-
PDF
- Split View
-
Views
-
Cite
Cite
Alex Shoung, Nicholas Shoung, Rachael Hii, Nitesh Nerlekar, Peter R Ebeling, Alexander J Rodríguez, Electrocardiogram Changes Following Intravenous Bisphosphonate Infusion: A Systematic Review and Meta‐Analysis, Journal of Bone and Mineral Research, Volume 38, Issue 11, 1 November 2023, Pages 1679–1688, https://doi.org/10.1002/jbmr.4911
Close - Share Icon Share
ABSTRACT
Bisphosphonates are first‐line treatments for several bone and mineral disorders. Studies have reported an increased incidence of serious atrial fibrillation in patients receiving bisphosphonates; however, uncertainty remains as to whether electrical disturbances are precipitated by bisphosphonates. We aimed to review the literature for studies reporting electrocardiogram (ECG) findings in patients receiving intravenous bisphosphonates for any indication. We searched MEDLINE and EMBASE from inception until January 14, 2023, for studies reporting ECG parameters after intravenous bisphosphonate infusion. We excluded studies that only reported atrial fibrillation. Study quality was assessed using the Newcastle‐Ottawa scale. Continuous data were meta‐analyzed if reported in at least two studies. Random‐effects models were fitted and reported as standardized mean difference (SMD) with 95% confidence intervals (95% CIs). We found 1083 unique records, of which 11 met our inclusion and exclusion criteria. Studies had a low to low/moderate risk of bias. Six prospective cohort studies were included in the meta‐analysis. Five studies used zoledronic acid, whereas one study used pamidronate. Most studies (n = 4) were conducted in postmenopausal women with osteoporosis, one study was conducted in patients with bone metastases, and one study in children with osteoporosis secondary to cerebral palsy. Study populations ranged from n = 15 to n = 116. Heart rate–corrected QT (QTc) was significantly longer post‐infusion (SMD = 0.46 ms [95% CI 0.80 to 0.11]; n = 67 patients, k = 2 studies, τ2 = 0). There were no differences in heart rate, P wave (maximum), P wave (minimum), P wave dispersion, PR interval, QRS duration, QTc, QTc (maximum), QTc (minimum), and QTc dispersion. The correlation between pre‐ and post‐infusion QTc was not significant (p = 0.93). Overall, there is a weak association between intravenous bisphosphonate infusion and a QTc interval prolongation. However, there is insufficient evidence to support an association between intravenous bisphosphonate and any ECG variable changes, which may precipitate atrial fibrillation. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Introduction
Aminobisphosphonates are first‐line agents used in the management of osteoporosis, hypercalcemia, Paget's disease of bone, and skeletal complications of malignancy,(1) exerting their effects by inhibiting farnesyl pyrophosphate synthase, an enzyme required for cholesterol synthesis and cell wall integrity in osteoclasts.(1) They are commonly prescribed because of their efficacy in decreasing osteoclast‐mediated bone resorption and are generally well tolerated in clinical practice.(2) However, the HORIZON Pivotal Fracture Trial (HORIZON‐PFT), the seminal trial in the clinical development of zoledronic acid (an intravenous [IV] bisphosphonate), reported an increased incidence of serious atrial fibrillation (AF) associated with its administration.(3) Since then, large population and registry‐based studies have demonstrated mixed results concerning bisphosphonates and AF, with some showing increased incidence,(4‐7) some decreased incidence,(8,9) and others showing no association.(10) Pharmacovigilance studies have also demonstrated an increased incidence of AF with bisphosphonates.(11,12)
With the varied results thus far, it is also possible that IV bisphosphonates have no effect on AF. There was no change in total prevalence of AF in the HORIZON‐PFT, and with most serious AF cases occurring more than 30 days after administration, it is unlikely that this can be attributed to acute effects from bisphosphonates, given that they remain in circulation for less than 24 hours and do not typically deposit in cardiac tissue.(1,13)
Contrary, the mechanisms by which bisphosphonates may precipitate AF are not completely understood but have been hypothesized to involve disturbances to cardiomyocyte calcium activity.(3,14,15) On this assumption, it can be hypothesized that bisphosphonates may also precipitate other arrhythmias that are also dependent on cardiomyocyte calcium and electrolyte activity. Few studies have directly explored this relationship and whether such abnormalities may be detected through an electrocardiogram (ECG). As such, this systematic review and meta‐analysis will explore reported changes in ECG parameters after bisphosphonate administration.
Materials and Methods
Data sources and searches
MEDLINE and EMBASE databases were searched from inception to January 14, 2023, without language restrictions. A title and abstract search were performed (Supplemental Table S1). A citation manager was used to store records and remove duplicates. Records were screened based on titles and abstracts, and full texts were then evaluated for eligibility. Reference lists of eligible studies were also hand‐searched and similar meta‐analyses were evaluated for potentially relevant articles missing from the primary search.
Study selection
This systematic review and meta‐analysis was performed in accordance with the PRISMA statement and is registered with PROSPERO (CRD42021273998).(16) Studies were included if patients received IV bisphosphonates and at least one ECG parameter (other than AF) was reported. Exclusion criteria included non‐human studies, systematic reviews, narrative reviews, conference abstracts, letters, editorials, and studies that included patients with pre‐existing cardiovascular disease or electrolyte abnormalities.
Data extraction and quality assessment
Data extraction was performed by a single reviewer (AS) and verified by another (AJR) for accuracy. Data extracted included the total number of patients, average age, proportion of women, average body mass index, length of time for follow‐up (post‐infusion ECG), and the mean and standard deviation of pre‐infusion and post‐infusion values for ECG parameters reported.
Risk of bias was assessed using the Newcastle‐Ottawa quality assessment scale for cohort studies.(17) This scale provides a score out of 14 and assesses for bias across three domains: selection of the cohort, comparability of cohorts, and evaluation of outcome. All included studies were independently reviewed by two reviewers (AS and AJR) and overall risk of bias was determined with consensus between both reviewers. Studies were judged as having an overall low risk of bias if the overall score on the assessment scale was 13 or higher and as low/moderate risk if the score was between 10 and 12.
Data synthesis and statistical analysis
For ECG measures, estimates were expressed as standardized mean difference (SMD) with 95% confidence intervals (95% CIs) to account for reporting differences. Data were meta‐analyzed in an inverse‐variance random‐effects model using the DerSimonian–Laird estimator to calculate between‐study variance (τ2), with smaller τ2 and I2 values indicating less variance in between‐study heterogeneity. All analyses were computed using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/) and considered statistically significant when the bounds of the 95% CI did not cross zero for SMD.
Results
Literature search
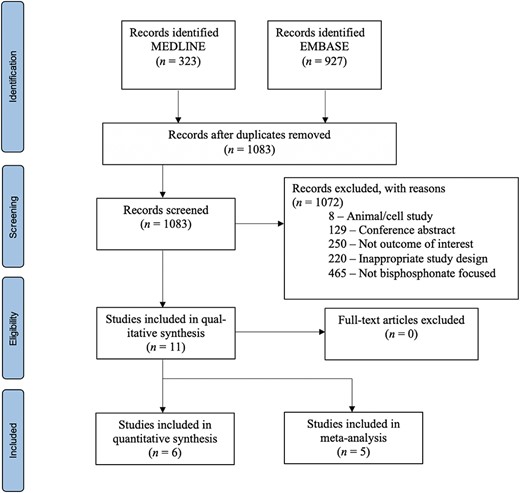
A total of 1250 records were identified from database searches. One hundred sixty‐seven duplicates were removed, resulting in 1083 unique records for screening. Of these, 1072 records were excluded, with the main reason for exclusion being that the study did not use a bisphosphonate as the intervention (n = 465). Other common reasons included not reporting an outcome of interest, namely ECG parameters (n = 250), inappropriate study design (n = 220), and conference abstracts (n = 129). In total, 11 records were included in this study (Fig. 1).
Details of prospective cohort studies included in meta‐analysis
Six prospective cohort studies were reviewed (Table 1). The largest cohort studied consisted of 116 patients and the smallest of 15 patients.(18,19) The mean age of cohorts ranged from 13.0 to 73.3 years. Women comprised the majority of the cohorts, making up 87.5% of the total population of meta‐analyzed data. Indications for bisphosphonates varied; four studies focused on patients with primary osteoporosis,(18‐21) one on new diagnoses of bone metastases,(22) and one on osteoporosis secondary to cerebral palsy in children.(23) All studies provided pre‐ and post‐infusion outcome data. Five of the studies used zoledronic acid,(18‐22) whereas one used pamidronate.(23) Length of follow‐up for ECG monitoring varied greatly between studies and ranged from 24 hours to 30 days post‐bisphosphonate infusion. Patients in four of six cohorts received vitamin D and calcium supplements during the study.(18,19,21,23) The most frequently reported outcome was P wave dispersion (Pwd), with four studies recording it.(18,20‐22)
| Study (year), country | Cohort (sex, diagnosis, mean age [years] [±SD]) | Total population (n) | Bisphosphonate | Dose | Electrolytes reported (before/after infusion) | Inclusion (I)/exclusion (E) criteria |
| Zhuang et al. (2021), China | 100% women, OP, 69.6 ± 9.5 | 116 | ZA | 5 mg | Yes/no | I: primary osteoporosis, aged ≥50 years E: secondary osteoporosis, bone metastases, severe arrhythmias, antiarrhythmic therapy, severe ischemic heart disease |
| Aktas et al. (2016), Turkey | 91% women, OP, 70.5 ± 11.6 | 100 | ZA | N/A | Yes/no | I: N/A E: chronic AF, valvular heart disease, chronic renal failure, serious electrolyte imbalance, thyroid disease, antiarrhythmic therapy, previous IV bisphosphonate therapy, oral bisphosphonates within past 3 months |
| Güzelant et al. (2016), Turkey | 100% women, OP, 73.3 ± 6.9 | 33 | ZA | 5 mg | Yes/yes | I: osteoporosis, aged ≥65 years E: male, history of treatment with bisphosphonate, cardiac pathology, undefinable P waves in baseline ECG, primary biliary cirrhosis, renal pathology, anemia |
| Cipriani et al. (2015), Italy | 100% women, OP or fragility fractures, 70.7 ± 6.9 | 15 | ZA | 5 mg | Yes/yes | I: osteoporosis, fragility fractures E: history of paroxysmal or permanent AF, left bundle branch block, pacemakers, ventricular pre‐excitation, hypertrophic cardiomyopathy, severe valvular disease |
| Rothenbuhler et al. (2010), France | 50% girls, children with OP secondary to cerebral palsy, 13.0 (9.0–15.7) | 34 | Pamidronate | 3 mg/kg/year | Yes/yes | N/A |
| Demirtas et al. (2017), Turkey | 57% women, cancer with new bone metastases, 53.70 ± 14.1 | 37 | ZA | N/A | Yes/no | I: diagnosis of cancer and new bone metastases E: malignant hypercalcemia requiring dialysis, hyperkalemia, previous antiarrhythmic or antihypertensive therapy, uncontrolled diabetes mellitus, ischemic heart disease. |
| Study (year), country | Cohort (sex, diagnosis, mean age [years] [±SD]) | Total population (n) | Bisphosphonate | Dose | Electrolytes reported (before/after infusion) | Inclusion (I)/exclusion (E) criteria |
| Zhuang et al. (2021), China | 100% women, OP, 69.6 ± 9.5 | 116 | ZA | 5 mg | Yes/no | I: primary osteoporosis, aged ≥50 years E: secondary osteoporosis, bone metastases, severe arrhythmias, antiarrhythmic therapy, severe ischemic heart disease |
| Aktas et al. (2016), Turkey | 91% women, OP, 70.5 ± 11.6 | 100 | ZA | N/A | Yes/no | I: N/A E: chronic AF, valvular heart disease, chronic renal failure, serious electrolyte imbalance, thyroid disease, antiarrhythmic therapy, previous IV bisphosphonate therapy, oral bisphosphonates within past 3 months |
| Güzelant et al. (2016), Turkey | 100% women, OP, 73.3 ± 6.9 | 33 | ZA | 5 mg | Yes/yes | I: osteoporosis, aged ≥65 years E: male, history of treatment with bisphosphonate, cardiac pathology, undefinable P waves in baseline ECG, primary biliary cirrhosis, renal pathology, anemia |
| Cipriani et al. (2015), Italy | 100% women, OP or fragility fractures, 70.7 ± 6.9 | 15 | ZA | 5 mg | Yes/yes | I: osteoporosis, fragility fractures E: history of paroxysmal or permanent AF, left bundle branch block, pacemakers, ventricular pre‐excitation, hypertrophic cardiomyopathy, severe valvular disease |
| Rothenbuhler et al. (2010), France | 50% girls, children with OP secondary to cerebral palsy, 13.0 (9.0–15.7) | 34 | Pamidronate | 3 mg/kg/year | Yes/yes | N/A |
| Demirtas et al. (2017), Turkey | 57% women, cancer with new bone metastases, 53.70 ± 14.1 | 37 | ZA | N/A | Yes/no | I: diagnosis of cancer and new bone metastases E: malignant hypercalcemia requiring dialysis, hyperkalemia, previous antiarrhythmic or antihypertensive therapy, uncontrolled diabetes mellitus, ischemic heart disease. |
Abbreviations: AF = atrial fibrillation; OP = osteoporosis; ZA = zoledronic acid.
| Study (year), country | Cohort (sex, diagnosis, mean age [years] [±SD]) | Total population (n) | Bisphosphonate | Dose | Electrolytes reported (before/after infusion) | Inclusion (I)/exclusion (E) criteria |
| Zhuang et al. (2021), China | 100% women, OP, 69.6 ± 9.5 | 116 | ZA | 5 mg | Yes/no | I: primary osteoporosis, aged ≥50 years E: secondary osteoporosis, bone metastases, severe arrhythmias, antiarrhythmic therapy, severe ischemic heart disease |
| Aktas et al. (2016), Turkey | 91% women, OP, 70.5 ± 11.6 | 100 | ZA | N/A | Yes/no | I: N/A E: chronic AF, valvular heart disease, chronic renal failure, serious electrolyte imbalance, thyroid disease, antiarrhythmic therapy, previous IV bisphosphonate therapy, oral bisphosphonates within past 3 months |
| Güzelant et al. (2016), Turkey | 100% women, OP, 73.3 ± 6.9 | 33 | ZA | 5 mg | Yes/yes | I: osteoporosis, aged ≥65 years E: male, history of treatment with bisphosphonate, cardiac pathology, undefinable P waves in baseline ECG, primary biliary cirrhosis, renal pathology, anemia |
| Cipriani et al. (2015), Italy | 100% women, OP or fragility fractures, 70.7 ± 6.9 | 15 | ZA | 5 mg | Yes/yes | I: osteoporosis, fragility fractures E: history of paroxysmal or permanent AF, left bundle branch block, pacemakers, ventricular pre‐excitation, hypertrophic cardiomyopathy, severe valvular disease |
| Rothenbuhler et al. (2010), France | 50% girls, children with OP secondary to cerebral palsy, 13.0 (9.0–15.7) | 34 | Pamidronate | 3 mg/kg/year | Yes/yes | N/A |
| Demirtas et al. (2017), Turkey | 57% women, cancer with new bone metastases, 53.70 ± 14.1 | 37 | ZA | N/A | Yes/no | I: diagnosis of cancer and new bone metastases E: malignant hypercalcemia requiring dialysis, hyperkalemia, previous antiarrhythmic or antihypertensive therapy, uncontrolled diabetes mellitus, ischemic heart disease. |
| Study (year), country | Cohort (sex, diagnosis, mean age [years] [±SD]) | Total population (n) | Bisphosphonate | Dose | Electrolytes reported (before/after infusion) | Inclusion (I)/exclusion (E) criteria |
| Zhuang et al. (2021), China | 100% women, OP, 69.6 ± 9.5 | 116 | ZA | 5 mg | Yes/no | I: primary osteoporosis, aged ≥50 years E: secondary osteoporosis, bone metastases, severe arrhythmias, antiarrhythmic therapy, severe ischemic heart disease |
| Aktas et al. (2016), Turkey | 91% women, OP, 70.5 ± 11.6 | 100 | ZA | N/A | Yes/no | I: N/A E: chronic AF, valvular heart disease, chronic renal failure, serious electrolyte imbalance, thyroid disease, antiarrhythmic therapy, previous IV bisphosphonate therapy, oral bisphosphonates within past 3 months |
| Güzelant et al. (2016), Turkey | 100% women, OP, 73.3 ± 6.9 | 33 | ZA | 5 mg | Yes/yes | I: osteoporosis, aged ≥65 years E: male, history of treatment with bisphosphonate, cardiac pathology, undefinable P waves in baseline ECG, primary biliary cirrhosis, renal pathology, anemia |
| Cipriani et al. (2015), Italy | 100% women, OP or fragility fractures, 70.7 ± 6.9 | 15 | ZA | 5 mg | Yes/yes | I: osteoporosis, fragility fractures E: history of paroxysmal or permanent AF, left bundle branch block, pacemakers, ventricular pre‐excitation, hypertrophic cardiomyopathy, severe valvular disease |
| Rothenbuhler et al. (2010), France | 50% girls, children with OP secondary to cerebral palsy, 13.0 (9.0–15.7) | 34 | Pamidronate | 3 mg/kg/year | Yes/yes | N/A |
| Demirtas et al. (2017), Turkey | 57% women, cancer with new bone metastases, 53.70 ± 14.1 | 37 | ZA | N/A | Yes/no | I: diagnosis of cancer and new bone metastases E: malignant hypercalcemia requiring dialysis, hyperkalemia, previous antiarrhythmic or antihypertensive therapy, uncontrolled diabetes mellitus, ischemic heart disease. |
Abbreviations: AF = atrial fibrillation; OP = osteoporosis; ZA = zoledronic acid.
We reasonably assume that the units of heart rate–corrected (QTc) data presented in Guzelant and colleagues(21) have been reported in error, as abnormally short QTc intervals were presented in the range of 40 to 50 ms, but the authors reported that ECG data were within normal limits. Nevertheless, as our meta‐analysis uses the SMD to measure effect size, the units of data become insignificant.
Literature quality
Four studies were judged as having a low/moderate risk of bias,(18,19,22,23) and two studies as low risk of bias (Table 2).(20,21) Studies generally scored poorly on representativeness of the exposed cohort, adequacy of follow‐up length, and comparability.
| Study (year) | Indication for BP | Representativeness of the exposed cohort (2) | Selection of the non‐exposed cohort (1) | Ascertainment of exposure (2) | Outcome of interest was not present at start of study (1) | Comparability (1) | Assessment of outcome (3) | Adequacy of follow‐up length (1) | Adequacy of follow‐up cohorts (3) | Risk of bias consensus |
| Cipriani et al. (2015) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Aktas et al. (2016) | Men and women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Güzelant et al. (2016) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Rothenbuhler et al. (2010) | Children with CP and OP | 1/0 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Demirtas et al. (2017) | Men and women with new metastatic bone cancer | 1/1 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Zhuang et al. (2020) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Study (year) | Indication for BP | Representativeness of the exposed cohort (2) | Selection of the non‐exposed cohort (1) | Ascertainment of exposure (2) | Outcome of interest was not present at start of study (1) | Comparability (1) | Assessment of outcome (3) | Adequacy of follow‐up length (1) | Adequacy of follow‐up cohorts (3) | Risk of bias consensus |
| Cipriani et al. (2015) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Aktas et al. (2016) | Men and women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Güzelant et al. (2016) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Rothenbuhler et al. (2010) | Children with CP and OP | 1/0 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Demirtas et al. (2017) | Men and women with new metastatic bone cancer | 1/1 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Zhuang et al. (2020) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Study (year) | Indication for BP | Representativeness of the exposed cohort (2) | Selection of the non‐exposed cohort (1) | Ascertainment of exposure (2) | Outcome of interest was not present at start of study (1) | Comparability (1) | Assessment of outcome (3) | Adequacy of follow‐up length (1) | Adequacy of follow‐up cohorts (3) | Risk of bias consensus |
| Cipriani et al. (2015) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Aktas et al. (2016) | Men and women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Güzelant et al. (2016) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Rothenbuhler et al. (2010) | Children with CP and OP | 1/0 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Demirtas et al. (2017) | Men and women with new metastatic bone cancer | 1/1 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Zhuang et al. (2020) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Study (year) | Indication for BP | Representativeness of the exposed cohort (2) | Selection of the non‐exposed cohort (1) | Ascertainment of exposure (2) | Outcome of interest was not present at start of study (1) | Comparability (1) | Assessment of outcome (3) | Adequacy of follow‐up length (1) | Adequacy of follow‐up cohorts (3) | Risk of bias consensus |
| Cipriani et al. (2015) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Aktas et al. (2016) | Men and women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Güzelant et al. (2016) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 1/1 | 3/3 | Low |
| Rothenbuhler et al. (2010) | Children with CP and OP | 1/0 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Demirtas et al. (2017) | Men and women with new metastatic bone cancer | 1/1 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
| Zhuang et al. (2020) | Postmenopausal women with OP | 2/2 | 1/1 | 2/2 | 1/1 | 0/0 | 3/3 | 0/0 | 3/3 | Low/moderate |
Meta‐analysis
Six studies with a total of 375 patients were eligible for meta‐analysis. Ten ECG parameters including heart rate, P wave (maximum), P wave (minimum), Pwd, PR interval, QRS duration, QTc, QTc (maximum), QTc (minimum), and QTc dispersion were reported in at least two studies each, enabling meta‐analysis. QTc was found to be significantly longer post‐infusion (SMD = 0.46 ms [95% CI 0.80 to 0.11]; n = 67 patients, k = 2 studies, I2 = 0%, τ2 = 0) (Fig. 2). No significant differences were found in any of the other ECG parameters pre‐ and post‐infusion.
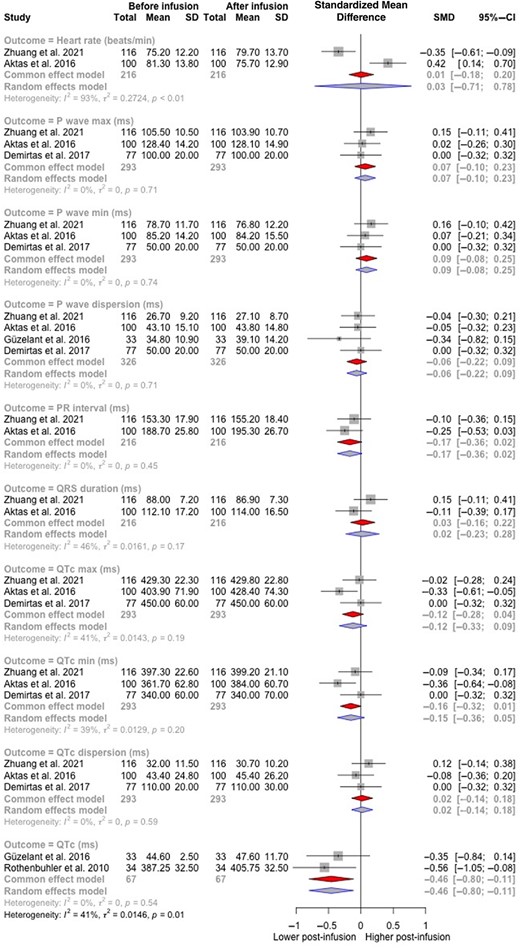
Forest plot of electrocardiogram parameters before and after bisphosphonate infusion.
Details of case studies included in qualitative synthesis
We identified five case reports that reported ECG changes after IV bisphosphonate administration (Table 3).(24‐28) The indication for treatment was malignancy or its complications in four studies(24,26‐28) and was unclear in another.(25) All cases reported QT segment prolongation. Four cases reported prolongation of at least 500 ms, and one did not specify length.(26) Four cases reported hypocalcemia,(24,26‐28) and one case reported normocalcemia.(25) Non‐sustained polymorphic ventricular tachycardia was identified on ECG in one case.(25)
| Study (year) | Age (years), sex | Bisphosphonate (dose) | Indication | Reported QT change | Calciuma | Time of BP infusion before ECG | Reported arrhythmia |
| Aldave & Jaiswal (2014) | 73, woman | ZA | Multiple myeloma | Prolongation (500 ms) | Hypocalcemia (0.66 mmol/L – ionized) | 11 days | None reported |
| Bonilla et al. (2014) | 55, woman | Ibandronate | N/A | Prolongation (575 ms) | Normal | 15 days | Non‐sustained polymorphic VT, ventricular ectopy |
| Mishra, Wong & Jonklaas (2001) | 62, woman | Pamidronate (90 mg) | Thyroid cancer with bone metastases | Prolongation (unknown) | Hypocalcemia (0.10 mmol/L – ionized) | 35 days | Junctional rhythm |
| Patel, Brahmbatt & Ramu (2005) | 74, man | ZA | Prostate cancer with bone metastases | Prolongation (>500 ms) | Hypocalcemia (0.19 mmol/L – serum) | 4 days | None reported |
| Varma & Kerrigan (1993) | 71, woman | Pamidronate (30 mg) | Hypercalcemia of malignancy | Prolongation (550 ms) | Hypocalcemia (1.8 mmol/L – serum)b | 15/19 daysc | Sinus rhythm |
| Study (year) | Age (years), sex | Bisphosphonate (dose) | Indication | Reported QT change | Calciuma | Time of BP infusion before ECG | Reported arrhythmia |
| Aldave & Jaiswal (2014) | 73, woman | ZA | Multiple myeloma | Prolongation (500 ms) | Hypocalcemia (0.66 mmol/L – ionized) | 11 days | None reported |
| Bonilla et al. (2014) | 55, woman | Ibandronate | N/A | Prolongation (575 ms) | Normal | 15 days | Non‐sustained polymorphic VT, ventricular ectopy |
| Mishra, Wong & Jonklaas (2001) | 62, woman | Pamidronate (90 mg) | Thyroid cancer with bone metastases | Prolongation (unknown) | Hypocalcemia (0.10 mmol/L – ionized) | 35 days | Junctional rhythm |
| Patel, Brahmbatt & Ramu (2005) | 74, man | ZA | Prostate cancer with bone metastases | Prolongation (>500 ms) | Hypocalcemia (0.19 mmol/L – serum) | 4 days | None reported |
| Varma & Kerrigan (1993) | 71, woman | Pamidronate (30 mg) | Hypercalcemia of malignancy | Prolongation (550 ms) | Hypocalcemia (1.8 mmol/L – serum)b | 15/19 daysc | Sinus rhythm |
Abbreviations: BP = bisphosphonate; ECG = electrocardiogram; VT = ventricular tachycardia; ZA = zoledronic acid.
Values that were provided in mg/dL were converted to mmol/L as 1 mg/dL = 0.055 mmol/L.
Patient received two doses of pamidronate (30 mg) 15 and 19 days before ECG.
Days 12 to 19 of admission.
| Study (year) | Age (years), sex | Bisphosphonate (dose) | Indication | Reported QT change | Calciuma | Time of BP infusion before ECG | Reported arrhythmia |
| Aldave & Jaiswal (2014) | 73, woman | ZA | Multiple myeloma | Prolongation (500 ms) | Hypocalcemia (0.66 mmol/L – ionized) | 11 days | None reported |
| Bonilla et al. (2014) | 55, woman | Ibandronate | N/A | Prolongation (575 ms) | Normal | 15 days | Non‐sustained polymorphic VT, ventricular ectopy |
| Mishra, Wong & Jonklaas (2001) | 62, woman | Pamidronate (90 mg) | Thyroid cancer with bone metastases | Prolongation (unknown) | Hypocalcemia (0.10 mmol/L – ionized) | 35 days | Junctional rhythm |
| Patel, Brahmbatt & Ramu (2005) | 74, man | ZA | Prostate cancer with bone metastases | Prolongation (>500 ms) | Hypocalcemia (0.19 mmol/L – serum) | 4 days | None reported |
| Varma & Kerrigan (1993) | 71, woman | Pamidronate (30 mg) | Hypercalcemia of malignancy | Prolongation (550 ms) | Hypocalcemia (1.8 mmol/L – serum)b | 15/19 daysc | Sinus rhythm |
| Study (year) | Age (years), sex | Bisphosphonate (dose) | Indication | Reported QT change | Calciuma | Time of BP infusion before ECG | Reported arrhythmia |
| Aldave & Jaiswal (2014) | 73, woman | ZA | Multiple myeloma | Prolongation (500 ms) | Hypocalcemia (0.66 mmol/L – ionized) | 11 days | None reported |
| Bonilla et al. (2014) | 55, woman | Ibandronate | N/A | Prolongation (575 ms) | Normal | 15 days | Non‐sustained polymorphic VT, ventricular ectopy |
| Mishra, Wong & Jonklaas (2001) | 62, woman | Pamidronate (90 mg) | Thyroid cancer with bone metastases | Prolongation (unknown) | Hypocalcemia (0.10 mmol/L – ionized) | 35 days | Junctional rhythm |
| Patel, Brahmbatt & Ramu (2005) | 74, man | ZA | Prostate cancer with bone metastases | Prolongation (>500 ms) | Hypocalcemia (0.19 mmol/L – serum) | 4 days | None reported |
| Varma & Kerrigan (1993) | 71, woman | Pamidronate (30 mg) | Hypercalcemia of malignancy | Prolongation (550 ms) | Hypocalcemia (1.8 mmol/L – serum)b | 15/19 daysc | Sinus rhythm |
Abbreviations: BP = bisphosphonate; ECG = electrocardiogram; VT = ventricular tachycardia; ZA = zoledronic acid.
Values that were provided in mg/dL were converted to mmol/L as 1 mg/dL = 0.055 mmol/L.
Patient received two doses of pamidronate (30 mg) 15 and 19 days before ECG.
Days 12 to 19 of admission.
Discussion
This systematic review and meta‐analysis evaluated changes in ECG parameters after IV bisphosphonate administration, hypothesizing that this may provoke arrhythmias. This phenomenon has been described in previous randomized controlled trials (RCTs) and several observational cohorts, but such an effect remains controversial. Though we did not find conclusive evidence of this phenomenon, our findings did reveal small effects on the QTc interval, noting that the magnitude of the SMD found in our study represents an approximate variation from normal of 10% and is thus of uncertain clinical significance. Published case reports have also indicated potential effects on the QT interval; however, those reports may have been published precisely because an arrhythmia was discovered, underscoring inherent publication bias of case reports, and may not be reflective of the typical patient undergoing bisphosphonate administration. Because there is limited evidence from this meta‐analysis supporting an effect of IV bisphosphonates on atrial electrophysiological parameters, we speculate previously reported associations between IV bisphosphonate administration and AF are potentially related to a direct effect that currently has an undescribed mechanism, that certain patients may be at increased risk of such effect after bisphosphonate exposure, or the alternative that there is no association. Regardless, given the potential for arrhythmias, clinicians should be aware of these effects and optimize patient factors such as monitoring renal function and use of concomitant nephrotoxic medications and ensuring replete vitamin D status and normal serum calcium levels before commencing treatment because of hypocalcemia risk.
The scientific and clinical motivation for this study were findings from the seminal trial of IV zoledronic acid, HORIZON, which demonstrated an increased incidence of serious AF.(3)
Given the uncertain relationship between bisphosphonates and cardiac electrophysiological abnormalities, our study explored the potential precipitants of AF, which may not be monitored in routine clinical practice or are impractical to systematically report in large cohorts. In this respect, we have analyzed information reported in several small cohorts and case reports, systematically reviewing ECGs both pre‐ and post‐infusion of IV bisphosphonates (predominantly zoledronic acid). Our meta‐analysis of six cohort studies showed that QTc intervals were on average longer after infusion, and is supported by evidence from the collective summary of the case reports, which tended to report that QT intervals were prolonged in those patients. In effect, we are observing the same phenomenon and hypothesize that there is either a direct effect of the bisphosphonate on these features or that changes in these features may be related to an autonomic effect post‐infusion. We have attempted to account for electrolyte abnormalities, which may explain any changes, but we were limited in the data reported to explore this. Given what the case reports demonstrated, careful examination of the patient characteristics of these patients will help inform physicians about the potential risk for similar adverse outcomes in their own patients. However, despite the meta‐analysis demonstrating prolonged QTc after bisphosphonate administration, the clinical significance of 0.46 ms is questionable.
It is unlikely that the small‐magnitude QTc prolongation identified in this meta‐analysis is of clinical importance. A normal QTc is between 350 and 450 ms for men and 360 and 460 ms for women and follows a normal distribution.(29) Although it is accepted that QTc prolongation is associated with increased mortality risk, there are no defined thresholds for risk stratification based on QT intervals.(29) This is also complicated in that up to 20% of otherwise healthy individuals will have a QTc interval outside of this normal range,(29) and while the upper limit of normal is 450 to 460 ms for adults, arrhythmias such as torsade de pointes are rarely associated with a QTc value less than 500 ms.(30) Furthermore, a threshold for which QT prolongation is free of proarrhythmic risk has not been established.(31)
Another possible reason for electrophysiological abnormalities from bisphosphonates could be related to direct electrochemical changes on the cell. An ex vivo animal perfusion study demonstrated increased Pwd after zoledronic acid infusion, and this was hypothesized to be involved in the pathogenesis of AF.(32) Pwd reflects inhomogeneous electrical conduction within the atria and is a predictor of AF.(33) However, our results contradict this, and our analysis found no significant difference in Pwd either before or after bisphosphonate infusion.
Our results and the described case reports suggest that bisphosphonates may be associated with QT interval prolongation. The likely mechanism behind this is the effect that bisphosphonates have on lowering the serum calcium concentration. Bisphosphonates cause osteoclast apoptosis, leading to decreased calcium release from bone and possible hypocalcemia,(34) a known cause for prolonged QT interval on ECG.(35) Hypocalcemia, which was observed in four of five described case studies,(24,26‐28) decreases influx of calcium into myocytes phase 2 of the cardiac action potential,(36,37) prolonging ventricular repolarization, which is reflected in an ECG as a prolonged QT interval. QT prolongation is a risk factor for ventricular arrhythmias, which prompts the important consideration of monitoring in patients receiving IV bisphosphonates. Long QT is not known to cause atrial tachycardias; however, etiologies of long QT, both congenital and acquired, have been suggested to be contributory to AF.(37,38) This could potentially be extended to IV bisphosphonate therapy.
An alternative explanation for the association between bisphosphonates and AF may be explained by inflammation.(14,39) Histopathology from atrial biopsies in patients with AF have shown chronic inflammatory changes(40) and bisphosphonate administration has been shown to increase release of pro‐inflammatory cytokines such as C‐reactive protein,(41,42) hypothesized to be involved in the pathogenesis AF.(43,44) Other inflammatory markers such as interleukin‐6 (IL‐6) have also been associated with AF;(45) however, there is conflicting evidence in literature with some studies finding bisphosphonates increasing IL‐6 levels(42,46) and others decreasing it.(47) Bisphosphonates have also been associated with an increase in tumor necrosis factor alpha,(46) another inflammatory marker associated with atrial remodeling and development of AF.(48)
Although the HORIZON‐PFT demonstrated an increased incidence of serious AF, a subsequent study of zoledronic acid in older patients with hip fractures reported no effects on AF and other tachyarrhythmias.(49) More recent trials in osteopenic patients have also found no association with AF,(50) leading us to speculate whether we are indeed observing a true effect. This is especially relevant given that observational studies have also demonstrated conflicting findings regarding electrophysical consequences of bisphosphonate exposure.(4,51)
The HORIZON‐PFT showed an increased incidence of AF reported as a serious adverse event increase but no change in the total prevalence.(3) AF in the study also occurred more than 30 days after zoledronic acid infusion in 47 of 50 patients.(3) However, it is unlikely that bisphosphonates still exert acute effects on cardiac tissue in this timeframe, given that bisphosphonates in circulation have a short half‐life (0.5–2 hours),(52) with drug not deposited within the skeleton being rapidly renally excreted,(1) and the amount of bisphosphonate deposited in noncalcified tissue such as cardiac tissue is negligible.(13) Given this, the possibility that IV bisphosphonates are unrelated to AF should be reconsidered.
The strengths of this review include interpreting data from a meta‐analysis of small observational cohort data alongside five case reports, strengthening the internal validity of the association between bisphosphonates and prolonged QT interval. We also looked at numerous different outcomes in terms of ECG parameters and had a broad approach to inclusion criteria to capture all relevant literature. The limitations of our systematic review was the use and availability of aggregate data, using only observational studies, small study sizes, and clinical heterogeneity for indications of bisphosphonates, along with limited reporting of electrolyte levels in patients. Further, other data reporting the effect that bisphosphonates have on ECG parameters have been found but have been published in a language other than English(53) or as conference abstracts only.(54‐58)
In conclusion, we showed there is some evidence of IV bisphosphonate administration prolonging the QT interval, which has been associated with ventricular arrhythmias and may also less commonly be associated with atrial arrhythmias. However, the meaningful collective of our analysis is that there is insufficient evidence to suggest IV bisphosphonates alter other cardiac electrophysiological parameters detectable on an ECG. To aid our understanding, future trials of bisphosphonates could consider including ECG measurements as part of their suite of safety outcomes. It would also be beneficial for existing trials to publish these data if available.
Acknowledgments
Open access publishing facilitated by Griffith University, as part of the Wiley ‐ Griffith University agreement via the Council of Australian University Librarians. [Correction added on 28 September 2023, after first online publication: CAUL funding statement has been added.]
Author Contributions
Alex Shoung: Writing – original draft; writing – review and editing; methodology; investigation. Nicholas Shoung: Writing – review and editing; supervision. Rachael Hii: Writing – review and editing. Nitesh Nerlekar: Writing – review and editing; supervision. Peter R Ebeling: Writing – review and editing; supervision. Alexander J Rodríguez: Conceptualization; supervision; writing – review and editing; formal analysis; methodology.
Disclosures
AJR and PRE are supported by a National Health and Medical Research Council Australia Investigator Grant (GNT1197958). PRE has received grant funding to his institution from Amgen, Sanofi, and Alexion, and honoraria from Amgen and Alexion.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/jbmr.4911.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.



