-
PDF
- Split View
-
Views
-
Cite
Cite
Aliya A. Khan, John P. Bilezikian, Maria Luisa Brandi, Bart L. Clarke, Neil J. Gittoes, Janice L. Pasieka, Lars Rejnmark, Dolores M. Shoback, John T. Potts, Gordon H. Guyatt, Michael Mannstadt, Evaluation and Management of Hypoparathyroidism Summary Statement and Guidelines from the Second International Workshop, Journal of Bone and Mineral Research, Volume 37, Issue 12, 1 December 2022, Pages 2568–2585, https://doi.org/10.1002/jbmr.4691
Close - Share Icon Share
ABSTRACT
This clinical practice guideline addresses the prevention, diagnosis, and management of hypoparathyroidism (HypoPT) and provides evidence‐based recommendations. The HypoPT task forces included four teams with a total of 50 international experts including representatives from the sponsoring societies. A methodologist (GG) and his team supported the taskforces and conducted the systematic reviews. A formal process following the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology and the systematic reviews provided the structure for seven of the guideline recommendations. The task force used a less structured approach based on narrative reviews for 20 non‐GRADEd recommendations. Clinicians may consider postsurgical HypoPT permanent if it persists for >12 months after surgery. To predict which patients will not develop permanent postsurgical HypoPT, we recommend evaluating serum PTH within 12 to 24 hours post total thyroidectomy (strong recommendation, moderate quality evidence). PTH > 10 pg/mL (1.05 pmol/L) virtually excludes long‐term HypoPT. In individuals with nonsurgical HypoPT, genetic testing may be helpful in the presence of a positive family history of nonsurgical HypoPT, in the presence of syndromic features, or in individuals younger than 40 years. HypoPT can be associated with complications, including nephrocalcinosis, nephrolithiasis, renal insufficiency, cataracts, seizures, cardiac arrhythmias, ischemic heart disease, depression, and an increased risk of infection. Minimizing complications of HypoPT requires careful evaluation and close monitoring of laboratory indices. In patients with chronic HypoPT, the panel suggests conventional therapy with calcium and active vitamin D metabolites as first‐line therapy (weak recommendation, low‐quality evidence). When conventional therapy is deemed unsatisfactory, the panel considers the use of PTH. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
SUMMARY OF RECOMMENDATIONS
The following recommendations are intended to guide practice and are not intended to be used for the development of reimbursement policies.
How should chronic HypoPT be diagnosed? (un‐GRADEd recommendation, i.e., not based on Grading of Recommendations, Assessment, Development and Evaluation)
- 1.1
Hypocalcemia (low ionized serum calcium or total serum calcium adjusted for albumin) in the presence of an undetectable, low or inappropriately normal intact PTH (utilizing either a second‐ or third‐generation assay) on two occasions at least 2 weeks apart confirms the diagnosis.
- 1.2
Additional abnormalities caused by low PTH that support the diagnosis: Elevation in serum phosphorus, reductions in 1,25‐dihydroxyvitamin D (1,25(OH)2D) and elevations in the urinary fractional excretion of calcium.
- 1.3
In patients with postsurgical HypoPT, panel members regard the condition as permanent if the HypoPT persists >12 months after surgery.
- 1.1
How can the risks of chronic postsurgical HypoPT be minimized? (un‐GRADEd recommendation)
The panel proposes avoiding accidental parathyroidectomy as well as intraoperative parathyroid autotransplantation during neck surgery and only utilizing this in the presence of inadvertent parathyroidectomy.
What is the value of determining serum calcium and PTH post‐thyroidectomy to predict future permanent postsurgical HypoPT? (GRADEd recommendation)
We recommend using PTH measurements early (12–24 hours) after total thyroidectomy for predicting which patients will not develop permanent postsurgical HypoPT
(strong recommendation, moderate quality evidence).
Comments: If PTH values are >10 pg/mL (1.05 pmol/L) 12–24 hours post surgery, the development of permanent HypoPT is unlikely, and therefore there is no long‐term need for treatment with active vitamin D and calcium supplements above the recommended daily allowance. Many patients with PTH values <10 pg/mL (1.05 pmol/L) 12–24 hours post surgery may still recover from temporary HypoPT.
What is the role of genetic testing in the diagnosis and evaluation of chronic HypoPT? (un‐GRADEd recommendations)
- 3.1
In patients with nonsurgical HypoPT who have a positive family history of nonsurgical HypoPT, present with syndromic features, or are younger than 40 years, panel members undertake genetic testing.
- 3.2
In patients with nonsurgical HypoPT who have other clinical features of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy syndrome (APECED), panel members undertake genetic testing for autoimmune regulator (AIRE) gene variants.
- 3.3
Panel members avoid the designation of “autoimmune HypoPT” for patients who do not have APECED because there are no definitive diagnostic tests for polygenic autoimmune HypoPT.
- 3.1
What are the most common symptoms and complications of chronic HypoPT reported in the literature? (GRADEd recommendation)
Observational studies comparing patients with HypoPT to controls with normal parathyroid function have identified the following complications associated with HypoPT (percentages represent the median among all studies): cataract (17%), infection (11%), nephrocalcinosis/nephrolithiasis (15%), renal insufficiency (12%), seizures (11%), depression (12%), ischemic heart disease (7%), and arrhythmias (7%).
What is the optimal monitoring strategy for chronic HypoPT?
- 5.1
(Systematic Current Practice Survey)*
New patient Follow‐up for stable patients** Serum creatinine, estimated glomerular filtration rate (eGFR), calcium (either ionized or albumin‐adjusted), magnesium, phosphorus √ Every 3–12 months 25‐hydroxyvitamin D √ Every 6–12 months 24‐hour urine for creatinine and calcium √ Every 6–24 months New patient Follow‐up for stable patients** Serum creatinine, estimated glomerular filtration rate (eGFR), calcium (either ionized or albumin‐adjusted), magnesium, phosphorus √ Every 3–12 months 25‐hydroxyvitamin D √ Every 6–12 months 24‐hour urine for creatinine and calcium √ Every 6–24 months *These are graded as low‐quality recommendations based on the practice of 70% of the respondents completing this at least 70% of the time.
**For unstable patients: Frequently measure serum calcium and phosphorus as clinically indicated.
The panel also proposes the following (non‐survey‐based):
- 5.2
Complete a baseline assessment for the presence of renal calcification or stones with renal imaging.
- 5.3
Monitor serum calcium (ionized or albumin‐adjusted) within several days of a significant change in medical treatment.
- 5.1
How are patients with HypoPT managed?
(GRADEd recommendations)
- 6.1
In patients with chronic HypoPT, the panel suggests conventional therapy as first‐line therapy (weak recommendation, low‐quality evidence).
Comment: When conventional therapy is deemed unsatisfactory, the panel considers the use of parathyroid hormone.
un‐GRADEd PANEL RECOMMENDATIONS FOR MANAGEMENT
In patients with HypoPT, the panel proposes:
- 6.2
Treat with calcium and an active vitamin D analogue, with the goal of raising serum calcium to the target range, i.e., the lower half of the normal reference range or just below the normal reference range. At this time, it is not clear how to best balance the doses of calcium relative to those of the active vitamin D analogue.
- 6.3
Alleviate symptomatic hypocalcemia while avoiding hypercalciuria.
- 6.4
Avoid hypercalciuria when titrating calcium and active vitamin D analogue therapy, aiming for low normal plasma calcium levels.
The panel proposes achieving a 24‐hour urinary calcium level of <6.25 mmol/24 hours or 250 mg/24 hours for adult women and <7.5 mmol/24 hours or 300 mg/24 hours for adult men. Data from the general population have shown a relationship between hypercalciuria and the development of renal stones—such data do not exist in patients with HypoPT. However, panel members infer that hypercalciuria may be associated with a higher risk of renal stones in patients with HypoPT as well and thus seek to avoid hypercalciuria.
- 6.5
Avoid hyperphosphatemia. Panel members prescribe calcium supplements with meals to serve as phosphate binders, implement a low‐phosphate diet in adults if needed, and judiciously use active vitamin D analogue therapy. No data are available on the use of other types of phosphate binders in HypoPT. Hyperphosphatemia may be associated with an increased incidence of ectopic calcification, but currently there is no evidence of this in HypoPT.
- 6.6
Treat to normalize plasma magnesium levels. Magnesium supplements can be used as tolerated by the patient.
- 6.7
Aim to achieve a 25‐hydroxyvitamin D (25(OH)D) level in the normal reference range (75–125 nmol/L).
- 6.8
Consider treating hypercalciuria with thiazide diuretics in conjunction with a low‐sodium diet with careful monitoring of blood pressure (BP), serum magnesium, potassium, and renal function.
- 6.9
Consider PTH replacement therapy in patients who are not adequately controlled on conventional therapy. Inadequate control is considered to be any one of the following: (i) symptomatic hypocalcemia, (ii) hyperphosphatemia, (iii) renal insufficiency, (iv) hypercalciuria, or (v) poor quality of life.
- 6.10
Individuals with poor compliance or malabsorption or who are intolerant of large doses of calcium and active vitamin D may also benefit from PTH therapy. Individuals requiring high doses of conventional therapy (i.e., calcium >2 g/day or active vitamin D > 2 μg/day) may also benefit from PTH therapy.
- 6.1
un‐GRADEd CONSENSUS MANAGEMENT RECOMMENDATIONS DURING PREGNANCY AND LACTATION
In pregnant women with HypoPT, the panel proposes the following:
- 7.1
Aim to achieve serum calcium (ionized or albumin adjusted) in the mid to low normal reference range throughout pregnancy.
- 7.2
Aim to achieve serum phosphorus, magnesium, and 25OHD levels in the normal reference range.
- 7.3
Closely monitor serum calcium (ionized or albumin‐adjusted) every 3–4 weeks during pregnancy and lactation, with increased frequency in the months preceding and following parturition as well as in the presence of symptoms of hypercalcemia or hypocalcemia.
- 7.4
Work closely with the obstetrician to optimize pregnancy outcomes. Coordinate with the pediatric team to ensure appropriate postnatal monitoring for transient neonatal hypo‐ or hypercalcemia.
- 7.5
Avoid using thiazide diuretics and PTH or PTH analogues during pregnancy.
- 7.1
Introduction
An international task force on hypoparathyroidism (HypoPT) was convened to review new findings and insights and to develop updated evidence‐based guidelines on the diagnosis, evaluation, and management of HypoPT. Over the past 5 years, significant advances have been made in our understanding of the multisystem complications of HypoPT and, in particular, the skeletal and renal manifestations of this disorder. New treatment options are being developed with improvements in our understanding of calcium homeostasis and pharmacological approaches to intervention.
The international task force, consisting of 50 international experts in HypoPT and general endocrinologists from 15 countries, met over 24 months to review key issues pertaining to the diagnosis, prevention, evaluation, and management of HypoPT.
A methods team, using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology, completed four systematic reviews addressing the diagnosis, management, and complications of HypoPT. In addition, narrative reviews were completed regarding the epidemiology, financial burden, and etiology of HypoPT. A survey of panel members informed the recommendations for monitoring. The diagnosis and risk factors for the development of postsurgical HypoPT were reviewed with the development of strategies to help minimize postsurgical HypoPT. The role of genetic testing in determining the underlying etiology of HypoPT was highlighted and an approach to establishing the diagnosis presented. Calcium homeostasis in pregnancy and lactation was also reviewed with the development of strategies to optimize maternal and fetal outcomes. The risks and benefits of PTH replacement therapy in comparison to conventional therapy were evaluated with application of the GRADE methodology.
These new international guidelines on HypoPT have been endorsed by over 65 professional medical and surgical societies as well as patient advocacy organizations interested in advancing the care of individuals with HypoPT. These guidelines also highlight key areas for future research in HypoPT.
Methodology
A detailed discussion of the methodology is presented in an accompanying report.(1)
To summarize, four international task forces were formed with international experts in HypoPT addressing the following areas of review, with each task force publishing its findings as a separate manuscript in this issue of the JBMR.
Epidemiology and Financial Burden (cochairs BLC and NG)
Etiologies and Pathophysiology (cochairs JLP and DMS)
Genetics and Diagnosis (cochairs MM and MLB)
Evaluation and Management (cochairs AAK and LR)
Systematic reviews and narrative reviews were completed to inform the recommendations. GRADEd recommendations followed a structured process that included framing questions in patient/intervention/comparator/outcome format; conducting a systematic evidence search and associated summary, specifying values and preferences, and classifying and presenting recommendations as strong or weak with the corresponding quality of evidence. A strong recommendation was made when the desirable effects were much greater than undesirable effects or vice versa and is worded as “we recommend.”(2,3)
A weak recommendation was made if there was low certainty of evidence or a close balance between desirable and undesirable effects and is worded as “we suggest.” Each of the systematic reviews has also been published as a separate manuscript in this issue of JBMR.
The three systematic reviews evaluated (i) the value of measuring calcium and PTH 12–24 hours after total thyroidectomy for predicting which patient would develop chronic HypoPT, (ii) the prevalence of symptoms and complications of HypoPT, and (iii) the effects of therapy with PTH compared to conventional therapy.
Ungraded recommendations from the narrative reviews did not involve structured approaches and are presented as descriptions of the practice of the panelists in managing patients with HypoPT. Un‐GRADEd recommendations are presented as “we propose.” The intent was to achieve consensus on all recommendations. There was no provision for voting.
Following completion of the reviews, both systematic and narrative, the findings were presented to all the members of the HypoPT task forces and all members of the accompanying task forces on primary hyperparathyroidism (approximately 100 members in total) for their perspective and feedback at a recorded virtual meeting. The feedback and suggestions from these members were incorporated into each of the manuscripts. A second virtual meeting was held at which time the findings of the task forces were presented to representatives from global scientific societies and patient advocacy organizations for their feedback.
All task force members disclosed any potential conflicts of interest prior to participating in the development of the manuscripts and guidelines.
Funding was received in the form of unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. These companies had no input in the design of the project, conduct of the reviews, review of the data, content of the manuscripts, review of the manuscripts, or forthcoming recommendations.
Results
Diagnosis
The diagnosis of HypoPT is based on biochemical evaluation and is usually straightforward. Low or inappropriately normal PTH in the setting of hypocalcemia distinguishes the disease from secondary causes of hypocalcemia, in which PTH is elevated.
How should chronic HypoPT be diagnosed? (un‐GRADEd recommendation)
Hypocalcemia (low serum calcium adjusted for albumin or low ionized calcium) in the presence of an undetectable, low, or inappropriately normal intact PTH (utilizing either a second‐ or third‐generation assay) on two occasions at least 2 weeks apart confirms the diagnosis.
Additional abnormalities caused by low PTH, which support the diagnosis:Elevation in serum phosphorus, reductions in 1,25(OH)2D, and elevations in 24‐hour urinary calcium.
In patients with postsurgical HypoPT, panel members regard it as permanent if the HypoPT persists >12 months after surgery.
Epidemiology and Financial Burden
HypoPT is a rare condition with an estimated prevalence ranging from 6.4 to 37/100,000 person‐years and an incidence reported to be 0.8 to 2.3/100,000/person‐years.(4)
Postsurgical HypoPT constitutes approximately 75% of all cases. There are multiple reasons for a variable prevalence of postsurgical HypoPT in the literature, and these include variable timing of the biochemical monitoring, incomplete follow‐up, and variable definitions of permanent HypoPT with a lack of consensus in the literature. The literature is currently of low quality, with single‐institution results and retrospective series. The most commonly utilized definition of permanent HypoPT is a continued requirement for calcium and active vitamin D for 12 months or more following surgery.
The impact of HypoPT on mortality is not clear. Data based on five registries is inconsistent; mortality in HypoPT is reported to be increased in some studies(5,6) but not in others.(7‐9)
HypoPT is associated with a major financial burden owing to increased healthcare utilization. Individuals with chronic HypoPT have significant symptoms and comorbidity resulting in increased healthcare costs and utilize healthcare resources with increased numbers of outpatient visits and ER visits.(10) This was more frequently noted in individuals with poorly controlled HypoPT in a recent retrospective 1‐year review.(10)
A web‐based survey of 374 adult U.S. patients with chronic HypoPT also confirmed significant healthcare utilization with 79% of patients requiring hospitalization or ER visits during the year before the survey and 72% having experienced >10 symptoms during the year before the survey while receiving standard medical treatment. Symptoms were experienced for a mean of 13±9 hours each day.(11) HypoPT is associated with a major detrimental impact on the lives of patients with HypoPT.(11)
Etiology
Postsurgical HypoPT
Several risk factors have been identified for the development of postsurgical HypoPT. They include patient factors (i.e., vitamin D deficiency), the underlying disease (malignancy, thyrotoxicosis, size of the parathyroid glands identified during thyroidectomy), and operative factors (reoperation, extent of operation, surgeon's practice volume).
A recent meta‐analysis of 25 studies showed an increased risk of postsurgical HypoPT in those who underwent parathyroid autotransplantation.(12)
The number of autotransplanted glands correlates positively with the development of postsurgical HypoPT and may reflect the result of removal or devascularization of the glands.(13) It is advisable to leave all viable parathyroid in situ and not proceed with parathyroid autotransplantation. Autotransplantation should only be performed if parathyroidectomy has inadvertently occurred.(13) Emerging technologies may be of value in reducing the risk of inadvertent damage or removal of the parathyroid glands and are discussed in detail in an accompanying review.(13)
How can the risks of chronic postsurgical HypoPT be minimized? (un‐GRADEd recommendation)
The panel proposes avoiding parathyroid autotransplantation during neck surgery and only utilizing this in the presence of inadvertent parathyroidectomy.
Owing to improved surgical techniques, permanent HypoPT is uncommon after neck surgery. However, it would be desirable to predict postoperatively which patient is more or less likely to develop chronic HypoPT.
A systematic review was conducted to address the value of determining serum calcium and PTH post‐thyroidectomy to predict future permanent postsurgical HypoPT. If PTH values exceed 10 pg/mL (1.05 pmol/L) 12–24 hours post surgery, the development of permanent HypoPT is very unlikely, as is the long‐term need for treatment with active vitamin D and calcium supplements above the recommended daily allowance. Management in the immediate postoperative state should continue to be guided by serum calcium, symptoms, and clinical judgment.
PTH values <10 pg/mL (1.05 pmol/L) 12–24 hours post surgery have less predictive value. The possibility of developing permanent hypoPT still exists but is less than 50% (15).
What is the value of determining serum calcium and PTH post‐thyroidectomy to predict future permanent postsurgical HypoPT? (GRADEd recommendation)?
We recommend using PTH measurements after total thyroidectomy for predicting which patients will not develop permanent postsurgical HypoPT (strong recommendation, moderate‐quality evidence).
Nonsurgical HypoPT
The etiology of nonsurgical HypoPT requires careful evaluation with identification of the underlying cause. It may be due to genetic, autoimmune, and metabolic factors(13) (Table 1).(14)
|
|
Reproduced with permission from Khan et al., EJE 2019.(14)
|
|
Reproduced with permission from Khan et al., EJE 2019.(14)
Genetic disorders can present as isolated HypoPT or as part of a syndrome. Genetic HypoPT can be due to disorders of parathyroid gland formation, PTH secretion, and parathyroid gland damage.(15) Evaluation includes a thorough family history and attention to the presence of features of syndromic forms of HypoPT. Genetic tests can be employed examining each candidate gene at a time or a panel of implicated genes at once, or genome‐wide approaches can be used.
Autoimmune HypoPT may occur as an acquired disease or as a component of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy syndrome (APECED).(16)
Rare etiologies for nonsurgical HypoPT include deposition of copper, iron, or aluminum in parathyroid tissue or the invasion of the parathyroid glands by neoplastic diseases or granulomatous or inflammatory cells or by amyloid protein.(14,17)
Drugs can also rarely result in HypoPT. Cinacalcet, a calcimimetic agent, increases the sensitivity of the CaSR to extracellular calcium and reduces PTH and also serum calcium.(18)
Immune checkpoint inhibitors can also induce HypoPT in association with activating autoantibodies to the calcium‐sensing receptor.(19)
Permanent HypoPT should be differentiated from transient nonsurgical HypoPT, which can be caused by hypo‐ or hypermagnesemia.(20‐22) Maternal hypercalcemia can result in infantile hypocalcemia and infants of mothers with familial hypocalciuric hypercalcemia can also have HypoPT.(14,23,24)
Pseudo‐HypoPT or vitamin D disorders can also present with hypocalcemia; however, PTH is elevated. In pseudo‐HypoPT there is a target organ resistance to PTH, resulting in low serum calcium and high phosphate with a high PTH.(14) In vitamin D deficiency, calcium and phosphate absorption from the bowel is impaired and PTH is secondarily elevated.(14) These conditions require careful evaluation and close monitoring. If the cause of HypoPT is not identified, it is classified as idiopathic.(13)
What is the role of genetic testing in the diagnosis and evaluation of chronic HypoPT? (un‐GRADEd recommendation)
In patients with nonsurgical HypoPT who have a positive family history of nonsurgical HypoPT, present with syndromic features, or are younger than 40 years, the panel advises genetic testing.
In patients with nonsurgical HypoPT who have other clinical features of APECED, the panel advises genetic testing for autoimmune regulator gene (AIRE) gene variants.
Panel members advise avoiding the designation of “autoimmune HypoPT” for patients who do not have APECED because a definitive diagnostic test for polygenic autoimmune HypoPT is not currently available.
Autoimmune HypoPT
Autoimmune HypoPT may occur either in isolation or as part of APECED, which is also called autoimmune polyglandular syndrome type 1 (APS‐1). This syndrome is due to genetic mutations in the autoimmune regulator gene (AIRE).(13) APECED is associated with HypoPT owing to parathyroid damage by circulating autoantibodies and infiltration with lymphocytes.(14) The three major clinical features are chronic mucocutaneous candidiasis, HypoPT, and adrenal insufficiency. HypoPT is present in >80% of patients with APECED and may be the only endocrinopathy present.(14) Patients may also have minor features of APS1 (Table 2). The diagnosis of APS1 is probable in the presence of at least one major feature and positive antibodies to type 1 interferon (present in >95% of patients). The presence of auto‐antibodies to 21‐hydroxylase correlate with adrenal insufficiency.(16) The presence of antibodies to the antigen NALP5 correlate with developing hypoparathyroidism.(25) A molecular diagnosis can be confirmed with DNA analysis of the AIRE gene if pathogenic variants are present.
Major features
Minor features
|
Major features
Minor features
|
Reproduced with Permission from Khan et al., EJE 2019(14).
Major features
Minor features
|
Major features
Minor features
|
Reproduced with Permission from Khan et al., EJE 2019(14).
Activating antibodies to the calcium‐sensing receptor inhibiting PTH secretion have also been reported in individuals with autoimmune HypoPT.(26‐28) These antibodies have been reported in individuals with APS1 and may also occur in isolation without APS1.
Recovery over time has been reported in some individuals as the antibody titers decrease. Unfortunately, no standardized diagnostic tests for the presence of antibodies to the CaSR are currently available.
Functional HypoPT
Both hypomagnesemia and hypermagnesemia can impair parathyroid function, leading to functional HypoPT. Magnesium can activate the calcium‐sensing receptor and decrease PTH synthesis and secretion.(20) Hypomagnesemia also results in a resistance to PTH because intracellular magnesium is a cofactor for adenylate cyclase.(20) Abnormalities in serum magnesium require further evaluation to determine the cause, and correction is necessary in order to improve calcium homeostasis, especially in patients on PTH therapy.(13)
Complications
What are the most common complications of HypoPT? (systematic review)
HypoPT is associated with symptoms and complications affecting multiple organ systems. The methods team conducted a systematic review investigating the prevalence of symptoms and complications. Ninety‐three studies enrolling a total of 18,973 patients proved eligible. The review team developed the following two criteria to determine whether the complications and symptoms were caused by chronic HypoPT: (i) they were reported by at least three studies and (ii) they had statistically significantly greater pooled nonadjusted and adjusted relative estimates in comparison with individuals with normal parathyroid function. We identified the following eight most common complications that met these two criteria and identified their frequency in the median of all studies in which they were addressed.(29)
Other symptoms and complications
We identified 51 complications reported by one to two studies (see supplemental table), as predefined in the methods. Because only limited studies reported these complications and symptoms, we are uncertain as to whether they are caused by chronic HypoPT or are contributed to by comorbidities of other diseases. We therefore did not present these complications/symptoms in the main paper.
Renal complications reported in population studies
The prevalence of chronic kidney disease (CKD) in patients with HypoPT ranges from 2.5% to 41%, depending on the definition (eGFR < 60 mL/min/1.73 m2 International Classification of Diseases (ICD) codes or self‐report).(7,9,11,30‐38) Data from a large US managed care claims database (8097 cases, 40,485 controls) noted an increased risk of CKD (eGFR < 60) in HypoPT.(39)A population study from Denmark suggested that a longer disease duration,(36) higher median calcium‐phosphate product (>2.80 mmol2/L2), and a higher frequency of episodes of hypercalcemia were associated with an increased risk of CKD.(36)
Patients with nonsurgical HypoPT had an increased risk of CKD stage 4 (eGFR = 15–29 mL/minute) and 5 (eGFR<15 mL/minute) in comparison with matched controls.(38)
Skeletal complications
HypoPT is associated with bone mineral density (BMD) values that are above average(40‐43) in comparison to age, sex, and body mass index matched controls. Skeletal microstructure is abnormal with both cortical and cancellous compartments affected.(43‐46) Transiliac bone biopsies have demonstrated increased cortical thickness and cancellous bone volume.(47,48) Bone remodeling is significantly reduced,(47‐49) with each remodeling cycle being associated with a positive bone balance.(13) Trabecular bone score (TBS) is maintained as noted in a retrospective cohort study.(50)
The effects of these changes on bone strength are not fully understood at this time. Bone material strength index (BMSi) utilizing microindentation was found to be lower in comparison to controls and improved with recombinant human PTH (rhPTH) (1–84) therapy.(51) The effects of HypoPT on the risk of fracture has been evaluated; however, current data are limited by small sample sizes and study design. An increase in the overall risk of fracture incidence has not been consistently observed.(4,52)
There may also be differences in the response of the various skeletal sites to the effects of HypoPT.(8,9,38,42,52,53) Large fracture studies in HypoPT patients compared to controls have not yet been completed. The effects of HypoPT on the risk of fracture requires further study.
Cardiovascular complications
HypoPT can affect the cardiovascular system. Hypocalcemia can lead to electrocardiographic abnormalities, including prolongation of the corrected QT (QTc) interval. The rare occurrence of cardiomyopathy and congestive heart failure in the setting of hypocalcemia has been described in case reports. Data from the large Danish national registry evaluating180 nonsurgical HypoPT patients and 540 controls found an increased risk for cardiovascular disease; Hazard Ratio (HR) 1.91, 95% confidence interval (CI) 1.29–2.81, p = 0.01), ischemic heart disease (HR 2.01, 95% CI 1.31–3.09, p = 0.01), cardiac arrhythmias (HR 1.78, 95% CI 0.96–3.30, p = 0.03), and stroke (HR 1.84,95% CI 0.95–3.54, p = 0.03). Mortality was not increased. These findings are supported by a subgroup analysis(36) and by a population study from Korea.(8,36) Increased risk of cardiovascular disease in chronic HypoPT was associated with lower time‐weighted serum ionized calcium, increased number of hypercalcemic episodes, and longer duration of HypoPT.(36) Although hypocalcemia is the presumed etiological factor in several of these complications,(54‐56) it has also been hypothesized that the loss of PTH action on arterial vascular smooth muscle, endothelial cells, cardiomyocytes, and the cardiac conducting system may be implicated.(13)
Cataracts in HypoPT
Cataracts are seen more frequently as a complication of chronic HypoPT(8,38,57) and appear to have a two‐ to fourfold higher risk in chronic HypoPT. Surgery may be required at an earlier mean age of 35 years.(38,57,58)
The mechanisms leading to cataracts are not well established. Altered electrolyte composition of the aqueous humor may lead to a change in the solubility of the lens fibers.(59,60) Risk factors for the development of cataracts include the nonsurgical form of HypoPT as well as a longer duration of HypoPT.(61‐63)
Basal ganglia calcifications
Basal ganglia calcification (BGC) has been noted to occur more frequently in HypoPT than in the general population, where the prevalence of BGC appears to be approximately 12%.(47,64‐68) Studies completed to date have had limited sample size, and many have been retrospective. In a Canadian prospective observational study, BGCs were noted in 37% of nonsurgical patients and 15% of postsurgical patients with HypoPT.(68) The median prevalence of BGC was 28% based on 20 studies in a systematic review of the complications of HypoPT.(29)
BGC was, however, not identified as one of the most common complications among the 14 cohort studies comparing individuals with HypoPT to euparathyroid individuals and is not included in the eight most common complications of HypoPT.
The consequences of these observed intracranial calcifications are unclear at this time. An association with Parkinson's disease has been proposed; however, this requires further evaluation with higher‐quality evidence in order to determine causality.(69)
Neuropsychiatric complications and features
Data from national registries as well as population‐based cohort studies support an increased incidence of neuropsychiatric disease.(8,9,38,52) An increased incidence of anxiety, depression, and bipolar affective disorder has been observed in patients with HypoPT.(4)
Neuromuscular manifestations of HypoPT include seizures, tetany, and muscle stiffness, which are common presenting features in 40%–60% of patients with HypoPT.(66) Seizures are common in young patients, particularly those with nonsurgical disease.(70) The seizures may be generalized tonic–clonic (80%) but may also be petit mal, partial, or atonic.(70) Hypocalcemia leads to a decreased threshold for membrane depolarization, resulting in increased neuronal excitability.(71) Numbness and tingling in the face, hands, and feet are recognized as classical symptoms of hypocalcemia.
Neuropsychiatric complications: quality of life
Reductions in quality of life (QoL) with significant negative impact on physical, mental, or emotional health have been documented in several studies utilizing standardized instruments validated for chronic diseases such as the SF36.(37,62,72‐78) A disease‐specific instrument, the Hypoparathyroid Patient Experience Scale‐Symptom (HPES‐Symptom), was developed and validated for HypoPT.(79,80) Poor health‐related QoL has been noted in both postsurgical and nonsurgical HypoPT patients.(36,37,62,75)
Infections
Chronic HypoPT appears to be associated with an increased risk of infections(81). A higher incidence of all types of infections was noted in a Danish national cohort study in both nonsurgical and postsurgical patients with hypoparathyroidism.(52) Urinary tract infections and respiratory tract infections were seen more commonly in comparison to the general population.(52) Risk factors appear to be increased disease duration, hyperphosphatemia, and increased episodes of hypercalcemia.(36) Infections may be more prevalent in nonsurgical than in postsurgical HypoPT (HR 1.87, 95% CI, 1.20–2.92).(4,6,9,36,52) The mechanisms involved are unclear. Since calcium signaling plays an important role in the production of cytokines by mast cells, T cells and natural killer cells, in target cell lysis by cytotoxic T cells, and lymphocyte differentiation,(81) impaired immune function caused by abnormal serum calcium level may be involved. Impaired immune function in patients with chronic postsurgical hypoparathyroidism was noted by immune cell profiling,(81) A decrease in monocytes as well as regulatory, naïve, and total CD4 + T lymphocytes was noted and correlated with total calcium, ionized calcium, and PTH levels in patients with HypoPT.(81)
Evaluation and Management of HYPOPT
HypoPT requires careful clinical assessment of the underlying etiology and for the presence of complications.(82) Because of the limited number of studies, a systematic review to determine the optimal monitoring strategy for the avoidance of complications from HypoPT was not feasible. Therefore, we conducted a survey determining the practice pattern of the 97 experts serving on the parathyroid consensus panel.(82‐84) A practice recommendation was based on the practice pattern of 70% or more of the respondents if the pattern was adopted in at least 70% of their patients.(85) These recommendations are summarized below (Table 3).
Most common complications of chronic hypoparathyroidism reported in the literature (GRADEd recommendations)
| Complication | Prevalence (median %) |
| Cataract | 17 |
| Infection | 11 |
| Nephrocalcinosis/nephrolithiasis | 15 |
| Renal insufficiency | 12 |
| Seizures | 11 |
| Depression | 9 |
| Ischemic heart disease | 7 |
| Arrhythmias | 7 |
| Complication | Prevalence (median %) |
| Cataract | 17 |
| Infection | 11 |
| Nephrocalcinosis/nephrolithiasis | 15 |
| Renal insufficiency | 12 |
| Seizures | 11 |
| Depression | 9 |
| Ischemic heart disease | 7 |
| Arrhythmias | 7 |
Most common complications of chronic hypoparathyroidism reported in the literature (GRADEd recommendations)
| Complication | Prevalence (median %) |
| Cataract | 17 |
| Infection | 11 |
| Nephrocalcinosis/nephrolithiasis | 15 |
| Renal insufficiency | 12 |
| Seizures | 11 |
| Depression | 9 |
| Ischemic heart disease | 7 |
| Arrhythmias | 7 |
| Complication | Prevalence (median %) |
| Cataract | 17 |
| Infection | 11 |
| Nephrocalcinosis/nephrolithiasis | 15 |
| Renal insufficiency | 12 |
| Seizures | 11 |
| Depression | 9 |
| Ischemic heart disease | 7 |
| Arrhythmias | 7 |
What is the optimal monitoring strategy for chronic HypoPT? (systematic current practice survey)
Panel members complete a baseline laboratory profile, including calcium adjusted for albumin or ionized calcium, serum magnesium, phosphorus, creatinine and 25(OH)D, as well as 24‐hour urine for creatinine and calcium.(85) Stable patients are monitored with repeat laboratory profile every 6–12 months. Unstable patients are followed more closely to ensure that serum calcium does not fluctuate widely and to avoid the symptoms and the long‐term complications of HypoPT. Evaluation for the presence of nephrocalcinosis or nephrolithiasis can be performed by either an ultrasound or CT of the kidneys.(82,85)
Panel members refer patients for slit‐lamp examination searching for ocular complications in patients who are experiencing visual symptoms.
The panel proposes the following:
Evaluate and monitor the laboratory profile as described previously.
Monitor serum calcium (ionized or albumin‐adjusted) within several days of a significant change in medical treatment.
Complete a baseline assessment for the presence of nephrocalcinosis or nephrolithiasis with renal imaging.
Management
Conventional therapy consists of oral calcium and active vitamin D. In patients with low PTH levels following total thyroidectomy (<10 pg/mL (1.05 pmol/L), medical therapy is advised with 2–3 g of elemental calcium daily and 0.5–1.5 μg calcitriol/day.(13,86‐89) Approximately 70%–80% of individuals with postoperative parathyroid failure will recover within a month following thyroidectomy,(13,90) and medical therapy can be gradually withdrawn with close monitoring(13) (Table 4).
| Medication | Dose | Comments/half‐life |
| Calcium carbonate or calcium citrate | Ranges from 500–3000 mg three times daily preferably with meals to enhance phosphate binding effects | Calcium citrate preferred in presence of Proton Pump Inhibitor (PPI) use |
| Vitamin D3 (cholecalciferol) | 1000 IU/day to 100,000 IU/day based on 25‐hydroxy vitamin D level | 4–6 hours plasma half‐life |
| Vitamin D2 (ergocalciferol) | 50,000 IU weekly to daily based on 25‐hydroxyvitamin D levels | 4–6 hours plasma half‐life |
| Calcitriol | 0.25–3 μg /day total dose administered in divided doses | 5–8 hours plasma half‐life |
| Alfacalcidol | 0.5–6 μg/day | 3–6 hours plasma half‐life |
| Thiazide diuretics | 25–100 mg/day | 6–12 hours plasma half‐life |
| Medication | Dose | Comments/half‐life |
| Calcium carbonate or calcium citrate | Ranges from 500–3000 mg three times daily preferably with meals to enhance phosphate binding effects | Calcium citrate preferred in presence of Proton Pump Inhibitor (PPI) use |
| Vitamin D3 (cholecalciferol) | 1000 IU/day to 100,000 IU/day based on 25‐hydroxy vitamin D level | 4–6 hours plasma half‐life |
| Vitamin D2 (ergocalciferol) | 50,000 IU weekly to daily based on 25‐hydroxyvitamin D levels | 4–6 hours plasma half‐life |
| Calcitriol | 0.25–3 μg /day total dose administered in divided doses | 5–8 hours plasma half‐life |
| Alfacalcidol | 0.5–6 μg/day | 3–6 hours plasma half‐life |
| Thiazide diuretics | 25–100 mg/day | 6–12 hours plasma half‐life |
| Medication | Dose | Comments/half‐life |
| Calcium carbonate or calcium citrate | Ranges from 500–3000 mg three times daily preferably with meals to enhance phosphate binding effects | Calcium citrate preferred in presence of Proton Pump Inhibitor (PPI) use |
| Vitamin D3 (cholecalciferol) | 1000 IU/day to 100,000 IU/day based on 25‐hydroxy vitamin D level | 4–6 hours plasma half‐life |
| Vitamin D2 (ergocalciferol) | 50,000 IU weekly to daily based on 25‐hydroxyvitamin D levels | 4–6 hours plasma half‐life |
| Calcitriol | 0.25–3 μg /day total dose administered in divided doses | 5–8 hours plasma half‐life |
| Alfacalcidol | 0.5–6 μg/day | 3–6 hours plasma half‐life |
| Thiazide diuretics | 25–100 mg/day | 6–12 hours plasma half‐life |
| Medication | Dose | Comments/half‐life |
| Calcium carbonate or calcium citrate | Ranges from 500–3000 mg three times daily preferably with meals to enhance phosphate binding effects | Calcium citrate preferred in presence of Proton Pump Inhibitor (PPI) use |
| Vitamin D3 (cholecalciferol) | 1000 IU/day to 100,000 IU/day based on 25‐hydroxy vitamin D level | 4–6 hours plasma half‐life |
| Vitamin D2 (ergocalciferol) | 50,000 IU weekly to daily based on 25‐hydroxyvitamin D levels | 4–6 hours plasma half‐life |
| Calcitriol | 0.25–3 μg /day total dose administered in divided doses | 5–8 hours plasma half‐life |
| Alfacalcidol | 0.5–6 μg/day | 3–6 hours plasma half‐life |
| Thiazide diuretics | 25–100 mg/day | 6–12 hours plasma half‐life |
Cholecalciferol or ergocalciferol is also often required to maintain the 25(OH)D level within the normal range. Recommendations for management are derived from case series, consensus statements, guidelines, and standards of care.(14,25,91‐93)
Thiazide diuretics can be utilized to lower urine calcium losses as they enhance distal tubular renal calcium reabsorption when paired with low salt intake.(14) Potential adverse effects include hypokalemia, hypomagnesemia, and hyponatremia. Treatment with thiazides requires monitoring of electrolytes. Postural hypotension may limit its use, and thiazide diuretics are not advised in the presence of adrenal insufficiency. Thiazide diuretics should also be used carefully in the presence of autosomal dominant hypocalcemia as the urinary magnesium losses are further enhanced with thiazide diuretics(14) (Table 4).
Emergency management of severe acute hypocalcemia
Emergency management is advised in the presence of cardiac, respiratory, or significant neurologic symptoms of hypocalcemia or if the albumin‐adjusted calcium <7.0 mg/dL (1.75 mmol/L). Intravenous (iv) calcium bolus administration is given as 90–180 mg elemental calcium over 10–20 minutes and requires cardiac monitoring. This is followed by an iv calcium infusion and initiation of oral therapy with calcium and calcitriol.(14,25) Typically, the iv calcium bolus is followed by an iv calcium infusion. This may be prepared with 10 ampules (900 mg elemental calcium) in 1 L of 5% dextrose water or normal saline and initiate infusion at 50 mL/hour and titrate to serum calcium. The goal is to elevate serum calcium to just below the normal reference range. An elemental calcium iv dose of 15 mg/kg over the course of 4–6 hours is expected to elevate serum calcium by approximately 0.5–0.75 mmol/L.(25) Active vitamin D metabolites are also initiated with calcitriol orally (0.25 μg twice/day to 0.5 μg twice/day) or alfacalcidol, which is less potent than calcitriol.(14)
PTH replacement
Clinical trials of human PTH (1–34) in HypoPT have demonstrated the beneficial effects of PTH replacement therapy. Synthetic PTH (1–34) was effective in increasing serum calcium, lowering urine calcium excretion and increasing phosphate excretion.(94,95) Twice daily doses were of value because PTH (1–34) has a short half‐life of 1 hour and resulted in improved maintenance of eucalcemia over 24 hours with a lower total daily dose requirement in comparison to once daily regimens.(96,97) Administration of PTH (1–34) by continuous subcutaneous (sc) infusion pump in comparison to twice daily injections resulted in the normalization of serum calcium with less fluctuation in serum calcium, phosphorus, and magnesium and reduced urine calcium. Daily PTH (1–34) requirements were also lower when administered by pump compared to twice daily sc PTH (1–34) injections.(98,99)
The full‐length molecule, rhPTH (1–84), has been evaluated. The half‐life of sc rhPTH (1–84) is longer at 3 hours and can be administered as a once daily dose. rhPTH (1–84) is well tolerated, reduces the need for calcium and calcitriol, reduces serum phosphorus, and in long‐term studies lowered urinary calcium excretion. rhPTH (1–84) and PTH (1–34) have been associated with marked initial rises in bone turnover markers (BTMs). After reaching a plateau, BTMs decline but are maintained at a new steady state, higher than pretreatment values and well within the normal reference range.(98‐100) BMD appears to be stable at the hip and spine with decreases observed at the radial site.(82)
The REPLACE study was a blinded placebo‐controlled Phase 3 study conducted in 134 patients randomized to rhPTH (1–84) or placebo. In this study, 53% of patients receiving rhPTH (1–84) met the primary endpoint (≥50% reduction in oral calcium and calcitriol doses while maintaining normal serum calcium) in comparison to placebo, in whom only 2% achieved this endpoint.(101) Reductions in urine calcium were observed in both the rhPTH (1–84) and placebo arms and the difference was not statistically significant. Reductions in the dose of calcium and active vitamin D were also noted in other studies using rhPTH (1–84).(102,103)
In both short‐term and long‐term studies, renal function was stable with the use of rhPTH (1–84).(101‐104) Renal calcifications were not eliminated, and nephrolithiasis was still noted.(102,104)
Hypercalcemia was observed in rhPTH (1–84) studies, with 18% incidence reported in the REPLACE study.(101) In the Aarhus study, rhPTH (1–84) was given at a fixed dose of 100 μg/day initiated in addition to existing conventional therapy, and a higher incidence of hypercalcemia was observed, as expected, at 34%.(103) In the Columbia University studies, hypercalcemia was observed with rhPTH in 30% of the patients.(102,105)
Hypocalcemia in patients receiving rhPTH was also noted in the randomized control trials (RCTs).(82) TransCon PTH (TC PTH) is a prodrug consisting of PTH (1–34) transiently conjugated to polyethylene glycol carrier molecule providing stable PTH levels.(91,94,95) The carrier blocks the parent drug from binding to the PTH receptor and decreases renal clearance and enzymatic degradation.(106) Following sc injection with exposure to physiologic pH and temperature, the linker is cleaved, releasing PTH (1–34) in a controlled manner.(106) In a Phase 1 study, the effective half‐life of TC PTH was approximately 60 hours.(107,108) In a 4‐week blinded Phase 2 trial, TC PTH was compared to conventional therapy in 59 adults with chronic hypoPT; this was followed by a 26‐week open‐label extension period. TransCon PTH enabled cessation of active vitamin D with reductions in calcium supplements (≤500 mg/day) in 91% of subjects while achieving normal sCa, sP, uCa, and calcium phosphate product and demonstrating improved health‐related QoL. TC PTH was well tolerated with no adverse events of hypocalcemia or hypercalcemia requiring a visit to hospital, ER or urgent care (96).
A Phase 3 double‐blind, placebo‐controlled study with TransCon PTH 18 μg daily versus placebo recently evaluated safety and efficacy in 84 patients with chronic HypoPT. At 26 weeks, cessation of active vitamin D was possible in 93% of participants while maintaining normal serum calcium. Improvements in QoL as evaluated by the HPES and the SF36 scale were observed. Statistically significant reductions in 24‐hour urine calcium were also observed in comparison to placebo. Adverse events were mild or moderate, and no study drug‐related withdrawals occurred. TransCon PTH was safe and effective and well tolerated over 26 weeks.(109) These results support TransCon PTH as a potential hormone‐replacement therapy for adults with HypoPT.(106) Open‐label trials have reported improvements in QoL with both rhPTH (1–84)(72,73,110) and PTH (1–34).(111,112) PTH (1–84) replacement therapy was approved by the US Food and Drug Administration (FDA) in 2015 and the European Medicines Agency (EMA) for the management of HypoPT.
In 2019, the FDA recalled rhPTH (1–84) due to the possibility of the presence of rubber particles originating from the cartridge septum. A similar recall has not taken place in Europe.
PTH therapy in comparison to conventional therapy for managing chronic HypoPT (systematic review)
A systematic review and meta‐analysis of randomized trials from inception to May 2022 evaluated the benefits and harms of PTH therapy in comparison to conventional therapy in managing patients with chronic HypoPT.(116) Seven studies met the eligibility criteria.(74,94,101,103,106,113‐115,117,118) These studies were relatively small and of short duration and did not consistently report on the eight most common complications of HypoPT identified earlier in the systematic review.(82) These studies confirmed that PTH therapy permitted reductions in the dose of calcium and active vitamin D metabolites by 50% or more in a large number of patients. Statistically significant reductions in serum phosphorus were observed with PTH therapy in comparison to conventional therapy. Reductions in serum phosphorus may be of value in lowering the incidence of ectopic calcification and require further study in individuals with HypoPT. Small but statistically significant improvements in physical health related QoL were observed in the meta‐analysis evaluating PTH therapy in comparison to conventional therapy.(116) A significant reduction in pill burden was also noted in this meta‐analysis with PTH therapy(116) PTH therapy may not result in important adverse effects, but the quality of evidence is low(116) (Table 5).
Trials included in systematic review of PTH therapy in chronic hypoparathyroidism
| Reference study | Treatment | Control | Study duration | Conclusion |
| REPLACE(101,113,117) | 50–100 μg/day rhPTH (1–84); active vitamin D; calcium | Placebo; active vitamin D; calcium | 7 months | Eucalcemia maintained Calcium‐phosphate product and Serum phosphorus declined with PTH therapy Urine calcium declined in both PTH and control groups |
| Sikjaer, 2011–2014(74,103,114,115) | Recombinant human PTH (1–84) 100 μg/day; calcium, alfacalcidol/calcitriol/ergocalciferol | Placebo; calcium and alfacalcidol/calcitriol/ergocalciferol | 6 months | Eucalcemia maintained, Phosphorus declined with PTH therapy, no change in calcium phosphate product, and no change in urine calcium with PTH therapy |
| Winer, 2003(94) | PTH (1–34) 0.5 μg/kg/dose twice daily; elemental calcium 1000 mg/day | Calcitriol and calcium (0.91 ± 0.2 μg); calcium (1000 mg/day) | 36 months | Eucalcemia maintained, urine calcium declined with PTH therapy |
| Winer, 1996(95) | PTH (1–34) 0.5–3 μg/kg per day once daily; dietary elemental calcium 1–2 g/day | Calcitriol 0.5–6 μg/day; dietary elemental calcium 1–2 g/day; 1000 mg/day of calcium carbonate | 2.5 months | Eucalcemia maintained, Urine calcium declined with PTH therapy |
| Winer, 2010(118) | PTH (1–34) 0.4 μg/kg/dose twice daily; dietary elemental calcium 1–2 g/day; magnesium supplement | Twice‐daily calcitriol (initially 0.25 μg/dose); calcium (1200 mg/day) and cholecalciferol (800 IU/d); magnesium supplement | 36 months | Eucalcemia maintained, no change in urine calcium noted with PTH therapy |
| Khan, 2021(106) | TransCon PTH 15–21 μg per day; oral elemental calcium 1550 mg/day; active vitamin D | Placebo; oral elemental calcium 1200 mg/day; active vitamin D | 1 month | Eucalcemia maintained, Fractional excretion of calcium declined with PTH therapy |
| Khan, 2022(109) | TransCon PTH 18ug daily; calcium and active vitamin D | Placebo; oral calcium; active vitamin D | 6.5 months | Eucalcemia maintained, 24 hr urine calcium declined, QoL improved, pill burden declined |
| Reference study | Treatment | Control | Study duration | Conclusion |
| REPLACE(101,113,117) | 50–100 μg/day rhPTH (1–84); active vitamin D; calcium | Placebo; active vitamin D; calcium | 7 months | Eucalcemia maintained Calcium‐phosphate product and Serum phosphorus declined with PTH therapy Urine calcium declined in both PTH and control groups |
| Sikjaer, 2011–2014(74,103,114,115) | Recombinant human PTH (1–84) 100 μg/day; calcium, alfacalcidol/calcitriol/ergocalciferol | Placebo; calcium and alfacalcidol/calcitriol/ergocalciferol | 6 months | Eucalcemia maintained, Phosphorus declined with PTH therapy, no change in calcium phosphate product, and no change in urine calcium with PTH therapy |
| Winer, 2003(94) | PTH (1–34) 0.5 μg/kg/dose twice daily; elemental calcium 1000 mg/day | Calcitriol and calcium (0.91 ± 0.2 μg); calcium (1000 mg/day) | 36 months | Eucalcemia maintained, urine calcium declined with PTH therapy |
| Winer, 1996(95) | PTH (1–34) 0.5–3 μg/kg per day once daily; dietary elemental calcium 1–2 g/day | Calcitriol 0.5–6 μg/day; dietary elemental calcium 1–2 g/day; 1000 mg/day of calcium carbonate | 2.5 months | Eucalcemia maintained, Urine calcium declined with PTH therapy |
| Winer, 2010(118) | PTH (1–34) 0.4 μg/kg/dose twice daily; dietary elemental calcium 1–2 g/day; magnesium supplement | Twice‐daily calcitriol (initially 0.25 μg/dose); calcium (1200 mg/day) and cholecalciferol (800 IU/d); magnesium supplement | 36 months | Eucalcemia maintained, no change in urine calcium noted with PTH therapy |
| Khan, 2021(106) | TransCon PTH 15–21 μg per day; oral elemental calcium 1550 mg/day; active vitamin D | Placebo; oral elemental calcium 1200 mg/day; active vitamin D | 1 month | Eucalcemia maintained, Fractional excretion of calcium declined with PTH therapy |
| Khan, 2022(109) | TransCon PTH 18ug daily; calcium and active vitamin D | Placebo; oral calcium; active vitamin D | 6.5 months | Eucalcemia maintained, 24 hr urine calcium declined, QoL improved, pill burden declined |
Trials included in systematic review of PTH therapy in chronic hypoparathyroidism
| Reference study | Treatment | Control | Study duration | Conclusion |
| REPLACE(101,113,117) | 50–100 μg/day rhPTH (1–84); active vitamin D; calcium | Placebo; active vitamin D; calcium | 7 months | Eucalcemia maintained Calcium‐phosphate product and Serum phosphorus declined with PTH therapy Urine calcium declined in both PTH and control groups |
| Sikjaer, 2011–2014(74,103,114,115) | Recombinant human PTH (1–84) 100 μg/day; calcium, alfacalcidol/calcitriol/ergocalciferol | Placebo; calcium and alfacalcidol/calcitriol/ergocalciferol | 6 months | Eucalcemia maintained, Phosphorus declined with PTH therapy, no change in calcium phosphate product, and no change in urine calcium with PTH therapy |
| Winer, 2003(94) | PTH (1–34) 0.5 μg/kg/dose twice daily; elemental calcium 1000 mg/day | Calcitriol and calcium (0.91 ± 0.2 μg); calcium (1000 mg/day) | 36 months | Eucalcemia maintained, urine calcium declined with PTH therapy |
| Winer, 1996(95) | PTH (1–34) 0.5–3 μg/kg per day once daily; dietary elemental calcium 1–2 g/day | Calcitriol 0.5–6 μg/day; dietary elemental calcium 1–2 g/day; 1000 mg/day of calcium carbonate | 2.5 months | Eucalcemia maintained, Urine calcium declined with PTH therapy |
| Winer, 2010(118) | PTH (1–34) 0.4 μg/kg/dose twice daily; dietary elemental calcium 1–2 g/day; magnesium supplement | Twice‐daily calcitriol (initially 0.25 μg/dose); calcium (1200 mg/day) and cholecalciferol (800 IU/d); magnesium supplement | 36 months | Eucalcemia maintained, no change in urine calcium noted with PTH therapy |
| Khan, 2021(106) | TransCon PTH 15–21 μg per day; oral elemental calcium 1550 mg/day; active vitamin D | Placebo; oral elemental calcium 1200 mg/day; active vitamin D | 1 month | Eucalcemia maintained, Fractional excretion of calcium declined with PTH therapy |
| Khan, 2022(109) | TransCon PTH 18ug daily; calcium and active vitamin D | Placebo; oral calcium; active vitamin D | 6.5 months | Eucalcemia maintained, 24 hr urine calcium declined, QoL improved, pill burden declined |
| Reference study | Treatment | Control | Study duration | Conclusion |
| REPLACE(101,113,117) | 50–100 μg/day rhPTH (1–84); active vitamin D; calcium | Placebo; active vitamin D; calcium | 7 months | Eucalcemia maintained Calcium‐phosphate product and Serum phosphorus declined with PTH therapy Urine calcium declined in both PTH and control groups |
| Sikjaer, 2011–2014(74,103,114,115) | Recombinant human PTH (1–84) 100 μg/day; calcium, alfacalcidol/calcitriol/ergocalciferol | Placebo; calcium and alfacalcidol/calcitriol/ergocalciferol | 6 months | Eucalcemia maintained, Phosphorus declined with PTH therapy, no change in calcium phosphate product, and no change in urine calcium with PTH therapy |
| Winer, 2003(94) | PTH (1–34) 0.5 μg/kg/dose twice daily; elemental calcium 1000 mg/day | Calcitriol and calcium (0.91 ± 0.2 μg); calcium (1000 mg/day) | 36 months | Eucalcemia maintained, urine calcium declined with PTH therapy |
| Winer, 1996(95) | PTH (1–34) 0.5–3 μg/kg per day once daily; dietary elemental calcium 1–2 g/day | Calcitriol 0.5–6 μg/day; dietary elemental calcium 1–2 g/day; 1000 mg/day of calcium carbonate | 2.5 months | Eucalcemia maintained, Urine calcium declined with PTH therapy |
| Winer, 2010(118) | PTH (1–34) 0.4 μg/kg/dose twice daily; dietary elemental calcium 1–2 g/day; magnesium supplement | Twice‐daily calcitriol (initially 0.25 μg/dose); calcium (1200 mg/day) and cholecalciferol (800 IU/d); magnesium supplement | 36 months | Eucalcemia maintained, no change in urine calcium noted with PTH therapy |
| Khan, 2021(106) | TransCon PTH 15–21 μg per day; oral elemental calcium 1550 mg/day; active vitamin D | Placebo; oral elemental calcium 1200 mg/day; active vitamin D | 1 month | Eucalcemia maintained, Fractional excretion of calcium declined with PTH therapy |
| Khan, 2022(109) | TransCon PTH 18ug daily; calcium and active vitamin D | Placebo; oral calcium; active vitamin D | 6.5 months | Eucalcemia maintained, 24 hr urine calcium declined, QoL improved, pill burden declined |
In these randomized trials, PTH therapy was also associated with increases in the episodes of hypercalcemia in comparison to conventional therapy. In one of the seven studies, PTH therapy was added onto active vitamin D and hypercalcemia was expected. Gradual downward titration of the dose of the active vitamin D with cessation of active vitamin D occurred, as planned.(74,103,114,115) PTH therapy was well tolerated with very infrequent serious adverse effects. The quality of the evidence was, however, low.
PTH therapy can be considered in individuals who are inadequately controlled with conventional therapy. Individuals with fluctuating serum calcium requiring hospitalization for hypocalcemia or hypercalcemia, or those who have hyperphosphatemia, renal insufficiency, hypercalciuria, nephrocalcinosis, or nephrolithiasis may benefit from PTH therapy. Individuals with poor compliance, malabsorption, or gastrointestinal side effects from large doses of calcium, making it difficult to adhere to large frequent doses of calcium and active vitamin D, may also benefit from PTH therapy.
PTH therapy may also be appropriate in individuals with osteoporosis who require pharmacological intervention because antiresorptive therapy (i.e., denosumab) may result in significant hypocalcemia.
PTH therapy in the form of rhPTH (1–84) is initiated at 50 μg daily, and the dose is adjusted by 25 μg every 4 weeks up to a maximum dose of 100 μg daily. PTH (1–34) has not yet been approved for use in HypoPT.
How are patients with HypoPT managed? (GRADEd recommendations)
In patients with chronic HypoPT, the panel suggests conventional therapy as first‐line therapy rather than administration of parathyroid hormone. (weak recommendation, low‐quality evidence).
Comment: When conventional therapy is deemed unsatisfactory, the panel considers the use of parathyroid hormone.
Pregnancy and Lactation
Pregnancy is associated with changes in calcium‐regulating hormones and in calcium homeostasis. These changes may result in altered requirements for calcium and active vitamin D during pregnancy.(119) Reductions in the serum albumin in association with volume expansion result in reductions in measured total calcium and necessitate measurement of calcium adjusted for albumin or ionized calcium.(120‐122) Phosphorus and 25(OH)D remain unchanged during pregnancy. Levels of endogenous 1,25(OH)2D increase by two‐ to threefold as early as the first trimester, resulting in enhanced absorption of calcium from the bowel and increasing renal filtered calcium load, and urine calcium (Fig. 1).
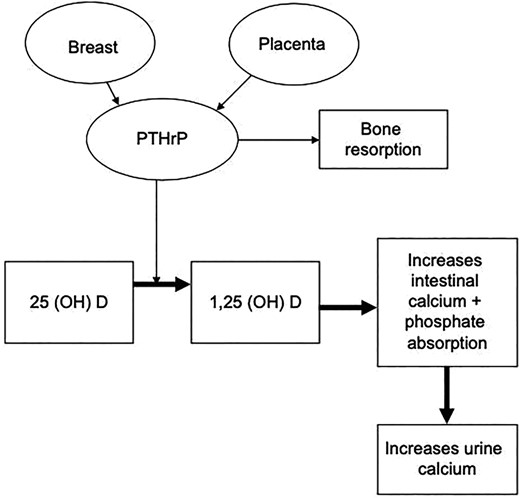
Role of PTH‐related peptide during pregnancy (reproduced with permission from Khan et al. EJE 2019).(119)
PTH‐related peptide (PTHrP) is produced by the placenta and breast tissue and begins to rise from the third to the thirteenth week of gestation and increases threefold by term(119) (Fig. 1). The significant rise in PTHrP may upregulate calcitriol.(123) Estradiol levels also rise by 100‐fold during pregnancy and stimulate Cyp27b1, further enhancing the formation of 1,25(OH)2D.(119) In women with residual parathyroid function, PTH is suppressed during pregnancy and subsequently rises into the midnormal reference range by the third trimester.(120‐122)
Due to increased endogenous production of 1,25(OH)2D and PTHrP during pregnancy, the requirements for calcium and active vitamin D analogues may decline. However, this may be offset by the increased requirements for calcium by the developing fetal skeleton as well as the increased maternal urinary calcium losses. In the presence of inadequate dietary calcium intake, active vitamin D requirements may increase during pregnancy. Thus, it is necessary to closely monitor serum calcium adjusted for albumin during pregnancy as the requirements for calcium and active vitamin D may increase, remain stable, or decline.(82) In a recent case series, the dose of the calcium and active vitamin D required adjustments by more than 20% in more than 50% of patients during pregnancy in order to maintain eucalcemia.(124)
Hypocalcemia during pregnancy is associated with uterine contractions and an increased risk of preterm labor or miscarriage.(125) Maternal hypocalcemia is also associated with stimulation of the fetal parathyroid glands and the development of secondary hyperparathyroidism in the fetus.(126‐129) Fetal secondary hyperparathyroidism can be associated with demineralization of the fetal skeleton and fractures in utero.(119)
Maternal hypercalcemia can suppress the development of the fetal parathyroid glands and result in transient hypocalcemia in the neonate.(130) Following birth, the neonate should be evaluated and closely followed to ensure that serum calcium is normal.
Following delivery, 1,25(OH)2D levels normalize postpartum, and serum calcium adjusted for albumin should be monitored closely.
The lactating breast produces PTHrP, and during lactation PTHrP levels rise and increase maternal bone resorption and enhance maternal renal calcium reabsorption.(131,132) The elevations in PTHrP seen during lactation may result in lower requirements for calcium and active vitamin D during lactation. If lactation is stopped, the requirements for calcium and active vitamin D may rise as PTHrP levels decline, and close follow‐up, particularly during the weaning period, is required to ensure that hypocalcemia does not develop in the mother.
Calcium, vitamin D, and active vitamin D analogues can be safely administered during pregnancy.(82) Thiazide diuretics and PTH therapy are not recommended for use during pregnancy due to lack of safety data and are considered FDA pregnancy risk category B and C respectively.(133)
During pregnancy and lactation, it is advised to maintain serum calcium adjusted for albumin in the low to midnormal reference range. Close monitoring with lab tests every 3–4 weeks is advised to ensure that hypocalcemia and hypercalcemia are avoided in the mother to optimize maternal and fetal outcomes. Changes in the dose of the calcitriol should be followed by a repeat serum calcium within 2–3 days.(119) A coordinated approach to care is advised, with close communication among the treating endocrinologist, obstetrician, and pediatrician.
What are additional recommendations for patients with HypoPT during pregnancy and lactation? (un‐GRADEd recommendation)
Closely monitor serum calcium (albumin‐adjusted or ionized) every 3–4 weeks during pregnancy and lactation, with increased frequency in the months preceding and following parturition as well as in the presence of symptoms of hypercalcemia or hypocalcemia.
Comment: The use of albumin‐adjusted or ionized calcium is essential during pregnancy as total calcium can be misleading. Close coordination between the obstetrician and pediatrician is advised in order to optimize pregnancy outcomes. Avoid using thiazide diuretics and PTH or analogues during pregnancy.
Approach to the Pediatric Patient with HypoPT
Children with HypoPT require careful evaluation to determine its underlying cause. A high prevalence of renal and basal ganglia calcification has been observed with conventional therapy.(82,134,135) Treatment with calcium and active vitamin D supplements are recommended as first‐line therapy. If these options are not effective or practical, then PTH therapy can be considered.(82) PTH (1–34) has been associated with improved metabolic control, and lower doses are required if administered as a continuous sc infusion.(100,136‐138) Clinical trials examining rhPTH (1–84) excluded children.
Emerging Therapies
New therapies are being developed targeting the PTH receptor (PTH1R) as well as the calcium sensing receptor, as summarized below.
1. Transcon PTH (1–34), discussed earlier, is in Phase 3 long‐term extension clinical trials at this time.
2. Long‐acting PTH analogue (LA‐PTH) is a hybrid molecule with both PTH and PTHrP homology and has been effective in increasing serum calcium and reducing serum phosphorus in animal studies.(139,140) Preliminary results from a Phase 1 trial with this drug (AZP‐3601) showed dose‐dependent increases in albumin‐adjusted serum calcium.(141)
3. PTHR1 agonist, an orally available agonist of PTHR1 (PCO371), was being evaluated in HypoPT patients in a Phase 1 study. The study was terminated due to increases in liver enzymes (AAK, personal communication with Chugai).
4. Oral PTH molecule with hPTH (1–34) complexed with excipients to facilitate small bowel absorption was evaluated in a 16‐week open‐label pilot study.(142) Early data indicate that it can maintain serum calcium and phosphorus in the target range.
5. Calcilytics are antagonists of the calcium‐sensing receptor and appear to be an attractive treatment option for individuals with autosomal dominant hypocalcemia type 1 (ADH1) due to gain‐of‐function mutations in the calcium‐sensing receptor gene. The calcilytic NPSP795 was shown to result in a dose‐dependent increase in PTH in patients with ADH1.(143) The calcilytic encaleret was recently shown to normalize serum calcium, phosphorus, and magnesium as well as urine calcium in an early Phase 2 study.(144)
Knowledge Gaps and Future Research Directions
Studies of the epidemiology of HypoPT published to date show that patients with nonsurgical HypoPT appear to have higher risks of complications than patients with postsurgical HypoPT. Future studies should address whether the differences seen are due to the longer duration of disease in those with nonsurgical disease or other factors that differ between these types of patients.
Future studies on the complications of HypoPT, including cardiovascular disease, cataracts, BGCs, infections, malignancy, and neuropsychiatric disorders, are needed.
Large multicenter prospective studies in pregnant patients with HypoPT are needed to better understand the impact of pregnancy on calcium homeostasis in HypoPT as well as requirements of conventional therapy and maternal and fetal outcomes.
Further studies are needed to establish the financial burden of HypoPT, given that healthcare resource utilization appears to be increased in these patients.
The effects of HypoPT on skeletal health and bone strength need to be evaluated.
Validated assays are needed to diagnose non‐APECED‐related autoimmune HypoPT, including the detection of antibodies against CaSR.
The impact of genetic testing on management/outcomes needs to be evaluated.
The best genetic testing modality needs to be determined.
Postsurgical diagnosis requires RCTs to test the utility of early predictors of permanent HypoPT.
Future trials should include patients with genetic disorders to clarify best management.
Prospective studies are needed in HypoPT in pregnancy to evaluate optimal management strategies.
Prospective controlled studies are needed to evaluate PTH versus conventional therapy on patient‐important outcomes.
Prospective controlled studies on the determinants of nephrocalcinosis and renal insufficiency in HypoPT are needed.
A controlled study should be conducted on phosphate restriction in children with severe hyperphosphatemia: Does dietary phosphate restriction do more harm than good?
Prospective long‐term large trials are needed to evaluate the risks and long‐term complications of chronic HypoPT and impact on QoL.
Summary
HypoPT is a rare disease associated with significant morbidity, poor QoL, and increased healthcare utilization. We have provided updated recommendations regarding the diagnosis and management of HypoPT. Careful evaluation to determine the underlying etiology is essential for optimal management. Early identification of HypoPT with initiation of effective conventional therapy with calcium and active vitamin D metabolites is helpful in improving symptoms. However, QoL remains poor in individuals receiving conventional therapy with significant complications. Unfortunately, conventional therapy is also associated with wide fluctuations in serum calcium. In these individuals, management may improve with PTH therapy. Pregnant and lactating women require close follow‐up because their requirements for calcium and active vitamin D may change due to changes in calcium homeostasis during pregnancy and lactation. To achieve optimal maternal and fetal outcomes, it is important to monitor patients closely and work effectively in a multidisciplinary team with an obstetrician and pediatrician. Emerging therapies show great promise in terms of refining and further improving the management of HypoPT in the near future.
Disclosures
AAK: Grants and/or Speaker for Alexion, Amgen, Amolyt, Ascendis, Chugai, Radius, Takeda, and Ultragenyx; consultant for Alexion, Amgen, Amolyt, Ascendis Takeda, and Ultragenyx
JPB: Consultant for Amgen, Radius, Ascendis, Calcilytix, Takeda, Amolyt, Rani Therapeutics, MBX, Novo‐Nordisk, and Ipsen
MLB has received honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB; grants or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, and UCB; consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB.
BLC: Consultant for Takeda/Shire, Amolyt Pharma, and Calcilytix; grants from Takeda/Shire and Ascendis.
LR: Speaker for Amgen, Lilly, Takeda, Alexion, Kyowa Kirin, Amolyt, Ascendis, and Ultragenyx; consultant for Amgen, Lilly, Takeda, Alexion, Kyowa Kirin, Amolyt, Ascendis, and Ultragenyx; grants from Takeda and Kyowa Kirin.
JTP: Consultant for Radius Pharma.
MM: Consultant for Takeda, Amolyt, and Chugai; grants from Takeda and Chugai.
We acknowledge unrestricted financial support from Amolyt, Ascendis, Calcilytix, and Takeda. They had no input into the planning or design of the project, the conduct of the reviews, evaluation of the data, or the writing or review of the manuscript, its contents, conclusions, or recommendations contained herein.
Acknowledgments
Author Roles:
Conceptualization, visualization: AAK, JPB, MLB, BLC, NJG, JLP, LR, DMS, JTP, GHG, MM. Data curation, formal analysis, investigation, methodology, validation, software: AAK, JPB, MLB, BLC, NJG, JLP, LR, DMS, JTP, GHG, MM. Project administration, funding acquisition, resources: AAK, JPB, MLB, BLC, JTP, MM. Supervision: AAK, JPB, MM. Writing—original draft: AAK. Writing—review and editing: AAK, JPB, MLB, BLC, NJG, JLP, LR, DMS, JTP, GHG, MM
In addition to the coauthors, the International Workshop on HypoPT was composed of the following individuals, whose major contributions are most appreciated and gratefully acknowledged: Dalal S. Ali, S Bjornsdottir, Luisella Cianferotti, Michael T Collins, Serge Cremers, Karel Dandurand, David Dempster, Seiji Fukumoto, Rachel Gafni, Ravinder Goswami, Francesca Guisti, Z Hassan‐Smith, Pascal Houiller, S Ing, E. Helen Kemp, Christian Koch, Michael A. Levine, DM Mitchell, Deborah Murphy, Iman M'Hiri, Jesse D. Pasternak, Nancy Perrier, Kelly Roszko, Mishaela Rubin, Robert Sanders, Jad Sfeir, Muhammed Shrayyef, Heide Siggelkow, T Sikjaer, Antonio Sitges‐Serra, Yu‐Kwang Donovan Tay, Rajesh Thakker, Gaia Tobacco, L Underbjerg, Stan Van Umm, Kelly Wentworth, Karen Winer, Weibo Xia, Liam Yao, and Caitlin T. Yeo. Following completion but prior to submission, we became aware of a publication by Bollerslev et al. on the European expert consensus on specific aspects of parathyroid disorders (European J Endocrinol, 12‐3‐2021).
Author Contributions
Aliya A. Khan: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing ‐ original draft; writing ‐ review and editing. John P. Bilezikian: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – review and editing. Maria Luisa Brandi: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – review and editing. Bart L. Clarke: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – review and editing. Neil Gittoes: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. Janice L Pasieka: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. Lars Rejnmark: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. Dolores Shoback: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. John T. Potts, Jr.: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – review and editing. Gordon Guyatt: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. Michael Mannstadt: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – review and editing.
Ethical Statement
These papers are retrospective reviews and did not require ethics committee approval.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4691.
Data Availability Statement
The data that support the findings in this study are openly available in PubMed, MEDLINE, EMBASE, and the Cochrane databases.
References
These guidelines have been endorsed by the following societies:
Academia Nacional de Medicina de Mexico
American Association of Clinical Endocrinology
American Association of Endocrine Surgeons
Asociación Argentina de Osteología y Metabolismo Mineral
Associação Brasileira de Avaliação Óssea e Osteometabolismo
Afghanistan Endocrine Society
Association for Multiple Endocrine Neoplasia Disorders
Australian and New Zealand Bone and Mineral Society
Armenian Osteoporosis Association
American Society for Bone and Mineral Research
Asociación Costarricense de Endocrinología
Bangladesh Endocrine Society
Brazilian Society of Endocrinology and Metabolism
Canadian Endocrine Update
Canadian Society of Endocrinology and Metabolism
Chilean Society of Endocrinology and Diabetes
Cyprus Endocrine Society
Dachverband Osteologie
Endocrine Chapter of the Academy of Medicine Singapore
Endocrine Society of India
Endocrinology and Diabetes Association of Mauritius
Endocrinology and Metabolism Research Institute
European Calcified Tissue Society
Federación Argentina de Sociedades de Endocrinología
FIRMO Foundation
Georgian Association of Skeletal Metabolism Diseases
German Society of Endocrinology
Hellenic Endocrine Society
Hellenic Society of Endocrine Surgeons
Hong Kong Society of Endocrinology, Metabolism and Reproduction
Hungarian Society for Endocrinology and Metabolism
HypoPara Support & Advocacy
HypoPARAthyroidism Association, Inc.
International Association of Endocrine Surgeons
Indonesian Society of Endocrinology
International Society of Endocrinology
Israel Endocrine Society
Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases
Italian Society of Endocrinology
Italian Society of Orthopedics, Medicine and Rare Skeleton Diseases
Japan Association of Endocrine Surgery
Japan Endocrine Society
Japanese Society for Bone and Mineral Research
Japanese Society for Pediatric Endocrinology
Jordanian Osteoporosis Society
Korean Endocrine Society
Kuwait Osteoporosis Society
Lebanese Society of Endocrinology Diabetes and Lipids
Parathyroid UK
Peruvian Diabetes Association
Peruvian Society of Endocrinology
Philippine Society of Endocrinology Diabetes and Metabolism
Qatar Osteoporosis Association
Romanian Society of Endocrinology
Russian Association of Endocrine Surgeons
Russian Association of Endocrinologists
Russian Association on Osteoporosis
Sociedad Argentina de Endocrinología
South Asian Federation of Endocrine Society
Sociedad Argentina de Osteoporosis
Saudi Osteoporosis Society
Sociedad Chilena de Osteología y Metabolismo Mineral
Sociedad Mexicana de Endocrinología Pediátrica
Sociedad Mexicana de Nutrición y Endocrinología
Society for Endocrinology
Society for Endocrinology and Metabolism of Turkey
WORLDMEN Int'l Conf Committee



