (95% CI 1.00–1.32) in high‐energy fractures. There were no significant associations in other age groups in any of the fracture groups. No statistically significant association between maternal smoking during pregnancy and offspring's risk of recurrent fractures was found. In conclusion, 5‐ to 15‐year‐olds whose mothers have smoked during pregnancy have an increased risk of bone fractures treated in specialty care. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Introduction
Bone fracture is a common injury among children. According to some references, up to 42% of boys and 27% of girls suffer a bone fracture by the age of 16 years.(1) The incidence of a fracture in children younger than 15 years varies between 75.8 and 163/10,000 person‐years in European populations.(2,3) Once a fracture has occurred, the risk of a recurrent fracture before the age of 18 years has been found to be 20%.(4) Key determinants of fracture risk include bone strength and behavioral characteristics predisposing to trauma. Bone structure and its fragility are affected by both parental and offspring characteristics such as diet, physical activity, sex, maternal age, and conditions during gestation.(5)
Although the global prevalence of smoking in pregnancy is estimated at 1.7%, the prevalence is much higher in Europe as a whole (8.1%) and in some individual European countries is over 30%.(6,7) In Finland in 2019, 10.7% of pregnant women smoked at the start of pregnancy and 4.9% continued after the first trimester.(8) In Finland between 1987 and 1991, the prevalence of smoking during pregnancy was 15%.(9)
Maternal smoking during pregnancy is associated with low birth weight of the offspring.(10) Also, bone and skeleton growth is sensitive to the effect of maternal smoking.(11) Smoking during pregnancy decreases maternal intestinal calcium absorption as well as oxygen and nutrient supply to the fetus.(12) In addition, some components of the tobacco smoke (eg, cadmium) may pose a direct toxic effect on the growing fetus.(13) A recent in vitro study showed that the osteogenic differentiation of the human embryonic stem cells was disturbed by tobacco smoke independently of nicotine.(14)
There are studies that have evaluated the association between maternal smoking during pregnancy and childhood fracture risk. These studies have presented somewhat conflicting results, and part of the studies are based on quite small populations. Jones and colleagues found no significant effect between maternal smoking during pregnancy and offspring's fracture risk at any age under 18 years, which is a conflicting result to almost all other studies in this field.(15) That study comprised 1139 children, who were followed up to age 18 years. Our previous study in the Northern Finland Birth Cohort, in turn, showed that there was almost twofold risk of fracture (1.83 odds ratio [OR]) among under 7‐year‐old children whose mother smoked during pregnancy.(16) In that cohort study of 6718 children, there were not enough subjects for subgroup analyses. Also, bone fractures were observed until the age of 7 years. Ayubi and colleagues(17) conducted a meta‐analysis that included approximately 1000 bone fracture cases. Maternal smoking was associated with a relative risk (RR) of 1.27, but the relatively wide 95% confidence interval (CI; 1.00–1.62) leaves room for statistical uncertainty.
We are aware of only two studies that have assessed the association between maternal smoking during pregnancy and offspring's fracture risk within a large population: one assessing fractures up to 1 year(18) and another up to 32 years of age.(19) They found an association between maternal smoking in pregnancy and higher risk of bone fracture among offspring, but the age at the first fracture varied between the studies. Högberg and colleagues(18) observed only fractures in infancy (0–1 year). Brand and colleagues addressed different age groups but found significant association, which survived within siblingship analysis, only in fractures suffered before the age of 1 year.(19) However, fractures in this age group are usually caused by obstetrical injuries, accidental falls, child abuse, and serious bone‐affecting illnesses (eg, osteogenesis imperfecta), rather than latent fragility of the bone.(20)
The aim of our study was to evaluate the association between maternal smoking during pregnancy and offspring's first fracture and recurrent fractures in childhood and adolescence. Previous studies mainly observed bone fractures in infancy or up to age 7 years. In our study, bone fractures in later childhood and adolescence were also included up to 15 years. The timing when the association of maternal smoking in pregnancy and the bone fracture differ between the previous studies. Further, the large size of the birth cohort allowed us to analyze whether there is a difference between high‐ and non‐high‐energy bone fractures or trabecular and vertebral fractures, which has not been done previously.
Materials and Methods
Study population
The initial study population was a birth cohort comprising all children born alive in Finland between January 1, 1987, and September 30, 1990 (N = 235,624). For this study, the cohort members were followed up until the age of 15 years.
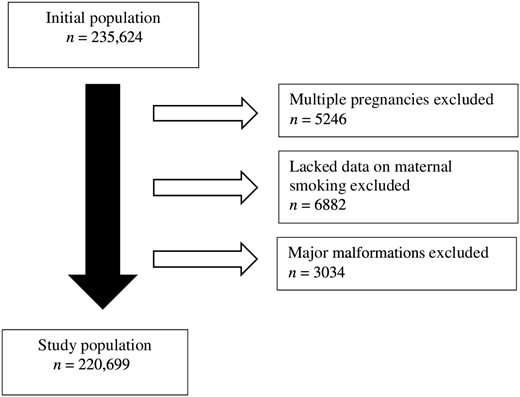
In this study, the multiple gestation pregnancies were excluded from the study population (n = 5246, 2.2%) because multiples are more likely to be born prematurely and to be small for gestational age(21,22) and could affect risk of fractures through a distinct mechanism.(23‐25) Further, such individuals who lacked data on maternal smoking during pregnancy (n = 6882, 2.9%) were excluded. Also, individuals who had major malformations, such as osteogenesis imperfecta, were excluded (n = 3034, 1.3%) because these conditions can affect the bone fragility. After the exclusions, total number of individuals in our analyses was 220,699, with 112,932 (51.2%) boys and 107,767 (48.8%) girls. The study population characteristics are shown in Table 1 and Figure 1 depicts the data collection flow chart.
| Character | All (%) | Mother non‐smoking (%) | Mother smoking (%) |
| No. of children | 220,699 | 186,957 (84.7) | 33,742 (15.3) |
| Sex | |||
| Boy | 112,932 (51.2) | 95,608 (51.1) | 17,343 (51.4) |
| Girl | 107,767 (48.8) | 91,349 (48.9) | 16,399 (48.6) |
| Fractures <15 years of age | |||
| High energy | 18,857 (8.5) | 15,678 (8.4) | 3179 (9.4) |
| Non‐high energy | 2544 (1.2) | 2089 (1.1) | 455 (1.3) |
| 16,313 (7.4) | 13,589 (7.3) | 2724 (8.1) | |
| Average age at first fracture (SD) <15 years | |||
| 10.3 (3.2) | 10.3 (3.2) | 10.3 (3.2) | |
| Maternal education | |||
| Upper secondary | 100,003 (45.3) | 82,465 (44.1) | 17,538 (51.9) |
| Post‐secondary | 47,814 (21.6) | 43,589 (23.3) | 4225 (12.5) |
| Lower tertiary | 20,238 (9.2) | 18,761 (10.0) | 1477 (4.4) |
| Upper tertiary or more | 22,639 (10.3) | 21,778 (11.6) | 861 (2.5) |
| Lower secondary or less or unknown | 30,005 (13.6) | 20,364 (11.0) | 9641 (28.7) |
| Paternal education | |||
| Upper secondary | 98,690 (44.7) | 82,135 (43.9) | 16,555 (49.1) |
| Post‐secondary | 30,862 (14.0) | 28,128 (15.0) | 2734 (8.1) |
| Lower tertiary | 17,345 (7.9) | 16,174 (8.7) | 1171 (3.5) |
| Upper tertiary or more | 23,140 (10.5) | 22,266 (11.9) | 874 (2.2) |
| Lower secondary or less or unknown | 50,662 (22.9) | 38,254 (20.5) | 12,408 (37.1) |
| Maternal age at birth (years) (SD) | 28.5 (5.1) | 28.7 (5.1) | 27.0 (5.5) |
| Gestational age (weeks) (SD) | 39.7 (1.7) | 39.7 (1.7) | 39.7 (1.9) |
| Parity | |||
| Primiparous | 67,098 (30.4) | 57,473 (30.7) | 9625 (28.5) |
| Multiparous | 83,548 (37.9) | 69,529 (37.2) | 14,019 (41.5) |
| Unknown | 70,53 (31.7) | 59,955 (32.1) | 10,098 (30.0) |
| Maternal fracture status | |||
| Fracture | 30,822 (14.0) | 24,334 (13.0) | 6488 (19.2) |
| No fracture | 189,877 (86.0) | 162,623 (87.0) | 27,254 (80.8) |
| Character | All (%) | Mother non‐smoking (%) | Mother smoking (%) |
| No. of children | 220,699 | 186,957 (84.7) | 33,742 (15.3) |
| Sex | |||
| Boy | 112,932 (51.2) | 95,608 (51.1) | 17,343 (51.4) |
| Girl | 107,767 (48.8) | 91,349 (48.9) | 16,399 (48.6) |
| Fractures <15 years of age | |||
| High energy | 18,857 (8.5) | 15,678 (8.4) | 3179 (9.4) |
| Non‐high energy | 2544 (1.2) | 2089 (1.1) | 455 (1.3) |
| 16,313 (7.4) | 13,589 (7.3) | 2724 (8.1) | |
| Average age at first fracture (SD) <15 years | |||
| 10.3 (3.2) | 10.3 (3.2) | 10.3 (3.2) | |
| Maternal education | |||
| Upper secondary | 100,003 (45.3) | 82,465 (44.1) | 17,538 (51.9) |
| Post‐secondary | 47,814 (21.6) | 43,589 (23.3) | 4225 (12.5) |
| Lower tertiary | 20,238 (9.2) | 18,761 (10.0) | 1477 (4.4) |
| Upper tertiary or more | 22,639 (10.3) | 21,778 (11.6) | 861 (2.5) |
| Lower secondary or less or unknown | 30,005 (13.6) | 20,364 (11.0) | 9641 (28.7) |
| Paternal education | |||
| Upper secondary | 98,690 (44.7) | 82,135 (43.9) | 16,555 (49.1) |
| Post‐secondary | 30,862 (14.0) | 28,128 (15.0) | 2734 (8.1) |
| Lower tertiary | 17,345 (7.9) | 16,174 (8.7) | 1171 (3.5) |
| Upper tertiary or more | 23,140 (10.5) | 22,266 (11.9) | 874 (2.2) |
| Lower secondary or less or unknown | 50,662 (22.9) | 38,254 (20.5) | 12,408 (37.1) |
| Maternal age at birth (years) (SD) | 28.5 (5.1) | 28.7 (5.1) | 27.0 (5.5) |
| Gestational age (weeks) (SD) | 39.7 (1.7) | 39.7 (1.7) | 39.7 (1.9) |
| Parity | |||
| Primiparous | 67,098 (30.4) | 57,473 (30.7) | 9625 (28.5) |
| Multiparous | 83,548 (37.9) | 69,529 (37.2) | 14,019 (41.5) |
| Unknown | 70,53 (31.7) | 59,955 (32.1) | 10,098 (30.0) |
| Maternal fracture status | |||
| Fracture | 30,822 (14.0) | 24,334 (13.0) | 6488 (19.2) |
| No fracture | 189,877 (86.0) | 162,623 (87.0) | 27,254 (80.8) |
| Character | All (%) | Mother non‐smoking (%) | Mother smoking (%) |
| No. of children | 220,699 | 186,957 (84.7) | 33,742 (15.3) |
| Sex | |||
| Boy | 112,932 (51.2) | 95,608 (51.1) | 17,343 (51.4) |
| Girl | 107,767 (48.8) | 91,349 (48.9) | 16,399 (48.6) |
| Fractures <15 years of age | |||
| High energy | 18,857 (8.5) | 15,678 (8.4) | 3179 (9.4) |
| Non‐high energy | 2544 (1.2) | 2089 (1.1) | 455 (1.3) |
| 16,313 (7.4) | 13,589 (7.3) | 2724 (8.1) | |
| Average age at first fracture (SD) <15 years | |||
| 10.3 (3.2) | 10.3 (3.2) | 10.3 (3.2) | |
| Maternal education | |||
| Upper secondary | 100,003 (45.3) | 82,465 (44.1) | 17,538 (51.9) |
| Post‐secondary | 47,814 (21.6) | 43,589 (23.3) | 4225 (12.5) |
| Lower tertiary | 20,238 (9.2) | 18,761 (10.0) | 1477 (4.4) |
| Upper tertiary or more | 22,639 (10.3) | 21,778 (11.6) | 861 (2.5) |
| Lower secondary or less or unknown | 30,005 (13.6) | 20,364 (11.0) | 9641 (28.7) |
| Paternal education | |||
| Upper secondary | 98,690 (44.7) | 82,135 (43.9) | 16,555 (49.1) |
| Post‐secondary | 30,862 (14.0) | 28,128 (15.0) | 2734 (8.1) |
| Lower tertiary | 17,345 (7.9) | 16,174 (8.7) | 1171 (3.5) |
| Upper tertiary or more | 23,140 (10.5) | 22,266 (11.9) | 874 (2.2) |
| Lower secondary or less or unknown | 50,662 (22.9) | 38,254 (20.5) | 12,408 (37.1) |
| Maternal age at birth (years) (SD) | 28.5 (5.1) | 28.7 (5.1) | 27.0 (5.5) |
| Gestational age (weeks) (SD) | 39.7 (1.7) | 39.7 (1.7) | 39.7 (1.9) |
| Parity | |||
| Primiparous | 67,098 (30.4) | 57,473 (30.7) | 9625 (28.5) |
| Multiparous | 83,548 (37.9) | 69,529 (37.2) | 14,019 (41.5) |
| Unknown | 70,53 (31.7) | 59,955 (32.1) | 10,098 (30.0) |
| Maternal fracture status | |||
| Fracture | 30,822 (14.0) | 24,334 (13.0) | 6488 (19.2) |
| No fracture | 189,877 (86.0) | 162,623 (87.0) | 27,254 (80.8) |
| Character | All (%) | Mother non‐smoking (%) | Mother smoking (%) |
| No. of children | 220,699 | 186,957 (84.7) | 33,742 (15.3) |
| Sex | |||
| Boy | 112,932 (51.2) | 95,608 (51.1) | 17,343 (51.4) |
| Girl | 107,767 (48.8) | 91,349 (48.9) | 16,399 (48.6) |
| Fractures <15 years of age | |||
| High energy | 18,857 (8.5) | 15,678 (8.4) | 3179 (9.4) |
| Non‐high energy | 2544 (1.2) | 2089 (1.1) | 455 (1.3) |
| 16,313 (7.4) | 13,589 (7.3) | 2724 (8.1) | |
| Average age at first fracture (SD) <15 years | |||
| 10.3 (3.2) | 10.3 (3.2) | 10.3 (3.2) | |
| Maternal education | |||
| Upper secondary | 100,003 (45.3) | 82,465 (44.1) | 17,538 (51.9) |
| Post‐secondary | 47,814 (21.6) | 43,589 (23.3) | 4225 (12.5) |
| Lower tertiary | 20,238 (9.2) | 18,761 (10.0) | 1477 (4.4) |
| Upper tertiary or more | 22,639 (10.3) | 21,778 (11.6) | 861 (2.5) |
| Lower secondary or less or unknown | 30,005 (13.6) | 20,364 (11.0) | 9641 (28.7) |
| Paternal education | |||
| Upper secondary | 98,690 (44.7) | 82,135 (43.9) | 16,555 (49.1) |
| Post‐secondary | 30,862 (14.0) | 28,128 (15.0) | 2734 (8.1) |
| Lower tertiary | 17,345 (7.9) | 16,174 (8.7) | 1171 (3.5) |
| Upper tertiary or more | 23,140 (10.5) | 22,266 (11.9) | 874 (2.2) |
| Lower secondary or less or unknown | 50,662 (22.9) | 38,254 (20.5) | 12,408 (37.1) |
| Maternal age at birth (years) (SD) | 28.5 (5.1) | 28.7 (5.1) | 27.0 (5.5) |
| Gestational age (weeks) (SD) | 39.7 (1.7) | 39.7 (1.7) | 39.7 (1.9) |
| Parity | |||
| Primiparous | 67,098 (30.4) | 57,473 (30.7) | 9625 (28.5) |
| Multiparous | 83,548 (37.9) | 69,529 (37.2) | 14,019 (41.5) |
| Unknown | 70,53 (31.7) | 59,955 (32.1) | 10,098 (30.0) |
| Maternal fracture status | |||
| Fracture | 30,822 (14.0) | 24,334 (13.0) | 6488 (19.2) |
| No fracture | 189,877 (86.0) | 162,623 (87.0) | 27,254 (80.8) |
Data sources
The data of the study were gathered from the Medical Birth Register (MBR), Statistics Finland and Care Register for Health Care (CRHC). The study protocol had been approved by an Ethics Committee, and register data were linked with permission from register keepers. The study followed the rules of Declaration of Helsinki. Data were linked using personal identification codes, which were first replaced by a non‐identifiable encrypted code before the authors had access to the data. Researchers had no access to data allowing direct identification of individuals.
The information on bone fracture was originated from the CRHC, which is maintained by Finnish Institute for Health and Welfare. This register includes information of in‐hospital treated fractures through the follow‐up period and fractures treated at a hospital outpatient clinic from 1998 onward.
Exposure
Maternal smoking during pregnancy was the exposure. Information of maternal smoking during pregnancy came from the Medical Birth Register, in which it is usually recorded at the first visit to the antenatal clinic as reported by the mother. In the main analysis, the smoking was handled as a dichotomous variable and the mothers were classified as smokers (n = 33,742) or non‐smokers (n = 186,957). The mothers were classified as smokers if they had smoked one or more cigarettes a day during any period of the pregnancy. In supplementary analyses, maternal smoking was treated as a categorical variable and for that the mothers were classified into three groups: non‐smoker, smoked 1–9 cigarettes per day (n = 17,553, 52.0%), and smoked more than 10 cigarettes per day (n = 16,189, 48.0%).
Outcome
In this study, we used three age groups depending on the year of the first fracture: under 1 year, 1–<5 years and 5–<15 years. Bone fracture(s) under the age of 15 years was the outcome. The bone fractures after this age were not included in the study because it is likely that after puberty other factors (eg, hobbies, nutrition, personal cigarette smoking) than gestational environment affect the bone structure and fracture risk. A bone fracture was defined as present if any of the following ICD‐10 diagnoses (International Classification of Diseases, Tenth Edition) appeared in the CRHC between the birth of an individual and the end of the follow‐up: S02, S12, S22, S32, S42, S52, S62, S72, S82, S92, T02, T08, T12. Respective ICD‐9 and ICD‐8 (International Classification of Diseases, Ninth and Eighth Editions) diagnosis codes 800–829 were also employed in identifying care episodes associated with bone fractures. See the specific description in Supplemental Table S1. Repeated in‐hospital care episodes or outpatient clinic visits with the same ICD code within 6 months were considered as a single injury. We also conducted supplemental analysis including all bone fractures up to 18 years of age.
The fractures were categorized into high‐energy and non‐high‐energy fractures. The main idea was that high‐energy fractures can be recognized and separated in the analysis. Hypothesis was that a high‐energy trauma will cause the bone to break even though there is no underlying fragility in it. Also, there are studies where maternal smoking during pregnancy was found to increase offspring's risk for hyperkinetic disorders.(26) These disorders may increase the risk behavior and thus be the cause of high‐energy trauma. A fracture was classified into the high‐energy fracture group in the case of scull, spine, pelvis, and femur fractures, or if there were several simultaneous fractures, or if it was a traffic‐related injury. ICD‐codes V01‐V99 including transport accidents were used to search traffic‐related injuries from the CRHC. However, we also conducted an analysis where fractures were categorized into hip or vertebral fractures and into non‐hip or non‐vertebral fractures to see whether maternal smoking in pregnancy have an effect on more cortical or trabecular bone or both.
Confounders
The confounders were selected based on previous literature(5,22,25) and availability from the administrative registers. The sex of the offspring, low socioeconomic status of the family, mother's age at childbirth, mother's fracture status, parity, and child's year of birth were defined as potential confounders. The socioeconomic status of the family was determined by using the highest‐ever attained level of education of mother and father independently. The education level was classified in four categories based on the International Standard Classification of Education (ISCED) classification, categorized as follows: basic education, secondary level, tertiary level, and education unknown. If the education status was upper secondary, it was included in the secondary level and if it was higher than that (post‐secondary, lower tertiary, upper tertiary, or higher), it was included in the tertiary level group. The mean age of the mothers at childbirth was 28.5 years (standard deviation [SD] 5.1). The young age at childbirth group was defined as the mean age at childbirth minus two times the standard deviation (18.3 years) and the high age at childbirth group was determined as the mean age at childbirth plus two times the standard deviation (38.7 years). Maternal fracture status was considered as a confounder because of the high heritability of bone traits.(27,28) There were no missing data regarding sex of offspring. Three cohort members were missing the data on maternal age at birth, and these three individuals were not included in any of three categories presented above. Note that maternal and paternal education frequencies do not equal the total number of children because in the cohort, mothers and fathers can have more than one child.
We used two adjustment models: (i) adjusted for sex and year of birth and (ii) adjusted for sex, year of birth, parity, highest education level of the mother and father, and maternal age at childbirth.
Statistical analysis
The main outcome variable was the first fracture of offspring. Cox regression analysis was used to examine the relation between maternal smoking during pregnancy and the risk of bone fracture in childhood and adolescence. Poisson regression was used to analyze whether maternal smoking during pregnancy is associated with recurrent bone fractures of offspring. Those offspring that had died or emigrated during the follow‐up time were eliminated from the analysis in both Cox and Poisson regression analyses. The results are presented as hazard ratios (HRs) with 95% CIs for Cox regression and as incidence rate ratios (IRRs) with 95% CIs for Poisson regression. The statistical analyses were performed by using the IBM SPSS Statistics software version 28.0.0.0 (IBM Corp., Armonk, NY, USA).
Results
Bone fractures
A total 18,857 children (11,994, 10.6% boys and 6863, 6.4% girls) had a fracture between ages 0 and 14 years. According to the diagnosis codes, of the first fractures, there were 4691 lower limb fractures, 12,777 upper limb fractures, 0 skull fractures, and 322 pelvic and spine fractures. A total of 1067 children had other first fracture diagnoses. Mean age at the first fracture at the age under 15 years was 10.3 years (SD 3.2), 10.6 (SD 3.3) for males and 9.8 (SD 3.2) for females. The associations between maternal smoking (yes versus no) and offspring fracture risk were similar across the two sexes for all of the age categories: p for interaction of less than 0.26 among those less than 1 year of age, and less than 0.73 and less than 0.02 among children aged 1–4 years and 5–14 years, respectively. We therefore report the estimates pooled for both sexes.
There were 2544 first high‐energy fractures and 16,313 first non‐high‐energy fractures. High‐energy fracture included scull, spine, pelvis, and femur fractures (numbers shown above). If several simultaneous fractures were present (n = 1494), they were taken as high‐energy fractures. Traffic‐related fractures (n = 762) were also included in the high‐energy fracture group.
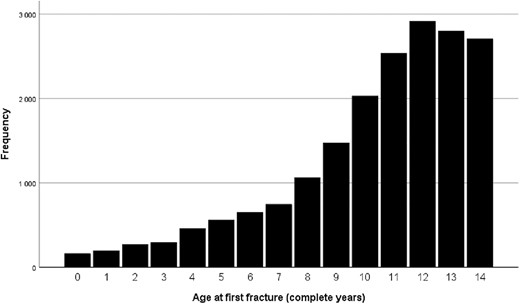
There were 161 first fractures in the under 1 year age group, 1219 first fractures in the 1–<5 years age group, and 17,466 first fractures in the 5–<15 years age group. Figure 2 shows the age‐related frequencies of fractures. Incidence was 55/10,000 for bone fractures under 15 years of age (7/10,000 for <1 year, 13/10,000 for 1–<5 years, and 77/10,000 for 5–<15 years).
Maternal smoking during pregnancy and bone fractures
We tested the association between maternal smoking during pregnancy and child's first bone fracture in three different age groups following the grouping of Brand and colleagues(19): under 1 year of age, 1–4 completed years, and 5–14 completed years. The results in these three age groups and in three fracture groups in both adjustment models are shown in Table 2.
Cox Regression Results Comparing Offspring of Mothers Who Smoked During Pregnancy With Mothers Who Did Not Smoke
| HR (95% CI) model 1 | HR (95% CI) model 2 | |
| Any fracture <1 year | 1.21 (0.81–1.81) | 1.08 (0.65–1.80) |
| Any fracture 1–4 years | 1.01 (0.86–1.18) | 0.87 (0.74–1.09) |
| Any fracture 5–14 years | 1.13 (1.08–1.18) | 1.12 (1.06–1.17) |
| Non‐high‐energy fracture <1 year | 1.40 (0.53–3.73) | 0.60 (0.13–2.75) |
| Non‐high‐energy fracture 1–4 years | 0.97 (0.81–1.17) | 0.93 (0.74–1.16) |
| Non‐high‐energy fracture 5–14 years | 1.13 (1.08–1.18) | 1.13 (1.07–1.19) |
| High‐energy fracture <1 year | 1.22 (0.78–1.89) | 1.22 (0.71–2.10) |
| High‐energy fracture 1–4 years | 1.17 (0.88–1.54) | 0.86 (0.60–1.24) |
| High‐energy fracture 5–14 years | 1.25 (1.12–1.40) | 1.15 (1.00–1.32) |
| HR (95% CI) model 1 | HR (95% CI) model 2 | |
| Any fracture <1 year | 1.21 (0.81–1.81) | 1.08 (0.65–1.80) |
| Any fracture 1–4 years | 1.01 (0.86–1.18) | 0.87 (0.74–1.09) |
| Any fracture 5–14 years | 1.13 (1.08–1.18) | 1.12 (1.06–1.17) |
| Non‐high‐energy fracture <1 year | 1.40 (0.53–3.73) | 0.60 (0.13–2.75) |
| Non‐high‐energy fracture 1–4 years | 0.97 (0.81–1.17) | 0.93 (0.74–1.16) |
| Non‐high‐energy fracture 5–14 years | 1.13 (1.08–1.18) | 1.13 (1.07–1.19) |
| High‐energy fracture <1 year | 1.22 (0.78–1.89) | 1.22 (0.71–2.10) |
| High‐energy fracture 1–4 years | 1.17 (0.88–1.54) | 0.86 (0.60–1.24) |
| High‐energy fracture 5–14 years | 1.25 (1.12–1.40) | 1.15 (1.00–1.32) |
Note: Model 1: adjusted for sex and year of birth. Model 2: adjusted for sex, year of birth, maternal age, maternal education, paternal education, parity, and maternal fracture status.
Abbreviations: CI = confidence interval; HR = hazard ratio.
Cox Regression Results Comparing Offspring of Mothers Who Smoked During Pregnancy With Mothers Who Did Not Smoke
| HR (95% CI) model 1 | HR (95% CI) model 2 | |
| Any fracture <1 year | 1.21 (0.81–1.81) | 1.08 (0.65–1.80) |
| Any fracture 1–4 years | 1.01 (0.86–1.18) | 0.87 (0.74–1.09) |
| Any fracture 5–14 years | 1.13 (1.08–1.18) | 1.12 (1.06–1.17) |
| Non‐high‐energy fracture <1 year | 1.40 (0.53–3.73) | 0.60 (0.13–2.75) |
| Non‐high‐energy fracture 1–4 years | 0.97 (0.81–1.17) | 0.93 (0.74–1.16) |
| Non‐high‐energy fracture 5–14 years | 1.13 (1.08–1.18) | 1.13 (1.07–1.19) |
| High‐energy fracture <1 year | 1.22 (0.78–1.89) | 1.22 (0.71–2.10) |
| High‐energy fracture 1–4 years | 1.17 (0.88–1.54) | 0.86 (0.60–1.24) |
| High‐energy fracture 5–14 years | 1.25 (1.12–1.40) | 1.15 (1.00–1.32) |
| HR (95% CI) model 1 | HR (95% CI) model 2 | |
| Any fracture <1 year | 1.21 (0.81–1.81) | 1.08 (0.65–1.80) |
| Any fracture 1–4 years | 1.01 (0.86–1.18) | 0.87 (0.74–1.09) |
| Any fracture 5–14 years | 1.13 (1.08–1.18) | 1.12 (1.06–1.17) |
| Non‐high‐energy fracture <1 year | 1.40 (0.53–3.73) | 0.60 (0.13–2.75) |
| Non‐high‐energy fracture 1–4 years | 0.97 (0.81–1.17) | 0.93 (0.74–1.16) |
| Non‐high‐energy fracture 5–14 years | 1.13 (1.08–1.18) | 1.13 (1.07–1.19) |
| High‐energy fracture <1 year | 1.22 (0.78–1.89) | 1.22 (0.71–2.10) |
| High‐energy fracture 1–4 years | 1.17 (0.88–1.54) | 0.86 (0.60–1.24) |
| High‐energy fracture 5–14 years | 1.25 (1.12–1.40) | 1.15 (1.00–1.32) |
Note: Model 1: adjusted for sex and year of birth. Model 2: adjusted for sex, year of birth, maternal age, maternal education, paternal education, parity, and maternal fracture status.
Abbreviations: CI = confidence interval; HR = hazard ratio.
We also categorized the bone fractures into hip or vertebral fractures and into non‐hip or non‐vertebral fractures to see whether maternal smoking in pregnancy have an effect on more cortical or trabecular bone or both. There was a statistically significant result in non‐vertebral or non‐hip fracture group in the 5–14 years age group (HR = 1.11 [95% CI 1.06–1.17]), when in the vertebral or hip fracture group, results were not statistically significant (HR = 1.29 [95% CI 0.90–1.85]). There were no statistically significant results in other age groups. See the results in Supplemental Table S4.
Results with all fractures
With all fractures as outcome, the maternal smoking during pregnancy was associated with higher fracture risk in the 5–14 years age group; the HR was 1.13 (95% CI 1.08–1.18) after adjustment for the sex and birth year (model 1). Adjustment with model 2 was similar: HR = 1.12 (95% CI 1.06–1.17). In the <1 year and 1–4 years age groups, the results did not reach statistical significance (Table 2).
Non‐high‐energy fractures
When only non‐high‐energy fractures were considered, results for the 5–14 years age group again reached statistical significance. The results in this age group were HR = 1.13 (95% CI 1.08–1.18) for model 1 and 1.13 (95% CI 1.07–1.19) for model 2. The results in age groups <1 year and 1–4 years were not statistically significant. These results are presented in Table 2.
High‐energy fractures
When only the high‐energy fractures were under consideration, the statistical significance was again reached in the 5–14 years age group. The HR was 1.25 (95% CI 1.12–1.40) for model 1 and 1.15 (95% CI 1.00–1.32) for model 2. The results of age groups <1 year and 1–4 years are available in Table 2.
Smoking as a three‐category variable
When the smoking of the mother was classified in three categories (non‐smoker, <10 cigarettes/d and >10 cigarettes/d), there was a dose–response relationship between number of cigarettes and HR for bone fracture for the 5–14 years age group. For light smokers, when all fractures were considered, the HR was 1.11 (95% CI 1.05–1.17) after adjustment for model 1 and 1.09 (95% CI 1.02–1.17) after adjustment for model 2. For heavy smokers, the HR was 1.15 (95% CI 1.09–1.21) for model 1 and 1.14 (95% CI 1.07–1.22) for model 2. The dose‐dependent relationship was not found when non‐high‐energy and high‐energy fractures served as outcomes. The full results are presented in Supplemental Table S2.
The results in age groups <1 year and 1–4 years were not statistically significant. These results are also presented also in Supplemental Table S2.
Supplemental analyses
We conducted analyses in children up to 17 completed years. In total, there were 26,174 (12%) bone fractures. The incidence was 62/10,000. In association between maternal smoking during pregnancy and offspring's bone fracture, there were no associations in that age group in any of the fracture groups (Supplemental Table S3).
Maternal smoking during pregnancy and recurrent bone fractures
We also tested whether maternal smoking during pregnancy was associated with recurrent bone fractures among the offspring. There were 5987 (2.7%) children who had more than one fracture under the age of 15 years. Number of fractures ranged from 2 to 15 fractures.
Results with all fractures
Poisson regression results within the under 1‐year‐old group was 1.38 (95% CI 0.42–4.47), in 1–4‐year‐old group 1.08 (95% CI 0.78–1.49), and in 5–14‐year‐old group 1.00 (95% CI 0.92–1.09) when adjusted for all the covariates (model 2). None of these were statistically significant (Table 3).
Poisson Regression Results With Offspring's Recurrent Fracture as a Predictor (Maternal Smoking as Dichotomous Variable)
| IRR (95% CI) adjustment model 1 | IRR (95% CI) adjustment model 2 | |
| Any fracture <1 year | 1.02 (0.74–1.41) | 1.38 (0.42–4.47) |
| Any fracture 1–4 years | 1.02 (0.90–1.16) | 1.08 (0.78–1.49) |
| Any fracture 5–14 years | 1.02 (0.98–1.05) | 1.00 (0.92–1.09) |
| Non‐high‐energy fracture <1 year | 1.54 (0.67–2.94) | 0.94 (0.20–4.47) |
| Non‐high‐energy fracture 1–4 years | 0.99 (0.85–1.15) | 0.93 (0.64–1.35) |
| Non‐high‐energy fracture 5–14 years | 1.02 (0.99–1.06) | 1.00 (0.92–1.10) |
| High‐energy fracture <1 year | 0.94 (0.66–1.33) | 1.43 (0.44–4.67) |
| High‐energy fracture 1–4 years | 1.10 (0.88–1.38) | 1.61 (0.81–3.22) |
| High‐energy fracture 5–14 years | 0.97 (0.89–1.06) | 1.01 (0.78–1.29) |
| IRR (95% CI) adjustment model 1 | IRR (95% CI) adjustment model 2 | |
| Any fracture <1 year | 1.02 (0.74–1.41) | 1.38 (0.42–4.47) |
| Any fracture 1–4 years | 1.02 (0.90–1.16) | 1.08 (0.78–1.49) |
| Any fracture 5–14 years | 1.02 (0.98–1.05) | 1.00 (0.92–1.09) |
| Non‐high‐energy fracture <1 year | 1.54 (0.67–2.94) | 0.94 (0.20–4.47) |
| Non‐high‐energy fracture 1–4 years | 0.99 (0.85–1.15) | 0.93 (0.64–1.35) |
| Non‐high‐energy fracture 5–14 years | 1.02 (0.99–1.06) | 1.00 (0.92–1.10) |
| High‐energy fracture <1 year | 0.94 (0.66–1.33) | 1.43 (0.44–4.67) |
| High‐energy fracture 1–4 years | 1.10 (0.88–1.38) | 1.61 (0.81–3.22) |
| High‐energy fracture 5–14 years | 0.97 (0.89–1.06) | 1.01 (0.78–1.29) |
Note: Model 1: adjusted for sex and year of birth. Model 2: adjusted for sex, year of birth, maternal age, maternal education, paternal education, parity, and maternal fracture status.
Abbreviations: CI = confidence interval; IRR = incidence rate ratio.
Poisson Regression Results With Offspring's Recurrent Fracture as a Predictor (Maternal Smoking as Dichotomous Variable)
| IRR (95% CI) adjustment model 1 | IRR (95% CI) adjustment model 2 | |
| Any fracture <1 year | 1.02 (0.74–1.41) | 1.38 (0.42–4.47) |
| Any fracture 1–4 years | 1.02 (0.90–1.16) | 1.08 (0.78–1.49) |
| Any fracture 5–14 years | 1.02 (0.98–1.05) | 1.00 (0.92–1.09) |
| Non‐high‐energy fracture <1 year | 1.54 (0.67–2.94) | 0.94 (0.20–4.47) |
| Non‐high‐energy fracture 1–4 years | 0.99 (0.85–1.15) | 0.93 (0.64–1.35) |
| Non‐high‐energy fracture 5–14 years | 1.02 (0.99–1.06) | 1.00 (0.92–1.10) |
| High‐energy fracture <1 year | 0.94 (0.66–1.33) | 1.43 (0.44–4.67) |
| High‐energy fracture 1–4 years | 1.10 (0.88–1.38) | 1.61 (0.81–3.22) |
| High‐energy fracture 5–14 years | 0.97 (0.89–1.06) | 1.01 (0.78–1.29) |
| IRR (95% CI) adjustment model 1 | IRR (95% CI) adjustment model 2 | |
| Any fracture <1 year | 1.02 (0.74–1.41) | 1.38 (0.42–4.47) |
| Any fracture 1–4 years | 1.02 (0.90–1.16) | 1.08 (0.78–1.49) |
| Any fracture 5–14 years | 1.02 (0.98–1.05) | 1.00 (0.92–1.09) |
| Non‐high‐energy fracture <1 year | 1.54 (0.67–2.94) | 0.94 (0.20–4.47) |
| Non‐high‐energy fracture 1–4 years | 0.99 (0.85–1.15) | 0.93 (0.64–1.35) |
| Non‐high‐energy fracture 5–14 years | 1.02 (0.99–1.06) | 1.00 (0.92–1.10) |
| High‐energy fracture <1 year | 0.94 (0.66–1.33) | 1.43 (0.44–4.67) |
| High‐energy fracture 1–4 years | 1.10 (0.88–1.38) | 1.61 (0.81–3.22) |
| High‐energy fracture 5–14 years | 0.97 (0.89–1.06) | 1.01 (0.78–1.29) |
Note: Model 1: adjusted for sex and year of birth. Model 2: adjusted for sex, year of birth, maternal age, maternal education, paternal education, parity, and maternal fracture status.
Abbreviations: CI = confidence interval; IRR = incidence rate ratio.
Results with non‐high‐energy fractures
Poisson regression results in the under 1‐year‐old group was 0.94 (95% CI 0.20–4.47), in 1–4‐year‐old group 0.93 (95% CI 0.64–1.35), and in 5–14‐year‐old group 1.00 (95% CI 0.92–1.10) when adjusted for all the covariates (model 2). There were no statistically significant results in any age group (Table 3).
Results with high‐energy fractures
Poisson regression results in the under 1‐year‐old group was 1.43 (95% CI 0.44–4.67), in 1–4‐year‐old group 1.61 (95% CI 0.81–3.22), and in 5–14‐year‐old group 1.01 (95% CI 0.78–1.29) when adjusted for all the covariates (model 2). There were no statistically significant results in any age group (Table 3).
Discussion
Main findings
The comprehensive birth cohort study establishes that maternal smoking during pregnancy is associated with increased bone fracture risk of the offspring between the ages of 5 and 14 years but not before 5 years of age. The association survived adjustment for sex, year of birth, maternal age, parity, mother's fracture status, and both maternal and paternal education. The increase in the hazard was found to be 12%, which is a notable rise when population level is considered. The associations were similar across the two sexes for all the age categories.
The statistical association in the 5–14 years age group was also found when only non‐high‐energy fractures were taken into consideration. The HR was 1.13 with adjustment model 1 and 1.13 with adjustment model 2. These numbers are similar to the case where all fractures were included. On the other hand, when only the high‐energy fractures were taken into account, the statistical association in the 5–14 years age group was somewhat stronger. When adjusted with sex and year of birth, the HR was 1.25, but after adjustment with maternal age, parity, parent's level of education, in addition to sex and year of birth, the HR leveled off to ths same order of magnitude as when all fractures were included (HR = 1.15).
When the fractures were divided into hip or vertebral fractures and into non‐hip or non‐vertebral fractures, the statistical association in the 5–14 years age group was found in non‐hip or non‐vertebral fracture group (HR = 1.11 [95% CI 1.06–1.17]) and in hip or vertebral not. This result could deduce that maternal smoking during pregnancy affects more cortical bone than trabecular bone in this age group.
There was also a dose–response relationship between the amount of maternal smoking and later bone fracture risk of the offspring when all fractures were considered. The offspring of those mothers who were heavy smokers (>10 cigarettes/d) were somewhat more likely to experience a fracture than those whose mothers were light smokers (1–9 cigarettes/d). However, the dose–response relationship was not found in high‐energy and non‐high‐energy fracture groups.
We could not find a statistically significant association between maternal smoking during pregnancy and recurrent fractures. This implies that children with recurrent bone fractures have underlying bone‐affecting diseases, hobbies, or other lifestyle factors that affect the fracture risk more than the gestational environment.
Strengths and weaknesses of the study
A large birth cohort population with a long follow‐up time is one of the strengths of this study. The Care Register for Health Care is comprehensive and considered a valid tool in population health research. Also, the size of the cohort allowed the execution of analyses in different fracture subgroups. In our previous study, the outpatient‐treated fractures were missing from the data, but in this current study, they are included from 1998 onward.(16) One limitation is that the outpatient fractures were missing from the data between 1987 and 1998.
The Care Register for Health Care does not include diagnoses given in primary health care or in the private sector. Uncomplicated fractures with straightforward conservative treatment may thus be underrepresented. However, high‐standard public health care services are available for all in the study country. Primary health care and private sector have a minimal role in the inpatient treatment of childhood bone fractures.
One source of bias might be that people living in rural areas might not end up going to hospital for treatment compared with people living in urban areas. However, in Finland the number of bone fractures is smaller in the rural areas than in the vicinity of cities.(29,30)
The register data did not include any information on measurements of bone strength or mineral content; therefore, comparison to previous studies, focusing on bone quality, could not be made. In addition, we had no access to other medical information of the cohort members, and therefore we could not take into account the possible bone‐affecting diseases or medications, such as glucocorticoid exposure. However, they would be expected to confound or mediate the associations only if they also were associated with maternal smoking in pregnancy. Still, we ruled out from the analysis cohort members with major malformations, such as osteogenesis imperfecta, that could cause fragility of bones. We did not have information of offspring's body mass index (BMI). In future studies, it would be interesting to analyze whether low BMI, as a sign of malabsorption, could increase fracture risk regardless of smoking during pregnancy.
The exposure to smoke has been shown to be a risk factor for low vitamin D levels for the mother and the developing fetus as well.(31,32) On the other hand, the maternal vitamin D insufficiency has been linked to offspring's reduced bone mineral content during childhood.(33) Unfortunately, we did not have information on the vitamin D levels of the mothers. However, vitamin D supplementation has been provided to children in Finland since the 1940s.(34) Also, development of children in Finland is followed regularly from birth until graduation from junior high school by regular nurse and doctor visits at a child welfare clinic of school health care attended by virtually all children. Therefore, nutritional deficiencies are rare.(35)
Our data on maternal smoking in pregnancy, originating from the MBR, may be subject to some level of underreporting because it is based on maternal self‐reports. In a sample selected from the MBR in 1987–2011, the smoking information was validated against cotinine measurements from maternal first‐trimester serum samples. In the sample, 16.2% reported smoking and had high cotinine, whereas 7.7% reported no smoking but had high cotinine and were classified as undisclosed smokers.(36) We were unable to assess undisclosed smoking, which is likely to lead to them to be erroneously classified as nonsmokers. However, we would rather expect such exposure misclassification to lead to a more conservative estimate in the association between maternal smoking and offspring fractures. Moreover, 2.9% of the cohort members had no information on the maternal smoking during pregnancy and they were excluded from this study.
Earlier studies have found that nicotine exposure prenatally is linked to neurobehavioral problems later in life.(37‐40) These defects, including attention‐deficit/hyperactivity disorder (ADHD), learning disabilities, and increased risk of behavioral problems, may all influence the fracture risk in childhood because of behavioral factors. The study by Hurtig and colleagues(41) found a link between ADHD and hospital‐treated injuries. Because we did not have knowledge on the other diagnoses of the cohort members, we could not consider the effect of these conditions in the analysis.
There are studies that have evaluated the association of maternal smoking during pregnancy with bone mineral density (BMD) and bone mass of the child. Some studies have found an association between maternal smoking and lower neonatal bone mass.(42,43) When the associations of smoking during pregnancy have been evaluated later in childhood and in adolescence, the results are mixed.(44,45) Some studies have found an association between bone mineral density and risk of bone fractures,(46,47) whereas others have not.(48,49) We did not have information on offspring's BMD or other factors of bone quality in this study.
It is notable that smoking during pregnancy is a well‐established risk factor for preterm birth and low birth weight.(10) Preterm birth, in turn, increases the risk of bone fractures at 0 to 12 years of age.(25) However, in the study by Michaud and colleagues,(22) the higher risk of fracture hospitalizations was explained by the fractures occurring in the first 18 months of life. There was no significant relationship between gestational age and bone fractures thereafter. We considered gestational age and birth weight as potential mediating variables and, therefore, did not consider it appropriate to include them in the regression models.
Comparison with previous studies
Results of the studies that have evaluated the association between maternal smoking during pregnancy and bone health of offspring seem to be inconclusive. Ma and Jones did not find a statistically significant association between maternal smoking in pregnancy and later fracture risk among 324 children at 8 years of age or younger.(50) A study by Manias and colleagues among 150 children aged 4 to 16 years suggests that smoking during pregnancy by itself does not predict increased risk of bone fractures, but in combination with nutritional shortcomings in childhood (eg, no breastfeeding), maternal smoking can increase the risk of single or recurrent fractures.(5) Parviainen and colleagues found in a study among more than 6700 children a statistically significant association between maternal smoking in pregnancy and increased fracture risk of the offspring before 7 years of age, but the size of the study population did not allow for subgrouping of the fracture data.(16)
Högberg and colleagues and Brand and colleagues conducted the studies based on larger populations.(18,19) Högberg and colleagues established that maternal smoking in late pregnancy predicts an increased risk of fracture in infants under the age of 6 months.(18) Brand and colleagues found that the risk of fracture is elevated in children under the age of 5 years if the mother had smoked during the pregnancy. However, when the offspring exposed to maternal smoking during pregnancy were compared with siblings not exposed to maternal smoking, this association vanished, and a statistically significant association was found only in the age group under 1 year old.(19) A common feature in sibling‐comparison studies of maternal smoking during pregnancy is that associations that exist in comparison with unrelated controls attenuate or vanish in comparison with sibling controls.(51) This has been interpreted as a sign of unmeasured confounding by genetic or environmental factors shared within a family.(51) However, sibling comparisons are also particularly prone to exposure misclassification(52) that is common in studies of maternal smoking during pregnancy.(36)
The population size of our study is comparable to the studies of Brand and colleagues(19) and Högberg and colleagues.(18) Still, our findings differ from these two studies. Both Brand and colleagues and Högberg and colleagues found that bone fracture risk is increased in infants. Fractures in infants have different risk factors that include underlying bone fragility and physical abuse.(53,54) Our current study does not include the within‐sibship analysis. In the future, this could give interesting knowledge on the possible confounding aspect of the familial factors.
The bone fracture incidence in our population was 55/10,000 person‐years in under 15‐year‐old children. This is a bit lower compared with the literature. However, it was about the same magnitude in the Orton and colleagues study, 75/10,000 person‐years.(2) Mäyräpää and colleagues had higher incidence, 163/10,000 person‐years, but in their study, the population was smaller (1373), and data were collected from urban area.(3)
Our results reinforce arguments toward reducing smoking during pregnancy as a key target in perinatal care.
This large cohort study shows that maternal smoking during pregnancy is associated with increased risk of the offspring to have a register record on bone fracture between preschool age and adolescence.
Author Contributions
Emil Kääntä: Writing – original draft; formal analysis; data curation; conceptualization; investigation; visualization. Roope Parviainen: Conceptualization; methodology; writing – original draft; writing – review and editing; investigation; software; validation. Marjaana Tikanmäki: Conceptualization; resources; writing – review and editing. Suvi Alenius: Methodology; resources; software; data curation; writing – review and editing. Juha‐Jaakko Sinikumpu: Conceptualization; methodology; validation; supervision; project administration; writing – review and editing; resources. Eero Kajantie: Conceptualization; methodology; validation; supervision; project administration; writing – review and editing; resources.
Acknowledgments
The data were provided by the Finnish Institute of Health and Welfare and Statistics Finland.
The study was supported by grants from Academy of Finland, European Commission (733280 RECAP Research on Children and Adults Bron Preterm), Sigrid Jusélius Foundation, Foundation for Pediatric Research, Signe and Ane Gyllenberg Foundation, and Novo Nordisk Foundation.
Disclosures
All authors state that they have no conflicts of interests.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/jbmr.4923.
Data Availability Statement
The data that support the findings are national healthcare data that comprise confidential health data of individuals. The data are not publicly available due to privacy or ethical restrictions. Permission to access the data can be requested through http://www.findata.fi and http://www.stat.fi.
References
Ministry of Social Affairs and Health. Maternity and child health clinics [Internet];



